Current Insights into Monitoring, Bioaccumulation, and Potential Health Effects of Microplastics Present in the Food Chain
Abstract
1. Introduction
2. Occurrence and Concentrations in the Food Production Chain
3. In Vivo Uptake and Bioaccumulation Kinetics
Rodent Studies
4. In Vivo Effects
4.1. Rodent Studies
4.2. Supporting Documentation from Studies in Zebrafish
5. In Vitro Experiments with Human Cells
6. Methods for Detection and Identification
6.1. Elements in Methodology: Sample Preparation, MP Detection, and Identification
6.1.1. Isolation of MPs
6.1.2. Detection Techniques
6.1.3. Identification of MPs
6.2. Quality Assurance and Quality Control
7. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- European Commission. Closing the Loop—An EU Action Plan for the Circular Economy. Communication No. 52015DC0614 of 2 December 2015. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52015DC0614 (accessed on 4 December 2019).
- European Commission. Directive No 2018/851 of the European Parliament and of the Council of 30 May 2018 amending Directive 2008/98/EC on waste. Off. J. Eur. Union 2018, 150, 109–140. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv:OJ.L_.2018.150.01.0109.01.ENG (accessed on 4 December 2019).
- European Commission. European Council Decision No 2018/340 of 6 March 2018 establishing the list of projects to be developed under PESCO. Off. J. Eur. Union 2018, 65, 24. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32018D0340 (accessed on 4 December 2019).
- Karbalaei, S.; Hanachi, P.; Walker, T.R.; Cole, M. Occurrence, sources, human health impacts and mitigation of microplastic pollution. Environ. Sci. Pollut. Res. Int. 2018, 25, 36046–36063. [Google Scholar] [CrossRef] [PubMed]
- Talvitie, J.; Mikola, A.; Koistinen, A.; Setälä, O. Solutions to microplastic pollution—Removal of microplastics from wastewater effluent with advanced wastewater treatment technologies. Water Res. 2017, 123, 401–407. [Google Scholar] [CrossRef]
- Schwabl, P.; Koppel, S.; Konigshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of Various Microplastics in Human Stool: A Prospective Case Series. Ann. Intern. Med. 2019. [Google Scholar] [CrossRef] [PubMed]
- Lusher, A.L.; Hollman, P.C.; Mendoza-Hill, J. Microplastic in Fisheries and Aquaculture; FAO Fisheries and Aquaculture Technical Paper; FAO: Rome, Italy, 2017; Volume 615. [Google Scholar]
- Yurtsever, M. Glitters as a Source of Primary Microplastics: An Approach to Environmental Responsibility and Ethics. J. Agric. Environ. Ethic 2019, 32, 459–478. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Presence of microplastics and nanoplastics in food, with particular focus on seafood. EFSA J. 2016, 14, e04501. [Google Scholar] [CrossRef]
- Hartmann, N.B.; Huffer, T.; Thompson, R.C.; Hassellov, M.; Verschoor, A.; Daugaard, A.E.; Rist, S.; Karlsson, T.; Brennholt, N.; Cole, M.; et al. Are We Speaking the Same Language? Recommendations for a Definition and Categorization Framework for Plastic Debris. Environ. Sci. Technol. 2019, 53, 1039–1047. [Google Scholar] [CrossRef]
- Verschoor, A.; de Poorter, L.; Droege, R.; Kuenen, J.; de Valk, E. Emission of Microplastics and Potential Mitigation Measures: Abrasive Cleaning Agents, Paints and Tyre Wear; 2016-0026; Netherlands National Institute for Public Health and the Environment: Bilthofen, The Netherlands, 2016; Available online: https://www.rivm.nl/bibliotheek/rapporten/2016-0026.pdf (accessed on 4 December 2019).
- Hantoro, I.; Lohr, A.J.; Van Belleghem, F.; Widianarko, B.; Ragas, A.M.J. Microplastics in coastal areas and seafood: Implications for food safety. Food Addit Contam A Chem. Anal. Control Expo. Risk Assess 2019, 36, 674–711. [Google Scholar] [CrossRef]
- Toussaint, B.; Raffael, B.; Angers-Loustau, A.; Gilliland, D.; Kestens, V.; Petrillo, M.; Rio-Echevarria, I.M.; Van den Eede, G. Review of micro- and nanoplastic contamination in the food chain. Food Addit. Contam A Chem. Anal. Control Expo. Risk Assess 2019, 36, 639–673. [Google Scholar] [CrossRef]
- Van Raamsdonk, L.W.D.; Rijk, R.; Schouten, G.P.J.; Mennes, W.; Meijer, G.A.L.; van der Poel, A.F.B.; de Jong, J. A risk Evaluation of Traces of Packaging Materials in Former Food Products Intended as Feed Materials; 2011.002; Wageningen Food Safety Research Report; Wageningen University and Research Centre: Wageningen, The Netherlands, 2011; Available online: https://wur.on.worldcat.org/oclc/731944123 (accessed on 4 December 2019).
- Karami, A.; Golieskardi, A.; Choo, C.K.; Larat, V.; Karbalaei, S.; Salamatinia, B. Microplastic and mesoplastic contamination in canned sardines and sprats. Sci. Total Environ. 2018, 612, 1380–1386. [Google Scholar] [CrossRef] [PubMed]
- European Academies. A Scientific Perspective on Microplastics in Nature and Society; Science Advice for Policy by European Academies; 978-3-9820301-0-4; European Academies: Berlin, Germany, 2019; Available online: https://www.sapea.info/topics/microplastics/ (accessed on 4 December 2019).
- Maes, T.; Jessop, R.; Wellner, N.; Haupt, K.; Mayes, A.G. A rapid-screening approach to detect and quantify microplastics based on fluorescent tagging with Nile Red. Sci. Rep. 2017, 7, 44501. [Google Scholar] [CrossRef] [PubMed]
- Shim, W.J.; Hong, S.H.; Eo, S.E. Identification methods in microplastic analysis: A review. Anal. Methods 2017, 9, 1384–1391. [Google Scholar] [CrossRef]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the Marine Environment: A Review of the Methods Used for Identification and Quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef] [PubMed]
- Koelmans, A.A.; Mohamed Nor, N.H.; Hermsen, E.; Kooi, M.; Mintenig, S.M.; De France, J. Microplastics in freshwaters and drinking water: Critical review and assessment of data quality. Water Res. 2019, 155, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Lu, S.; Song, Y.; Lei, L.; Hu, J.; Lv, W.; Zhou, W.; Cao, C.; Shi, H.; Yang, X.; et al. Microplastic and mesoplastic pollution in farmland soils in suburbs of Shanghai, China. Environ. Pollut. 2018, 242, 855–862. [Google Scholar] [CrossRef]
- Marsden, P.; Koelmans, A.; Bourdon-Lacombe, J.; Gouin, T.; D‘Anglada, L.; Cunliffe, D.; Jarvis, P.; Fawell, J.; De France, J. Microplastics in Drinking-Water; World Health Organisation: Geneva, Switzerland, 2019; ISBN 978-92-4-151619-8. Available online: https://apps.who.int/iris/bitstream/handle/10665/326499/9789241516198-eng.pdf?ua=1 (accessed on 4 December 2019).
- Oliveira, M.; Almeida, M. The why and how of micro(nano)plastic research. TrAC Trends Anal. Chem. 2019, 114, 196–201. [Google Scholar] [CrossRef]
- Pagter, E.; Frias, J.; Nash, R. Microplastics in Galway Bay: A comparison of sampling and separation methods. Mar. Pollut. Bull. 2018, 135, 932–940. [Google Scholar] [CrossRef]
- Bové, H.; Bongaerts, E.; Slenders, E.; Bijnens, E.M.; Saenen, N.D.; Gyselaers, W.; Van Eyken, P.; Plusquin, M.; Roeffaers, M.B.J.; Ameloot, M.; et al. Ambient black carbon particles reach the fetal side of human placenta. Nat. Commun. 2019, 10, 3866. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Fu, W.; Xia, X.; Liu, C.; Min, J.; Zhang, W.; Crittenden, J.C. Interactions between nano/micro plastics and suspended sediment in water: Implications on aggregation and settling. Water Res. 2019, 161, 486–495. [Google Scholar] [CrossRef]
- Klein, S.; Worch, E.; Knepper, T.P. Occurrence and Spatial Distribution of Microplastics in River Shore Sediments of the Rhine-Main Area in Germany. Environ. Sci. Technol. 2015, 49, 6070–6076. [Google Scholar] [CrossRef] [PubMed]
- Leslie, H.A.; Brandsma, S.H.; van Velzen, M.J.M.; Vethaak, A.D. Microplastics en route: Field measurements in the Dutch river delta and Amsterdam canals, wastewater treatment plants, North Sea sediments and biota. Environ. Int. 2017, 101, 133–142. [Google Scholar] [CrossRef]
- He, B.; Goonetilleke, A.; Ayoko, G.A.; Rintoul, L. Abundance, distribution patterns, and identification of microplastics in Brisbane River sediments, Australia. Sci. Total Environ. 2019, 700, 134467. [Google Scholar] [CrossRef]
- Weithmann, N.; Möller, J.N.; Löder, M.G.J.; Piehl, S.; Laforsch, C.; Freitag, R. Organic fertilizer as a vehicle for the entry of microplastic into the environment. Sci. Adv. 2018, 4, eaap8060. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C.; Lehmann, A.; de Souza Machado, A.A.; Yang, G. Microplastic effects on plants. New Phytol. 2019, 223, 1066–1070. [Google Scholar] [CrossRef] [PubMed]
- Sundbæk, K.B.; Koch, I.D.W.; Villaro, C.G.; Rasmussen, N.S.; Holdt, S.L.; Hartmann, N.B. Sorption of fluorescent polystyrene microplastic particles to edible seaweed Fucus vesiculosus. J. Appl. Phycol. 2018, 30, 2923–2927. [Google Scholar] [CrossRef]
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human Consumption of Microplastics. Environ. Sci. Technol. 2019, 53, 7068–7074. [Google Scholar] [CrossRef]
- Mason, S.A.; Welch, V.G.; Neratko, J. Synthetic Polymer Contamination in Bottled Water. Front. Chem. 2018, 6, 407. [Google Scholar] [CrossRef]
- Li, J.; Green, C.; Reynolds, A.; Shi, H.; Rotchell, J.M. Microplastics in mussels sampled from coastal waters and supermarkets in the United Kingdom. Environ. Pollut. 2018, 241, 35–44. [Google Scholar] [CrossRef]
- Teng, J.; Wang, Q.; Ran, W.; Wu, D.; Liu, Y.; Sun, S.; Liu, H.; Cao, R.; Zhao, J. Microplastic in cultured oysters from different coastal areas of China. Sci. Total Environ. 2019, 653, 1282–1292. [Google Scholar] [CrossRef]
- Baechler, B.R.; Granek, E.F.; Hunter, M.V.; Conn, K.E. Microplastic concentrations in two Oregon bivalve species: Spatial, temporal, and species variability. Limnol. Oceanogr. Lett. 2019. [Google Scholar] [CrossRef]
- Oβmann, B.E.; Sarau, G.; Holtmannspotter, H.; Pischetsrieder, M.; Christiansen, S.H.; Dicke, W. Small-sized microplastics and pigmented particles in bottled mineral water. Water Res. 2018, 141, 307–316. [Google Scholar] [CrossRef]
- Pivokonsky, M.; Cermakova, L.; Novotna, K.; Peer, P.; Cajthaml, T.; Janda, V. Occurrence of microplastics in raw and treated drinking water. Sci. Total Environ. 2018, 643, 1644–1651. [Google Scholar] [CrossRef] [PubMed]
- Schymanski, D.; Goldbeck, C.; Humpf, H.U.; Furst, P. Analysis of microplastics in water by micro-Raman spectroscopy: Release of plastic particles from different packaging into mineral water. Water Res. 2018, 129, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Kosuth, M.; Mason, S.A.; Wattenberg, E.V. Anthropogenic contamination of tap water, beer, and sea salt. PLoS ONE 2018, 13, e0194970. [Google Scholar] [CrossRef] [PubMed]
- Wiggin, K.J.; Holland, E.B. Validation and application of cost and time effective methods for the detection of 3–500 μm sized microplastics in the urban marine and estuarine environments surrounding Long Beach, California. Mar. Pollut. Bull. 2019, 143, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, L.M.; Xu, E.G.; Larsson, H.C.E.; Tahara, R.; Maisuria, V.B.; Tufenkji, N. Plastic Teabags Release Billions of Microparticles and Nanoparticles into Tea. Environ. Sci. Technol. 2019, 53, 12300–12310. [Google Scholar] [CrossRef]
- International Organization for Standardization (ISO) 3103. Tea—Preparation of Liquor for Use in Sensory Tests; International Organization for Standardization: Geneva, Switzerland, 1980. [Google Scholar]
- Bergmann, M.; Mützel, S.; Primpke, S.; Tekman, M.B.; Trachsel, J.; Gerdts, G. White and wonderful? Microplastics prevail in snow from the Alps to the Arctic. Sci. Adv. 2019, 5, eaax1157. [Google Scholar] [CrossRef]
- Rose, C.; Parker, A.; Jefferson, B.; Cartmell, E. The Characterization of Feces and Urine: A Review of the Literature to Inform Advanced Treatment Technology. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1827–1879. [Google Scholar] [CrossRef]
- Bruinink, A.; Wang, J.; Wick, P. Effect of particle agglomeration in nanotoxicology. Arch. Toxicol. 2015, 89, 659–675. [Google Scholar] [CrossRef]
- Revel, M.; Châtel, A.; Mouneyrac, C. Micro(nano)plastics: A threat to human health? Curr. Opin. Environ. Sci. Health 2018, 1, 17–23. [Google Scholar] [CrossRef]
- Wright, S.L.; Kelly, F.J. Plastic and Human Health: A Micro Issue? Environ. Sci. Technol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef] [PubMed]
- Walczak, A.P.; Hendriksen, P.J.; Woutersen, R.A.; van der Zande, M.; Undas, A.K.; Helsdingen, R.; van den Berg, H.H.; Rietjens, I.M.; Bouwmeester, H. Bioavailability and biodistribution of differently charged polystyrene nanoparticles upon oral exposure in rats. J. Nanopart. Res. 2015, 17, 231. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, Y.; Lemos, B.; Ren, H. Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci. Rep. 2017, 7, 46687. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhang, Y. Response to Uptake of microplastics and related health effects: A critical discussion of Deng et al., Scientific reports 7: 46687, 2017. Arch. Toxicol. 2019, 93, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Braeuning, A. Uptake of microplastics and related health effects: A critical discussion of Deng et al., Scientific reports 7:46687, 2017. Arch. Toxicol. 2019, 93, 219–220. [Google Scholar] [CrossRef] [PubMed]
- Böhmert, L.; Stock, V.; Braeuning, A. Plausibility of microplastic uptake in a paper by Deng et al., Scientific reports 7:46687, 2017. Arch. Toxicol. 2019, 93, 217–218. [Google Scholar] [CrossRef]
- Stock, V.; Böhmert, L.; Lisicki, E.; Block, R.; Cara-Carmona, J.; Pack, L.K.; Selb, R.; Lichtenstein, D.; Voss, L.; Henderson, C.J.; et al. Uptake and effects of orally ingested polystyrene microplastic particles in vitro and in vivo. Arch. Toxicol. 2019, 93, 1817–1833. [Google Scholar] [CrossRef]
- Hoogenboom, L.A.P.; (Wageningen Food Safety Research, part of Wageningen University & Research, Wageningen, The Netherlands); Braeuning, A.; (German Federal Institute for Risk Assessment, Berlin, Germany). Personal communication, 2019.
- Hoogenboom, L.A.P.; (Wageningen Food Safety Research, part of Wageningen University & Research, Wageningen, The Netherlands); Zhang, Y.; (State Key Laboratory of Pollution Control and Resource Reuse, School of the Environment, Nanjing University, Nanjing, China). Personal communication, 2019.
- Lu, L.; Wan, Z.; Luo, T.; Fu, Z.; Jin, Y. Polystyrene microplastics induce gut microbiota dysbiosis and hepatic lipid metabolism disorder in mice. Sci. Total Environ. 2018, 631–632, 449–458. [Google Scholar] [CrossRef]
- Jin, Y.; Lu, L.; Tu, W.; Luo, T.; Fu, Z. Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Sci. Total Environ. 2019, 649, 308–317. [Google Scholar] [CrossRef]
- Luo, T.; Zhang, Y.; Wang, C.; Wang, X.; Zhou, J.; Shen, M.; Zhao, Y.; Fu, Z.; Jin, Y. Maternal exposure to different sizes of polystyrene microplastics during gestation causes metabolic disorders in their offspring. Environ. Pollut. 2019, 255, 113122. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Wang, C.; Pan, Z.; Jin, C.; Fu, Z.; Jin, Y. Maternal Polystyrene Microplastic Exposure during Gestation and Lactation Altered Metabolic Homeostasis in the Dams and Their F1 and F2 Offspring. Environ. Sci. Technol. 2019, 53, 10978–10992. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Almeida, M.; Miguel, I. A micro(nano)plastic boomerang tale: A never ending story? TrAC Trend Anal. Chem. 2019, 112, 196–200. [Google Scholar] [CrossRef]
- Qiao, R.; Sheng, C.; Lu, Y.; Zhang, Y.; Ren, H.; Lemos, B. Microplastics induce intestinal inflammation, oxidative stress, and disorders of metabolome and microbiome in zebrafish. Sci. Total Environ. 2019, 662, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Cole, M. A novel method for preparing microplastic fibers. Sci. Rep. 2016, 6, 34519. [Google Scholar] [CrossRef] [PubMed]
- Kzhyshkowska, J.; Gudima, A.; Riabov, V.; Dollinger, C.; Lavalle, P.; Vrana, N.E. Macrophage responses to implants: Prospects for personalized medicine. J. Leukoc. Biol. 2015, 98, 953–962. [Google Scholar] [CrossRef]
- Rainieri, S.; Conlledo, N.; Larsen, B.K.; Granby, K.; Barranco, A. Combined effects of microplastics and chemical contaminants on the organ toxicity of zebrafish (Danio rerio). Environ. Res. 2018, 162, 135–143. [Google Scholar] [CrossRef]
- Hesler, M.; Aengenheister, L.; Ellinger, B.; Drexel, R.; Straskraba, S.; Jost, C.; Wagner, S.; Meier, F.; von Briesen, H.; Buchel, C.; et al. Multi-endpoint toxicological assessment of polystyrene nano- and microparticles in different biological models in vitro. Toxicol. In Vitro 2019, 61, 104610. [Google Scholar] [CrossRef]
- Schirinzi, G.F.; Perez-Pomeda, I.; Sanchis, J.; Rossini, C.; Farre, M.; Barcelo, D. Cytotoxic effects of commonly used nanomaterials and microplastics on cerebral and epithelial human cells. Environ. Res. 2017, 159, 579–587. [Google Scholar] [CrossRef]
- Hwang, J.; Choi, D.; Han, S.; Choi, J.; Hong, J. An assessment of the toxicity of polypropylene microplastics in human derived cells. Sci. Total Environ. 2019, 684, 657–669. [Google Scholar] [CrossRef]
- Huang, Z.; Ma, T.; Ren, P.G.; Smith, R.L.; Goodman, S.B. Effects of orthopedic polymer particles on chemotaxis of macrophages and mesenchymal stem cells. J. Biomed. Mater. Res. A 2010, 94, 1264–1269. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Wang, S.; Yue, B.; Wang, Y.; Wang, Y. Effects of wear particles of polyether-ether-ketone and cobalt-chromium-molybdenum on CD4- and CD8-T-cell responses. Oncotarget 2017, 9, 11197–11208. [Google Scholar] [CrossRef] [PubMed]
- Zarfl, C. Promising techniques and open challenges for microplastic identification and quantification in environmental matrices. Anal. Bioanal. Chem. 2019, 411, 3743–3756. [Google Scholar] [CrossRef]
- Corami, F.; Rosso, B.; Bravo, B.; Gambaro, A.; Barbante, C. A novel method for purification, quantitative analysis and characterization of microplastic fibers using Micro-FTIR. Chemosphere 2020, 238, 124564. [Google Scholar] [CrossRef] [PubMed]
- Dehaut, A.; Cassone, A.L.; Frere, L.; Hermabessiere, L.; Himber, C.; Rinnert, E.; Riviere, G.; Lambert, C.; Soudant, P.; Huvet, A.; et al. Microplastics in seafood: Benchmark protocol for their extraction and characterization. Environ. Pollut. 2016, 215, 223–233. [Google Scholar] [CrossRef]
- Hurley, R.R.; Lusher, A.L.; Olsen, M.; Nizzetto, L. Validation of a Method for Extracting Microplastics from Complex, Organic-Rich, Environmental Matrices. Environ. Sci. Technol. 2018, 52, 7409–7417. [Google Scholar] [CrossRef]
- Miller, M.E.; Kroon, F.J.; Motti, C.A. Recovering microplastics from marine samples: A review of current practices. Mar. Pollut. Bull. 2017, 123, 6–18. [Google Scholar] [CrossRef]
- Catarino, A.I.; Thompson, R.; Sanderson, W.; Henry, T.B. Development and optimization of a standard method for extraction of microplastics in mussels by enzyme digestion of soft tissues. Environ. Toxicol. Chem. 2017, 36, 947–951. [Google Scholar] [CrossRef]
- Thiele, C.J.; Hudson, M.D.; Russell, A.E. Evaluation of existing methods to extract microplastics from bivalve tissue: Adapted KOH digestion protocol improves filtration at single-digit pore size. Mar. Pollut. Bull. 2019, 142, 384–393. [Google Scholar] [CrossRef]
- Löder, M.G.J.; Imhof, H.K.; Ladehoff, M.; Loschel, L.A.; Lorenz, C.; Mintenig, S.; Piehl, S.; Primpke, S.; Schrank, I.; Laforsch, C.; et al. Enzymatic Purification of Microplastics in Environmental Samples. Environ. Sci. Technol. 2017, 51, 14283–14292. [Google Scholar] [CrossRef]
- Quinn, B.; Murphy, F.; Ewins, C. Validation of density separation for the rapid recovery of microplastics from sediment. Anal. Methods 2017, 9, 1491–1498. [Google Scholar] [CrossRef]
- Osipov, V.I. Density of clay minerals. Soil Mech. Found. Eng. 2012, 48, 231–240. [Google Scholar] [CrossRef]
- Shim, W.J.; Song, Y.K.; Hong, S.H.; Jang, M. Identification and quantification of microplastics using Nile Red staining. Mar. Pollut. Bull. 2016, 113, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Tamminga, M. Nile Red Staining as a Subsidiary Method for Microplastic Quantifica-tion: A Comparison of Three Solvents and Factors Influencing Application Reliability. SDRP JESES 2017, 2. [Google Scholar] [CrossRef]
- Catarino, A.I.; Macchia, V.; Sanderson, W.G.; Thompson, R.C.; Henry, T.B. Low levels of microplastics (MP) in wild mussels indicate that MP ingestion by humans is minimal compared to exposure via household fibres fallout during a meal. Environ. Pollut. 2018, 237, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Erni-Cassola, G.; Gibson, M.I.; Thompson, R.C.; Christie-Oleza, J.A. Lost, but Found with Nile Red: A Novel Method for Detecting and Quantifying Small Microplastics (1 mm to 20 mum) in Environmental Samples. Environ. Sci. Technol. 2017, 51, 13641–13648. [Google Scholar] [CrossRef] [PubMed]
- Hitchcock, J.N.; Mitrovic, S.M. Microplastic pollution in estuaries across a gradient of human impact. Environ. Pollut. 2019, 247, 457–466. [Google Scholar] [CrossRef]
- Käppler, A.; Fischer, D.; Oberbeckmann, S.; Schernewski, G.; Labrenz, M.; Eichhorn, K.J.; Voit, B. Analysis of environmental microplastics by vibrational microspectroscopy: FTIR, Raman or both? Anal. Bioanal. Chem. 2016, 408, 8377–8391. [Google Scholar] [CrossRef]
- Primpke, S.; Lorenz, C.; Rascher-Friesenhausen, R.; Gerdts, G. An automated approach for microplastics analysis using focal plane array (FPA) FTIR microscopy and image analysis. Anal. Methods 2017, 9, 1499–1511. [Google Scholar] [CrossRef]
- Simon, M.; van Alst, N.; Vollertsen, J. Quantification of microplastic mass and removal rates at wastewater treatment plants applying Focal Plane Array (FPA)-based Fourier Transform Infrared (FT-IR) imaging. Water Res. 2018, 142, 1–9. [Google Scholar] [CrossRef]
- Zada, L.; Leslie, H.A.; Vethaak, A.D.; Tinnevelt, G.H.; Jansen, J.J.; de Boer, J.F.; Ariese, F. Fast microplastics identification with stimulated Raman scattering microscopy. J. Raman Spectrosc. 2018, 49, 1136–1144. [Google Scholar] [CrossRef]
- Araujo, C.F.; Nolasco, M.M.; Ribeiro, A.M.P.; Ribeiro-Claro, P.J.A. Identification of microplastics using Raman spectroscopy: Latest developments and future prospects. Water Res. 2018, 142, 426–440. [Google Scholar] [CrossRef]
- Gillibert, R.; Balakrishnan, G.; Deshoules, Q.; Tardivel, M.; Magazzu, A.; Donato, M.G.; Marago, O.M.; Lamy de La Chapelle, M.; Colas, F.; Lagarde, F.; et al. Raman Tweezers for Small Microplastics and Nanoplastics Identification in Seawater. Environ. Sci. Technol. 2019, 53, 9003–9013. [Google Scholar] [CrossRef]
- Hermabessiere, L.; Himber, C.; Boricaud, B.; Kazour, M.; Amara, R.; Cassone, A.L.; Laurentie, M.; Paul-Pont, I.; Soudant, P.; Dehaut, A.; et al. Optimization, performance, and application of a pyrolysis-GC/MS method for the identification of microplastics. Anal. Bioanal. Chem. 2018, 410, 6663–6676. [Google Scholar] [CrossRef]
- Alexy, P.; Anklam, E.; Emans, T.; Furfari, A.; Galgani, F.; Hanke, G.; Koelmans, A.; Pant, R.; Saveyn, H.; Sokull Kluettgen, B. Managing the analytical challenges related to micro- and nanoplastics in the environment and food: Filling the knowledge gaps. Food Addit. Contam A Chem. Anal. Control Expo. Risk Assess 2019, 1–10. [Google Scholar] [CrossRef]
- European Commission Decision No 2002/657 of 12 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results (Text with EEA relevance). Off. J. Eur. Union 2002, L 221, 0008–0036. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX:32002D0657 (accessed on 4 December 2019).
- Currie, L.A. Nomenclature in evaluation of analytical methods including detection and quantification capabilities. Anal. Chim. Acta 1999, 391, 105–126. [Google Scholar] [CrossRef]
- Thompson, M.; Ellison, S.; Wood, R. Harmonized guidelines for single-laboratory validation of methods of analysis (IUPAC Technical Report). Pure Appl. Chem. 2002, 74, 835–855. [Google Scholar] [CrossRef]
- AOAC International. Guidelines for validation of botanical identification methods. J AOAC Int. 2012, 95, 268–272. [Google Scholar] [CrossRef]
- International Organization for Standardization (ISO) 17025. General Requirements for the Competence of Testing and Calibration Laboratories; International Organization for Standardization: Geneva, Switzerland, 2017. [Google Scholar]
- Thompson, M.; Wood, R. Harmonized Guidelines for Internal Quality Control in Analytical Chemistry Laboratories. Pure Appl. Chem. 1995, 67, 649–666. [Google Scholar] [CrossRef]
- International Organization for Standardization (ISO) 20813. Molecular Biomarker Analysis—Methods of Analysis for the Detection and Identification of Animal Species in Foods and Food Products (Nucleic Acid-Based Methods)—General Requirements and Definitions; International Organization for Standardization: Geneva, Switzerland, 2019. [Google Scholar]
- International Organization for Standardization (ISO) 24276. I. Foodstuffs—Methods of Analysis for the Detection of Genetically Modified Organisms and Derived Products—General Requirements and Definitions; International Organization for Standardization: Geneva, Switzerland, 2006. [Google Scholar]
- Hermsen, E.; Mintenig, S.M.; Besseling, E.; Koelmans, A.A. Quality Criteria for the Analysis of Microplastic in Biota Samples: A Critical Review. Environ. Sci. Technol. 2018, 52, 10230–10240. [Google Scholar] [CrossRef]
- Editorial, No authors Listed. The future of plastic. Nat. Commun. 2018, 9, 2157. [Google Scholar] [CrossRef]
- Gewert, B.; Plassmann, M.M.; MacLeod, M. Pathways for degradation of plastic polymers floating in the marine environment. Environ. Sci. Process. Impacts 2015, 17, 1513–1521. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Van Raamsdonk, L.W.D.; Pinckaers, V.G.Z.; Scholtens, I.M.J.; Prins, T.W.; van der Voet, H.; Vliege, J.J.M. IAG Ring Test Animal Proteins 2014; Wageningen Food Safety Research Report 2014.011; Wageningen Food Safety Research: Wageningen, The Netherlands, 2014; Available online: https://edepot.wur.nl/323884 (accessed on 4 December 2019).
- Cameron, A.C.; Trivedi, P.K. Regression Analysis of Count Data; Cambridge University Press: Cambridge, UK, 1998. [Google Scholar]
- Johnson, N.L.; Kemp, A.W.; Kotz, S. Univariate Discrete Distributions, 3rd ed.; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- Kappel, R.M.B.; Boer, L.L.; Dijkman, H. Gel Bleed and Rupture of Silicone Breast Implants Investigated by Light-, Electron Microscopy and Energy Dispersive X-ray Analysis of Internal Organs and Nervous Tissue. Clin. Med. Rev. Case Rep. 2016. [Google Scholar] [CrossRef]
- International Association for Feedingstuff Analysis. Available online: www.iag-micro.org (accessed on 4 December 2019).
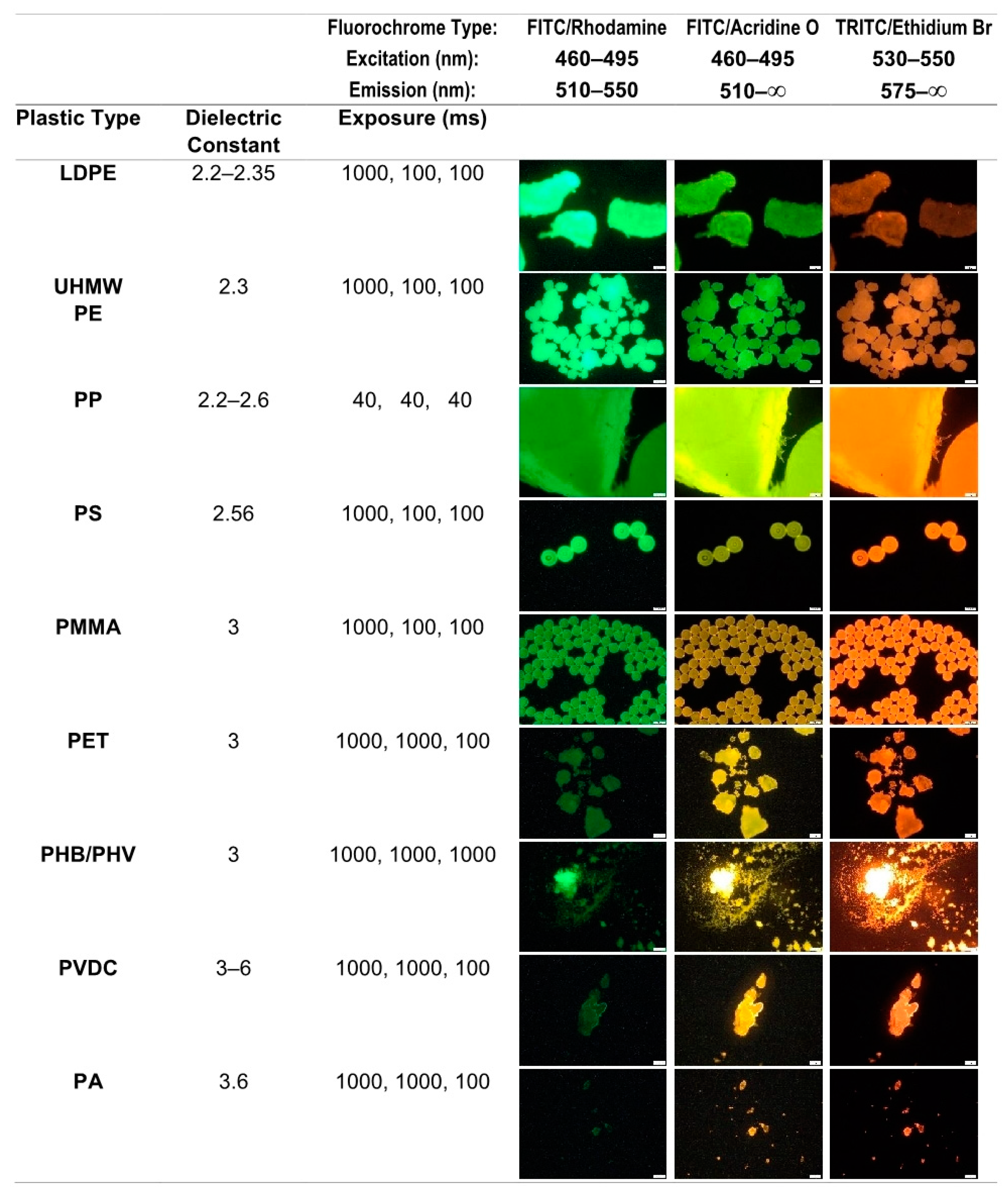
| Bottle Type | Oβmann et al. [38] | Schymansky et al. [40] | |||
|---|---|---|---|---|---|
| n ± SD | % > 5 µm | n>5 µm | n ± SD | Min–Max Range | |
| Single use PET | 2649 ± 2857 | 1.7% | 45 | 14 ± 14 | 2–44 |
| Reusable PET | 4889 ± 5432 | 4.6% | 224 | 118 ± 88 | 28–241 |
| Glass | 6292 ± 10521 | 22.3% | 1403 | 50 ± 52 | 4–156 |
| Study, Type a and Age of Animals Used | Experiment | Exposure Scheme | MP Type, Concentration | Tissues, Readout | Reported Result | Comments |
|---|---|---|---|---|---|---|
| Deng et al., 2017 [51]: ICR mice, aged 5 weeks | Bio-accumulation Effects | Oral gavage, 0.1 mg per day, 28 days and a wash-out group of 7 days Oral gavage, 0.01, 0.1, and 0.5 mg per day, 28 days | Pristine fluorescent PS MPs: 5 µm: 1.46 × 106 particles per day; 20 µm: 2.27×104 particles per day Pristine fluorescent PS MPs: 5 µm: 1 × 105, 1 × 106, and 5 × 106 particles per day; 20 µm: 2 × 103, 2 × 104, and 1 × 105 particles per day | Liver, kidney, and gut; fluorescence spectroscopy after 1, 2, 4, 7, 14, 21, and 28 days of exposure. Liver: histology. Serum: biomarkers and metabolomic analysis | Accumulation in all tested organs of both MPs. MPs were still present in all tissues after a wash-out period of 7 days. Liver inflammation and presence of lipid droplets. Disturbance of energy and lipid metabolism, oxidative stress, and neurotoxic responses | Unclear whether the gut was washed before measurement. Measured MP levels below standardized calibration curves. High accumulation; results point toward 100% bioavailability |
| Stock et al., 2019 [55]: Male HMOX1 reporter mice (C57BL/6NTac), aged 16–20 weeks | Bio-accumulation Effects | Oral gavage, mixture of three MP sizes, approximately 1.25, 25, and 34 mg kg−1 bw for 1, 4, and 10 µm particles, three times per week for 28 days. Animals sacrificed 3 days after last dosing. Similar to bioaccumulation | Carboxylated fluorescent PS MPs (1 µm), PS MPs (4, 10 µm). Per treatment: 1 µm: 4.55 × 107 particles; 4 µm: 4.55 × 107 particles; 10 µm: 1.49 × 106 particles | Intestine, liver, spleen, and kidney: fluorescence microscopy. Duodenum, ileum, jejunum, large intestine, liver, testes, lung, heart, spleen, and kidney: histology (H&E staining and β-galactosidase staining) | Few MPs in intestinal cell layers, no MPs in liver, spleen, and kidney. No evidence of inflammation and/or oxidative stress (no induction of β-galactosidase expression) | Administration was 3× per week and animals were sacrificed 3 days after last dosing, so (some) clearance and recovery between and after last exposure would have been possible. Mice were older than in the other studies and had a different genetic background |
| Lu et al., 2018 [58]: ICR mice, aged 5 weeks | Effects | Exposure through drinking water (unlimited supply), 100 µg MPs per liter and 1000 µg MPs per liter, 35 days | Pristine MPs: 0.5 µm: 1.456 × 1010 particles per liter; 50 µm: 1.456 × 104 particles per liter. Particle numbers correspond to the high dose | Colon: histology (mucus staining). Liver and serum: biomarkers. Microbiome composition (qPCR, sequencing) | Reduced body and liver weight for the high dose. Colon: reduced mucin excretion. Liver and serum: decreased serum indices (indicating decreased fat metabolism). Altered microbiota composition | Water intake not reported. Unknown amount of MP intake. Reduced body and organ weight at a high dose might be an experimental artefact |
| Jin et al., 2019 [59]: ICR mice, aged 5 weeks | Bio-accumulation Effects | Exposure through drinking water (unlimited supply), 1000 µg per liter, 42 days 100 µg per liter and 1000 µg per liter, oral gavage, continuous, 42 days | Fluorescent PS MPs: 5 µm: 1.456 × 107 particles per liter. Pristine PS MPs: 5 µm: 1.456 × 106 and 1.456 × 107 particles per liter | Colon: fluorescence microscopy. Colon: histology (mucus staining), transporter protein expression. Liver, colon and, ileum: gene expression. Liver and serum: biomarkers. Serum: measurement of amino acids, carnitine, and succinylacetone. Bile acid: measurement of bile acids. Microbiome composition (qPCR, sequencing) | Presence of MPs in colon: decreased secretion of mucus, down-regulation of genes/proteins involved in ion transport. Altered amino acid and bile acid metabolism. Altered microbiota composition | Unknown amount of MP intake. Unknown amount of MP consumption |
| Luo et al., 2019a [60]: ICR mice, aged 7 weeks | Effects in offspring (F1) | Exposure through drinking water (unlimited supply) F0: 100 and 1000 µg per liter, exposure during gestation F1: no exposure | Pristine PS MPs: 0.5 µm: no particle concentration; 50 µm: no particle concentration. See Lu et al., 2018 [58] | F1 liver and serum: biomarkers. F1 liver: gene expression (fatty acid metabolism). F1 serum: measurement of amino acids, carnitine, and succinylacetone | Altered amino acid, carnitine, and fatty acid metabolism in the offspring without direct exposure to MPs | Unknown amount of MP consumption |
| Luo et al., 2019b [61]: ICR mice, aged 7 weeks | Effects in offspring (F1 and F2) | Exposure through drinking water (unlimited supply) F0: 100 µg per liter and 1000 µg per liter, exposure during gestation and lactation (approximately 42 days) F1: no exposure; offspring from F0 1000 µg per liter and control group used for production of F2 F2: no exposure | Pristine PS MPs: 5 µm: no counts. See Jin et al., 2019 [59] | Colon: histology (mucus and transporter staining). Liver: histology (H&E staining), biomarkers, and transcriptome analysis. Serum: biomarkers, measurement of amino acids, carnitine, and succinylacetone. Microbiome composition (qPCR, sequencing) | F0: altered gut barrier composition, altered hepatic gene expression, and modified gut microbiota. F1 (post-natal day 42): modified hepatic and serum metabolite levels, gut microbiota not altered. F1 (post-natal day 280): potential effects on lipid metabolism. F2 (post-natal day 42): few effects | Unknown amount of MP consumption. Assumed modification of the glycolipid metabolism is hypothetical. Only dams evaluated for some parameters |
| Material | Particle Size (µm) | Concentration (per L or Cup) | Estimated Daily Consumption | Exposure (Day−1) | Estimated Exposure (kg−1 bw Day−1) |
|---|---|---|---|---|---|
| Water, mice [58] | 0.5 | 1.5 × 109 | 4 mL | 5.8 × 106 | 1.5 × 108 |
| Gavage, mice [55] | 1 | 4.6 × 107 | 4.9 × 108 | ||
| Gavage, mice [55] | 4 | 4.6 × 107 | 4.9 × 108 | ||
| Water, mice [59] | 5 | 1.5 × 106 | 4 mL | 5.8 × 103 | 1.5 × 105 |
| Gavage, mice [51] | 5 | 1.0 × 105 | 2.5 × 106 | ||
| Gavage, mice [55] | 10 | 1.5 × 106 | 3.7 × 106 | ||
| Gavage, mice [51] | 20 | 2.0 × 103 | 5.0 × 104 | ||
| Water, mice [58] | 50 | 1.5 × 103 | 4 mL | 6 | 146 |
| Diet, food, maximum [33] | Depending on source | 142 | |||
| Diet, food and bottled water, maximum [33] | Depending on source | 389 | |||
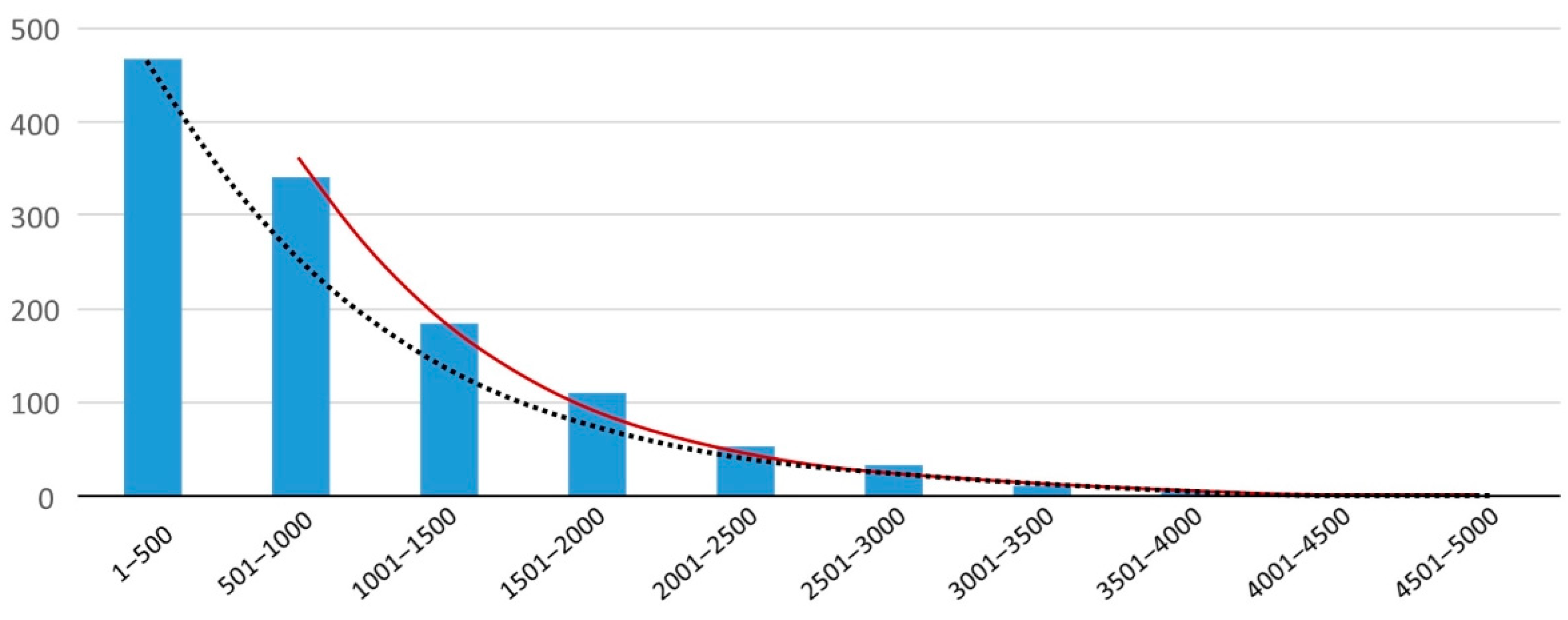
| Stool, median [6] | 50–500 | 256 | |||
| Tap water, average [39] | 1 and larger | 470 | 2 L | 940 | 13 |
| Bottled water, average [38] | 1 and larger | 3.8 × 103 | 2 L | 7.5 × 103 | 108 |
| Bottled water, average [40] | 5 and larger | 66 | 2 L | 132 | 2 |
| Bottled water, average [34] | 6.5–100 | 325 | 2 L | 650 | 9 |
| Bottled water, maximum [38] | 1 and larger | 1.7 × 104 | 2 L | 3.3 × 104 | 475 |
| Bottled water, maximum [34] | 6.5–100 | 1.0 × 104 | 2 L | 2.1 × 104 | 297 |
| Tea per cup [43] | 2.5 and larger | 2.3 × 106 | 2 cups | 4.6 × 106 | 6.5 × 104 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Raamsdonk, L.W.D.; van der Zande, M.; Koelmans, A.A.; Hoogenboom, R.L.A.P.; Peters, R.J.B.; Groot, M.J.; Peijnenburg, A.A.C.M.; Weesepoel, Y.J.A. Current Insights into Monitoring, Bioaccumulation, and Potential Health Effects of Microplastics Present in the Food Chain. Foods 2020, 9, 72. https://doi.org/10.3390/foods9010072
van Raamsdonk LWD, van der Zande M, Koelmans AA, Hoogenboom RLAP, Peters RJB, Groot MJ, Peijnenburg AACM, Weesepoel YJA. Current Insights into Monitoring, Bioaccumulation, and Potential Health Effects of Microplastics Present in the Food Chain. Foods. 2020; 9(1):72. https://doi.org/10.3390/foods9010072
Chicago/Turabian Stylevan Raamsdonk, Leonard W. D., Meike van der Zande, Albert A. Koelmans, Ron L. A. P. Hoogenboom, Ruud J. B. Peters, Maria J. Groot, Ad A. C. M. Peijnenburg, and Yannick J. A. Weesepoel. 2020. "Current Insights into Monitoring, Bioaccumulation, and Potential Health Effects of Microplastics Present in the Food Chain" Foods 9, no. 1: 72. https://doi.org/10.3390/foods9010072
APA Stylevan Raamsdonk, L. W. D., van der Zande, M., Koelmans, A. A., Hoogenboom, R. L. A. P., Peters, R. J. B., Groot, M. J., Peijnenburg, A. A. C. M., & Weesepoel, Y. J. A. (2020). Current Insights into Monitoring, Bioaccumulation, and Potential Health Effects of Microplastics Present in the Food Chain. Foods, 9(1), 72. https://doi.org/10.3390/foods9010072





