Acacia Gum as a Natural Anti-Plasticizer for the Production of Date Syrup Powder: Sorption Isotherms, Physicochemical Properties, and Data Modeling
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Date Syrup
2.2. Preliminary Studies
2.3. Sample Preparation
2.4. Chemical Composition
2.5. Sugar Analysis
2.6. Bulk Density
2.7. Caking Degree
2.8. Glass Transition Temperature
2.9. Color Evaluation
2.10. Determination of Isotherms
2.11. Data Modeling
2.12. Statistical Analysis
3. Results and Discussion
3.1. Chemical Properties of the Date Syrup
3.2. Physical Properties of the Date Syrup Powders
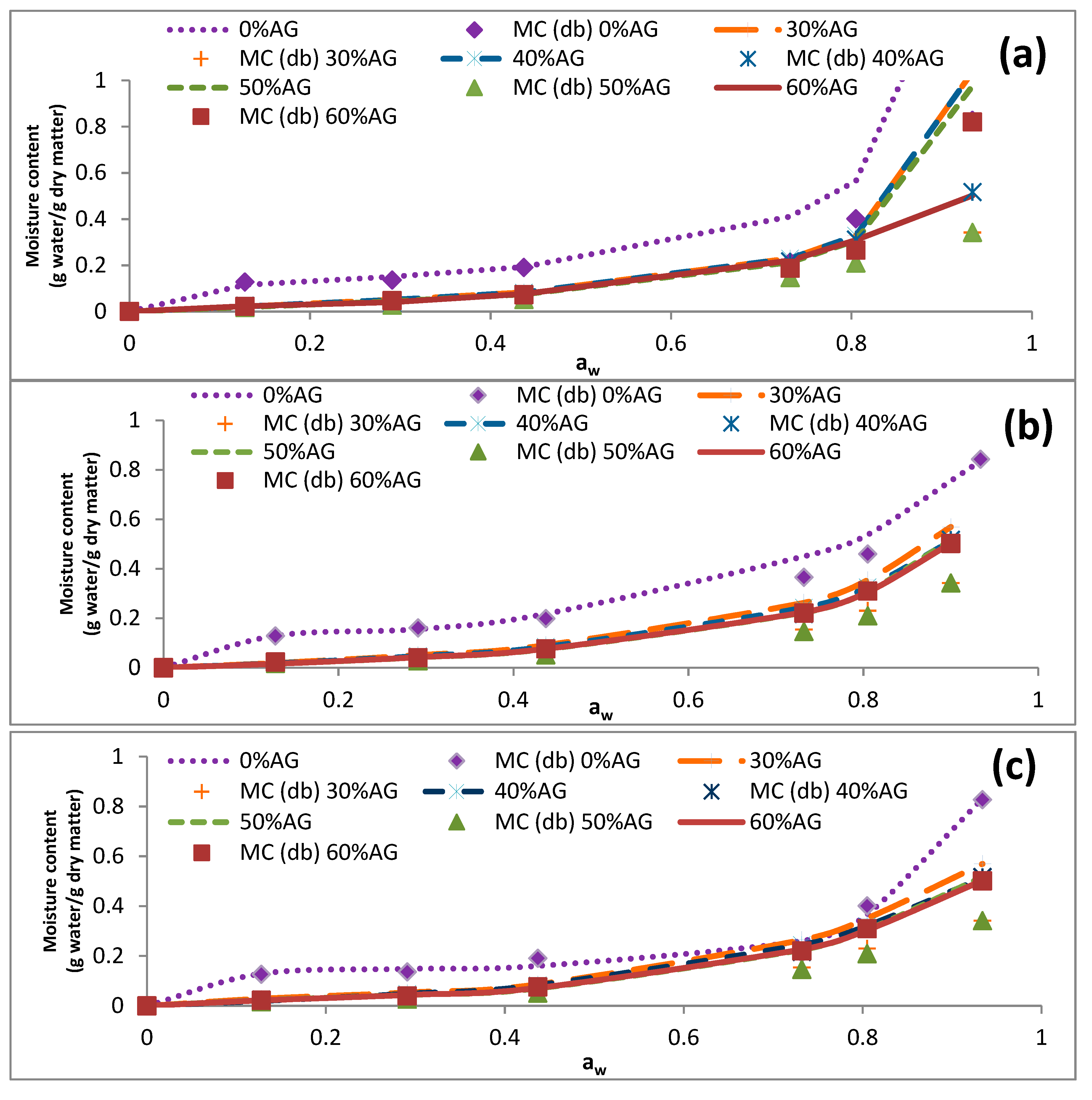
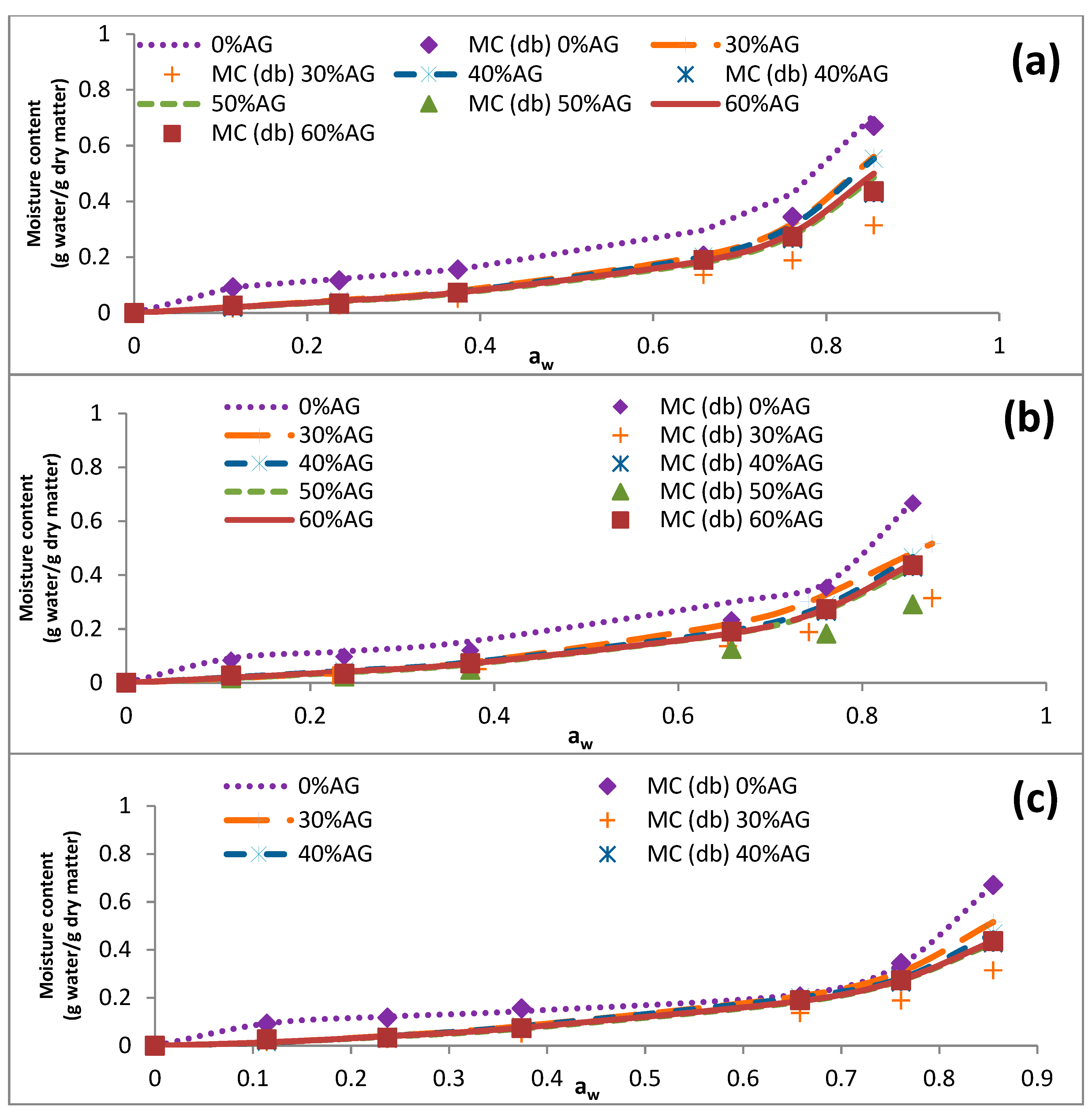
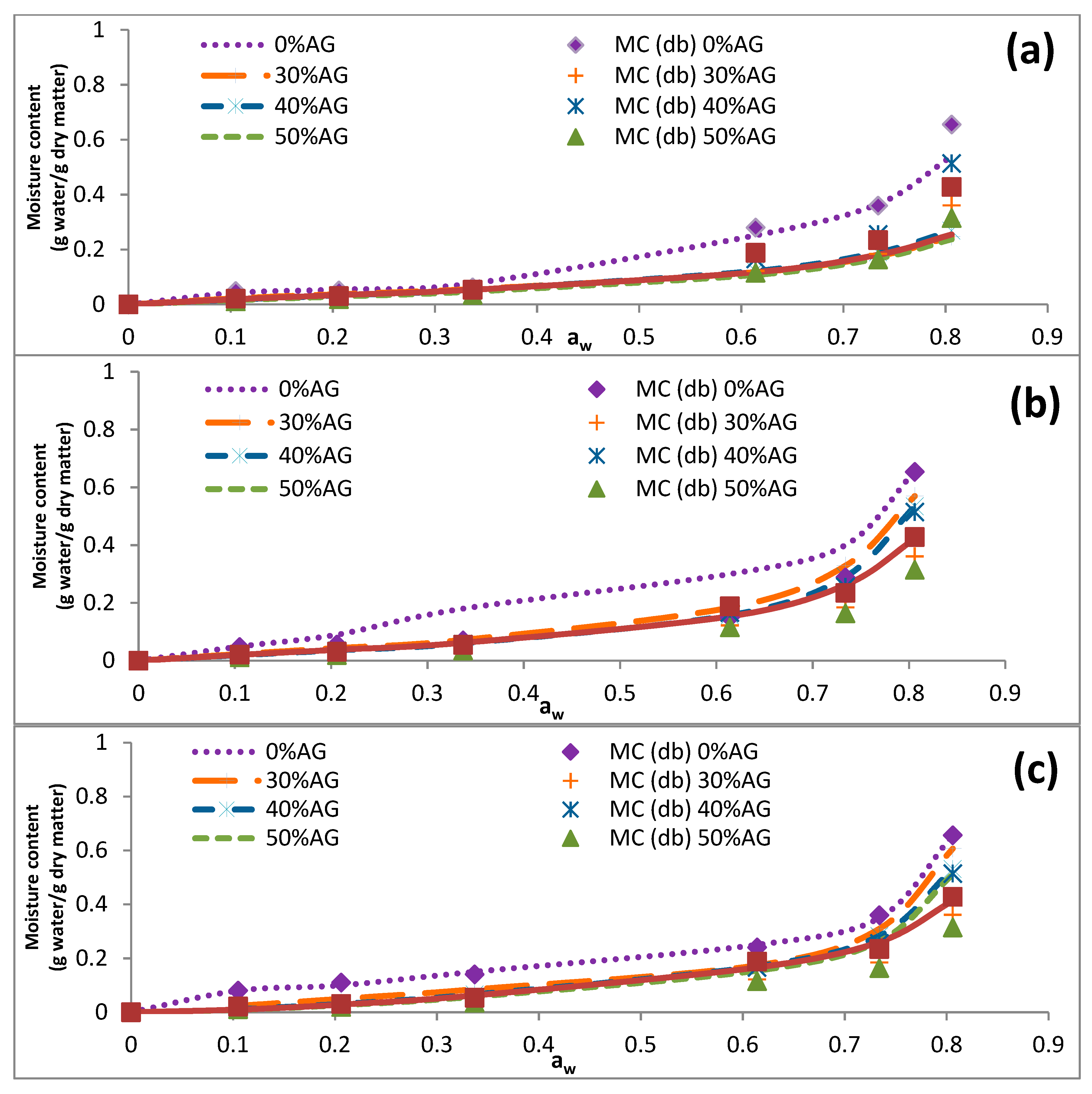
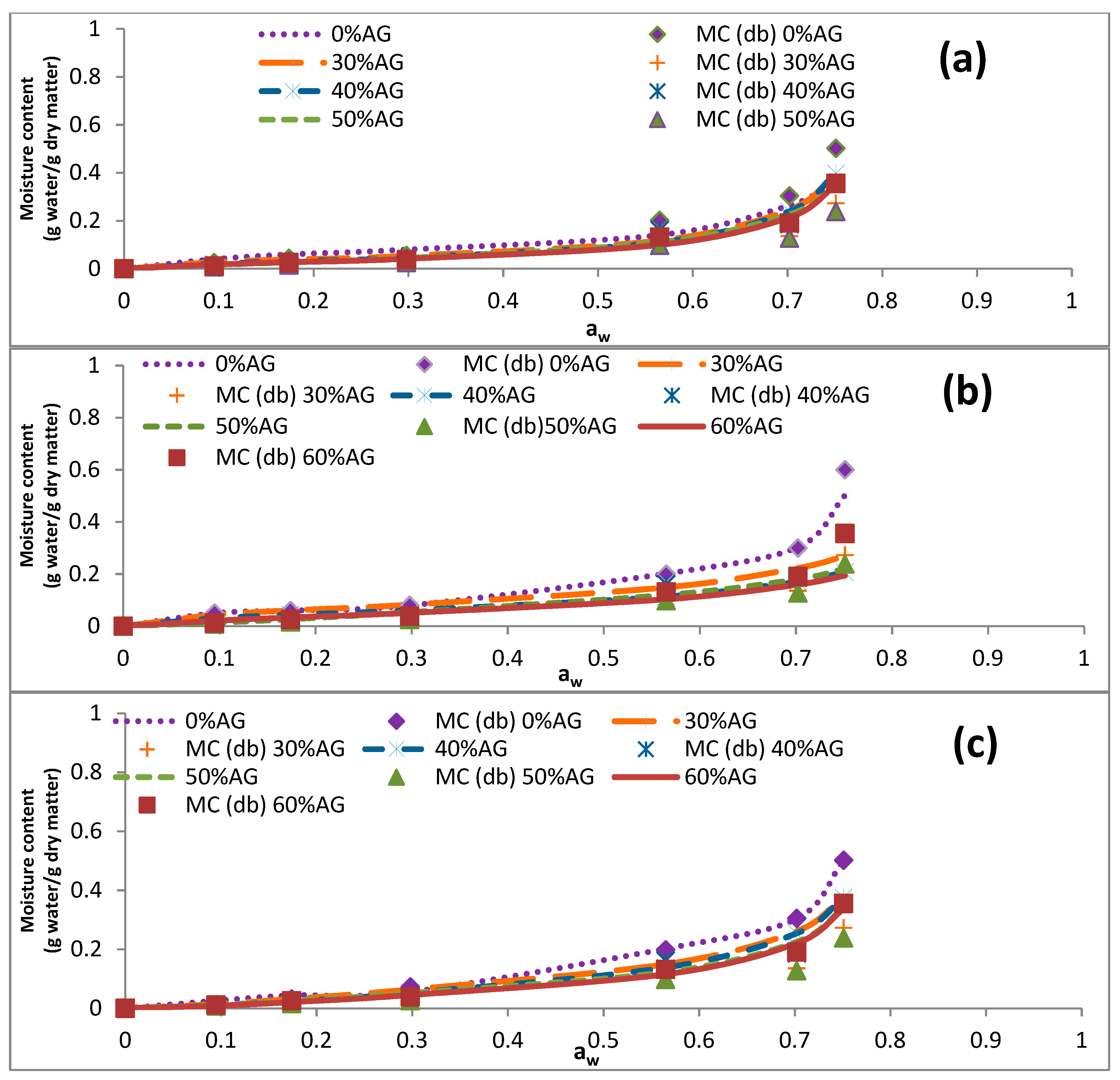
3.3. Sorption Isotherms
3.4. Data Modeling
4. Conclusions
Abbreviations and Nomenclatures
| °Bx | degrees Brix |
| 0% AG | date syrup with no acacia gum |
| 30% AG | powder samples containing 70% date syrup and 30% acacia gum |
| 40% AG | powder samples containing 60% date syrup and 40% acacia gum |
| 50% AG | powder samples containing 50% date syrup and 50% acacia gum |
| 60% AG | powder samples containing 40% date Syrup and 60% acacia |
| AOAC | Association of Official Analytical Chemists |
| AG | acacia gum |
| aw | water activity |
| C | a constant in GAB and BET models |
| CD | caking degree |
| DS | date syrup |
| DS-AG | date syrup- acacia gum mixtures |
| EMC | equilibrium moisture content |
| exp | experimental |
| GAB | Guggenheim-Anderson-de Boer |
| H | the height of the vessel used for bulk density analysis |
| HPLC | high-performance liquid chromatography |
| IUPAC | International Union of Pure and Applied Chemistry |
| K | a constant in GAB model |
| k1 | a Peleg model constant |
| k2 | a Peleg model constant |
| M0 | monolayer moisture content |
| Me | mean relative percentage deviation modulus |
| n1 | a Peleg model constant |
| n2 | a Peleg model constant |
| pb | bulk density |
| Pre | predicted |
| R | radius of the vessel used for bulk density analysis |
| R2 | correlation coefficient |
| RSS | residual sum of squares |
| SEE | standard error of estimate |
| Temp. | temperature |
| Tg | glass transition temperature |
| Tgc | endset glass transition |
| Tgi | onset glass transition |
| Tgm | middle glass transition |
| V | volume of the bulk density measurement vessel |
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Martín-Sánchez, A.M.; Ciro-Gómez, G.; Vilella-Esplá, J.; Pérez-Álvarez, J.Á.; Sayas-Barberá, E. Physicochemical and sensory characteristics of spreadable liver pâtés with annatto extract (bixa orellana L.) and date palm co-products (phoenix dactylifera L.). Foods 2017, 6, 94. [Google Scholar] [CrossRef] [PubMed]
- Siddeeg, A.; Zeng, X.A.; Ammar, A.F.; Han, Z. Sugar profile, volatile compounds, composition and antioxidant activity of Sukkari date palm fruit. J. Food Sci. Technol. 2019, 56, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Ben-Amor, R.; Dhouibi, M.H.; Aguayo, E. Hot water treatments combined with cold storage as a tool for ectomyelois ceratoniae mortality and maintenance of deglet noor palm date quality. Postharvest Biol. Technol. 2016, 112, 247–255. [Google Scholar] [CrossRef]
- Seerangurayar, T.; Manickavasagan, A.; Al-Ismaili, A.M.; Al-Mulla, Y.A. Effect of carrier agents on physicochemical properties of foam-mat freeze-dried date powder. Dry. Technol. 2017, 1–12. [Google Scholar] [CrossRef]
- Eid, N.M.S.; Al-Awadi, B.; Vauzour, D.; Oruna-Concha, M.J.; Spencer, J.P.E. Effect of cultivar type and ripening on the polyphenol content of date palm fruit. J. Agric. Food Chem. 2013, 61, 2453–2460. [Google Scholar] [CrossRef]
- Ghnimi, S.; Umer, S.; Karim, A.; Kamal-Eldin, A. Date fruit (Phoenix dactylifera L.): An underutilized food seeking industrial valorization. NFS J. 2017, 6, 1–10. [Google Scholar] [CrossRef]
- Truong, V.; Bhandari, B.R.; Howes, T. Optimization of co-current spray drying process of sugar-rich foods. Part I—Moisture and glass transition temperature profile during drying. J. Food Eng. 2005, 71, 55–65. [Google Scholar] [CrossRef]
- Ahmed, J.; Ramaswamy, H.S. Physico-chemical properties of commercial date pastes (Phoenix dactylifera). J. Food Eng. 2006, 76, 348–352. [Google Scholar] [CrossRef]
- Jaya, S.; Das, H. Effect of maltodextrin, glycerol monostearate and tricalcium phosphate on vacuum dried mango powder properties. J. Food Eng. 2004, 63, 125–134. [Google Scholar] [CrossRef]
- Nieto Calvache, J.E.; Soria, M.; DeEscalada Pla, M.F.; Gerschenson, L.N. Optimization of the production of dietary fiber concentrates from by-products of papaya (Carica papaya L. Var. Formosa) with microwave assistance. Evaluation of its physicochemical and functional characteristics. J. Food Process. Preserv. 2017, 41, e13071. [Google Scholar]
- Ganesan, V.; Rosentrater, K.A.; Muthukumarappan, K. Flowability and handling characteristics of bulk solids and powders—A review with implications for DDGS. Biosyst. Eng. 2008, 101, 425–435. [Google Scholar] [CrossRef]
- Mosquera, L.H.; Moraga, G.; Martínez-Navarrete, N. Critical water activity and critical water content of freeze-dried strawberry powder as affected by maltodextrin and arabic gum. Food Res. Int. 2012, 47, 201–206. [Google Scholar] [CrossRef]
- Musa, H.H.; Ahmed, A.A.; Musa, T.H. Chemistry, biological, and pharmacological properties of gum arabic. In Bioactive Molecules in Food; Springer: Berlin, Germany, 2018; pp. 1–18. [Google Scholar]
- Erben, M.; Pérez, A.A.; Osella, C.A.; Alvarez, V.A.; Santiago, L.G. Impact of gum arabic and sodium alginate and their interactions with whey protein aggregates on bio-based films characteristics. Int. J. Biol. Macromol. 2019, 125, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Gavahian, M.; Chen, Y.M.; Mousavi Khaneghah, A.; Barba, F.J.; Yang, B.B. In-pack sonication technique for edible emulsions: Understanding the impact of acacia gum and lecithin emulsifiers and ultrasound homogenization on salad dressing emulsions stability. Food Hydrocoll. 2018, 83, 79–87. [Google Scholar] [CrossRef]
- Patel, S.; Goyal, A. Applications of natural polymer gum Arabic: A review. Int. J. Food Prop. 2015, 18, 986–998. [Google Scholar] [CrossRef]
- Samborska, K.; Gajek, P.; Kamińska-Dwórznicka, A. Spray drying of honey: The effect of drying agents on powder properties. Polish J. Food Nutr. Sci. 2015, 65, 109–118. [Google Scholar] [CrossRef]
- Fikry, M.; Al-Awaadh, A.M. Characteristics of dynamics sorption isotherms of date flesh powder rich in fiber. Int. J. Food Eng. 2016, 12, 469–480. [Google Scholar] [CrossRef]
- Farahnaky, A.; Mansoori, N.; Majzoobi, M.; Badii, F. Physicochemical and sorption isotherm properties of date syrup powder: Antiplasticizing effect of maltodextrin. Food Bioprod. Process. 2016, 98, 133–141. [Google Scholar] [CrossRef]
- Gao, J.; Brennan, M.A.; Mason, S.L.; Brennan, C.S. Effects of sugar substitution with “Stevianna” on the sensory characteristics of muffins. J. Food Qual. 2017, 2017, 1–11. [Google Scholar] [CrossRef]
- Sablani, S.S.; Shrestha, A.K.; Bhandari, B.R. A new method of producing date powder granules: Physicochemical characteristics of powder. J. Food Eng. 2008, 87, 416–421. [Google Scholar] [CrossRef]
- Angel, R.C.M.; Espinosa-Muñoz, L.C.; Aviles-Aviles, C.; González-García, R.; Moscosa-Santillán, M.; Grajales-Lagunes, A.; Abud-Archila, M. Spray-drying of passion fruit juice using lactose-maltodextrin blends as the support material. Brazilian Arch. Biol. Technol. 2009, 52, 1011–1018. [Google Scholar] [CrossRef]
- Afshari-Jouybari, H.; Farahnaky, A. Evaluation of photoshop software potential for food colorimetry. J. Food Eng. 2011, 106, 170–175. [Google Scholar] [CrossRef]
- Gavahian, M.; Farahnaky, A.; Javidnia, K.; Majzoobi, M. Comparison of ohmic-assisted hydrodistillation with traditional hydrodistillation for the extraction of essential oils from thymus vulgaris L. Innov. Food Sci. Emerg. Technol. 2012, 14, 85–91. [Google Scholar] [CrossRef]
- Rizvi, S.S.H. Thermodynamic properties of foods in dehydration. In Engineering Properties of Foods; Taylor and Francis: New York, NY, USA, 1995; Volume 2, p. 123. [Google Scholar]
- Gavahian, M.; Chu, Y.H.; Farahnaky, A. Effects of ohmic and microwave cooking on textural softening and physical properties of rice. J. Food Eng. 2019, 243, 114–124. [Google Scholar] [CrossRef]
- Chowdhury, M.M.I.; Huda, M.D.; Hossain, M.A. Moisture sorption isotherms for mungbean (Vigna radiata L). J. Food Eng. 2006, 74, 462–467. [Google Scholar] [CrossRef]
- Al-Muhtaseb, A. Water sorption isotherms of starch powders Part 1: Mathematical description of experimental data. J. Food Eng. 2004, 61, 297–307. [Google Scholar] [CrossRef]
- Muthukumarappan, K.; Swamy, G.J. Glass transition thermodynamics and kinetics. In Glass Transition and Phase Transitions in Food and Biological Materials; Ahmed, J., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 31–47. ISBN 9781118935682. [Google Scholar]
- Al-Hooti, S.N.; Sidhu, J.S.; Al-Saqer, J.M.; Al-Othman, A. Chemical composition and quality of date syrup as affected by pectinase/cellulase enzyme treatment. Food Chem. 2002, 79, 215–220. [Google Scholar] [CrossRef]
- Gabas, A.L.; Telis, V.R.N.; Sobral, P.J.A.; Telis-Romero, J. Effect of maltodextrin and arabic gum in water vapor sorption thermodynamic properties of vacuum dried pineapple pulp powder. J. Food Eng. 2007, 82, 246–252. [Google Scholar] [CrossRef]
- Pérez-Alonso, C.; Beristain, C.I.; Lobato-Calleros, C.; Rodríguez-Huezo, M.E.; Vernon-Carter, E.J. Thermodynamic analysis of the sorption isotherms of pure and blended carbohydrate polymers. J. Food Eng. 2006, 77, 753–760. [Google Scholar] [CrossRef]
- Righetto, A.M.; Netto, F.M. Effect of encapsulating materials on water sorption, glass transition and stability of juice from immature acerola. Int. J. Food Prop. 2005, 8, 337–346. [Google Scholar] [CrossRef]
- Ayranci, E.; Duman, O. Moisture sorption isotherms of cowpea (Vigna unguiculata L. Walp) and its protein isolate at 10, 20 and 30 °C. J. Food Eng. 2005, 70, 83–91. [Google Scholar] [CrossRef]
- Farahnaky, A.; Ansari, S.; Majzoobi, M. Effect of glycerol on the moisture sorption isotherms of figs. J. Food Eng. 2009, 93, 468–473. [Google Scholar] [CrossRef]
- Hazaveh, P.; Mohammadi Nafchi, A.; Abbaspour, H. The effects of sugars on moisture sorption isotherm and functional properties of cold water fish gelatin films. Int. J. Biol. Macromol. 2015, 79, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Aviara, N.A.; Ajibola, O.O.; Aregbesola, O.A.; Adedeji, M.A. Moisture sorption isotherms of sorghum malt at 40 and 50 °C. J. Stored Prod. Res. 2006, 42, 290–301. [Google Scholar] [CrossRef]
- Alhamdan, A.M.; Hassan, B.H. Water sorption isotherms of date pastes as influenced by date cultivar and storage temperature. J. Food Eng. 1999, 39, 301–306. [Google Scholar] [CrossRef]
| Type of Saturated Salt Solution | Temperature (°C) | |||
|---|---|---|---|---|
| (IUPAC ID/Chemical Formula) | 5 | 25 | 40 | 60 |
| Lithium chloride (LiCl) | 0.128 | 0.114 | 0.105 | 0.095 |
| Potassium acetate (CH3COOK) | 0.291 | 0.237 | 0.206 | 0.174 |
| Sodium iodide (NaI) | 0.437 | 0.374 | 0.337 | 0.298 |
| Sodium nitrite (NaNO2) | 0.732 | 0.658 | 0.614 | 0.565 |
| Sodium chloride (NaCl) | 0.805 | 0.761 | 0.734 | 0.702 |
| Potassium chloride (KCl) | 0.934 | 0.855 | 0.806 | 0.751 |
| Component | DS * | AG |
|---|---|---|
| Moisture content (% w/w) | 14.42 ± 1.95 a | 9.17 ± 0.19 b |
| Protein (% w/w) | 0.76 ± 0.06 b | 2.50 ± 1.07 a |
| Fat (% w/w) | 0.10 ± 0.00 a | 0.14 ± 0.01 a |
| Ash (% w/w) | 2.23 ± 0.17 b | 3.11 ± 0.17 a |
| Glucose (% w/w) | 39.63 ± 0.08 a | NA b |
| Fructose (% w/w) | 33.68 ± 0.14 a | NA b |
| Sucrose (% w/w) | 1.25 ± 0.00 a | NA b |
| °Bx | 82.00 ± 0.05 a | NA b |
| Total carbohydrates ** (% w/w) | ~74.56 | ~85.08 ** |
| Sample | pb (kg·m−3) | Color Values * | Tg (°C) | |||||
|---|---|---|---|---|---|---|---|---|
| L | a | b | CD (%) | Tgi | Tgm | Tgc | ||
| 0% AG | - | 11.66 ± 3.05 c | 7.33 ± 0.57 a | 3.33 ± 0.5 f | - | −8.00 ± 188 f | −1.27 ± 97 f | 5.19 ± 2.16 f |
| 30% AG | 590 ± 1 c | 64.33 ± 0.57 b | 4.66 ± 0.57 a | 47.66 ± 1.52 a | 54.82 ± 0.23 a | 5.06 ± 0.21 e | 10.22 ± 0.16 e | 15.73 ± 0.33 e |
| 40% AG | 610 ± 0 c | 66.00 ± 1.00 a,b | 4.66 ± 0.57 a | 44.00 ± 1.00 b | 3.11 ± 0.25 b | 9.94 ± 0.38 d | 17.43 ± 0.35 d | 25.81 ± 1.17 d |
| 50% AG | 690 ± 0 b | 67.53 ± 1.00 a | 0.33 ± 0.57 b | 42.33 ± 0.57 c | 0.32 ± 0.03 c | 14.84 ± 0.06 c | 28.25 ± 0.21 c | 41.29 ± 0.34 c |
| 60% AG | 730 ± 3 a | 67.66 ± 1.15 a | 0.66 ± 0.57 b | 36.33 ± 0.57 d | 0.08 ± 0.00 d | 28.09 ± 0.82 b | 40.87 ± 0.58 b | 52.88 ± 0.26 b |
| AG | 730 ± 1 a | 68.33 ± 0.57 a | −9.00 ± 0.00 c | 17.33 ± 0.57 e | 0.00 ± 0.00 d | 76.90 ± 0.93 a | 84.73 ± 1.89 a | 91.47 ± 1.72 a |
| Temperature (°C) | aw | EMC of Samples (g Water/g Dry Matter) | ||||
|---|---|---|---|---|---|---|
| 0% AG | 30% AG | 40% AG | 50% AG | 60% AG | ||
| 5 | 0.128 | 0.127 ± 0.001 | 0.031 ± 0.002 | 0.022 ± 0.001 | 0.025 ± 0.002 | 0.023 ± 0.000 |
| 0.291 | 0.136 ± 0.003 | 0.045 ± 0.000 | 0.040 ± 0.000 | 0.037 ± 0.002 | 0.040 ± 0.002 | |
| 0.437 | 0.192 ± 0.004 | 0.098 ± 0.000 | 0.093 ± 0.001 | 0.077 ± 0.002 | 0.076 ± 0.000 | |
| 0.732 | 0.353 ± 0.005 | 0.242 ± 0.015 | 0.228 ± 0.003 | 0.217 ± 0.009 | 0.220 ± 0.001 | |
| 0.805 | 0.402 ± 0.005 | 0.372 ± 0.010 | 0.337 ± 0.006 | 0.312 ± 0.009 | 0.310 ± 0.001 | |
| 0.934 | 0.828 ± 0.016 | 0.566 ± 0.009 | 0.560 ± 0.008 | 0.516 ± 0.003 | 0.502 ± 0.012 | |
| 25 | 0.114 | 0.092 ± 0.006 | 0.023 ± 0.001 | 0.022 ± 0.003 | 0.021 ± 0.000 | 0.027 ± 0.00 |
| 0.237 | 0.117 ± 0.011 | 0.037 ± 0.001 | 0.040 ± 0.000 | 0.031 ± 0.001 | 0.034 ± 0.002 | |
| 0.374 | 0.155 ± 0.004 | 0.081 ± 0.001 | 0.077 ± 0.001 | 0.078 ± 0.000 | 0.073 ± 0.001 | |
| 0.658 | 0.305 ± 0.011 | 0.209 ± 0.007 | 0.203 ± 0.002 | 0.188 ± 0.004 | 0.190 ± 0.002 | |
| 0.761 | 0.384 ± 0.005 | 0.308 ± 0.002 | 0.281 ± 0.002 | 0.267 ± 0.004 | 0.273 ± 0.002 | |
| 0.855 | 0.670 ± 0.010 | 0.516 ± 0.004 | 0.469 ± 0.008 | 0.429 ± 0.010 | 0.436 ± 0.008 | |
| 40 | 0.105 | 0.049 ± 0.003 | 0.022 ± 0.001 | 0.016 ± 0.001 | 0.014 ± 0.004 | 0.021 ± 0.001 |
| 0.206 | 0.052 ± 0.001 | 0.039 ± 0.000 | 0.030 ± 0.004 | 0.026 ± 0.001 | 0.031 ± 0.002 | |
| 0.337 | 0.062 ± 0.001 | 0.056 ± 0.000 | 0.0584 ± 0.001 | 0.054 ± 0.004 | 0.054 ± 0.001 | |
| 0.614 | 0.280 ± 0.006 | 0.234 ± 0.003 | 0.179 ± 0.002 | 0.163 ± 0.007 | 0.188 ± 0.002 | |
| 0.734 | 0.360 ± 0.006 | 0.293 ± 0.004 | 0.275 ± 0.003 | 0.255 ± 0.002 | 0.234 ± 0.004 | |
| 0.806 | 0.655 ± 0.008 | 0.577 ± 0.012 | 0.537 ± 0.009 | 0.513 ± 0.003 | 0.428 ± 0.005 | |
| 60 | 0.095 | 0.026 ± 0.000 | 0.021 ± 0.001 | 0.010 ± 0.001 | 0.011 ± 0.000 | 0.012 ± 0.001 |
| 0.174 | 0.044 ± 0.005 | 0.034 ± 0.000 | 0.032 ± 0.001 | 0.018 ± 0.001 | 0.026 ± 0.004 | |
| 0.298 | 0.056 ± 0.003 | 0.047 ± 0.002 | 0.037 ± 0.001 | 0.037 ± 0.000 | 0.0384 ± 0.001 | |
| 0.565 | 0.202 ± 0.004 | 0.186 ± 0.004 | 0.166 ± 0.005 | 0.161 ± 0.000 | 0.132 ± 0.001 | |
| 0.702 | 0.304 ± 0.006 | 0.193 ± 0.002 | 0.199 ± 0.008 | 0.185 ± 0.002 | 0.191 ± 0.008 | |
| 0.751 | 0.502 ± 0.003 | 0.409 ± 0.011 | 0.408 ± 0.004 | 0.356 ± 0.005 | 0.355 ± 0.005 | |
| Samples | Temperature (°C) | BET | GAB | Peleg | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M0 (%) | C | M0 (%) | C | K | k1 | k2 | n1 | n2 | ||
| 0% AG | 5 | 11.1 | 53.1 | 37.54 | 0.29 | 0.82 | 1.14 | 0.18 | 8.26 | 0.16 |
| 30% AG | 5 | 7.16 | 3.23 | 13.32 | 1.13 | 0.89 | 0.6 | 0.11 | 4.04 | 0.67 |
| 40% AG | 5 | 7.01 | 2.70 | 12.92 | 1.07 | 0.88 | 0.39 | 1.64 | 3.1 | 0.95 |
| 50% AG | 5 | 6.74 | 2.22 | 10.19 | 1.16 | 0.92 | 0.56 | 0.1 | 4.39 | 0.71 |
| 60% AG | 5 | 6.61 | 1.69 | 10.85 | 1.12 | 0.91 | 0.53 | 0.1 | 4.23 | 0.76 |
| 0% AG | 25 | 10.25 | 28.89 | 18.42 | 0.6 | 0.93 | 2.21 | 0.2 | 9.79 | 0.36 |
| 30% AG | 25 | 7.54 | 2.75 | 9.62 | 1.84 | 0.97 | 1.22 | 0.35 | 10.5 | 1.48 |
| 40% AG | 25 | 6.89 | 3.31 | 9.04 | 2.1 | 0.96 | 1.53 | 0.37 | 13.82 | 1.55 |
| 50% AG | 25 | 6.18 | 1.99 | 9.66 | 1.59 | 0.94 | 1.00 | 0.33 | 11.39 | 1.55 |
| 60% AG | 25 | 6.15 | 3.81 | 9.43 | 1.79 | 0.94 | 0.77 | 0.28 | 8.44 | 1.36 |
| 0% AG | 40 | 9.27 | 2.34 | 8.80 | 0.52 | 1.1 | 7.42 | 0.28 | 13.45 | 1.19 |
| 30% AG | 40 | 5.12 | 5.47 | 6.89 | 3.25 | 1.96 | 6.9 | 0.27 | 13.41 | 1.05 |
| 40% AG | 40 | 5.65 | 3.60 | 5.70 | 3.22 | 1.11 | 6.61 | 0.22 | 13.37 | 1.04 |
| 50% AG | 40 | 5.25 | 3.44 | 5.07 | 3.29 | 1.12 | 6.01 | 0.23 | 13.35 | 1.21 |
| 60% AG | 40 | 5.08 | 4.29 | 6.11 | 3.25 | 1.06 | 3.2 | 0.32 | 13.45 | 1.46 |
| 0% AG | 60 | 6.1 | 3.23 | 6.49 | 2.54 | 1.11 | 8.9 | 0.26 | 13.38 | 1.2 |
| 30% AG | 60 | 4.21 | 8.13 | 4.06 | 11.78 | 1.19 | 7.89 | 0.29 | 13.43 | 1.26 |
| 40% AG | 60 | 3.93 | 5.43 | 3.86 | 5.5 | 1.15 | 7.93 | 0.31 | 13.41 | 1.49 |
| 50% AG | 60 | 4.80 | 2.50 | 4.18 | 4.33 | 1.17 | 7.8 | 0.25 | 13.42 | 1.32 |
| 60% AG | 60 | 4.82 | 3.64 | 3.46 | 6.7 | 1.2 | 7.85 | 0.25 | 13.41 | 1.4 |
| RSS | SEE | Me (%) | R2 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | 5 | 25 | 40 | 60 | 5 | 25 | 40 | 60 | 5 | 25 | 40 | 60 | Overall% | 5 | 25 | 40 | 60 | |
| Sample | Models | |||||||||||||||||
| 0% AG | BET | 3.46 × 10−2 | 3.02 × 10−2 | 1.29 × 10−2 | 5.08 × 10−2 | 0.07030 | 0.02070 | 0.04280 | 0.02430 | 15.80 | 11.10 | 17.23 | 17.37 | 15.38 | 0.971 | 0.980 | 0.955 | 0.973 |
| GAB | 1.46 × 10−2 | 2.47 × 10−3 | 1.36 × 10−3 | 7.08 × 10−5 | 0.04570 | 0.01877 | 0.01401 | 0.00318 | 8.66 | 9.22 | 7.00 | 8.97 | 8.46 | 0.988 | 0.992 | 0.995 | 0.999 | |
| Peleg | 4.59 × 10−3 | 2.50 × 10−4 | 1.33 × 10−3 | 4.08 × 10−5 | 0.02559 | 0.00597 | 0.01375 | 0.00241 | 7.31 | 8.63 | 7.75 | 8.60 | 8.07 | 0.989 | 0.999 | 0.995 | 0.999 | |
| 30% AG | BET | 5.00 × 10−3 | 1.00 × 10−3 | 5.92 × 10−3 | 3.30 × 10−2 | 0.0850 | 0.03820 | 0.02910 | 0.02910 | 18.27 | 17.25 | 18.79 | 14.60 | 17.23 | 0.980 | 0.975 | 0.962 | 0.983 |
| GAB | 9.55 × 10−4 | 1.80 × 10−4 | 4.11 × 10−3 | 7.78 × 10−3 | 0.01168 | 0.00506 | 0.02423 | 0.00333 | 10.22 | 5.89 | 7.83 | 8.16 | 8.03 | 0.996 | 0.999 | 0.984 | 0.997 | |
| Peleg | 1.17 × 10−4 | 1.16 × 10−4 | 3.69 × 10−3 | 7.23 × 10−3 | 0.01292 | 0.00406 | 0.02350 | 0.00320 | 7.56 | 7.95 | 7.59 | 8.80 | 7.98 | 0.995 | 0.999 | 0.980 | 0.996 | |
| 40% AG | BET | 2.31 × 10−3 | 2.03 × 10−3 | 4.00 × 10−3 | 3.50 × 10−3 | 0.05550 | 0.01890 | 0.07801 | 0.02240 | 19.23 | 15.19 | 16.78 | 14.10 | 16.33 | 0.969 | 0.970 | 0.968 | 0.909 |
| GAB | 5.05 × 10−4 | 1.96 × 10−4 | 7.44 × 10−4 | 5.33 × 10−3 | 0.00849 | 0.00528 | 0.00103 | 0.00270 | 8.06 | 8.76 | 8.61 | 9.84 | 8.82 | 0.997 | 0.998 | 0.996 | 0.996 | |
| Peleg | 4.36 × 10−4 | 1.07 × 10−4 | 2.49 × 10−4 | 5.27 × 10−3 | 0.00102 | 0.00390 | 0.00596 | 0.00274 | 7.51 | 7.06 | 7.82 | 8.46 | 7.71 | 0.998 | 0.999 | 0.998 | 0.998 | |
| 50% AG | BET | 1.22 × 10−3 | 9.34 × 10−3 | 1.00 × 10−3 | 3.00 × 10−3 | 0.03820 | 0.03732 | 0.04410 | 0.07002 | 19.25 | 18.10 | 19.11 | 19.69 | 19.04 | 0.972 | 0.980 | 0.970 | 0.967 |
| GAB | 2.35 × 10−4 | 1.28 × 10−4 | 8.78 × 10−4 | 4.55 × 10−4 | 0.00579 | 0.00427 | 0.03526 | 0.02548 | 8.60 | 6.95 | 8.29 | 6.08 | 7.48 | 0.999 | 0.999 | 0.986 | 0.980 | |
| Peleg | 2.00 × 10−4 | 8.14 × 10−5 | 1.95 × 10−4 | 3.61 × 10−4 | 0.00535 | 0.00341 | 0.00527 | 0.02271 | 5.84 | 7.43 | 7.86 | 5.83 | 6.74 | 0.999 | 0.999 | 0.999 | 0.997 | |
| 60% AG | BET | 5.47 × 10−3 | 1.40 × 10−3 | 1.22 × 10−3 | 1.00 × 10−3 | 0.02713 | 0.04522 | 0.01401 | 0.0121 | 19.00 | 18.16 | 18.10 | 21.57 | 19.21 | 0.985 | 0.970 | 0.970 | 0.970 |
| GAB | 8.44 × 10−5 | 1.82 × 10−4 | 2.12 × 10−4 | 1.68 × 10−4 | 0.0034 | 0.00510 | 0.00738 | 0.00551 | 5.66 | 8.89 | 8.33 | 8.29 | 7.79 | 0.999 | 0.998 | 0.985 | 0.983 | |
| Peleg | 9.90 × 10−5 | 2.16 × 10−4 | 1.61 × 10−4 | 1.58 × 10−4 | 0.00377 | 0.00555 | 0.00151 | 0.00150 | 3.32 | 2.11 | 4.80 | 3.28 | 3.38 | 0.999 | 0.998 | 0.989 | 0.984 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansoori, N.; Majzoobi, M.; Gavahian, M.; Badii, F.; Farahnaky, A. Acacia Gum as a Natural Anti-Plasticizer for the Production of Date Syrup Powder: Sorption Isotherms, Physicochemical Properties, and Data Modeling. Foods 2020, 9, 50. https://doi.org/10.3390/foods9010050
Mansoori N, Majzoobi M, Gavahian M, Badii F, Farahnaky A. Acacia Gum as a Natural Anti-Plasticizer for the Production of Date Syrup Powder: Sorption Isotherms, Physicochemical Properties, and Data Modeling. Foods. 2020; 9(1):50. https://doi.org/10.3390/foods9010050
Chicago/Turabian StyleMansoori, Nasim, Mahsa Majzoobi, Mohsen Gavahian, Fojan Badii, and Asgar Farahnaky. 2020. "Acacia Gum as a Natural Anti-Plasticizer for the Production of Date Syrup Powder: Sorption Isotherms, Physicochemical Properties, and Data Modeling" Foods 9, no. 1: 50. https://doi.org/10.3390/foods9010050
APA StyleMansoori, N., Majzoobi, M., Gavahian, M., Badii, F., & Farahnaky, A. (2020). Acacia Gum as a Natural Anti-Plasticizer for the Production of Date Syrup Powder: Sorption Isotherms, Physicochemical Properties, and Data Modeling. Foods, 9(1), 50. https://doi.org/10.3390/foods9010050








