Antiproliferative Effect and Mediation of Apoptosis in Human Hepatoma HepG2 Cells Induced by Djulis Husk and Its Bioactive Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
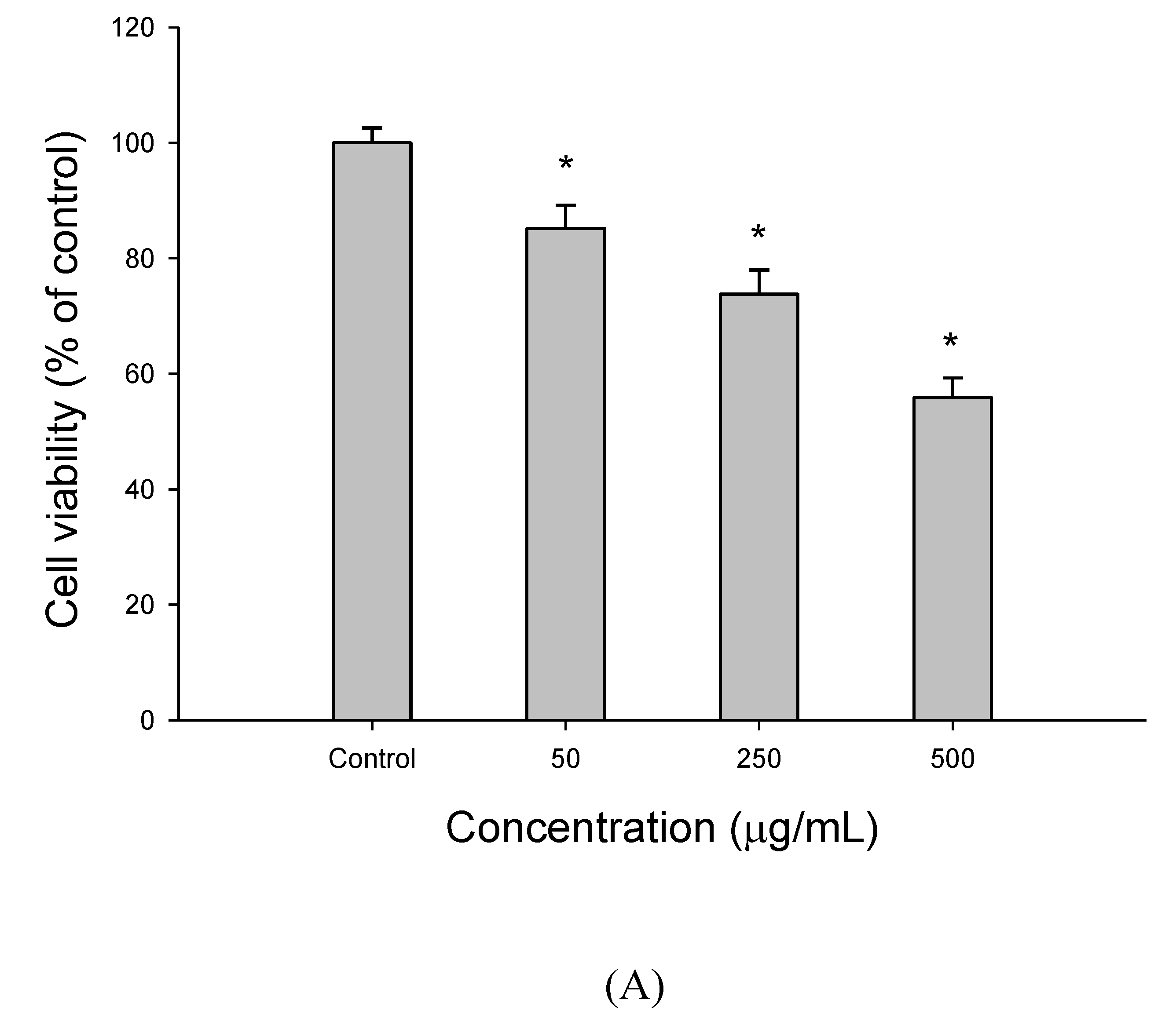
2.3. Cell Viability Assay
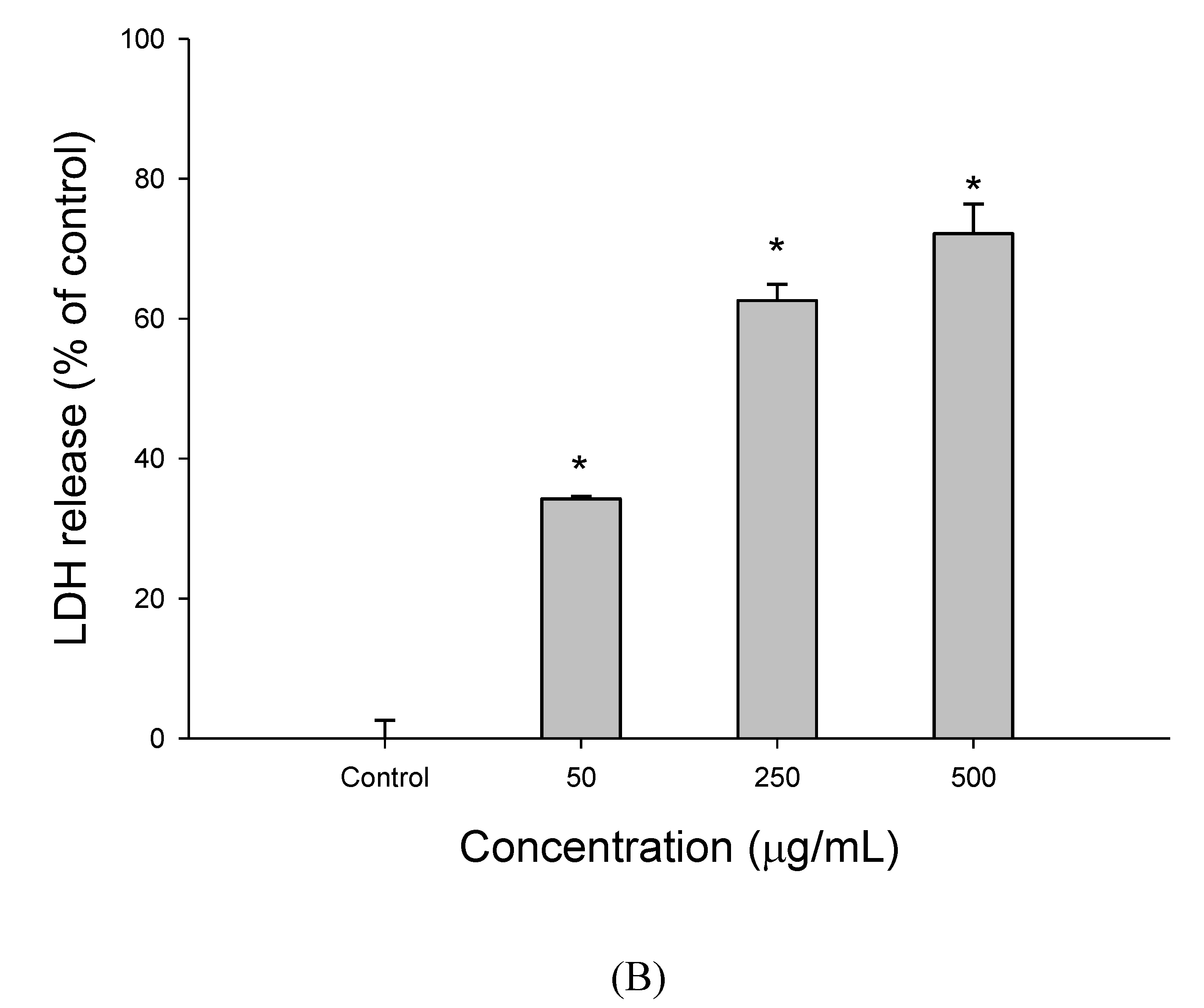
2.4. Lactate Dehydrogenase (LDH) Assay
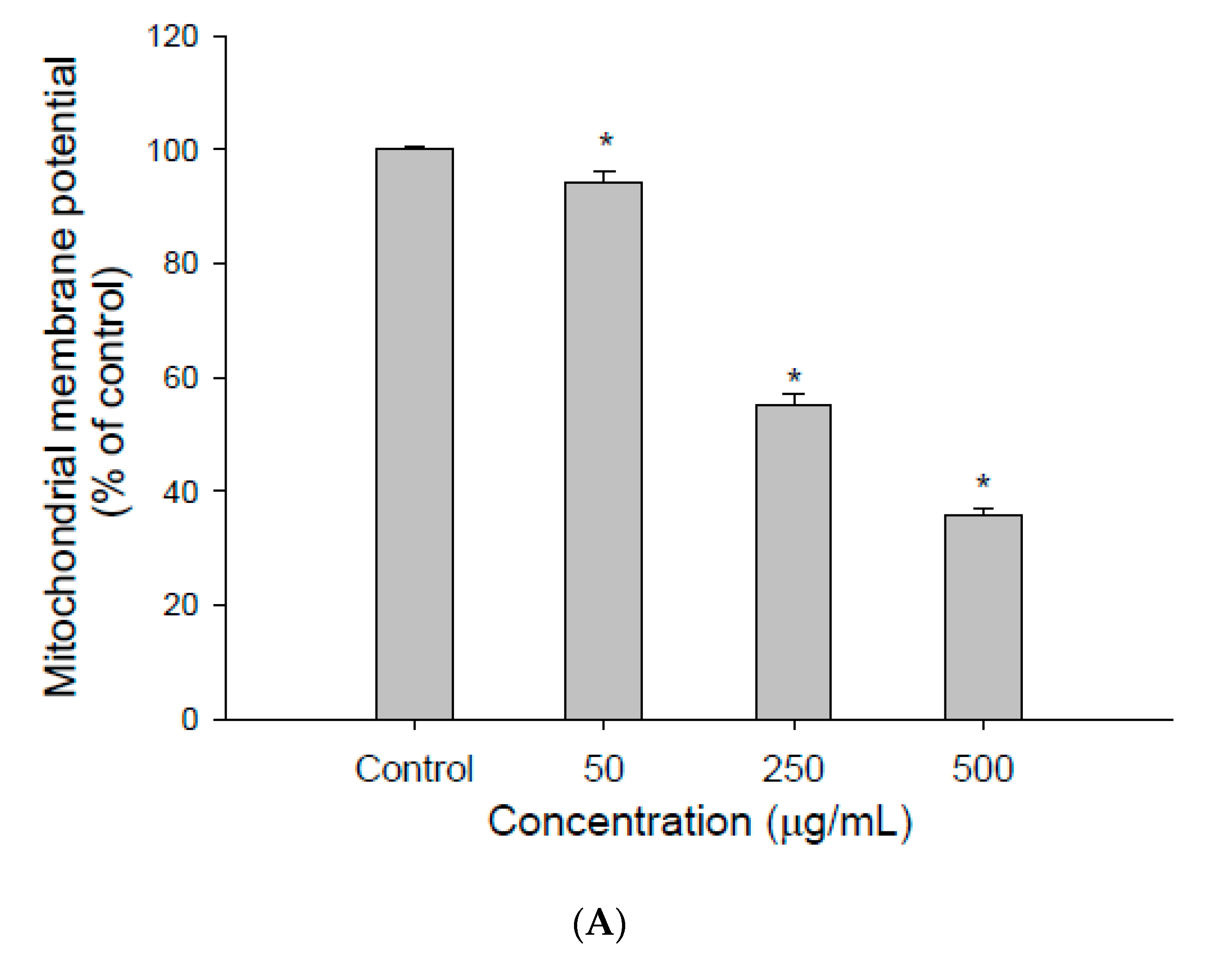
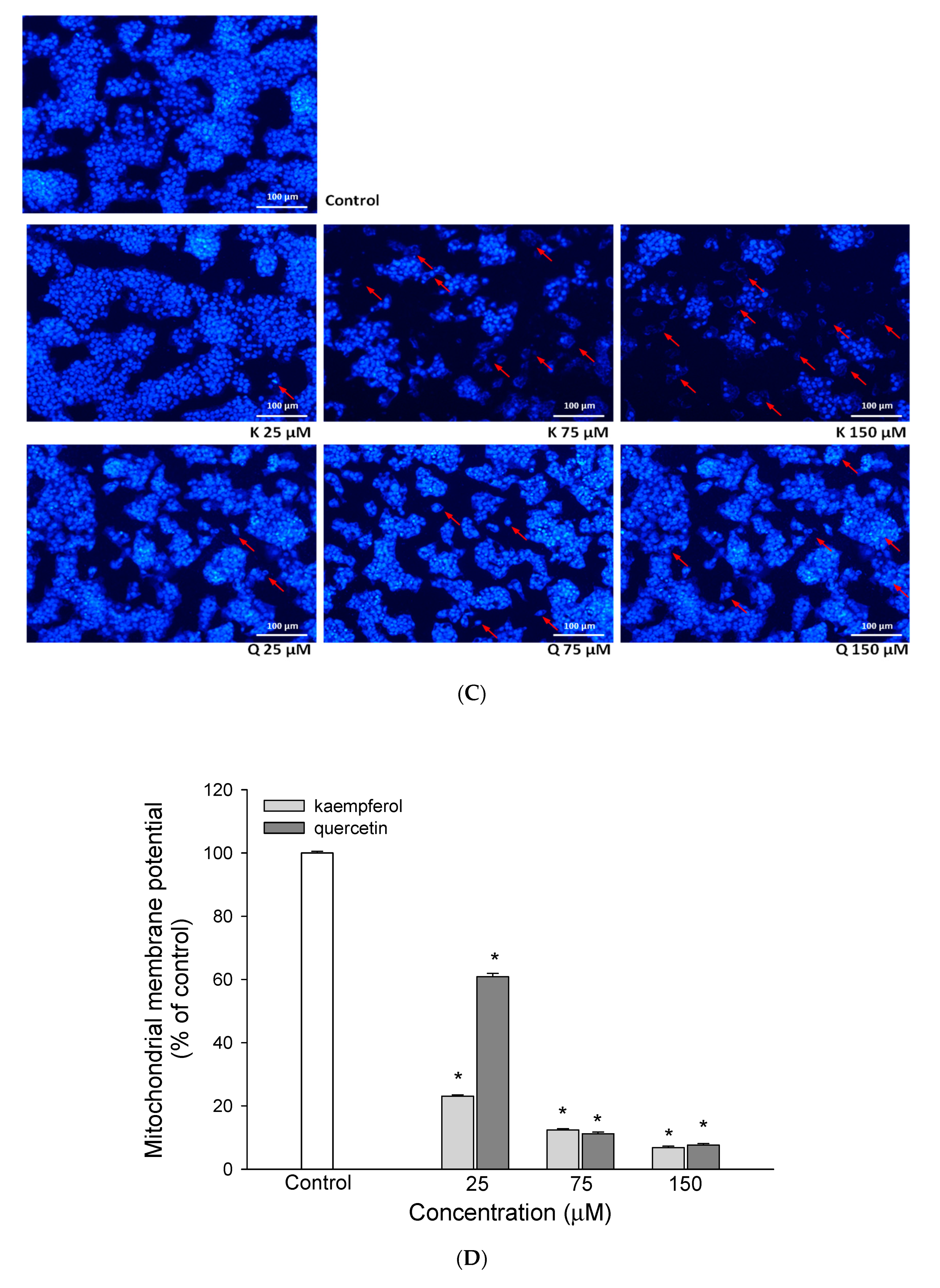
2.5. Measurement of Mitochondrial Membrane Potential (ΔΨm)
2.6. Measurement of Caspase-3 Activity
2.7. Evaluation of Reactive Oxygen Species (ROS) in HepG2 Cells
2.8. Apoptosis Assay by Flow Cytometry
2.9. DAPI Nucleic Acid Staining
2.10. Cell Cycle Analysis
2.11. Western Blot Analysis
2.12. The HPLC-Tandem MS Analysis of EEDH
2.13. Statistical Analysis
3. Results
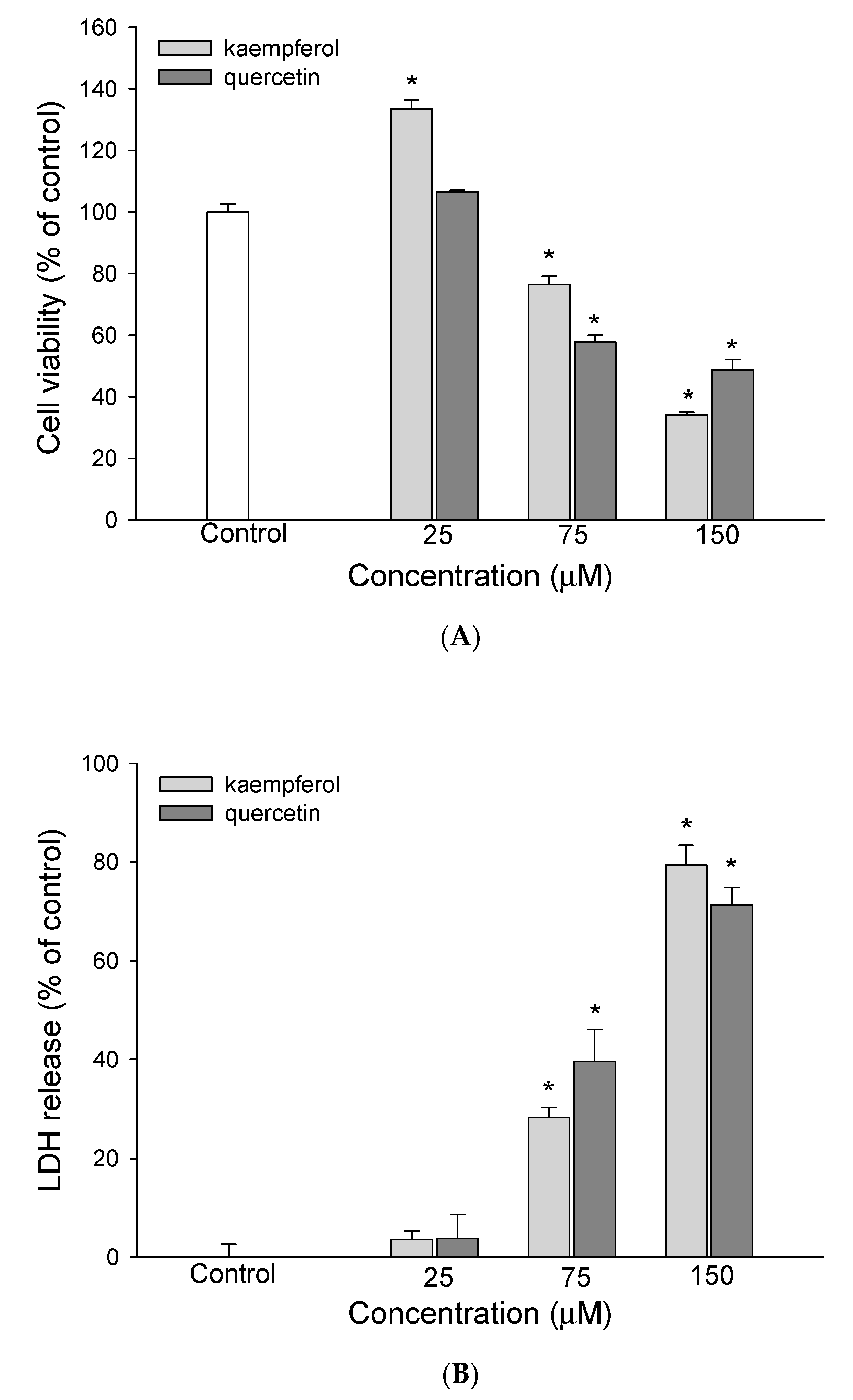
3.1. The Effect of EEDH on HepG2 Cell Growth and Induction of Apoptosis
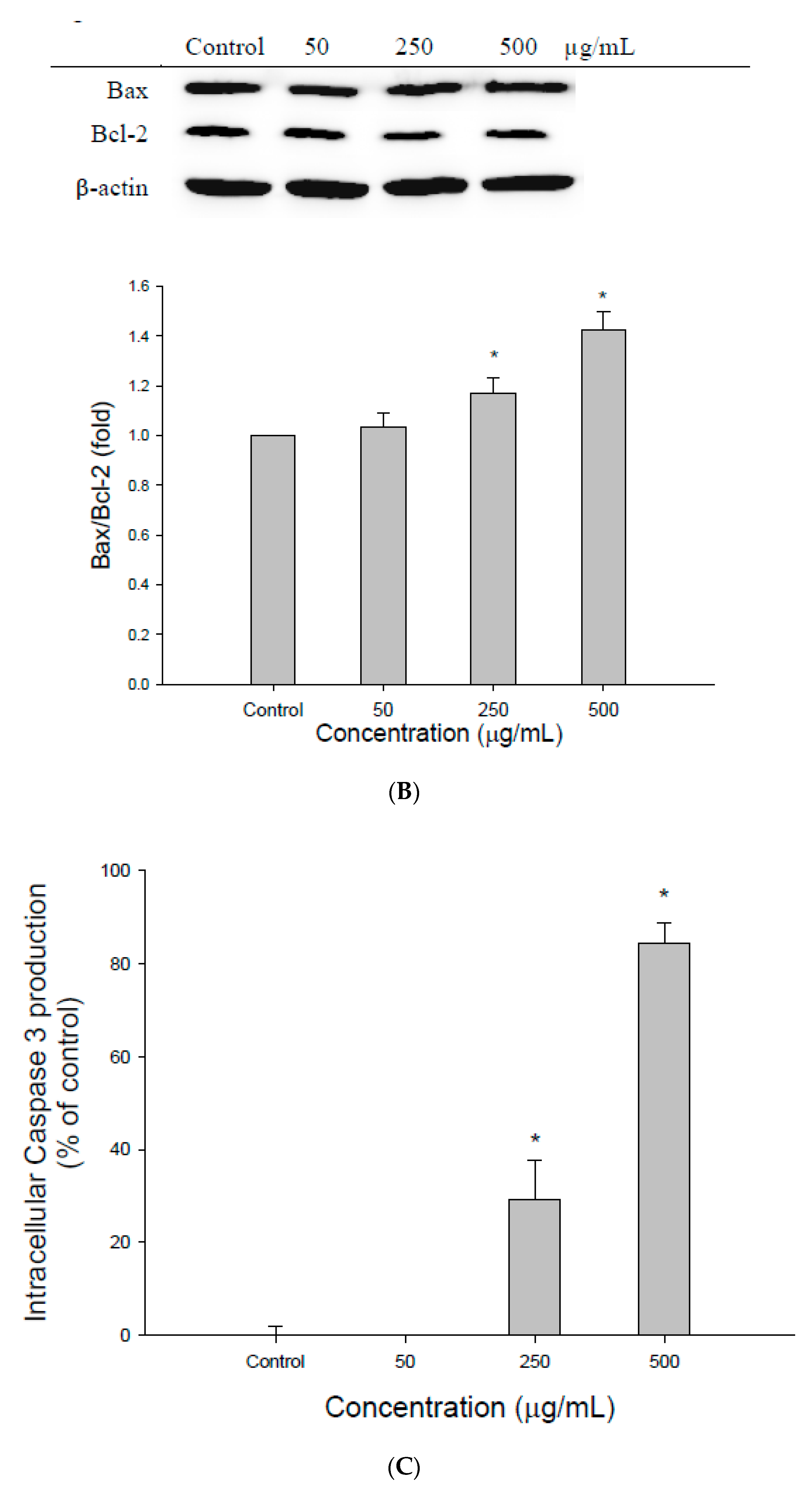
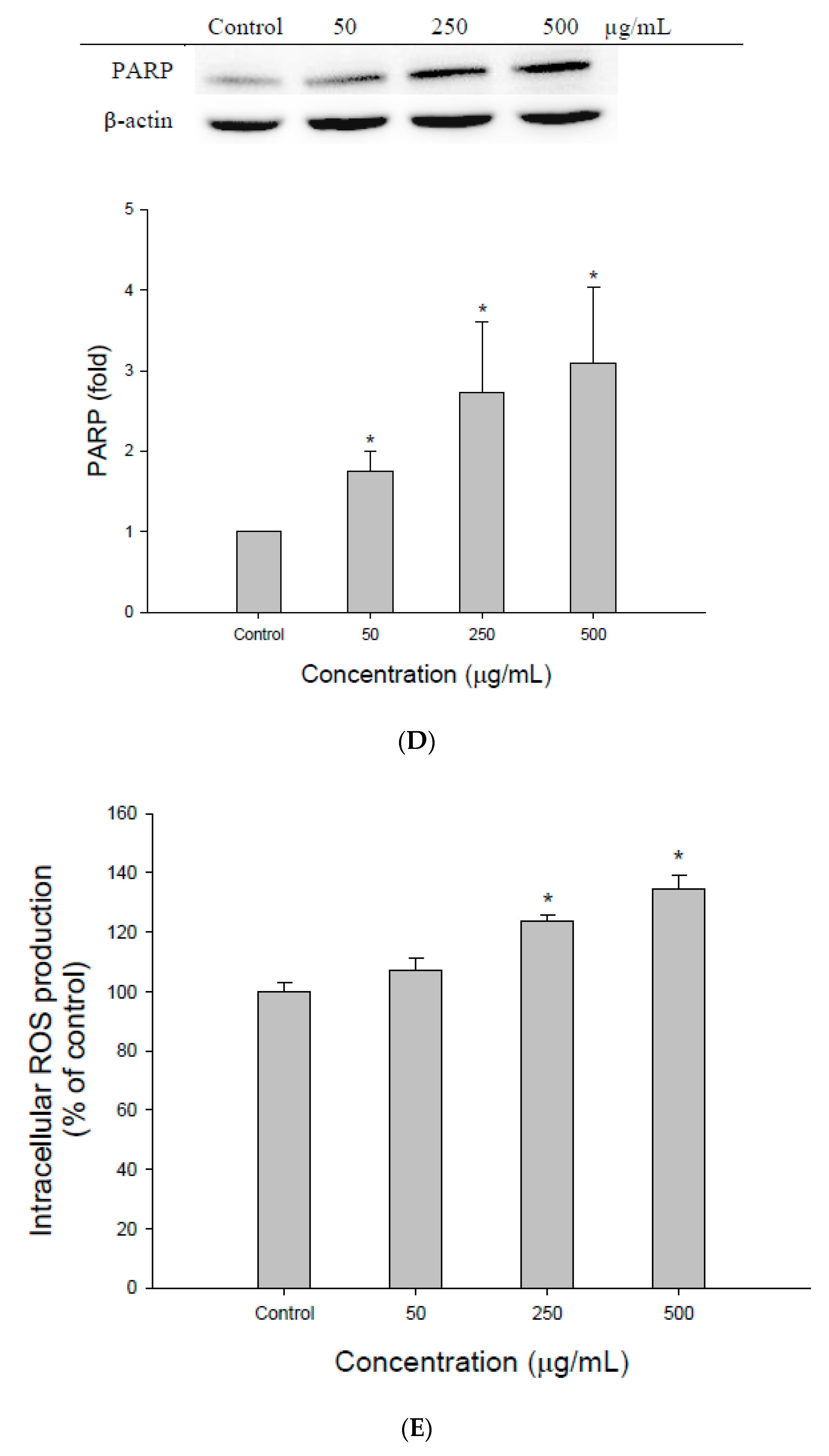
3.2. The Effect of EEDH on Apoptosis in Cells
3.3. ROS Generation Contributes to Apoptosis
3.4. Bioactive Compounds Presented in EEDH
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. Cancer. 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 12 September 2018).
- Kannan, A.; Hettiarachchy, N.; Johnson, M.G.; Nannapaneni, R. Human colon and liver cancer cell proliferation inhibition by peptide hydrolysates derived from heat-stabilized defatted rice bran. J. Agric. Food Chem. 2008, 56, 11643–11647. [Google Scholar] [CrossRef]
- Ness, A.R.; Powles, J.W. Fruit and vegetables, and cardiovascular disease: A review. Int. J. Epidemiol. 1997, 26, 1–13. [Google Scholar] [CrossRef]
- Chyau, C.C.; Chu, C.C.; Chen, S.Y.; Duh, P.D. Djulis (Chenopodium formosaneum) and its bioactive compounds protect against oxidative stress in human HepG2 cells. J. Funct. Foods 2015, 18, 159–170. [Google Scholar] [CrossRef]
- Chen, S.Y.; Chu, C.C.; Chyau, C.C.; Yang, J.W.; Duh, P.D. Djulis (Chenopodium formosanum) and its bioactive compounds affect vasodilation, angiotensin converting enzyme activity, and hypertension. Food Biosci. 2019, 32, 100469. [Google Scholar] [CrossRef]
- Chyau, C.C.; Chu, C.C.; Chen, S.Y.; Duh, P.D. The inhibitory effects of djulis (Chenopodium formosanum) and its bioactive compounds on adipogenesis in 3T3-L1 adipocytes. Molecules 2018, 23, 1780. [Google Scholar] [CrossRef]
- Chen, S.Y.; Chu, C.C.; Chyau, C.C.; Fu, Z.H.; Duh, P.D. Effect of water extract of Djulis (Chenopodium formosaneum) and its bioactive compounds on alcohol-induced liver damage in rats. Int. J. Food Nutr. Sci. 2018, 5, 55–63. [Google Scholar] [CrossRef]
- Lin, Y.H.; Lin, Y.K.; Chan, S.T.; Chun, Y.M.; Lin, Y.T.; Kan, K.W.; Yu, C.H. Anticutaneous aging effect of red djulis 2 (Chenopodium formosanum) extract on gene 3 expression of human dermal fibroblast. Prepr. Org. 2019, 2019090028. [Google Scholar] [CrossRef]
- Moure, A.; Cruz, J.M.; Franco, D.; Dominguez, J.M.; Sineiro, J.; Dominguez, H.; Nunez, M.J.; Parajo, J.C. Natural antioxidants from residual sources. Food Chem. 2001, 72, 145–171. [Google Scholar] [CrossRef]
- Chen, H.C. Analyses of Nutritional and Functional Components of Djulis (Chenopodium formosanum Koidz.). 2015. Available online: http://hdl.handle.net/11455/90154 (accessed on 1 January 2015).
- Hsu, B.Y.; Pan, S.Y.; Wu, L.Y.; Ho, C.T.; Hwang, L.S. Hypoglycemic activity of Chenopodium formosanum Koidz. components using a glucose uptake assay with 3T3-L1 adipocytes. Food Biosci. 2018, 24, 9–16. [Google Scholar] [CrossRef]
- Alia, M.; Mateos, R.; Ramos, S.; Lecumberri, E.; Bravo, L.; Goya, L. Influence of quercetin and rutin on growth and the antioxidant defense system in a human hepatoma cell line (HepG2). Eur. J. Nutr. 2006, 45, 19–28. [Google Scholar] [CrossRef]
- Wang, H.; Joseph, J.A. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic. Biol. Med. 1999, 27, 612–616. [Google Scholar] [CrossRef]
- Park, K.W.; Kim, S.Y.; Jeong, I.Y.; Byun, K.H.; Tamada, K.; Eso, K.I. Cytotoxic and antitumor activities of thiosulfinates from Allium tuberosum L. J. Agric. Food Chem. 2007, 55, 7957–7961. [Google Scholar] [CrossRef]
- Chu, C.C.; Chen, S.Y.; Chyau, C.C.; Fu, Z.H.; Liu, C.C.; Duh, P.D. Protective effect of Djulis (Chenopodium formosaneum) and its bioactive compounds protect against carbon tetrachloride-induced liver injury, in vivo. J. Funct. Foods 2016, 26, 585–597. [Google Scholar] [CrossRef]
- Calderón-Montaño, J.M.; Burgos-Morón, E.; Pérez-Guerrero, C.; López-Lázaro, M. A review on the dietary flavonoid kaempferol. Mini Rev. Med. Chem. 2011, 11, 298–344. [Google Scholar] [CrossRef]
- Yang, J.; Lee, H.; Sung, J.; Kim, Y.; Jeong, H.S.; Lee, J. Conversion of rutin to quercetin by acid treatment in relation to biological activities. Prev. Nutr. Food Sci. 2019, 24, 313–320. [Google Scholar] [CrossRef]
- Yao, S.W.; Wen, X.X.; Huang, R.Q.; He, R.R.; Ou, S.Y.; Shen, W.Z.; Huang, C.H.; Peng, X.C. Protection of feruloylated oligosaccharides from corn bran against oxidative stress in PC 12 cells. J. Agric. Food Chem. 2014, 62, 668–674. [Google Scholar] [CrossRef]
- Chidambara Murthy, K.N.; Kim, J.; Vikram, A.; Patil, B.S. Differential inhibition of human colon cancer cells by structurally similar flavonoids of citrus. Food Chem. 2012, 132, 27–34. [Google Scholar] [CrossRef]
- Chen, T.; Hao, J.; He, J.; Zhang, J.; Li, Y.; Liu, R.; Li, L. Cannabisin B induces autophagic cell death by inhibiting the AKT/mTOR pathway and S phase cell cycle arrest in HepG2 cells. Food Chem. 2013, 138, 1034–1041. [Google Scholar] [CrossRef]
- Li, T.; Zhu, J.; Guo, L.; Shi, X.; Liu, Y.; Yang, X. Differential effects of polyphenols-enriched extracts from hawthorn fruit peels and fleshes on cell cycle and apoptosis in human MCF-7 breast carcinoma cells. Food Chem. 2013, 141, 1008–1018. [Google Scholar] [CrossRef]
- Gómez-Alonso, S.; Collins, V.J.; Vauzour, D.; Rodríguez-Mateos, A.; Corona, G.; Spencer, J.P.E. Inhibition of colon adenocarcinoma cell proliferation by flavonols is linked to a G2/M cell cycle block and reduction in cyclin D1 expression. Food Chem. 2012, 130, 493–500. [Google Scholar] [CrossRef]
- Tripathi, M.; Singh, B.K.; Kakkar, P. Glycyrrhizic acid modulates t-BHP induced apoptosis in primary rat hepatocytes. Food Chem. Toxicol. 2009, 47, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.H.; Chen, Z.T.; Duh, P.D. Cytotoxic effect of Eucalyptus citriodora resin on human hepatoma HepG2 cells. Am. J. Chin. Med. 2012, 40, 399–413. [Google Scholar] [CrossRef]
- Danial, N.N.; Korsmeyer, S.J. Cell death: Critical control points. Cell 2004, 116, 205–219. [Google Scholar] [CrossRef]
- Hsieh, Y.C.; Rao, Y.K.; Whang-Peng, J.; Huang, C.Y.F.; Shyue, S.K.; Hsu, S.L.; Tzeng, Y.M. Antcin B and its ester derivative from Antrodia camphorata induce apoptosis in hepatocellular carcinoma cells involves enhancing oxidative stress coincident with activation of intrinsic and extrinsic apoptotic pathway. J. Agric. Food Chem. 2011, 59, 10943–10954. [Google Scholar] [CrossRef] [PubMed]
- Barillari, J.; Iori, R.; Papi, A.; Orlandi, M.; Bartolini, G.; Gabbanini, S.; Pedulli, G.F.; Valgimigli, L. Kaiware Daikon (Raphanus sativus L.) extract: A naturally multipotent chemopreventive agent. J. Agric. Food Chem. 2008, 56, 7823–7830. [Google Scholar] [CrossRef] [PubMed]
- Papi, A.; Orlandi, M.; Bartolini, G.; Barillari, J.; Iori, R.; Paolini, M.; Ferroni, F.; Fumo, M.G.; Pedulli, G.F.; Valgimigli, L. Cytotoxic and antioxidant activity of 4-methylthio-3-butenyl isothiocyanate from Raphanus sativus L. (Kaiware Daikon) sprouts. J. Agric. Food Chem. 2008, 56, 875–883. [Google Scholar] [CrossRef]
- Ji, B.C.; Hsu, W.H.; Yang, J.S.; Hsia, T.C.; Lu, C.C.; Chiang, J.H.; Yang, J.L.; Lin, C.H.; Lin, J.J.; Wu Suen, L.J.; et al. Gallic aid induces apoptosis via caspase-3 and mitochondrion-dependent pathways in vitro and suppresses lung xenograft tumor growth in vivo. J. Agric. Food Chem. 2009, 57, 7596–7604. [Google Scholar] [CrossRef]
- Liu, C.; Lin, J.J.; Yang, Z.Y.; Tsai, C.C.; Hsu, J.L.; Wu, Y.J. Proteomic study reveals a co-occurrence of gallic acid-induced apoptosis and glycolysis in B16F10 melanoma cells. J. Agric. Food Chem. 2014, 62, 11672–11680. [Google Scholar] [CrossRef]
- Dai, F.; Wang, Q.; Fan, G.J.; Du, Y.T.; Zhou, B. ROS-driven and preferential killing of HepG2 over L-02 cells by a short-term cooperation of Cu(II) and a catechol-type resveratrol analog. Food Chem. 2018, 250, 213–220. [Google Scholar] [CrossRef]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Ansary, J.; Gil, E.; Adolfo, E.; Amici, A.; Bompadre, S.; Simal-Gandara, J.; Giampieri, F.; Battino, M. Phenolic compounds from Mediterranean foods as nutraceutical tools for the prevention of cancer: The effect of honey polyphenols on colorectal cancer stem-like cells from spheroids. Food Chem. 2020, 325, 1268812. [Google Scholar] [CrossRef]
- Alasalvar, C.; Salvadó, J.S.; Ros, E. Bioactives and health benefits of nuts and dried fruits. Food Chem. 2020, 314, 126192. [Google Scholar] [CrossRef] [PubMed]
- Esatbeyoglu, T.; Wagner, A.E.; Schini-Kerth, V.B.; Rimbach, G. Betanin—A food colorant with biological activity. Mol. Nutr. Food Res. 2015, 59, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Gorelick-Feldman, J.; Maclean, D.; Ilic, N.; Poulev, A.; Lila, M.A.; Cheng, D.; Raskin, I. Phytoecdysteroids increase protein synthesis in skeletal muscle cells. J. Agric. Food Chem. 2008, 56, 3532–3537. [Google Scholar] [CrossRef]
- Jayameena, P.; Sivakumari, K.; Ashok, K.; Rajesh, S. Rutin: A potential anticancer drug against human colon cancer (HCT116) cells. Int. J. Biol. Pharm. Allied Sci. 2018, 7, 1731–1745. [Google Scholar] [CrossRef]
- Craig, S.A.S. Betaine in human nutrition. Am. J. Clin. Nutr. 2004, 80, 539–549. [Google Scholar] [CrossRef] [PubMed]

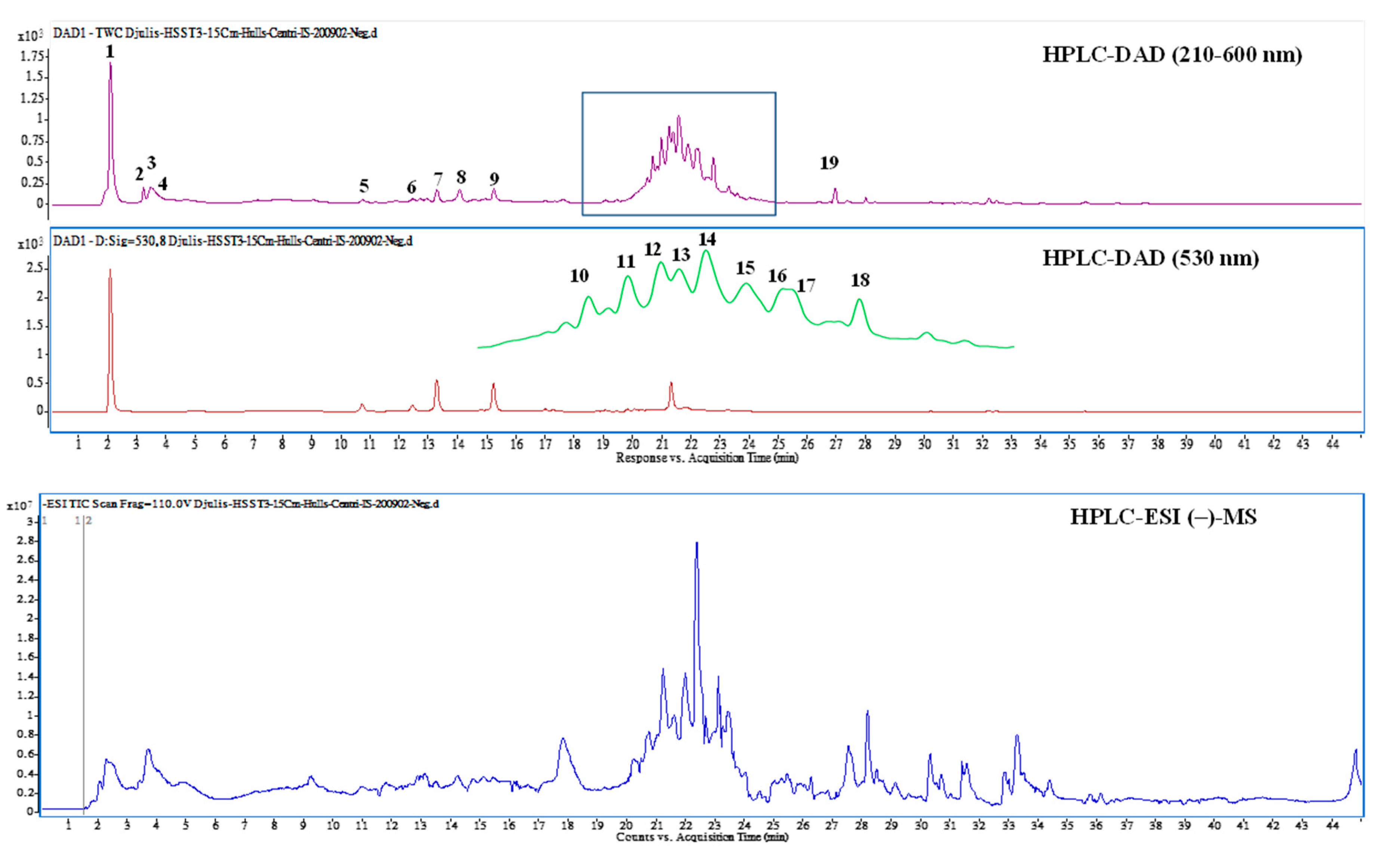

| Peak No. | RT (min) | Compound Name | λmax (nm) | [M + H]+/[M − H]−, m/z | MS/MS m/z c | Content (μg/g) |
|---|---|---|---|---|---|---|
| 1 | 2.04 | Betaine * | 226, 278, 532 | 118b/ | 58, 59 | 2902.7 ± 118.3 |
| 2 | 3.17 | Betaxanthins derivatives * | 222, 264, 482 | 280/ | 216, 84, 198, 97 | 220.1 ± 38.5 |
| 3 | 3.34 | 3,4-Dihydroxy-L-phenylalanine | 224, 280 | 198/ | 152, 135, 107, 139 | 392.6 ± 35.8 |
| 4 | 3.61 | Unknown | 224, 280 | 360/ | 152, 164, 296, 139 | 174.0 ± 20.9 |
| 5 | 10.67 | Amaranthin | 268, 536 | 727/ | 389 | 72.4 ± 6.5 |
| 6 | 12.39 | Isoamaranthin | 264, 530 | 727/ | 389 | 62.4 ± 18.5 |
| 7 | 13.23 | Betanin a | 260, 290sh, 538 | 551/ | 389 | 290.0 ± 21.8 |
| 8 | 14.19 | Isodopaxanthin | 260, 472 | 391/389 | 255, 150, 345, 347 | 314.1 ± 24.2 |
| 9 | 15.24 | Isobetanin | 268, 290sh, 532 | 551/ | 389 | 220.9 ± 45.9 |
| 10 | 20.68 | Dihydroxybenzoic acid-O-dipentoside | 224, 260, 332 | 417 | 152, 108 | 439.8 ± 101.5 |
| 11 | 20.97 | Unknown | 228, 254, 344 | 449/ | 337, 199, 127, 215 | 595.5 ± 71.6 |
| 12 | 21.24 | Quercetin-3-trisaccharide | 228, 254, 322 | 743/ | 303 | 680.3 ± 45.3 |
| 13 | 21.39 | Amaranthin isomer * | 224, 328, 528 | 727/ | 389 | 292.3 ± 64.7 |
| 14 | 21.59 | Rutin a | 254, 352 | 611/609 | 301 | 909.9 ± 102.4 |
| 15 | 21.93 | 20-Hydroxyecdysone | 246, 316, 422 | 481 | 165, 371, 301, 173 | 505.5 ± 25.5 |
| 16 | 22.01 | Kaempferol 3-O-β-rutinoside | 228, 266, 316 | 595/593 | 287 | 625.5 ± 267.2 |
| 17 | 22.15 | Unknown | 228, 282, 350 | 291/289 | 159, 130, 185, 227 | 326.3 ± 28.3 |
| 18 | 22.77 | Unknown | 224, 312, 410 | 677/675 | 319, 599, 643, 557 | 448.7 ± 46.5 |
| 19 | 26.93 | Internal standard | 220, 272, 312 | 255/ | 151, 131, 103, 209 | 250 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, D.-G.; Chyau, C.-C.; Chen, S.-Y.; Chu, H.-L.; Wang, S.-C.; Duh, P.-D. Antiproliferative Effect and Mediation of Apoptosis in Human Hepatoma HepG2 Cells Induced by Djulis Husk and Its Bioactive Compounds. Foods 2020, 9, 1514. https://doi.org/10.3390/foods9101514
Tu D-G, Chyau C-C, Chen S-Y, Chu H-L, Wang S-C, Duh P-D. Antiproliferative Effect and Mediation of Apoptosis in Human Hepatoma HepG2 Cells Induced by Djulis Husk and Its Bioactive Compounds. Foods. 2020; 9(10):1514. https://doi.org/10.3390/foods9101514
Chicago/Turabian StyleTu, Dom-Gene, Charng-Cherng Chyau, Shih-Ying Chen, Heuy-Ling Chu, Shu-Chen Wang, and Pin-Der Duh. 2020. "Antiproliferative Effect and Mediation of Apoptosis in Human Hepatoma HepG2 Cells Induced by Djulis Husk and Its Bioactive Compounds" Foods 9, no. 10: 1514. https://doi.org/10.3390/foods9101514
APA StyleTu, D.-G., Chyau, C.-C., Chen, S.-Y., Chu, H.-L., Wang, S.-C., & Duh, P.-D. (2020). Antiproliferative Effect and Mediation of Apoptosis in Human Hepatoma HepG2 Cells Induced by Djulis Husk and Its Bioactive Compounds. Foods, 9(10), 1514. https://doi.org/10.3390/foods9101514