Chemical Composition and Bioactive Properties of Commercial and Non-Commercial Purple and White Açaí Berries
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Amaterial (E. oleracea)
2.2. Extraction of Açaí Samples
2.3. Chemical Analysis and Antioxidant Activity
2.3.1. Chemicals
2.3.2. Total Polyphenol Content by Folin–Ciocalteu Assay
2.3.3. Total Flavonoid Content by AlCl3 Assay
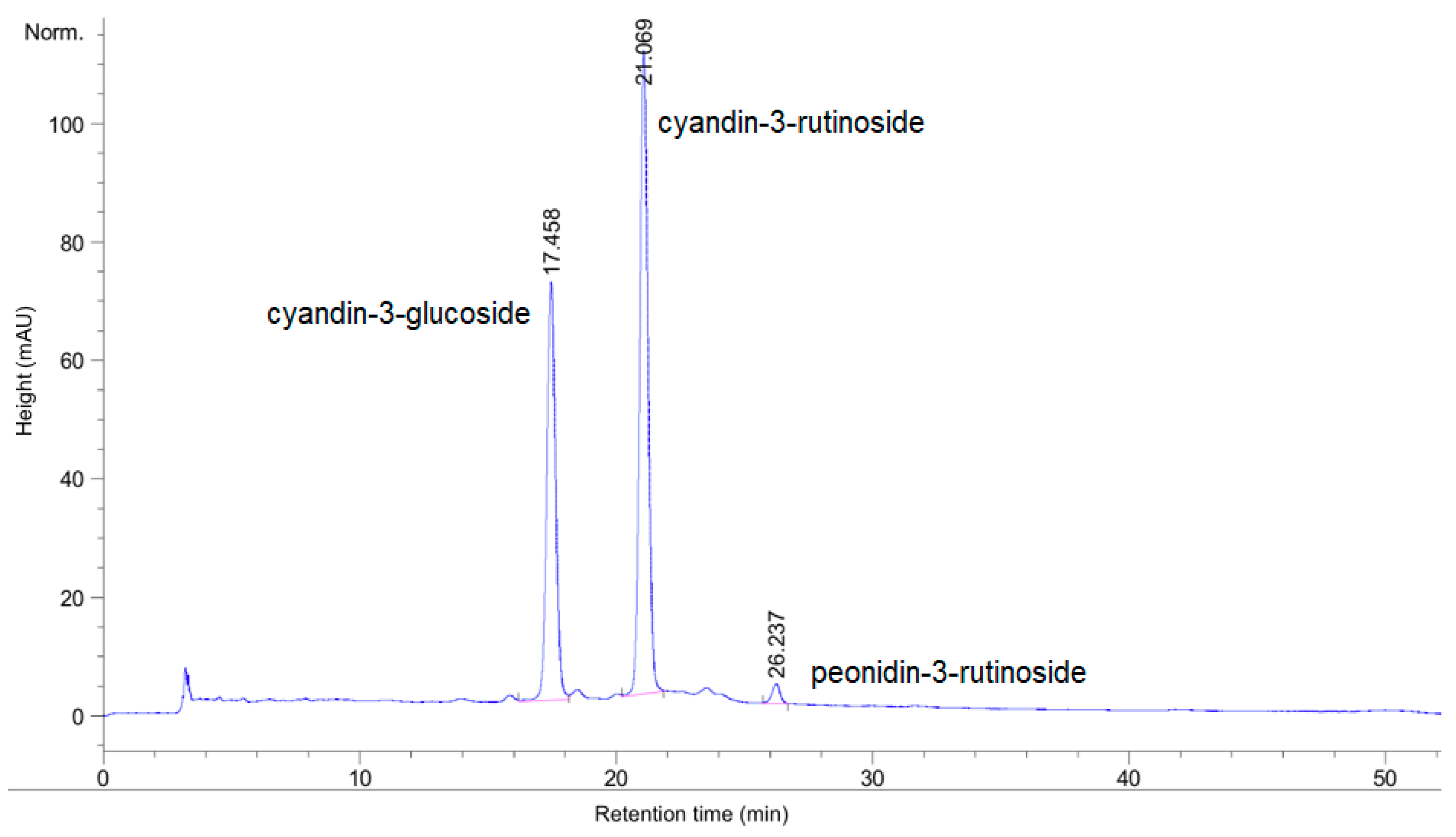
2.3.4. Total Anthocyanin Content by HPLC
2.3.5. Total Proanthocyanidin Content (PAC) via DMAC Assay
2.3.6. Antioxidant Activity by ABTS and DPPH Assay
2.4. Elemental Analysis
2.5. Biological Assays on RAW 264.7 Macrophage Cells
2.5.1. Macrophage Cell Culture
2.5.2. Cell Viability Assay
2.5.3. Nitric Oxide Radical Inhibition Assay
2.5.4. Reactive Oxygen Species Assay
2.5.5. Cell Migration Assay
2.6. Statistical Analysis
3. Results and Discussion
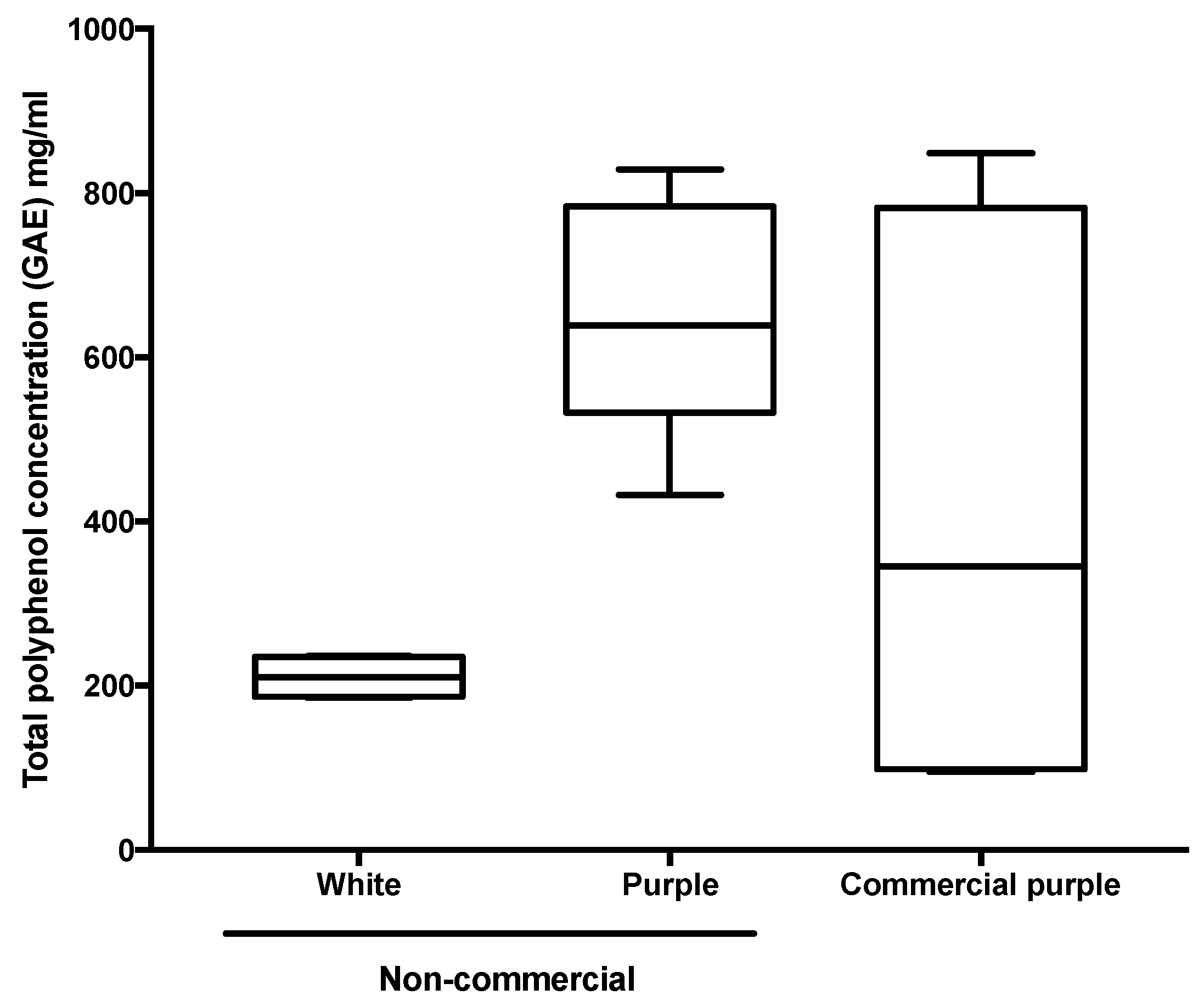
3.1. Chemical Analysis and Antioxidant Activity
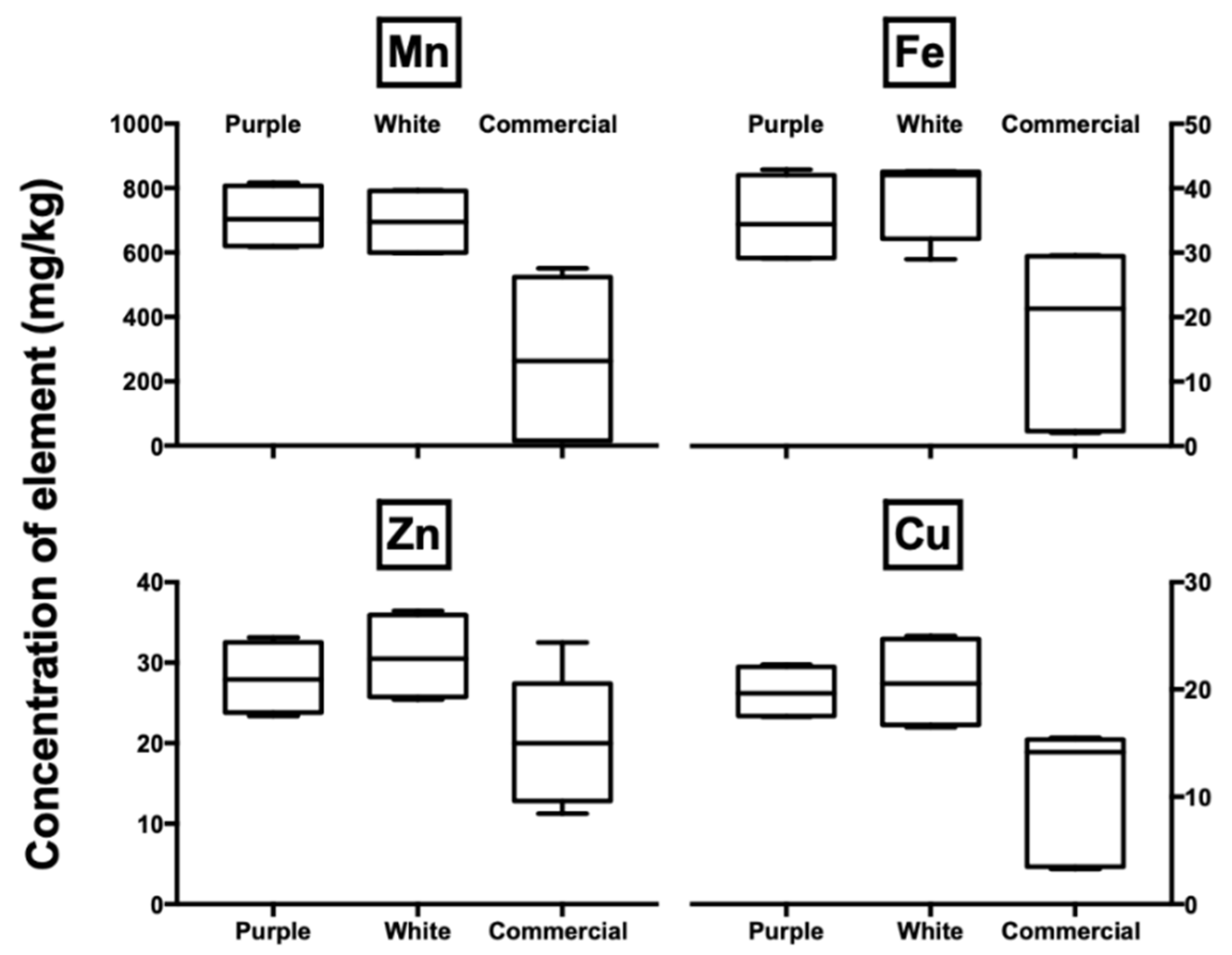
3.2. Elemental Analysis
3.3. Biological Assays
3.3.1. Cell Viability Assay with MTT
3.3.2. Nitric Oxide (NO) Inhibition
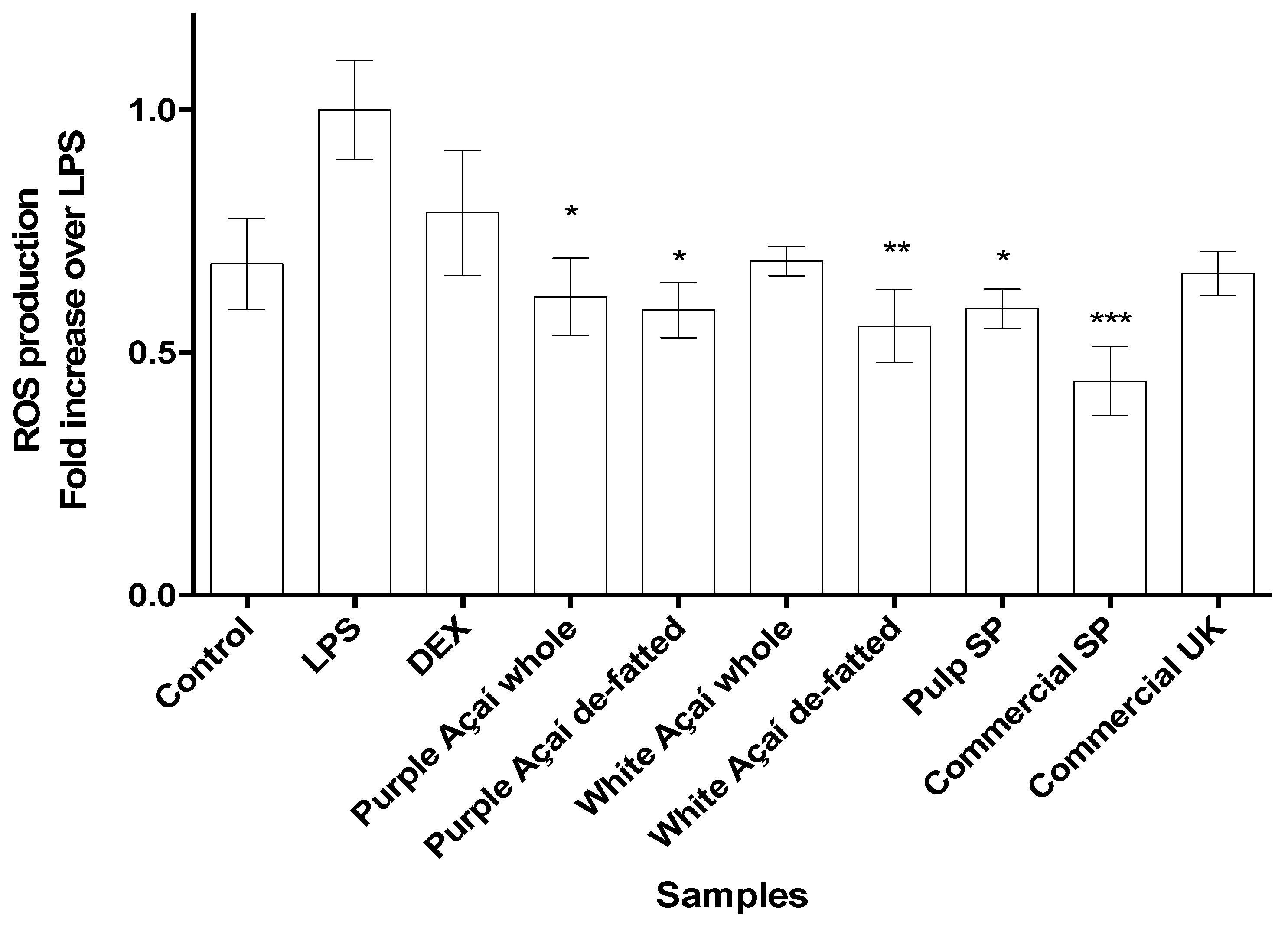
3.3.3. Radical Oxygen Species (ROS)
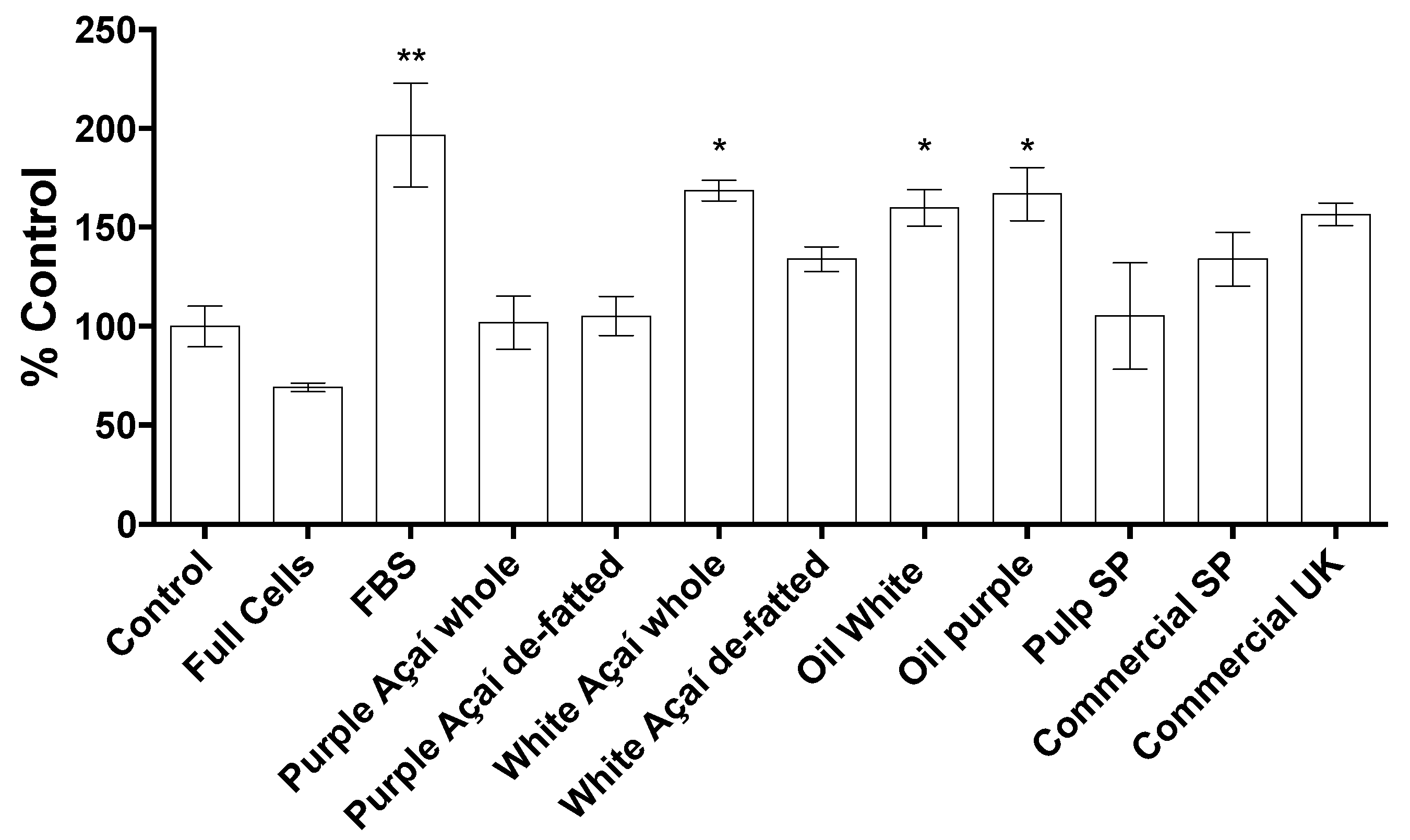
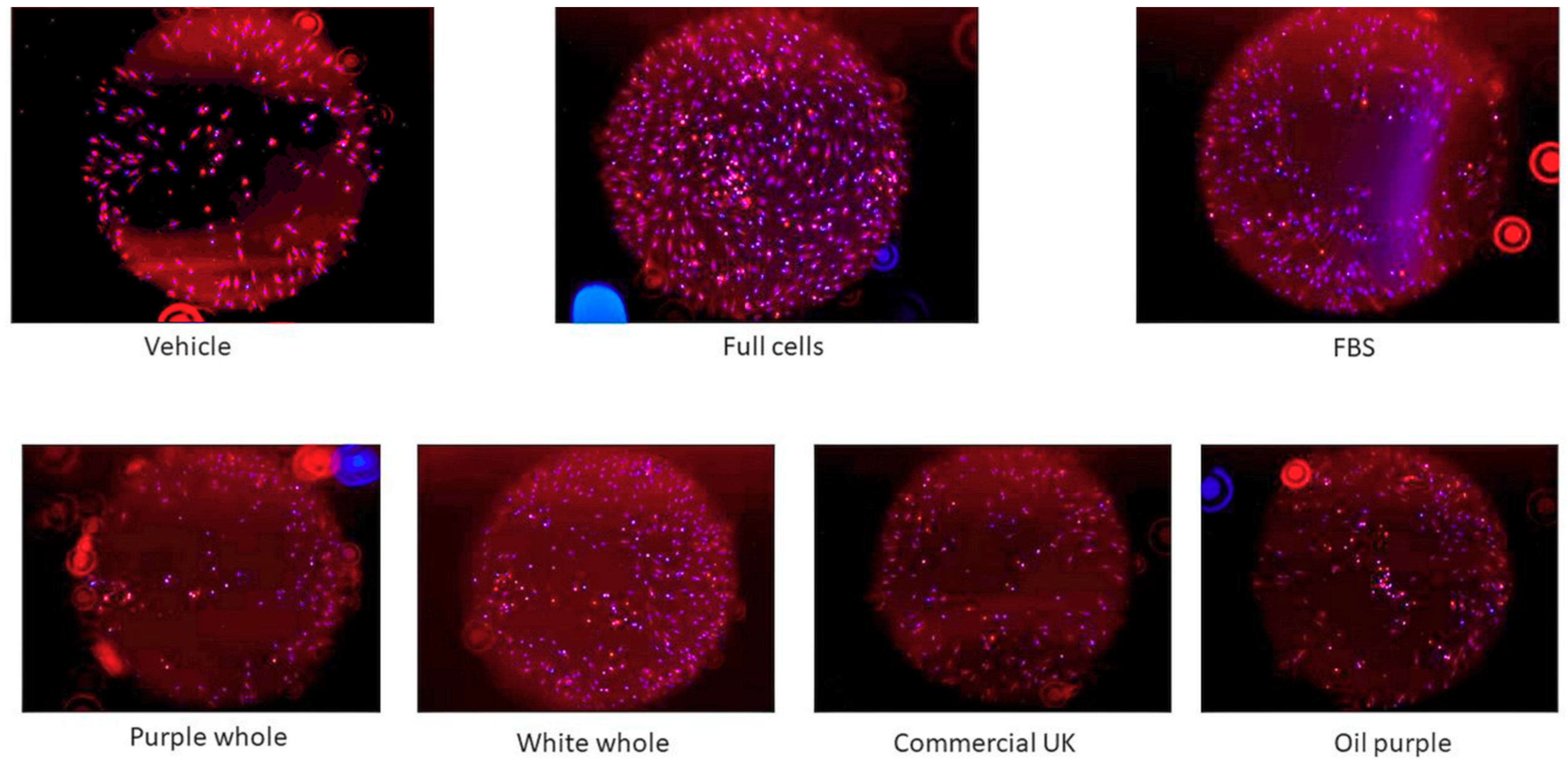
3.3.4. Cell Migration Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yamaguchi, K.K.D.L.; Pereira, L.F.R.; Lamarao, C.V.; Lima, E.S.; da Veiga-Junior, V.F. Amazon acai: Chemistry and biological activities: A review. Food Chem. 2015, 179, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Palencia, L.A.; Mertens-Talcott, S.; Talcott, S.T. Chemical composition, antioxidant properties, and thermal stability of a phytochemical enriched oil from Acai (Euterpe oleracea Mart.). J. Agric. Food Chem. 2008, 56, 4631–4636. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.; Balick, M.J. Palms, people, and health. Explore 2008, 4, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Pompeu, D.R.; Silva, E.M.; Rogez, H. Optimisation of the solvent extraction of phenolic antioxidants from fruits of Euterpe oleracea using response surface methodology. Bioresour. Technol. 2009, 100, 6076–6082. [Google Scholar] [CrossRef]
- Da Silveira, T.F.F.; De Souza, T.; Carvalho, A.V.; Ribeiro, A.B.; Kuhnle, G.G.; Godoy, H.T. White açaí juice (Euterpe oleracea): Phenolic composition by LC-ESI-MS/MS, antioxidant capacity and inhibition effect on the formation of colorectal cancer related compounds. J. Funct. Foods 2017, 36, 215–223. [Google Scholar] [CrossRef]
- de Sousa, A.M.; Oliveira, M.; de Farias Neto, J.T. Variabilidade Genética Entre Progênies de Açaí Branco Para Caracteres da Planta. Embrapa Amazônia Oriental-Artigo em Anais de Congresso (ALICE); SEMINÁRIO DE INICIAÇÃO CIENTÍFICA, 19; SEMINÁRIO DE PÓS-GRADUAÇÃO DA EMBRAPA: Belém, PA, Brazil, 2015. [Google Scholar]
- Lichtenthäler, R.; Rodrigues, R.B.; Maia, J.G.S.; Papagiannopoulos, M.; Fabricius, H.; Marx, F. Total oxidant scavenging capacities of Euterpe oleracea Mart.(Acai) fruits. Int. J. Food Sci. Nutr. 2005, 56, 53–64. [Google Scholar] [CrossRef]
- Heinrich, M.; Dhanji, T.; Casselman, I. Açai (Euterpe oleracea Mart.)-A phytochemical and pharmacological assessment of the species’ health claims. Phytochem. Lett. 2011, 4, 10–21. [Google Scholar] [CrossRef]
- Schauss, A.G.; Wu, X.; Prior, R.L.; Ou, B.; Huang, D.; Owens, J.; Agarwal, A.; Jensen, G.S.; Hart, A.N.; Shanbrom, E. Antioxidant capacity and other bioactivities of the freeze-dried Amazonian palm berry, Euterpe oleraceae mart. (acai). J. Agric. Food Chem. 2006, 54, 8604–8610. [Google Scholar] [CrossRef]
- Lobato, K.B.D.S.; Alamar, P.D.; Caramês, E.T.S.; Pallone, J.A.L. Authenticity of freeze-dried açai pulp by near-infrared spectroscopy. J. Food Eng. 2018, 224, 105–111. [Google Scholar] [CrossRef]
- Xiong, J.; Matta, F.V.; Grace, M.; Lila, M.A.; Ward, N.I.; Felipe-Sotelo, M.; Esposito, D. Phenolic content, anti-inflammatory, and dermal wound repair properties of industrially processed and non-processed acai from the Brazilian Amazon. Food Funct. 2020, 11, 4903–4914. [Google Scholar] [CrossRef]
- de Souza, M.O.; Silva, M.; Silva, M.E.; de Paula Oliveira, R.; Pedrosa, M.L. Diet supplementation with acai (Euterpe oleracea Mart.) pulp improves biomarkers of oxidative stress and the serum lipid profile in rats. Nutrition 2010, 26, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Rocha, A.; Carvalho, L.; Sousa, M.; Madeira, S.F.; Sousa, P.; Tano, T.; Schini-Kerth, V.B.; Resende, A.C.; De Moura, R.S. Endothelium-dependent vasodilator effect of Euterpe oleracea Mart.(Acai) extracts in mesenteric vascular bed of the rat. Vasc. Pharmacol. 2007, 46, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Mertens-Talcott, S.U.; Rios, J.; Jilma-Stohlawetz, P.; Pacheco-Palencia, L.A.; Meibohm, B.; Talcott, S.T.; Derendorf, H. Pharmacokinetics of anthocyanins and antioxidant effects after the consumption of anthocyanin-rich acai juice and pulp (Euterpe oleracea Mart.) in human healthy volunteers. J. Agric. Food Chem. 2008, 56, 7796–7802. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.C.; Antunes, L.M.G.; Aissa, A.F.; Darin, J.D.C.; De Rosso, V.V.; Mercadante, A.Z.; Bianchi, M.D.L.P. Evaluation of the genotoxic and antigenotoxic effects after acute and subacute treatments with açai pulp (Euterpe oleracea Mart.) on mice using the erythrocytes micronucleus test and the comet assay. Mutat. Res./Genet. Toxicol. Environ. Mutagenesis 2010, 695, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Schreckinger, M.E.; Lotton, J.; Lila, M.A.; de Mejia, E.G. Berries from South America: A comprehensive review on chemistry, health potential, and commercialization. J. Med. Food 2010, 13, 233–246. [Google Scholar] [CrossRef]
- Menezes, E.M.D.S.; Torres, A.T.; Sabaa Srur, A.U. Valor nutricional da polpa de açaí (Euterpe oleracea Mart) liofilizada. Acta Amaz. 2008, 38, 311–316. [Google Scholar] [CrossRef]
- Kang, M.H.; Choi, S.; Kim, B. Skin wound healing effects and action mechanism of acai berry water extracts. Toxicol. Res. 2017, 33, 149. [Google Scholar] [CrossRef]
- Kang, M.H.; Kim, B. Oral wound healing effects of acai berry water extracts in rat oral mucosa. Toxicol. Res. 2018, 34, 97. [Google Scholar] [CrossRef]
- Wild, T.; Rahbarnia, A.; Kellner, M.; Sobotka, L.; Eberlein, T. Basics in nutrition and wound healing. Nutrition 2010, 26, 862–866. [Google Scholar] [CrossRef]
- Sanchez, L.W.; Watson, R.R. Fruits, Vegetables, and Herbs: Bioactive Foods Promoting Wound Healing; Fruits, Vegetables, and Herbs; Elsevier: Cambridge, MA, USA, 2016; pp. 451–464. [Google Scholar]
- Esposito, D.; Overall, J.; Grace, M.; Komarnytsky, S.; Lila, M.A. Alaskan berry extracts promote dermal wound repair through modulation of bioenergetics and integrin signaling. Front. Pharmacol. 2019, 10, 1058. [Google Scholar] [CrossRef]
- Van de Velde, F.; Esposito, D.; Grace, M.H.; Pirovani, M.E.; Lila, M.A. Anti-inflammatory and wound healing properties of polyphenolic extracts from strawberry and blackberry fruits. Food Res. Int. 2019, 121, 453–462. [Google Scholar] [CrossRef]
- Pessoa, J.D.C.; Teixeira, G.d.A. Tecnologias Para Inovação nas Cadeias Euterpe; Embrapa Instrumentação-Livros científicos (ALICE): Belém, PA, Brazil, 2013. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Xiong, J.; Grace, M.H.; Esposito, D.; Wang, F.; Lila, M.A. Phytochemical characterization and anti-inflammatory properties of Acacia mearnsii leaves. Nat. Prod. Commun. 2016, 11, 1934578X1601100524. [Google Scholar] [CrossRef]
- Hoskin, R.T.; Xiong, J.; Lila, M.A. Comparison of berry juice concentrates and pomaces and alternative plant proteins to produce spray dried protein-polyphenol food ingredients. Food Funct. 2019, 10, 6207–6948. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Fan, E.; Ji, H.; Howell, A.; Nio, C.; Payne, M.J.; Reed, J. Multi-laboratory validation of a standard method for quantifying proanthocyanidins in cranberry powders. J. Sci. Food Agric. 2010, 90, 1473–1478. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Esposito, D.; Chen, A.; Grace, M.H.; Komarnytsky, S.; Lila, M.A. Inhibitory effects of wild blueberry anthocyanins and other flavonoids on biomarkers of acute and chronic inflammation in vitro. J. Agric. Food Chem. 2014, 62, 7022–7028. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Hwang, J.; Ko, H.; Park, J.; Kim, S. Nobiletin from citrus fruit peel inhibits the DNA-binding activity of NF-κB and ROS production in LPS-activated RAW 264.7 cells. J. Ethnopharmacol. 2007, 113, 149–155. [Google Scholar] [CrossRef]
- Augusti, P.R.; Torma, P.; Carvalho, A.V.; Flôres, S.H.; Rios, A.d.O. Compostos Bioativos e Atividade Antioxidante de Genótipos de Açaí (Euterpe Oleracea); Embrapa Amazônia Oriental-Artigo Em Anais de congresso (ALICE); Congresso Brasileiro de Ciência E Tecnologia de Alimentos: Gramado, RS, Brazil, 2016; p. 25. [Google Scholar]
- Gordon, A.; Cruz, A.P.G.; Cabral, L.M.C.; de Freitas, S.C.; Taxi, C.M.A.D.; Donangelo, C.M.; de Andrade Mattietto, R.; Friedrich, M.; da Matta, V.M.; Marx, F. Chemical characterization and evaluation of antioxidant properties of Açaí fruits (Euterpe oleraceae Mart.) during ripening. Food Chem. 2012, 133, 256–263. [Google Scholar] [CrossRef]
- Maria do Socorro, M.R.; Alves, R.E.; de Brito, E.S.; Pérez-Jiménez, J.; Saura-Calixto, F.; Mancini-Filho, J. Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chem. 2010, 121, 996–1002. [Google Scholar]
- Tonon, R.V.; Baroni, A.F.; Brabet, C.; Gibert, O.; Pallet, D.; Hubinger, M.D. Water sorption and glass transition temperature of spray dried açai (Euterpe oleracea Mart.) juice. J. Food Eng. 2009, 94, 215–221. [Google Scholar] [CrossRef]
- Rothwell, J.A.; Perez-Jimenez, J.; Neveu, V.; Medina-Remon, A.; M’Hiri, N.; García-Lobato, P.; Manach, C.; Knox, C.; Eisner, R.; Wishart, D.S. Phenol-Explorer 3.0: A major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database 2013. [Google Scholar] [CrossRef]
- Horszwald, A.; Andlauer, W. Characterisation of bioactive compounds in berry juices by traditional photometric and modern microplate methods. J. Berry Res. 2011, 1, 189–199. [Google Scholar] [CrossRef]
- de Rosso, V.V.; Hillebrand, S.; Montilla, E.C.; Bobbio, F.O.; Winterhalter, P.; Mercadante, A.Z. Determination of anthocyanins from acerola (Malpighia emarginata DC.) and açai (Euterpe oleracea Mart.) by HPLC–PDA–MS/MS. J. Food Compos. Anal. 2008, 21, 291–299. [Google Scholar] [CrossRef]
- Gouvêa, A.C.M.S.; Araujo, M.C.P.D.; Schulz, D.F.; Pacheco, S.; Godoy, R.L.D.O.; Cabral, L.M.C. Anthocyanins standards (cyanidin-3-O-glucoside and cyanidin-3-O-rutinoside) isolation from freeze-dried açaí (Euterpe oleraceae Mart.) by HPLC. Food Sci. Technol. 2012, 32, 43–46. [Google Scholar] [CrossRef]
- Del Pozo-Insfran, D.; Brenes, C.H.; Talcott, S.T. Phytochemical composition and pigment stability of Açai (Euterpe oleracea Mart.). J. Agric. Food Chem. 2004, 52, 1539–1545. [Google Scholar] [CrossRef]
- Earling, M.; Beadle, T.; Niemeyer, E.D. Açai Berry (Euterpe oleracea) dietary supplements: Variations in anthocyanin and flavonoid concentrations, phenolic contents, and antioxidant properties. Plant Foods Hum. Nutr. 2019, 74, 421–429. [Google Scholar] [CrossRef]
- He, F.; Pan, Q.; Shi, Y.; Duan, C. Chemical synthesis of proanthocyanidins in vitro and their reactions in aging wines. Molecules 2008, 13, 3007–3032. [Google Scholar] [CrossRef]
- Crozier, S.J.; Preston, A.G.; Hurst, J.W.; Payne, M.J.; Mann, J.; Hainly, L.; Miller, D.L. Cacao seeds are a “Super Fruit”: A comparative analysis of various fruit powders and products. Chem. Central J. 2011, 5, 1–6. [Google Scholar] [CrossRef]
- Garzón, G.A.; Narváez-Cuenca, C.; Vincken, J.; Gruppen, H. Polyphenolic composition and antioxidant activity of açai (Euterpe oleracea Mart.) from Colombia. Food Chem. 2017, 217, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Unicamp, N. Tabela Brasileira de Composição de Alimentos–TACO. UNICAMP; NEPA: Campinas, Brazil, 2011; p. 57. [Google Scholar]
- Santos, V.S.; Nardini, V.; Cunha, L.C., Jr.; Barbosa, F., Jr.; Teixeira, G.H.D.A. Identification of species of the Euterpe genus by rare earth elements using inductively coupled plasma mass spectrometry and linear discriminant analysis. Food Chem. 2014, 153, 334–339. [Google Scholar] [CrossRef]
- Gonzálvez, A.; Llorens, A.; Cervera, M.L.; Armenta, S.; De la Guardia, M. Elemental fingerprint of wines from the protected designation of origin Valencia. Food Chem. 2009, 112, 26–34. [Google Scholar] [CrossRef]
- Bannerman, D.D.; Goldblum, S.E. Mechanisms of bacterial lipopolysaccharide-induced endothelial apoptosis. Am. J. Physiol. Cell. Mol. Physiol. 2003, 284, L899–L914. [Google Scholar] [CrossRef]
- Matheus, M.E.; de Oliveira Fernandes, S.B.; Silveira, C.S.; Rodrigues, V.P.; de Sousa Menezes, F.; Fernandes, P.D. Inhibitory effects of Euterpe oleracea Mart. on nitric oxide production and iNOS expression. J. Ethnopharmacol. 2006, 107, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Azofeifa, G.; Quesada, S.; Pérez, A.M.; Vaillant, F.; Michel, A. Pasteurization of blackberry juice preserves polyphenol-dependent inhibition for lipid peroxidation and intracellular radicals. J. Food Compos. Anal. 2015, 42, 56–62. [Google Scholar] [CrossRef]
- Van de Velde, F.; Grace, M.H.; Esposito, D.; Pirovani, M.É.; Lila, M.A. Quantitative comparison of phytochemical profile, antioxidant, and anti-inflammatory properties of blackberry fruits adapted to Argentina. J. Food Compos. Anal. 2016, 47, 82–91. [Google Scholar] [CrossRef]
- Tattini, M.; Remorini, D.; Pinelli, P.; Agati, G.; Saracini, E.; Traversi, M.L.; Massai, R. Morpho-anatomical, physiological and biochemical adjustments in response to root zone salinity stress and high solar radiation in two Mediterranean evergreen shrubs, Myrtus communis and Pistacia lentiscus. New Phytol. 2006, 170, 779–794. [Google Scholar] [CrossRef]
- Minighin, E.C. Composição centesimal, perfil de ácidos graxos e efeitos da digestão in vitro sobre o teor de minerais, compostos fenólicos e atividade antioxidante de polpas comerciais de açaí (Euterpe oleracea Mart.) roxo e branco; Universidade Federal de Minas Gerais: Belo Horizonte, MG, Brazil, 2019. [Google Scholar]
- Moore, E.M.; Wagner, C.; Komarnytsky, S. The Enigma of Bioactivity and Toxicity of Botanical Oils for Skin Care. Front. Pharmacol. 2020, 11, 11. [Google Scholar] [CrossRef]
- Weimann, E.; Silva, M.B.B.; Murata, G.M.; Bortolon, J.R.; Dermargos, A.; Curi, R.; Hatanaka, E. Topical anti-inflammatory activity of palmitoleic acid improves wound healing. PLoS ONE 2018, 13, e0205338. [Google Scholar] [CrossRef]
- Yang, B.; Kalimo, K.O.; Mattila, L.M.; Kallio, S.E.; Katajisto, J.K.; Peltola, O.J.; Kallio, H.P. Effects of dietary supplementation with sea buckthorn (Hippophae rhamnoides) seed and pulp oils on atopic dermatitis. J. Nutr. Biochem. 1999, 10, 622–630. [Google Scholar] [CrossRef]
- Monge-Fuentes, V.; Muehlmann, L.A.; Longo, J.P.F.; Silva, J.R.; Fascineli, M.L.; de Souza, P.; Faria, F.; Degterev, I.A.; Rodriguez, A.; Carneiro, F.P. Photodynamic therapy mediated by acai oil (Euterpe oleracea Martius) in nanoemulsion: A potential treatment for melanoma. J. Photochem. Photobiol. B Biol. 2017, 166, 301–310. [Google Scholar] [CrossRef] [PubMed]
| Samples | Total Phenolics 1 | Total Flavonoids 2 | Total Anthocyanins 3 | Total Proanthocyanidins 4 | ABTS 5 | DPPH 6 | ||
|---|---|---|---|---|---|---|---|---|
| Non-commercial | Purple açaí whole | 32.00 ± 1.03 c | 6.39 ± 1.23 b | 10.20 ± 0.24 b | 6.10 ± 2.09 a | 438.0 ± 17.5 b | 336.0 ± 72.0 ab | |
| Purple açaí de-fatted | 39.40 ± 1.67 b | 8.05 ± 0.81 a | 14.33 ± 0.58 a | 5.06 ± 0.68 ab | 529.0 ± 57.0 a | 419.0 ± 69.5 a | ||
| White açaí whole | 9.40 ± 0.70 f | 2.12 ± 0.27 d | <0.01 | 3.96 ± 2.39 ab | 83.0 ± 9.7 de | 53.2 ± 15.1 d | ||
| White açaí de-fatted | 11.70 ± 0.24 e | 2.38 ± 0.35 d | <0.01 | 2.60 ± 0.49 ab | 101.0 ± 16.2 de | 67.4 ± 10.7 d | ||
| Oil white | 2.74 ± 1.13 g | 1.42 ± 0.96 de | <0.01 | 1.54 ± 0.22 ab | <15.3 | <7.4 | ||
| Oil purple | 1.68 ± 0.52 g | 0.87 ± 0.38 e | <0.01 | 3.40 ± 1.42 ab | <15.3 | <7.4 | ||
| Commercial | Pulp SP | 28.30 ± 0.64 e | 5.00 ± 0.68 c | 3.59 ± 0.12 c | 4.75 ± 1.58 ab | 310.0 ± 41.4 c | 222.0 ± 38.6 c | |
| Powder SP | 42.40 ± 1.53 a | 6.07 ± 1.45 bc | 4.70 ± 0.01 c | 4.47 ± 0.54 ab | 316.0 ± 39.1 c | 304.0 ± 43.2 bc | ||
| Powder UK | 5.09 ± 0.42 d | 1.88 ± 0.73 de | <0.01 | 5.48 ± 2.20 ab | 55.2 ± 31.4 e | <7.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matta, F.V.; Xiong, J.; Lila, M.A.; Ward, N.I.; Felipe-Sotelo, M.; Esposito, D. Chemical Composition and Bioactive Properties of Commercial and Non-Commercial Purple and White Açaí Berries. Foods 2020, 9, 1481. https://doi.org/10.3390/foods9101481
Matta FV, Xiong J, Lila MA, Ward NI, Felipe-Sotelo M, Esposito D. Chemical Composition and Bioactive Properties of Commercial and Non-Commercial Purple and White Açaí Berries. Foods. 2020; 9(10):1481. https://doi.org/10.3390/foods9101481
Chicago/Turabian StyleMatta, Fernanda V., Jia Xiong, Mary Ann Lila, Neil I. Ward, Mónica Felipe-Sotelo, and Debora Esposito. 2020. "Chemical Composition and Bioactive Properties of Commercial and Non-Commercial Purple and White Açaí Berries" Foods 9, no. 10: 1481. https://doi.org/10.3390/foods9101481
APA StyleMatta, F. V., Xiong, J., Lila, M. A., Ward, N. I., Felipe-Sotelo, M., & Esposito, D. (2020). Chemical Composition and Bioactive Properties of Commercial and Non-Commercial Purple and White Açaí Berries. Foods, 9(10), 1481. https://doi.org/10.3390/foods9101481