Effect of the Intake of a Snack Containing Dietary Fiber on Postprandial Glucose Levels
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Snack Contents
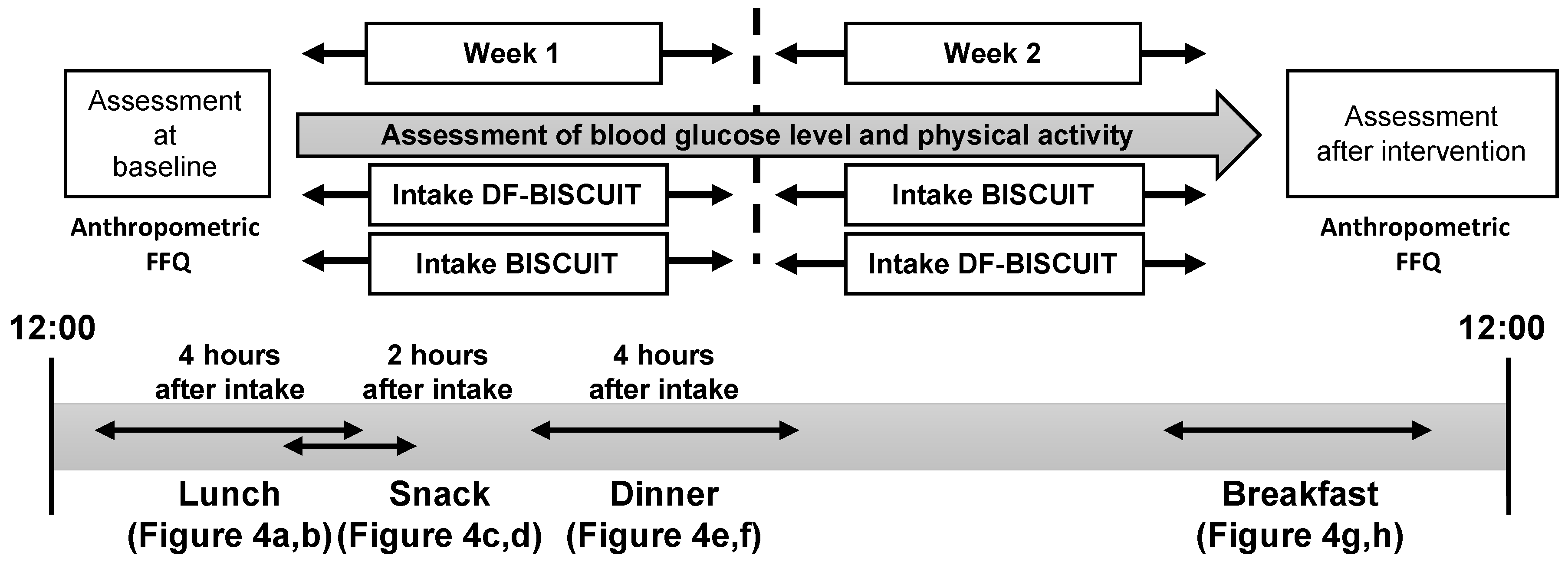
2.3. Main Trials
2.4. Blood Glucose Level and Analysis
2.5. Standardization of the Meal and Physical Activity
2.6. Statistical Analysis
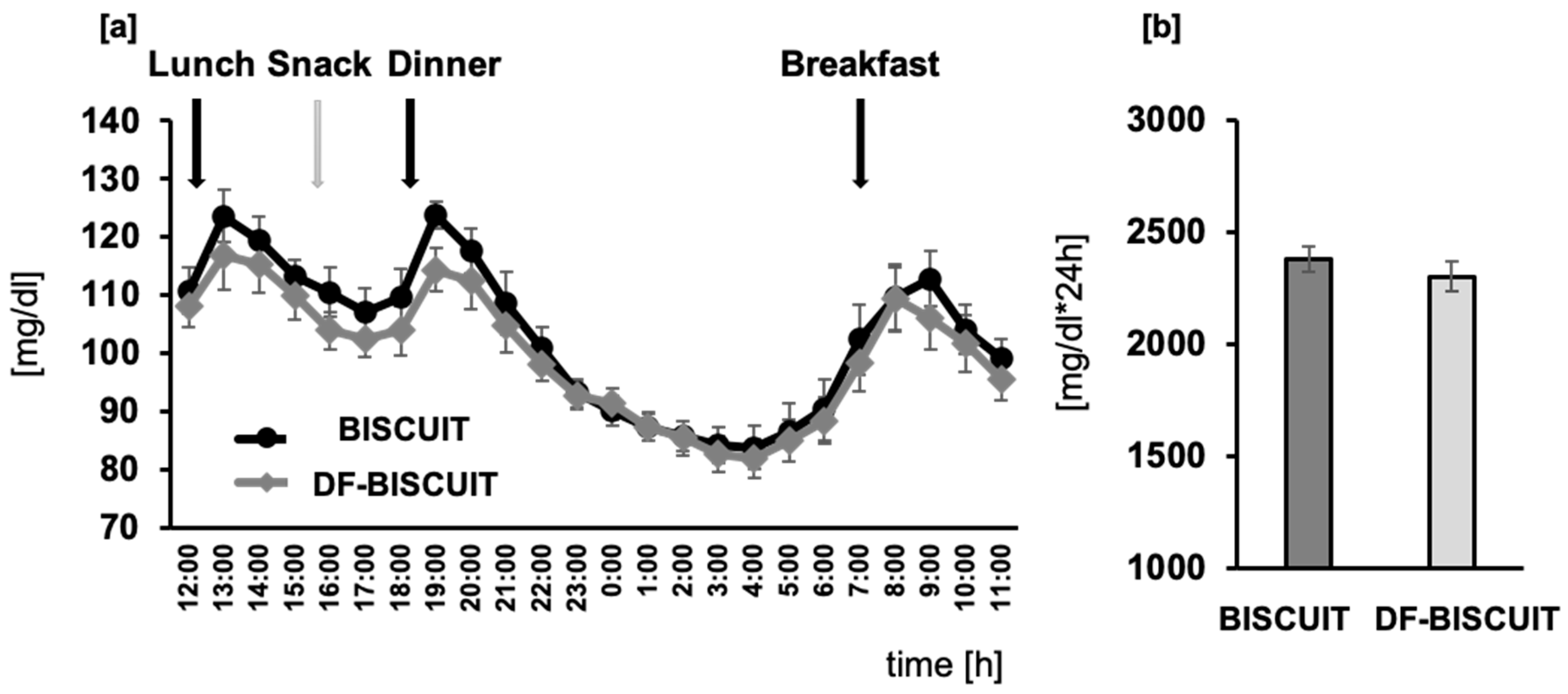
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nakagami, T.; Group, D.S. Hyperglycaemia and mortality from all causes and from cardiovascular disease in five populations of Asian origin. Diabetologia 2004, 47, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Glucose tolerance and cardiovascular mortality: Comparison of fasting and 2-hour diagnostic criteria. Arch. Intern. Med. 2001, 161, 397–405. [CrossRef] [PubMed]
- Glucose tolerance and mortality: Comparison of WHO and American Diabetes Association diagnostic criteria. The DECODE study group. European Diabetes Epidemiology Group. Diabetes Epidemiology: Collaborative analysis Of Diagnostic criteria in Europe. Lancet 1999, 354, 617–621.
- Lopez-Minguez, J.; Gomez-Abellan, P.; Garaulet, M. Timing of Breakfast, Lunch, and Dinner. Effects on Obesity and Metabolic Risk. Nutrients 2019, 11, 2624. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Tinsley, G.; Bianco, A.; Moro, T. The Influence of Meal Frequency and Timing on Health in Humans: The Role of Fasting. Nutrients 2019, 11, 719. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.J.; Yang, J.N.; Garcia, J.I.; Myers, S.; Bozzi, I.; Wang, W.; Buxton, O.M.; Shea, S.A.; Scheer, F.A. Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc. Natl. Acad. Sci. USA 2015, 112, E2225–E2234. [Google Scholar] [CrossRef]
- Bo, S.; Fadda, M.; Castiglione, A.; Ciccone, G.; De Francesco, A.; Fedele, D.; Guggino, A.; Parasiliti Caprino, M.; Ferrara, S.; Vezio Boggio, M.; et al. Is the timing of caloric intake associated with variation in diet-induced thermogenesis and in the metabolic pattern? A randomized cross-over study. Int. J. Obes. 2015, 39, 1689–1695. [Google Scholar] [CrossRef]
- Bandin, C.; Scheer, F.A.; Luque, A.J.; Avila-Gandia, V.; Zamora, S.; Madrid, J.A.; Gomez-Abellan, P.; Garaulet, M. Meal timing affects glucose tolerance, substrate oxidation and circadian-related variables: A randomized, crossover trial. Int. J. Obes. 2015, 39, 828–833. [Google Scholar] [CrossRef]
- Kant, A.K.; Graubard, B.I. 40-year trends in meal and snack eating behaviors of American adults. J. Acad. Nutr. Diet. 2015, 115, 50–63. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Majima, S.; Fukuda, T.; Matsumoto, S.; Ushigome, E.; Tanaka, M.; Asano, M.; Yamazaki, M.; Furusawa, K.; Fukui, M. Investigation of the lifestyles of Japanese type 2 diabetes patients: Regarding diet and exercise habits. Ther. Res. 2017, 38, 77–88. [Google Scholar]
- Howarth, L.; Petrisko, Y.; Furchner-Evanson, A.; Nemoseck, T.; Kern, M. Snack selection influences nutrient intake, triglycerides, and bowel habits of adult women: A pilot study. J. Am. Diet. Assoc. 2010, 110, 1322–1327. [Google Scholar] [CrossRef] [PubMed]
- Mekary, R.A.; Giovannucci, E.; Willett, W.C.; van Dam, R.M.; Hu, F.B. Eating patterns and type 2 diabetes risk in men: Breakfast omission, eating frequency, and snacking. Am. J. Clin. Nutr. 2012, 95, 1182–1189. [Google Scholar] [CrossRef] [PubMed]
- Imai, S.; Kajiyama, S.; Hashimoto, Y.; Nitta, A.; Miyawaki, T.; Matsumoto, S.; Ozasa, N.; Tanaka, M.; Kajiyama, S.; Fukui, M. Consuming snacks mid-afternoon compared with just after lunch improves mean amplitude of glycaemic excursions in patients with type 2 diabetes: A randomized crossover clinical trial. Diabetes Metab. 2018, 44, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Weickert, M.O.; Pfeiffer, A.F.H. Impact of Dietary Fiber Consumption on Insulin Resistance and the Prevention of Type 2 Diabetes. J. Nutr. 2018, 148, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.L.; Otto, B.; Reich, S.C.; Weickert, M.O.; Steiniger, J.; Machowetz, A.; Rudovich, N.N.; Mohlig, M.; Katz, N.; Speth, M.; et al. Arabinoxylan consumption decreases postprandial serum glucose, serum insulin and plasma total ghrelin response in subjects with impaired glucose tolerance. Eur. J. Clin. Nutr. 2007, 61, 334–341. [Google Scholar] [CrossRef]
- Dikeman, C.L.; Murphy, M.R.; Fahey, G.C., Jr. Dietary fibers affect viscosity of solutions and simulated human gastric and small intestinal digesta. J. Nutr. 2006, 136, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Nitta, A.; Imai, S.; Kajiyama, S.; Miyawaki, T.; Matsumoto, S.; Ozasa, N.; Kajiyama, S.; Hashimoto, Y.; Tanaka, M.; Fukui, M. Impact of different timing of consuming sweet snack on postprandial glucose excursions in healthy women. Diabetes Metab. 2019, 45, 369–374. [Google Scholar] [CrossRef]
- Mâsse, L.C.; Fuemmeler, B.F.; Anderson, C.B.; Matthews, C.E.; Trost, S.G.; Catellier, D.J.; Treuth, M. Accelerometer data reduction: A comparison of four reduction algorithms on select outcome variables. Med. Sci. Sports Exerc. 2005, 37, S544–S554. [Google Scholar] [CrossRef]
- Meyer, K.A.; Kushi, L.H.; Jacobs, D.R., Jr.; Slavin, J.; Sellers, T.A.; Folsom, A.R. Carbohydrates, dietary fiber, and incident type 2 diabetes in older women. Am. J. Clin. Nutr. 2000, 71, 921–930. [Google Scholar] [CrossRef]
- Streppel, M.T.; Ocke, M.C.; Boshuizen, H.C.; Kok, F.J.; Kromhout, D. Dietary fiber intake in relation to coronary heart disease and all-cause mortality over 40 y: The Zutphen Study. Am. J. Clin. Nutr. 2008, 88, 1119–1125. [Google Scholar] [CrossRef]
- Bonuccelli, S.; Muscelli, E.; Gastaldelli, A.; Barsotti, E.; Astiarraga, B.D.; Holst, J.J.; Mari, A.; Ferrannini, E. Improved tolerance to sequential glucose loading (Staub-Traugott effect): Size and mechanisms. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E532–E537. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Staub, H. Examination of sugar metabolism in humans. Z. Klin. Med. 1921, 91, 44–48. [Google Scholar]
- Traugott, K. In reference to the reactions of blood sugar levels in repeated and varied types of enteral sugar increases and their significance in liver function. Klin. Wochenschr. 1922, 1, 892–894. [Google Scholar] [CrossRef]
- Wolever, T.M.; Jenkins, D.J.; Ocana, A.M.; Rao, V.A.; Collier, G.R. Second-meal effect: Low-glycemic-index foods eaten at dinner improve subsequent breakfast glycemic response. Am. J. Clin. Nutr. 1988, 48, 1041–1047. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Wolever, T.M.; Taylor, R.H.; Griffiths, C.; Krzeminska, K.; Lawrie, J.A.; Bennett, C.M.; Goff, D.V.; Sarson, D.L.; Bloom, S.R. Slow release dietary carbohydrate improves second meal tolerance. Am. J. Clin. Nutr. 1982, 35, 1339–1346. [Google Scholar] [CrossRef]
- Liljeberg, H.G.; Akerberg, A.K.; Bjorck, I.M. Effect of the glycemic index and content of indigestible carbohydrates of cereal-based breakfast meals on glucose tolerance at lunch in healthy subjects. Am. J. Clin. Nutr. 1999, 69, 647–655. [Google Scholar] [CrossRef]
- Meng, H.; Matthan, N.R.; Ausman, L.M.; Lichtenstein, A.H. Effect of prior meal macronutrient composition on postprandial glycemic responses and glycemic index and glycemic load value determinations. Am. J. Clin. Nutr. 2017, 106, 1246–1256. [Google Scholar] [CrossRef]
- Lee, S.H.; Tura, A.; Mari, A.; Ko, S.H.; Kwon, H.S.; Song, K.H.; Yoon, K.H.; Lee, K.W.; Ahn, Y.B. Potentiation of the early-phase insulin response by a prior meal contributes to the second-meal phenomenon in type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E984–E990. [Google Scholar] [CrossRef]
- Rahat-Rozenbloom, S.; Fernandes, J.; Cheng, J.; Gloor, G.B.; Wolever, T.M. The acute effects of inulin and resistant starch on postprandial serum short-chain fatty acids and second-meal glycemic response in lean and overweight humans. Eur. J. Clin. Nutr. 2017, 71, 227–233. [Google Scholar] [CrossRef]
- Sadakiyo, T.; Ishida, Y.; Inoue, S.I.; Taniguchi, Y.; Sakurai, T.; Takagaki, R.; Kurose, M.; Mori, T.; Yasuda-Yamashita, A.; Mitsuzumi, H.; et al. Attenuation of postprandial blood glucose in humans consuming isomaltodextrin: Carbohydrate loading studies. Food Nutr. Res. 2017, 61, 1325306. [Google Scholar] [CrossRef][Green Version]
- Delzenne, N.M.; Daubioul, C.; Neyrinck, A.; Lasa, M.; Taper, H.S. Inulin and oligofructose modulate lipid metabolism in animals: Review of biochemical events and future prospects. Br. J. Nutr. 2002, 87 (Suppl. S2), S255–S259. [Google Scholar] [CrossRef]
- Gibson, G.R.; Beatty, E.R.; Wang, X.; Cummings, J.H. Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology 1995, 108, 975–982. [Google Scholar] [CrossRef]
- Brighenti, F.; Casiraghi, M.C.; Canzi, E.; Ferrari, A. Effect of consumption of a ready-to-eat breakfast cereal containing inulin on the intestinal milieu and blood lipids in healthy male volunteers. Eur. J. Clin. Nutr. 1999, 53, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Bouhnik, Y.; Raskine, L.; Simoneau, G.; Paineau, D.; Bornet, F. The capacity of short-chain fructo-oligosaccharides to stimulate faecal bifidobacteria: A dose-response relationship study in healthy humans. Nutr. J. 2006, 5, 8. [Google Scholar] [CrossRef]
- Diamant, M.; Blaak, E.E.; de Vos, W.M. Do nutrient-gut-microbiota interactions play a role in human obesity, insulin resistance and type 2 diabetes? Obes. Rev. 2011, 12, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Lattimer, J.M.; Haub, M.D. Effects of dietary fiber and its components on metabolic health. Nutrients 2010, 2, 1266–1289. [Google Scholar] [CrossRef]
- Maki, K.C.; Davidson, M.H.; Witchger, M.S.; Dicklin, M.R.; Subbaiah, P.V. Effects of high-fiber oat and wheat cereals on postprandial glucose and lipid responses in healthy men. Int. J. Vitam. Nutr. Res. 2007, 77, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Lightowler, H.J.; Henry, C.J. Glycemic response of mashed potato containing high-viscocity hydroxypropylmethylcellulose. Nutr. Res. 2009, 29, 551–557. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Song, Y.H.; Zhao, R.; Xia, L.; Chen, Y.; Cui, Y.P.; Rao, Z.Y.; Zhou, Y.; Zhuang, W.; et al. Effects of the resistant starch on glucose, insulin, insulin resistance, and lipid parameters in overweight or obese adults: A systematic review and meta-analysis. Nutr. Diabetes 2019, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Denise Robertson, M. Metabolic cross talk between the colon and the periphery: Implications for insulin sensitivity. Proc. Nutr. Soc. 2007, 66, 351–361. [Google Scholar] [CrossRef] [PubMed]


| DF-BISCUIT | BISCUIT | |
|---|---|---|
| Energy (kcal/day) | 154 | 154 |
| Protein (g/day) | 2 | 1.7 |
| Fat (g/day) | 9.9 | 8.1 |
| Total carbohydrate (g/day) | 18.4 | 18.5 |
| Sugar (g/day) | 9.2 | 18.1 |
| Dietary fiber (g/day) | 9.2 | 0.4 |
| Isomaltodextrin (g/day) | 1.6 | 0 |
| Inulin (g/day) | 0.9 | 0 |
| Cellulose (g/day) | 0.3 | 0 |
| Others (g/day) | 6.4 | 0.4 |
| Sodium chloride equivalent (g/day) | 0.22 | 0.17 |
| Mealtime | ||
| Breakfast (h:min) | 7:19 ± 0:13 | 7:18 ± 0:11 |
| Lunch (h:min) | 12:20 ± 0:32 | 12:19 ± 0:10 |
| Snack (h:min) | 15:32 ± 0:09 | 15:35 ± 0:08 |
| Dinner (h:min) | 18:28 ± 0:12 | 18:30 ± 0:15 |
| Physical Activity | ||
| MVPA (min/day) | 86.0 ± 9.6 | 92.0 ± 12.2 |
| Step counts (step/day) | 8433.9 ± 1440.1 | 8260.9 ± 1100.1 |
| Before Intervention | After Intervention | |
|---|---|---|
| Age (years) | 76.9 ± 1.5 | 76.9 ± 1.6 |
| Height (cm) | 155.4 ± 2.7 | 155.4 ± 2.8 |
| Body weight (kg) | 52.9 ± 2.6 | 52.4 ± 2.5 |
| BMI (kg/m2) | 21.8 ± 0.6 | 21.1 ± 0.3 |
| Energy intake (kcal/day) | 2193.9 ± 288.9 | 2216.6 ± 255.8 |
| Dietary fiber intake (g/day) | 18.2 ± 2.1 | 16.9 ± 1.8 |
| DF-BISCUIT | BISCUIT | p | |
|---|---|---|---|
| SD (mg/dL) | 13.6 ± 0.6 | 15.8 ± 1.0 | 0.02 * |
| CV (%) | 13.7 ± 0.6 | 15.4 ± 1.2 | 0.08 |
| MAX (mg/dL) | 130.1 ± 3.7 | 134.5 ± 2.3 | 0.20 |
| MIN (mg/dL) | 80.9 ± 3.1 | 80.4 ± 3.4 | 0.85 |
| MAGE (mg/dL) | 49.1 ± 1.6 | 54.1 ± 3.8 | 0.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-K.; Nanba, T.; Ozaki, M.; Chijiki, H.; Takahashi, M.; Fukazawa, M.; Okubo, J.; Shibata, S. Effect of the Intake of a Snack Containing Dietary Fiber on Postprandial Glucose Levels. Foods 2020, 9, 1500. https://doi.org/10.3390/foods9101500
Kim H-K, Nanba T, Ozaki M, Chijiki H, Takahashi M, Fukazawa M, Okubo J, Shibata S. Effect of the Intake of a Snack Containing Dietary Fiber on Postprandial Glucose Levels. Foods. 2020; 9(10):1500. https://doi.org/10.3390/foods9101500
Chicago/Turabian StyleKim, Hyeon-Ki, Takuya Nanba, Mamiho Ozaki, Hanako Chijiki, Masaki Takahashi, Mayuko Fukazawa, Jin Okubo, and Shigenobu Shibata. 2020. "Effect of the Intake of a Snack Containing Dietary Fiber on Postprandial Glucose Levels" Foods 9, no. 10: 1500. https://doi.org/10.3390/foods9101500
APA StyleKim, H.-K., Nanba, T., Ozaki, M., Chijiki, H., Takahashi, M., Fukazawa, M., Okubo, J., & Shibata, S. (2020). Effect of the Intake of a Snack Containing Dietary Fiber on Postprandial Glucose Levels. Foods, 9(10), 1500. https://doi.org/10.3390/foods9101500





