Impact of Dietary Supplementation with Goji Berries (Lycium barbarum) on Microbiological Quality, Physico-Chemical, and Sensory Characteristics of Rabbit Meat
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Feeding
2.2. Rabbit Meat Microbiological Parameters
2.3. Rabbit Meat Physico-Chemical Measurements
2.4. Rabbit Meat Sensory Analysis
2.5. Data Analysis
3. Results and Discussion
3.1. Productive Performance
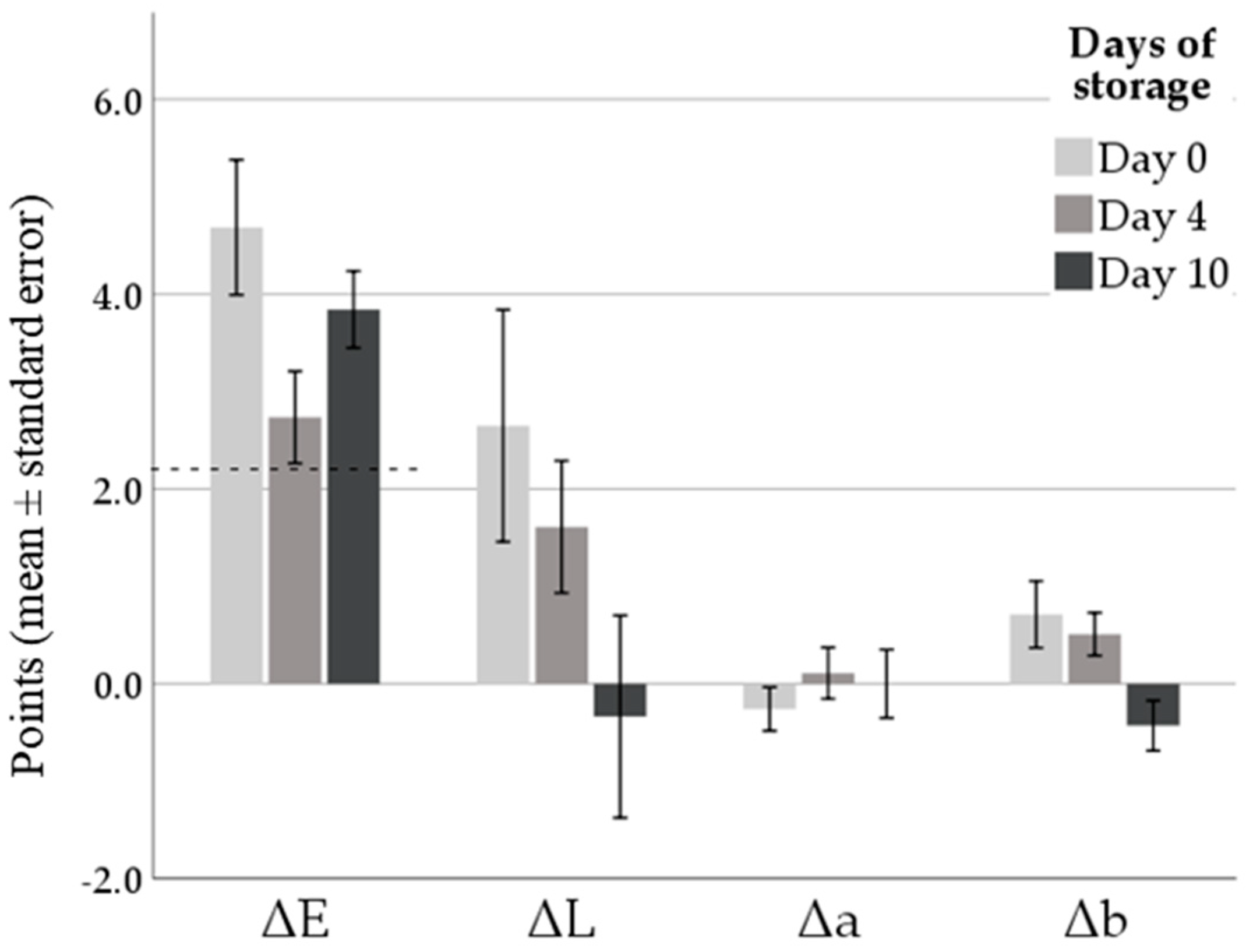
3.2. Color Values and pH
3.3. TBARS and TVBN
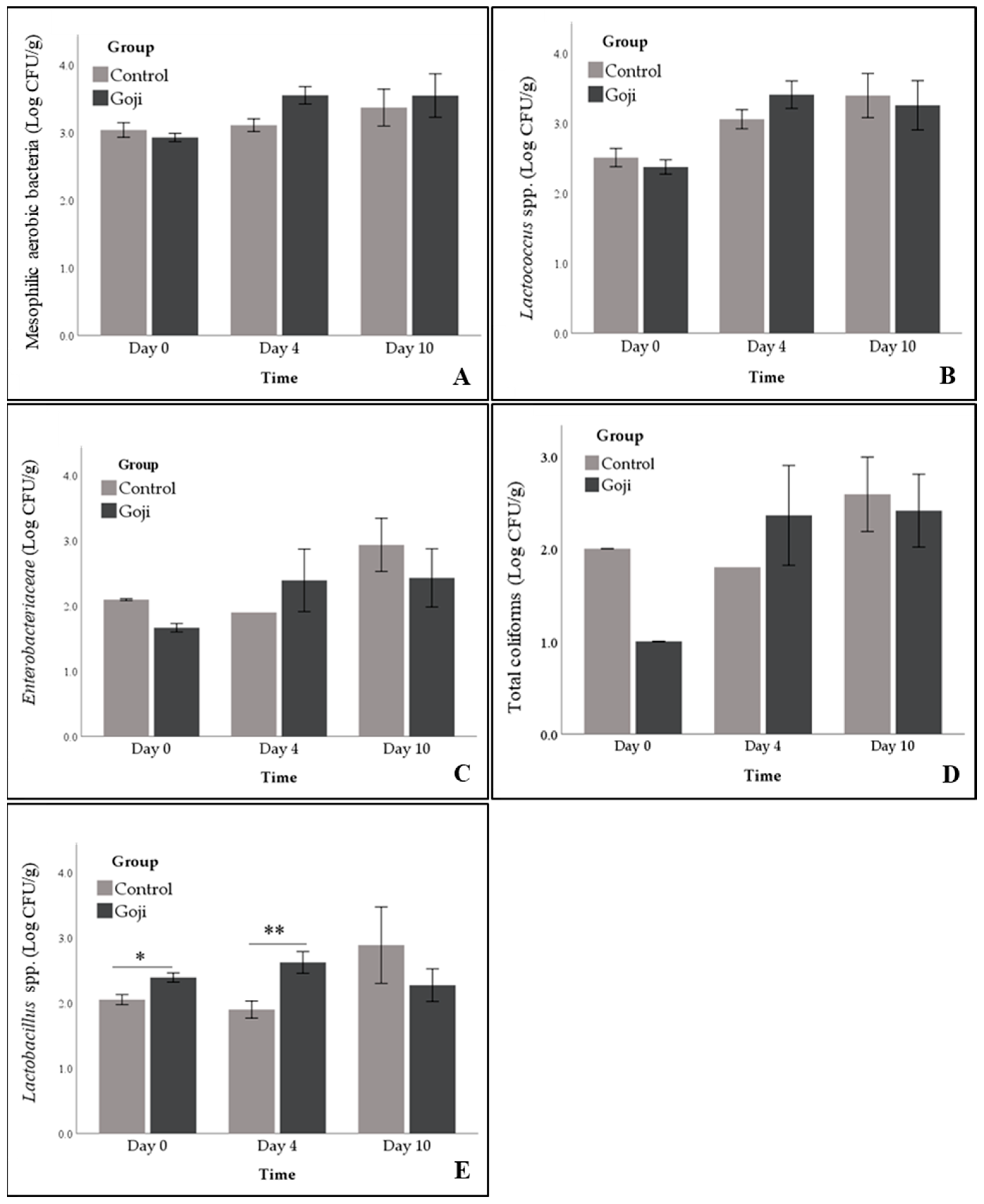
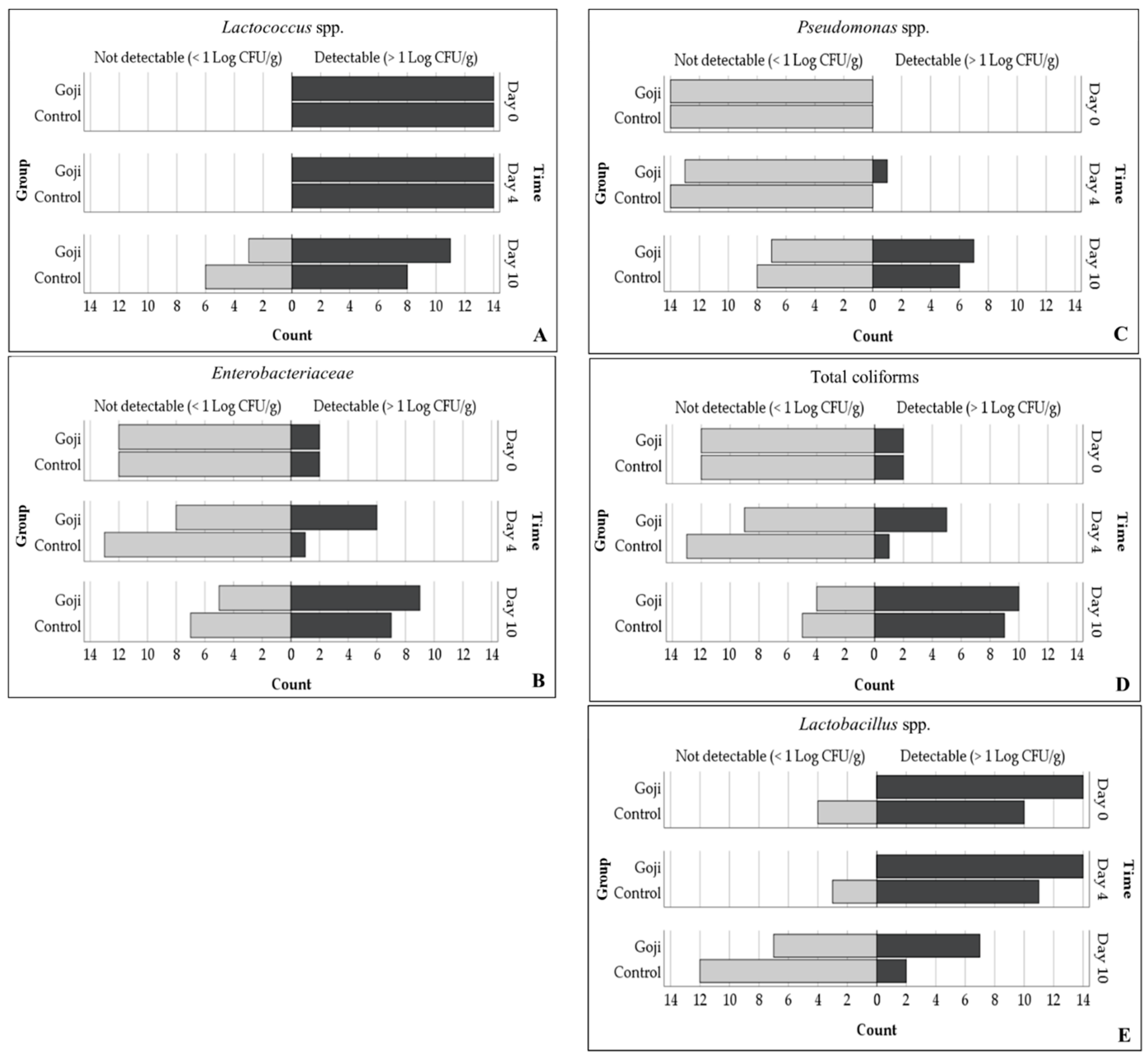
3.4. Meat Microbial Status
3.5. Sensory Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ma, Z.F.; Zhang, H.; Teh, S.S.; Wang, C.W.; Zhang, Y.; Hayford, F.; Wang, L.; Ma, T.; Dong, Z.; Zhang, Y.; et al. Goji berries as a potential natural antioxidant medicine: An insight into their molecular mechanisms of action. Oxidative Med. Cell. Longev. 2019, 2019, 2437397. [Google Scholar] [CrossRef] [PubMed]
- Potterat, O. Goji (Lycium barbarum and L. chinense): Phytochemistry, pharmacology and safety in the perspective of traditional uses and recent popularity. Planta Med. 2010, 76, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Carnés, J.; De Larramendi, C.H.; Ferrer, A.; Huertas, A.J.; López-Matas, M.A.; Pagán, J.A.; Navarro, L.A.; García-Abujeta, J.L.; Vicario, S.; Peña, M. Recently introduced foods as new allergenic sources: Sensitisation to Goji berries (Lycium barbarum). Food Chem. 2013, 137, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Peng, Y.; Xu, L.J.; Li, L.; Wu, Q.L.; Xiao, P.G. Phytochemical and biological studies of lycium medicinal plants. Chem. Biodivers. 2011, 8, 976–1010. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H.; Cheng, Q. Therapeutic roles of goji berry and ginseng in traditional Chinese. J. Nutr. Food Secur. 2019, 4, 293–305. [Google Scholar] [CrossRef]
- Gan, L.; Zhang, S.H.; Yang, X.L.; Xu, H.B. Immunomodulation and antitumor activity by a polysaccharide-protein complex from Lycium barbarum. Int. Immunopharmacol. 2004, 4, 563–569. [Google Scholar] [CrossRef]
- Cheng, D.; Kong, H. The effect of Lycium barbarum polysaccharide on alcohol-induced oxidative stress in rats. Molecules 2011, 16, 2542–2550. [Google Scholar] [CrossRef]
- Chen, S.; Liang, L.; Wang, Y.; Diao, J.; Zhao, C.; Chen, G.; He, Y.; Luo, C.; Wu, X.; Zhang, Y. Synergistic immunotherapeutic effects of Lycium barbarum polysaccharide and interferon-α2b on the murine Renca renal cell carcinoma cell line in vitro and in vivo. Mol. Med. Rep. 2015, 12, 6727–6737. [Google Scholar] [CrossRef]
- Flint, H.J.; Bayer, E.A.; Rincon, M.T.; Lamed, R.; White, B.A. Polysaccharide utilization by gut bacteria: Potential for new insights from genomic analysis. Nat. Rev. Microbiol. 2008, 6, 121–131. [Google Scholar] [CrossRef]
- Ding, Y.; Yan, Y.; Chen, D.; Ran, L.; Mi, J.; Lu, L.; Jing, B.; Li, X.; Zeng, X.; Cao, Y. Modulating effects of polysaccharides from the fruits of: Lycium barbarum on the immune response and gut microbiota in cyclophosphamide-treated mice. Food Funct. 2019, 10, 3671–3683. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, S.; Shen, Q.; Zhu, M.J. A metabolome explanation on beneficial effects of dietary Goji on intestine inflammation. J. Funct. Foods 2019, 53, 109–114. [Google Scholar] [CrossRef]
- Breme, K.; Guggenbühl, B. Aroma profile of a red-berries yoghurt drink by HS-SPME-GC-MS-O and influence of matrix texture on volatile aroma compound release of flavored dairy products. In Flavour Science; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Cruz, A.G.; Walter, E.H.M.; Cadena, R.S.; Faria, J.A.F.; Bolini, H.M.A.; Pinheiro, H.P.; Sant’Ana, A.S. Survival analysis methodology to predict the shelf-life of probiotic flavored yogurt. Food Res. Int. 2010, 43, 1444–1448. [Google Scholar] [CrossRef]
- Ścibisz, I.; Ziarno, M.; Mitek, M.; Zareba, D. Effect of probiotic cultures on the stability of anthocyanins in blueberry yoghurts. LWT Food Sci. Technol. 2012, 49, 208–212. [Google Scholar] [CrossRef]
- Chouchouli, V.; Kalogeropoulos, N.; Konteles, S.J.; Karvela, E.; Makris, D.P.; Karathanos, V.T. Fortification of yoghurts with grape (Vitis vinifera) seed extracts. LWT Food Sci. Technol. 2013, 53, 522–529. [Google Scholar] [CrossRef]
- Coda, R.; Lanera, A.; Trani, A.; Gobbetti, M.; Di Cagno, R. Yogurt-like beverages made of a mixture of cereals, soy and grape must: Microbiology, texture, nutritional and sensory properties. Int. J. Food Microbiol. 2012, 155, 120–127. [Google Scholar] [CrossRef]
- Rotar, A.M.; Vodnar, D.C.; Bunghez, F.; Cătunescu, G.M.; Pop, C.R.; Jimborean, M.; Semeniuc, C.A. Effect of goji berries and honey on lactic acid bacteria viability and shelf life stability of yoghurt. Not. Bot. Horti Agrobot. Cluj-Napoca 2015, 43, 196–203. [Google Scholar] [CrossRef]
- Ducruet, J.; Rébénaque, P.; Diserens, S.; Kosińska-Cagnazzo, A.; Héritier, I.; Andlauer, W. Amber ale beer enriched with goji berries—The effect on bioactive compound content and sensorial properties. Food Chem. 2017, 226, 109–118. [Google Scholar] [CrossRef]
- Shah, T.; Bule, M.; Niaz, K. Goji Berry (Lycium barbarum)- A Superfood. In Nonvitamin and Nonmineral Nutritional Supplements; Academic Press: Cambridge, MA, USA, 2018; ISBN 9780128124918. [Google Scholar]
- Bai, X.; Yan, X.; Xie, L.; Hu, X.; Lin, X.; Wu, C.; Zhou, N.; Wang, A.; See, M.T. Effects of pre-slaughter stressor and feeding preventative Chinese medicinal herbs on glycolysis and oxidative stability in pigs. Anim. Sci. J. 2016, 87, 1028–1033. [Google Scholar] [CrossRef]
- Menchetti, L.; Vecchione, L.; Filipescu, I.; Petrescu, V.F.; Fioretti, B.; Beccari, T.; Ceccarini, M.R.; Codini, M.; Quattrone, A.; Trabalza-Marinucci, M.; et al. Effects of Goji berries supplementation on the productive performance of rabbit. Livest. Sci. 2019, 220, 123–128. [Google Scholar] [CrossRef]
- Menchetti, L.; Brecchia, G.; Branciari, R.; Barbato, O.; Fioretti, B.; Codini, M.; Bellezza, E.; Trabalza-Marinucci, M.; Miraglia, D. The effect of Goji berries (Lycium barbarum) dietary supplementation on rabbit meat quality. Meat Sci. 2020, 161, 108018. [Google Scholar] [CrossRef] [PubMed]
- Cullere, M.; Zotte, A.D. Rabbit meat production and consumption: State of knowledge and future perspectives. Meat Sci. 2018, 143, 137–146. [Google Scholar] [CrossRef]
- Cullere, M.; Zotte, A.D.; Tasoniero, G.; Giaccone, V.; Szendrő, Z.; Szín, M.; Odermatt, M.; Gerencsér, Z.; Dal Bosco, A.; Matics, Z. Effect of diet and packaging system on the microbial status, pH, color and sensory traits of rabbit meat evaluated during chilled storage. Meat Sci. 2018, 141, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Menchetti, L.; Canali, C.; Castellini, C.; Boiti, C.; Brecchia, G. The different effects of linseed and fish oil supplemented diets on insulin sensitivity of rabbit does during pregnancy. Res. Vet. Sci. 2018, 118, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Menchetti, L.; Barbato, O.; Sforna, M.; Vigo, D.; Mattioli, S.; Curone, G.; Tecilla, M.; Riva, F.; Brecchia, G. Effects of diets enriched in linseed and fish oil on the expression pattern of toll-like receptors 4 and proinflammatory cytokines on gonadal axis and reproductive organs in rabbit buck. Oxidative Med. Cell. Longev. 2020, 2020, 4327470. [Google Scholar] [CrossRef]
- Maertens, L.; Moermans, R.; De Groote, G. Prediction of the apparent digestible energy content of commercial pelleted feeds for rabbits. J. Appl. Rabbit Res. 1988, 11, 60–67. [Google Scholar]
- Sharma, G. Digital Color Imaging Handbook; CRC Press: Boca Raton, FL, USA, 2017; ISBN 9781420041484. [Google Scholar]
- Mancini, S.; Mattioli, S.; Nuvoloni, R.; Pedonese, F.; Bosco, A.D.; Paci, G. Effects of garlic powder and salt on meat quality and microbial loads of rabbit burgers. Foods 2020, 9, 1022. [Google Scholar] [CrossRef]
- Tarladgis, B.G.; Watts, B.M.; Younathan, M.T.; Dugan, L. A distillation method for the quantitative determination of malonaldehyde in rancid foods. J. Am. Oil Chem. Soc. 1960, 37, 44–48. [Google Scholar] [CrossRef]
- FAO/WHO. Statistical Aspects of Microbiological Criteria Related to Foods; FAO: Rome, Italy, 2016. [Google Scholar]
- Koné, A.P.; Desjardins, Y.; Gosselin, A.; Cinq-Mars, D.; Guay, F.; Saucier, L. Plant extracts and essential oil product as feed additives to control rabbit meat microbial quality. Meat Sci. 2019, 150, 111–121. [Google Scholar] [CrossRef]
- Smeti, S.; Atti, N.; Mahouachi, M.; Munoz, F. Use of dietary rosemary (Rosmarinus officinalis L.) essential oils to increase the shelf life of Barbarine light lamb meat. Small Rumin. Res. 2013, 113, 340–345. [Google Scholar] [CrossRef]
- Nieto, G.; Díaz, P.; Bañón, S.; Garrido, M.D. Dietary administration of ewe diets with a distillate from rosemary leaves (Rosmarinus officinalis L.): Influence on lamb meat quality. Meat Sci. 2010, 84, 23–29. [Google Scholar] [CrossRef]
- Salueña, B.H.; Gamasa, C.S.; Rubial, J.M.D.; Odriozola, C.A. CIELAB color paths during meat shelf life. Meat Sci. 2019, 157, 107889. [Google Scholar] [CrossRef]
- Renerre, M. Factors involved in the discoloration of beef meat. Int. J. Food Sci. Technol. 1990, 25, 613–630. [Google Scholar] [CrossRef]
- Miraglia, D.; Ranucci, D.; Trabalza-Marinucci, M.; Acuti, G.; Forte, C.; Codini, M.; Roila, R.; Branciari, R. Microbiological, chemical-physical and sensory characteristics of Fabriano salami from pigs fed Oregano vulgaris extract. Ital. J. Food Saf. 2017, 6, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Balentine, C.W.; Crandall, P.G.; O’Bryan, C.A.; Duong, D.Q.; Pohlman, F.W. The pre- and post-grinding application of rosemary and its effects on lipid oxidation and color during storage of ground beef. Meat Sci. 2006, 73, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Ripoll, G.; Joy, M.; Muñoz, F. Use of dietary vitamin E and selenium (Se) to increase the shelf life of modified atmosphere packaged light lamb meat. Meat Sci. 2011, 87, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.I.; Gomaa, E.A.; Buckley, D.J. Oxidative quality and shelf life of meats. Meat Sci. 1996, 43, 111–123. [Google Scholar] [CrossRef]
- Wang, Z.; He, Z.; Zhang, D.; Li, H.; Wang, Z. Using oxidation kinetic models to predict the quality indices of rabbit meat under different storage temperatures. Meat Sci. 2020, 162, 108042. [Google Scholar] [CrossRef]
- Castrica, M.; Panseri, S.; Siletti, E.; Borgonovo, F.; Chiesa, L.; Balzaretti, C.M. Evaluation of smart portable device for food diagnostics: A preliminary study on Cape Hake fillets (M. Capensis and M. Paradoxus). J. Chem. 2019, 2019, 1–7. [Google Scholar] [CrossRef]
- Kyrana, V.R.; Lougovois, V.P. Sensory, chemical and microbiological assessment of farm-raised European sea bass (Dicentrarchus labrax) stored in melting ice. Int. J. Food Sci. Technol. 2002, 37, 319–328. [Google Scholar] [CrossRef]
- Byun, J.S.; Min, J.S.; Kim, I.S.; Kim, J.W.; Chung, M.S.; Lee, M. Comparison of indicators of microbial quality of meat during aerobic cold storage. J. Food Prot. 2003, 66, 1733–1737. [Google Scholar] [CrossRef]
- Pearson, A.M.; Gray, J.I.; Wolzak, A.M.; Horenstein, N.A. Safety implications of oxidized lipids in muscle foods. Food Technol. 1983, 37, 121–129. [Google Scholar]
- Ranucci, D.; Miraglia, D.; Marinucci, M.T.; Acuti, G.; Codini, M.; Ceccarini, M.R.; Forte, C.; Branciari, R. Dietary effects of oregano (Origanum vulgaris L.) plant or sweet chestnut (Castanea sativa Mill.) wood extracts on microbiological, chemical-physical characteristics and lipid oxidation of cooked ham during storage. Ital. J. Food Saf. 2015, 4, 5497. [Google Scholar] [CrossRef] [PubMed]
- Rouger, A.; Tresse, O.; Zagorec, M. Bacterial contaminants of poultry meat: Sources, species, and dynamics. Microorganisms 2017, 5, 50. [Google Scholar] [CrossRef] [PubMed]
- Hernández, P. Enhancement of nutritional quality and safety in rabbit meat. In Proceedings of the 9th World Rabbit Congress, Verona, Italy, 10–13 June 2008. [Google Scholar]
- Vannini, L.; Iucci, L.; Guerzoni, M.E. Risk assessment in rabbit meat products through the slaughtering/processing/storage phase. In Proceedings of the 2nd Meeting Working Group 5 “Meat quality and safety” COST Action 848, Athens, Greece, 11–14 April 2002; pp. 4–6. [Google Scholar]
- Vannini, L.; Sado, S.; Iucci, L.; Ndagijimana, M.; Guerzoni, M.E. The dietary use of linseed in growing rabbits: Effects on microbial population and spoilage patterns of meat products. In Proceedings of the 3rd Meeting Working Group 4 “Nutrition and pathology” and 5 “Meat quality and safety” COST Action 848, Prague, Czech Republic, 25–27 September 2003; pp. 32–33. [Google Scholar]
- Lavefve, L.; Howard, L.R.; Carbonero, F. Berry polyphenols metabolism and impact on human gut microbiota and health. Food Funct. 2020, 11, 45–65. [Google Scholar] [CrossRef] [PubMed]
- Chien, K.J.; Horng, C.T.; Huang, Y.S.; Hsieh, Y.H.; Wang, C.J.; Yang, J.S.; Lu, C.C.; Chen, F.A. Effects of Lycium barbarum (goji berry) on dry eye disease in rats. Mol. Med. Rep. 2018, 17, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Nardi, G.M.; De Farias Januário, A.G.; Freire, C.G.; Megiolaro, F.; Schneider, K.; Perazzoli, M.R.A.; Do Nascimento, S.R.; Gon, A.C.; Mariano, L.N.B.; Wagner, G.; et al. Anti-inflammatory activity of berry fruits in mice model of inflammation is based on oxidative stress modulation. Pharmacogn. Res. 2016, 8, S42–S49. [Google Scholar] [CrossRef]
- Soomro, A.H.; Masud, T.; Anwaar, K. Role of Lactic Acid Bacteria (LAB) in food preservation and human health—A review. Pak. J. Nutr. 2002, 1, 20–24. [Google Scholar] [CrossRef]
- Pothakos, V.; Devlieghere, F.; Villani, F.; Björkroth, J.; Ercolini, D. Lactic acid bacteria and their controversial role in fresh meat spoilage. Meat Sci. 2015, 109, 66–74. [Google Scholar] [CrossRef]
- Ce.I.R.S.A. Centro Interdipartimentale di ricerca e Documentazione sulla Sicurezza Alimentare. Linee Guida per l’analisi del Rischio nel Campo Della Microbiologia Degli Alimenti. 2017. Available online: https://www.ceirsa.org/matrice.php (accessed on 11 September 2020).
- Zhang, W.; Xiao, S.; Samaraweera, H.; Lee, E.J.; Ahn, D.U. Improving functional value of meat products. Meat Sci. 2010, 86, 15–31. [Google Scholar] [CrossRef]
- Zotte, A.D.; Szendro, Z. The role of rabbit meat as functional food. Meat Sci. 2011, 88, 319–331. [Google Scholar] [CrossRef]
| Unit | Group | ||
|---|---|---|---|
| Control | Goji | ||
| Ingredients | |||
| Wheat bran | % | 30.0 | 29.0 |
| Dehydrated alfalfa meal | % | 42.0 | 41.0 |
| Barley | % | 9.5 | 9.0 |
| Sunflower meal | % | 4.5 | 4.2 |
| Rice bran | % | 4.0 | 3.9 |
| Soybean meal | % | 4.0 | 3.9 |
| Calcium carbonate | % | 2.2 | 2.2 |
| Cane molasses | % | 2.0 | 2.0 |
| Vitamin–mineral premix 1 | % | 0.4 | 0.4 |
| Soybean oil | % | 0.4 | 0.4 |
| Salt | % | 0.3 | 0.3 |
| Goji berries | % | - | 3.0 |
| Analytical data | |||
| Crude Protein | % | 15.74 | 15.66 |
| Ether extract | % | 2.25 | 2.47 |
| Ash | % | 9.28 | 9.25 |
| Starch | % | 16.86 | 16.99 |
| NDF 3 | % | 38.05 | 37.49 |
| ADF 3 | % | 19.54 | 19.01 |
| ADL 3 | % | 4.01 | 3.98 |
| Digestible Energy 2 | MJ/kg | 10.3 | 10.3 |
| Parameter | Time (Day of Storage) | Group | p Value | |||
|---|---|---|---|---|---|---|
| Control | Goji | Group | Time | Group × Time | ||
| TBARS (mg MDA/kg) | Day 0 | 0.248 aA ± 0.048 | 0.135 bA ± 0.027 | <0.001 | 0.042 | 0.257 |
| Day 4 | 0.398 aB ± 0.050 | 0.144 bA ± 0.028 | ||||
| Day 10 | 0.374 aB ± 0.072 | 0.232 bA ± 0.045 | ||||
| TVBN (mg N/100 g) | Day 0 | 14.919 aA ± 0.327 | 15.823 bAB ± 0.336 | 0.024 | <0.001 | 0.204 |
| Day 4 | 15.140 aA ± 0.160 | 15.584 aA ± 0.151 | ||||
| Day 10 | 16.300 aB ± 0.339 | 16.320 aB ± 0.329 | ||||
| Attributes | Group | SEM | p Value | |
|---|---|---|---|---|
| Control | Goji | |||
| Color | 5.81 | 5.93 | 0.152 | 0.590 |
| Juiciness | 5.58 | 6.42 | 0.141 | <0.01 |
| Taste | 6.17 | 6.68 | 0.153 | 0.020 |
| Overall liking | 6.08 | 6.50 | 0.150 | 0.042 |
| Positive (Yes) Purchase Intent (%) | ||||
| Before information | 31.7 | 45.0 | - | 0.133 * |
| After information | 21.7 | 71.7 | - | <0.001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castrica, M.; Menchetti, L.; Balzaretti, C.M.; Branciari, R.; Ranucci, D.; Cotozzolo, E.; Vigo, D.; Curone, G.; Brecchia, G.; Miraglia, D. Impact of Dietary Supplementation with Goji Berries (Lycium barbarum) on Microbiological Quality, Physico-Chemical, and Sensory Characteristics of Rabbit Meat. Foods 2020, 9, 1480. https://doi.org/10.3390/foods9101480
Castrica M, Menchetti L, Balzaretti CM, Branciari R, Ranucci D, Cotozzolo E, Vigo D, Curone G, Brecchia G, Miraglia D. Impact of Dietary Supplementation with Goji Berries (Lycium barbarum) on Microbiological Quality, Physico-Chemical, and Sensory Characteristics of Rabbit Meat. Foods. 2020; 9(10):1480. https://doi.org/10.3390/foods9101480
Chicago/Turabian StyleCastrica, Marta, Laura Menchetti, Claudia M. Balzaretti, Raffaella Branciari, David Ranucci, Elisa Cotozzolo, Daniele Vigo, Giulio Curone, Gabriele Brecchia, and Dino Miraglia. 2020. "Impact of Dietary Supplementation with Goji Berries (Lycium barbarum) on Microbiological Quality, Physico-Chemical, and Sensory Characteristics of Rabbit Meat" Foods 9, no. 10: 1480. https://doi.org/10.3390/foods9101480
APA StyleCastrica, M., Menchetti, L., Balzaretti, C. M., Branciari, R., Ranucci, D., Cotozzolo, E., Vigo, D., Curone, G., Brecchia, G., & Miraglia, D. (2020). Impact of Dietary Supplementation with Goji Berries (Lycium barbarum) on Microbiological Quality, Physico-Chemical, and Sensory Characteristics of Rabbit Meat. Foods, 9(10), 1480. https://doi.org/10.3390/foods9101480












