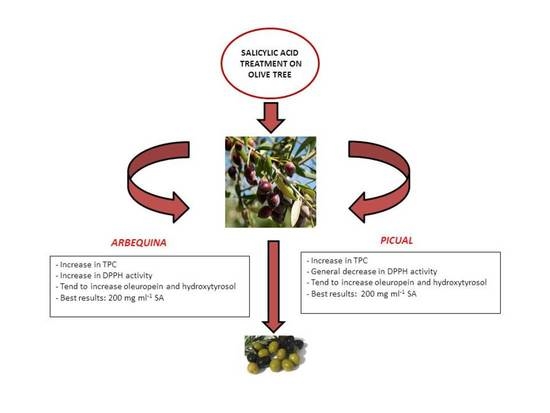
Enrichment of Olive Fruits in Antioxidant Content by Pre-Harvest Salicylic Acid Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Samples
2.2. Treatment of Olive Trees
2.3. Extraction
2.4. Analysis
2.4.1. Determination of TPC
2.4.2. Determination of Oleuropein and Hydroxytyrosol
2.4.3. 1,1-Diphenyl-2-Picrylhydrazyl FREE Radical (DPPH*) Scavenging Assay
2.5. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guasch-Ferré, M.; Hu, F.B.; Martínez-González, M.A.; Fitó, M.; Bulló, M.; Estruch, R.; Ros, E.; Corella, D.; Recondo, J.; Gómez-Gracia, E.; et al. Olive oil intake and risk of cardiovascular disease and mortality in the PREDIMED Study. BMC Med. 2014, 12, 78. [Google Scholar] [CrossRef] [PubMed]
- Owen, R.W.; Haubner, R.; Würtele, G.; Hull, W.E.; Spiegelhalder, B.; Bartsch, H. Olives and olive oil in cancer prevention. Eur. J. Cancer Prev. 2004, 13, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Wongwarawipat, T.; Papageorgiou, N.; Bertsias, D.; Siasos, G.; Tousoulis, D. Olive oil-related anti-inflammatory effects on aterosclerosis: Potential clinical implications. Endocr. Metab. Immune Dis. Drug Targets 2018, 18, 51–62. [Google Scholar]
- Kubo, A.; Lunde, S.C.; Kubo, I. Antimicrobial activity of the olive oil flavor compounds. J. Agric. Food Chem. 1995, 43, 1629–1633. [Google Scholar] [CrossRef]
- Teissedre, P.L.; Frankel, E.N.; Waterhouse, A.L.; Peleg, H.; Bruce, J. Inhibition of in vitro human LDL, oxidation by phenolic antioxidants from grapes and wines. J. Sci. Food Agric. 1996, 70, 55–61. [Google Scholar] [CrossRef]
- Schroder, H. Protective mechanism of the Mediterranean diet in obesity and type 2 diabetes. J. Nutr. Biochem. 2007, 18, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, G. Lipids and phenols in table olives. Eur. J. Lipid Sci. Technol. 2003, 105, 229–242. [Google Scholar] [CrossRef]
- Ben Othman, N.; Roblain, D.; Thonart, P.; Hamdi, M. Tunisian table olive phenolic compounds and their antioxidant capacity. J. Food Sci. 2008, 73, C235–C240. [Google Scholar] [CrossRef]
- Lalas, S.; Athanasiadis, V.; Gortzi, O.; Bounitsi, M.; Giovanoudis, I.; Tsaknis, J.; Bogiatzis, F. Enrichment of table olives with polyphenols extracted from olive leaves. Food Chem. 2011, 127, 1521–1525. [Google Scholar] [CrossRef]
- Ruiz-García, Y.; Gómez-Plaza, E. Elicitors: A tool for improving fruit phenolic content. Agriculture 2013, 3, 33–52. [Google Scholar] [CrossRef]
- Flores, G.; Blanch, G.P.; Ruiz del Castillo, M.L. Effect of postharvest methyl jasmonate treatment on fatty acid composition and phenolic acid content in olive fruits during storage. J. Sci. Food Agric. 2017, 97, 2767–2772. [Google Scholar] [CrossRef] [PubMed]
- Blanch, G.P.; Flores, G.; Gómez-Jiménez, M.C.; Ruiz del Castillo, M.L. Effect of the treatment of the olive tree (Olea europaea L.) on the phenolic content and antioxidant properties in olive fruits. J. Food Nutr. Res. 2018, 6, 49–55. [Google Scholar] [CrossRef]
- Blanch, G.P.; Flores, G.; Gómez-Jiménez, M.C.; Ruiz del Castillo, M.L. Abscisic acid sprayed on olive tree (Olea europaea L.) affects the phenolic composition of olive fruit cultivars. J. Agric. Sci. 2018, 10, 37–46. [Google Scholar] [CrossRef][Green Version]
- Flores, G.; Blanch, G.P.; Ruiz del Castillo, M.L. Abscisic acid treated olive seeds as a natural source of bioactive compounds. LWT-Food Sci. Technol. 2018, 90, 556–561. [Google Scholar] [CrossRef]
- Molinari, S. Salicylic acid as an elicitor of resistance to root-knot nematodes in tomato. Acta Hortic. 2008, 789, 119–126. [Google Scholar] [CrossRef]
- Durango, D.; Pulgarin, N.; Echeverri, F.; Escobar, G.; Quiñones, W. Effect of salicylic acid and structurally related compounds in the accumulation of phytoalexins in cotyledons of common bean (Phaseolus vulgaris L.) cultivars. Molecules 2013, 18, 10609–10628. [Google Scholar] [CrossRef]
- Gorni, P.H.; Pacheco, A.C. Growth promotion and elicitor activity of salicylic acid in Achillea millefolium L. Afr. J. Biotechnol. 2016, 15, 657–665. [Google Scholar]
- García-Pastor, M.E.; Zapata, P.J.; Castillo, S.; Martínez-Romero, D.; Guillén, F.; Valero, D.; Serrano, M. The effects of salicylic acid and its derivatives on increasing pomegranate fruit quality and bioactive compounds at harvest and during storage. Front. Plant Sci. 2020, 11, 668. [Google Scholar] [CrossRef]
- Brito, C.; Dinis, L.-T.; Meijón, M.; Ferreira, H.; Pinto, G.; Moutinho-Pereira, J.; Correira, C. Salicylic acid modulates olive tree physiological and growth responses to drought and rewatering events in a dose dependent manner. J. Plant Phys. 2018, 230, 21–32. [Google Scholar] [CrossRef]
- Vinha, A.F.; Ferreres, F.; Silva, B.M.; Valentao, P.; Gongalves, A.; Pereira, J.A.; Oliveria, M.B.; Seabre, R.M.; Andrade, P.B. Phenolic profiles of Portuguese olive fruits (Olea europaea L.): Influence of cultivar and geographical origin. Food Chem. 2005, 89, 561–568. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Smith, R.C.; Reeves, J.C.; Dage, R.C.; Schnettler, R.A. Antioxidant properties of 2-imidazolones and 2-imidazolthiones. Biochem. Pharm. J. 1987, 36, 1457–1460. [Google Scholar] [CrossRef]
- Mahmood, T.; Anwar, F.; Abbas, M.; Saari, N. Effect of maturity on phenolics (phenolic acids and flavonoids) profile of strawberry cultivars and mulberry species from Pakistan. Int. J. Mol. Sci. 2012, 13, 4591–4607. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Chen, F.; Wang, X.; Choi, J.H. Effect of methyl jasmonate on phenolics isothiocyanate and metabolic enzymes in radish sprout (Raphamus sativus L.). J. Agric. Food Chem. 2006, 54, 7263–7269. [Google Scholar] [CrossRef] [PubMed]
- Flores, G.; de la Peña Moreno, F.; Blanch, G.P.; Ruiz del Castillo, M.L. Phenylalanine ammonia-lyase, flavanone, 3β-hydroxylase and flavonol synthase enzyme activity by a new in vitro assay method in berry fruits. Food Chem. 2014, 153, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Demiray, S.; Pintado, M.E.; Castro, P.M.L. Evaluation of phenolic profiles and antioxidant activities of Turkish medicinal plants: Tilia argentea, Crataegi folium leaves and Polygonum bistorta roots. World Acad. Sci. Eng. Technol. 2009, 54, 312–317. [Google Scholar]
- Kanatt, S.R.; Chander, R.; Sharma, A. Antioxidant potential of mint (Mentha spicata L.) in radiation-processed lamb meat. Food Chem. 2007, 100, 451–458. [Google Scholar] [CrossRef]
- Umeno, A.; Takashima, M.; Murotomi, K.; Nakajima, Y. Radical-scavenging activity and antioxidative effects of olive leaf components oleuropein and hydroxytyrosol in comparison with homovanillic alcohol. J. Oleo Sci. 2015, 64, 793–800. [Google Scholar] [CrossRef]
- Pellegrini, N.; Visioli, E.; Buratti, S.; Brighenti, F. Direct analysis of total antioxidant activity of olive oil and studies on the influence of heating. J. Agric. Food Chem. 2001, 49, 2532–2538. [Google Scholar] [CrossRef]
- Zullo, B.A.; Ciafardini, G. The olive oil oxygen radical absorbance capacitiy (DPPH assay) as a quality indicator. Eur. J. Lipid Sci. Technol. 2008, 110, 428–434. [Google Scholar] [CrossRef]

| Cultivar | Arbequina | Picual | ||||
|---|---|---|---|---|---|---|
| Treatment | ABA | SA100 | SA200 | ABA | SA100 | SA200 |
| Harvest Day 3 | ||||||
| Control | 418 ± 0.6 AAa | 460 ± 0.5 AAa | 708 ± 0.8 BAa | 536 ± 0.6 AAa | 424 ± 0.5 AAa | 616 ± 0.7 BAa |
| Treated | 992 ± 0.8 Ab | 654 ± 0.7 Aa | 4629 ± 1.2 Ab | 1180 ± 0.8 Ab | 808 ± 0.9 Ab | 3718 ± 1.1 Ab |
| Harvest Day 6 | ||||||
| Control | 562 ± 0.7 AAa | 398 ± 0.6 AAa | 760 ± 0.8 BAa | 1718 ± 0.9 CBc | 1809 ± 0.7 CBa | 1901 ± 0.8 CBa |
| Treated | 1322 ± 0.9 Bb | 435 ± 0.7 Ba | 1763 ± 0.9 Bc | 2445 ± 1.0 Bd | 3019 ± 1.2 Bb | 3354 ± 1.0 Ab |
| SA200 Treatment | ||
|---|---|---|
| Cultivar | Arbequina | Picual |
| Harvest Day 3 | ||
| Control | 534 ± 0.8 AAa | 437 ± 0.9 AAa |
| SA200-Treated | 310 ± 0.6 Ab | 844 ± 0.8 Ab |
| Harvest Day 6 | ||
| Control | 176 ± 0.5 ABa | 308 ± 0.8 BAa |
| SA200-Treated | 353 ± 0.9 Ab | 383 ± 0.7 Ba |
| SA200 Treatment | ||
|---|---|---|
| Cultivar | Arbequina | Picual |
| Harvest Day 3 | ||
| Control | 276 ± 0.7 AAa | 211 ± 0.7 AAa |
| SA200-Treated | 271 ± 0.9 Aa | 601 ± 0.8 Ab |
| Harvest Day 6 | ||
| Control | 124 ± 0.7 ABa | 148 ± 0.5 AAa |
| SA200-Treated | 221 ± 0.8 Ab | 202 ± 0.6 Ba |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanch, G.P.; Gómez-Jiménez, M.C.; Ruiz del Castillo, M.L. Enrichment of Olive Fruits in Antioxidant Content by Pre-Harvest Salicylic Acid Treatment. Foods 2020, 9, 1513. https://doi.org/10.3390/foods9101513
Blanch GP, Gómez-Jiménez MC, Ruiz del Castillo ML. Enrichment of Olive Fruits in Antioxidant Content by Pre-Harvest Salicylic Acid Treatment. Foods. 2020; 9(10):1513. https://doi.org/10.3390/foods9101513
Chicago/Turabian StyleBlanch, Gracia Patricia, María C. Gómez-Jiménez, and María Luisa Ruiz del Castillo. 2020. "Enrichment of Olive Fruits in Antioxidant Content by Pre-Harvest Salicylic Acid Treatment" Foods 9, no. 10: 1513. https://doi.org/10.3390/foods9101513
APA StyleBlanch, G. P., Gómez-Jiménez, M. C., & Ruiz del Castillo, M. L. (2020). Enrichment of Olive Fruits in Antioxidant Content by Pre-Harvest Salicylic Acid Treatment. Foods, 9(10), 1513. https://doi.org/10.3390/foods9101513