Fresh Pasta Manufactured with Fermented Whole Wheat Semolina: Physicochemical, Sensorial, and Nutritional Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Preparation and Maintenance of Liquid Whole Wheat Semolina-Based Sourdough (LWS)
2.3. Physicochemical Analyses of Pasta and LWS
2.4. Pasta Making
2.5. Cooking Quality and Texture Analysis
2.6. In Vitro Starch Digestibility
2.7. Protein Digestibility and Free Amino Acid Analysis
2.8. Total Phenolic Content and Antioxidant Activity
2.9. Phytic Acid Determination
2.10. Consumer Testing
2.11. Statistical Analysis
3. Results
3.1. Physicochemical Characteristics and Cooking Properties
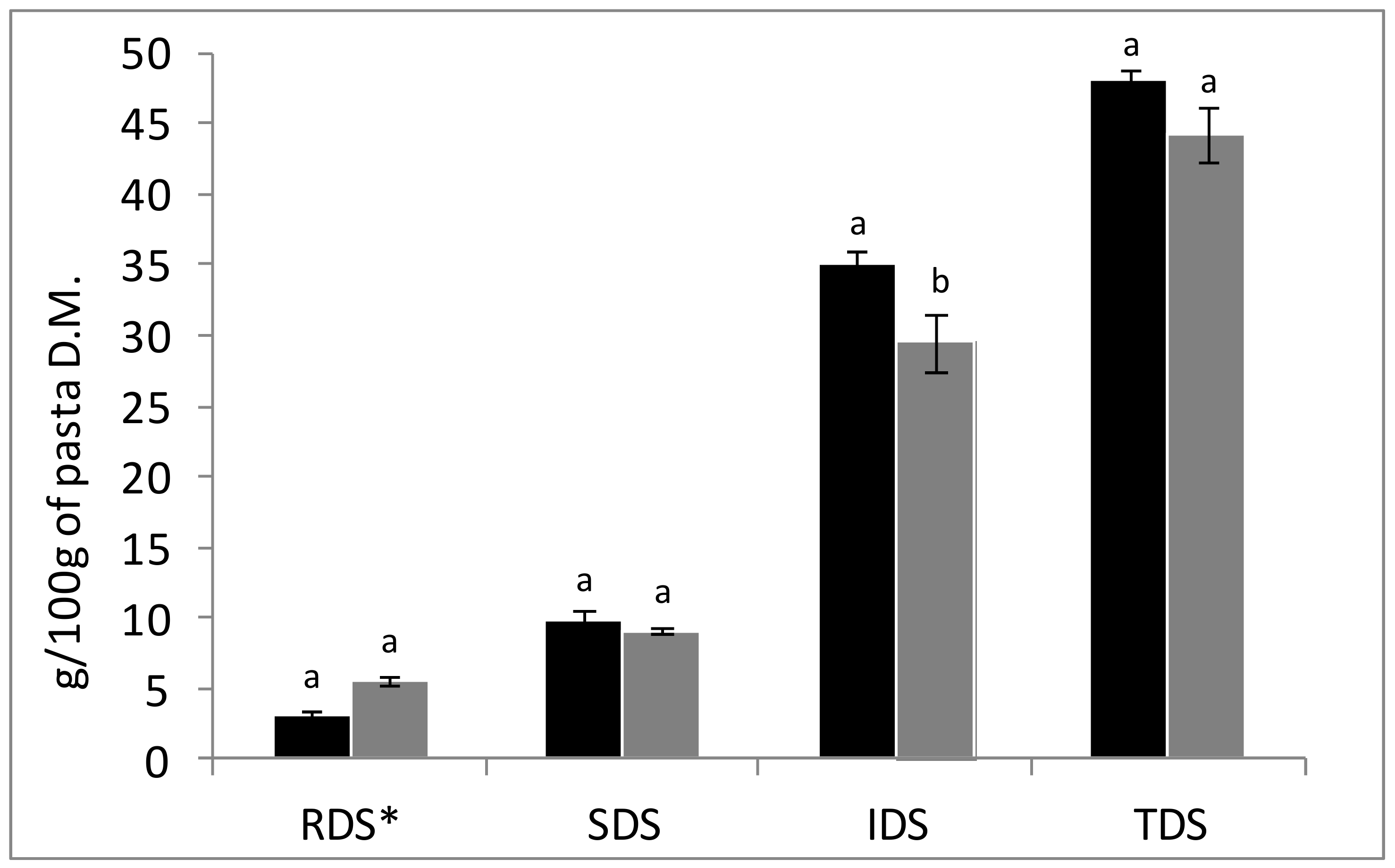
3.2. In Vitro Starch Digestibility
3.3. Protein Digestibility and Amino Acid Content
3.4. Total Phenolic Content, Antioxidant Activity, and Phytic Acid Content
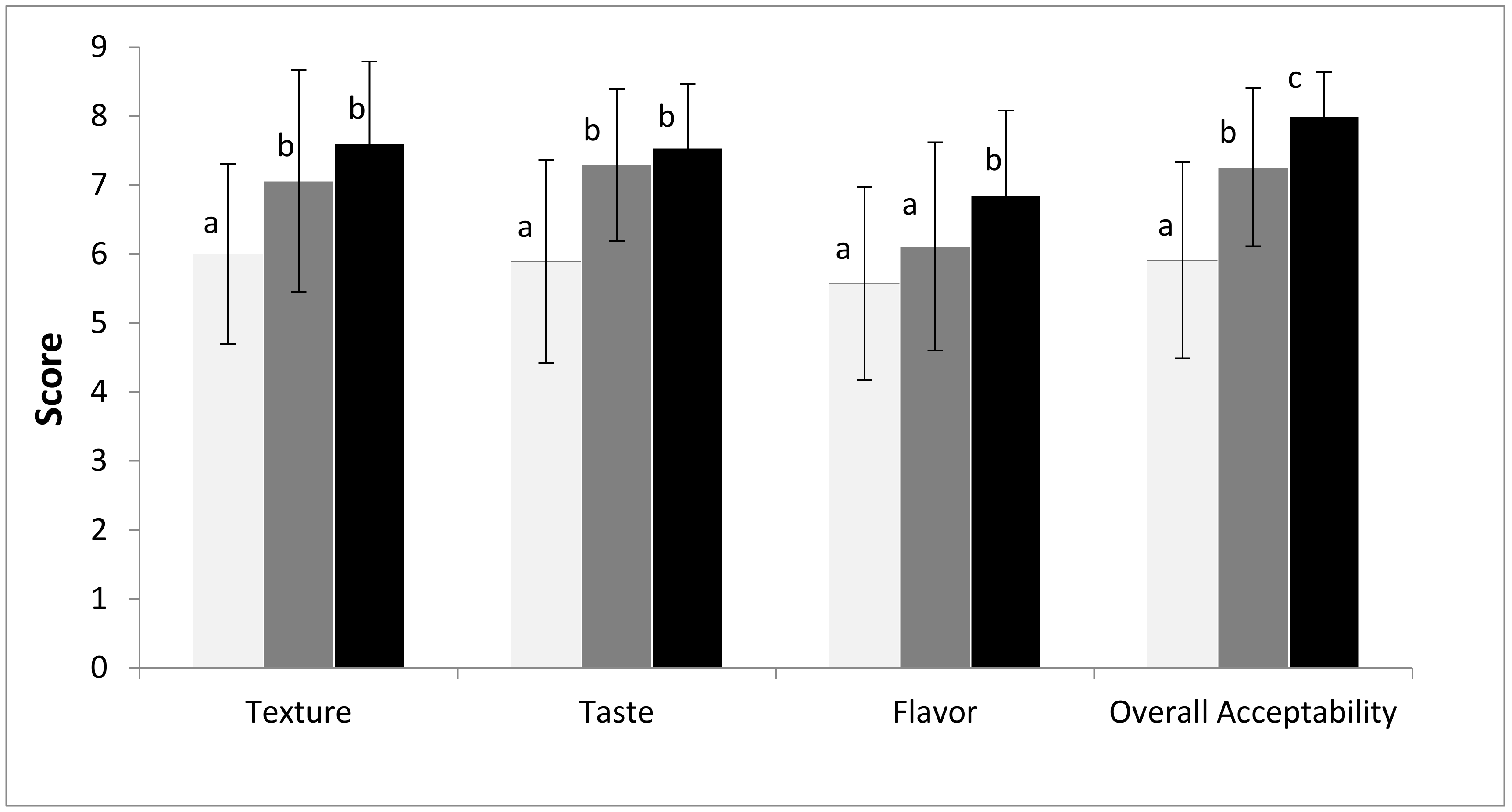
3.5. Consumer Testing
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aune, D.; Chan, D.S.M.; Lau, R.; Vieira, R.; Greenwood, D.C.; Kampman, E.; Norat, T. Dietary fibre, whole grains, and risk of colorectal cancer: Systematic review and dose-response meta-analysis of prospective studies. BMJ 2011, 343, d6617. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Yadava, R.K.; Gollen, B.; Kumar, S.; Verma, R.K.; Yadav, S. Nutritional Contents and Medicinal Properties of Wheat: A Review. Life Sci. Med. Res. 2011, 22, 1–10. [Google Scholar]
- Mongeau, R.; Brooks, S.P.J. Dietary fiber—properties and sources. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Academic Press: Cambridge, MA, USA, 2003; pp. 1813–1823. [Google Scholar]
- Hemery, Y.; Rouau, X.; Lullien-Pellerin, V.; Barron, C.; Abecassis, J. Dry processes to develop wheat fractions and products with enhanced nutritional quality. J. Cereal Sci. 2007, 46, 327–347. [Google Scholar] [CrossRef]
- Cheryan, M.; Anderson, F.W.; Grynspan, F. Magnesium-phytate complexes: Effect of pH and molar ratio on solubility characteristics. Cereal Chem. 1983, 60, 235–237. [Google Scholar]
- Hemdane, S.; Jacobs, P.J.; Dornez, E.; Verspreet, J.; Delcour, J.A.; Courtin, C.M. Wheat (Triticum aestivum L.) bran in bread making: A critical review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 28–42. [Google Scholar] [CrossRef]
- Aravind, N.; Sissons, M.; Egan, N.; Fellows, C. Effect of insoluble dietary fibre addition on technological, sensory, and structural properties of durum wheat spaghetti. Food Chem. 2012, 130, 299–309. [Google Scholar] [CrossRef]
- Vignola, M.B.; Bustos, M.C.; Pérez, G.T. Comparison of quality attributes of refined and whole wheat extruded pasta. LWT Food Sci. Technol. 2018, 89, 329–335. [Google Scholar] [CrossRef]
- Delcour, J.; Poutanen, K. Fibre-Enriched and Whole Grain Breads, 1st ed.; Martin Woodhead Publishing: Cambridge, UK, 2013. [Google Scholar]
- Servi, S.; Özkaya, H.; Colakoglu, A.S. Dephytinization of wheat bran by fermentation with bakers’ yeast, incubation with barley malt flour and autoclaving at different pH levels. J. Cereal Sci. 2008, 48, 471–476. [Google Scholar] [CrossRef]
- Coda, R.; Kärki, I.; Nordlund, E.; Heiniö, R.; Poutanen, K.; Katina, K. Influence of particle size on bioprocess induced changes on technological functionality of wheat bran. Food Microbiol. 2014, 37, 69–77. [Google Scholar] [CrossRef]
- Di Cagno, R.; De Angelis, M.; Alfonsi, G.; De Vincenzi, M.; Silano, M.; Vincentini, O.; Gobbetti, M. Pasta made from durum wheat semolina fermented with selected lactobacilli as a tool for potential decrease of the gluten intolerance. J. Agric. Food Chem. 2005, 53, 4393–4402. [Google Scholar] [CrossRef]
- Capozzi, V.; Menga, V.; Digesu, A.M.; De Vita, P.; van Sinderen, D.; Cattivelli, L.; Fares, C.; Spano, G. Biotechnological production of vitamin B2-enriched bread and pasta. J. Agric. Food Chem. 2011, 59, 8013–8020. [Google Scholar] [CrossRef] [PubMed]
- Fois, S.; Piu, P.P.; Sanna, M.; Roggio, T.; Catzeddu, P. Starch digestibility and properties of fresh pasta made with semolina-based liquid sourdough. LWT Food Sci. Technol. 2018, 89, 496–502. [Google Scholar] [CrossRef]
- Mokrzycki, W.S.; Tatol, M. Color difference Delta E—A survey. Mach. Graph. Vis. 2001, 20, 383–411. [Google Scholar]
- AACC. Approved Methods of the American Association of Cereal Chemist, 10th ed.; AACC Inc.: St. Paul, MN, USA, 2000. [Google Scholar]
- Sanna, M.; Fois, S.; Falchi, G.; Campus, M.; Roggio, T.; Catzeddu, P. Effect of liquid sourdough technology on the pre-biotic, texture, and sensory properties of a crispy flatbread. Food Sci. Biotechnol. 2019, 28, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.W.; Vavak, D.L.; Satterlee, L.D.; Miller, G.A. A multienzyme technique for estimating protein digestibility. J. Food Sci. 1977, 42, 1269–1273. [Google Scholar] [CrossRef]
- Desai, A.S.; Brennan, M.A.; Brennan, C.S. Effect of fortification with fish (Pseudophycis bachus) powder on nutritional quality of durum wheat pasta. Foods 2018, 7, 62. [Google Scholar] [CrossRef] [PubMed]
- Long, W. Automated Amino Acid Analysis Using an Agilent Poroshell HPH-C18 Column. 2015. Available online: http://www.agilent.com/cs/library/applications/5991-5571EN.pdf (accessed on 25 February 2019).
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Boroski, M.; de Aguiar, A.C.; Boeing, J.S.; Rotta, E.M.; Wibby, C.L.; Bonafé, E.G.; de Souza, N.E.; Visentainer, J.V. Enhancement of pasta antioxidant activity with oregano and carrot leaf. Food Chem. 2011, 125, 696–700. [Google Scholar] [CrossRef]
- Henrique, N.A.; Deliza, R.; Rosenthal, A. Consumer sensory characterization of cooked ham using the Check-All-That-Apply (CATA) methodology. Food Eng. Rev. 2015, 7, 265–273. [Google Scholar] [CrossRef]
- Moskowitz, H.R.; Beckley, H.; Resurreccion, A.V.A. Sensory and Consumer Research in Food Product Design and Development; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Coda, R.; Rizzello, C.G.; Pinto, D.; Gobbetti, M. Selected lactic acid bacteria synthesize antioxidant peptides during sourdough fermentation of cereal flours. Appl. Environ. Microbiol. 2012, 78, 1087–1096. [Google Scholar] [CrossRef]
- Kordonowy, R.K.; Youngs, V.L. Utilization of durum bran and its effect on spaghetti. Cereal Chem. 1985, 62, 301–308. [Google Scholar]
- Ӧstman, E. Fermentation as a Means of Optimizing the Glycaemic Index—Food Mechanisms and Metabolic Merits with Emphasis on Lactic Acid in Cereal Products. Ph.D. Thesis, Lund University, Lund, Sweden, 2003. [Google Scholar]
- Brennan, C.S.; Tudorica, C.M. Evaluation of potential mechanisms by which dietary fibre additions reduce the predicted glycaemic index of fresh pastas. Int. J. Food Sci. Technol. 2008, 43, 2151–2162. [Google Scholar] [CrossRef]
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R.T. Dietary fibre in foods: A review. J. Food Sci. Technol. 2012, 49, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Foschia, M.; Peressini, D.; Sensidoni, A.; Brennan, M.A.; Brennan, C.S. Synergistic effect of different dietary fibres in pasta on in vitro starch digestion? Food Chem. 2015, 172, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Lappi, J.; Selinheimo, E.; Schwab, U.; Katina, K.; Lehtinen, P.; Mykkänen, H.; Kolehmainen, M.; Poutanen, K. Sourdough fermentation of wholemeal wheat bread increases solubility of arabinoxylan and protein and decreases postprandial glucose and insulin responses. J. Cereal Sci. 2010, 51, 152–158. [Google Scholar] [CrossRef]
- Gänzle, M.; Gobbetti, M. Physiology and Biochemistry of Lactic Acid Bacteria. In Handbook on Sourdough Biotechnology; Gänzle, M., Gobetti, M., Eds.; Springer: New York, NY, USA, 2013; pp. 183–216. [Google Scholar]
- Teixeira, J.S.; Seeras, A.; Sanchez-Maldonado, A.F.; Zhang, C.; Su, M.S.; Gänzle, M.G. Glutamine, glutamate, and arginine-based acid resistance in Lactobacillus reuteri. Food Microbiol. 2014, 42, 172–180. [Google Scholar] [CrossRef]
- Zhao, C.J.; Kinner, M.; Wismer, W.; Gänzle, M.G. Effect of glutamate accumulation during sourdough fermentation with Lactobacillus reuteri on the taste of bread and sodium-reduced bread. Cereal Chem. 2015, 92, 224–230. [Google Scholar] [CrossRef]
- Diana, M.; Quílez, J.; Rafecas, M. Gamma-aminobutyric acid as a bioactive compound in foods: A review. J. Funct. Food. 2014, 10, 407–420. [Google Scholar] [CrossRef]
- Lamberts, L.; Joye, I.J.; Beliën, T.; Delcour, J.A. Dynamics of γ-aminobutyric acid in wheat flour bread making. Food Chem. 2012, 130, 896–901. [Google Scholar] [CrossRef]
- Hur, A.J.; Lee, S.Y.; Kim, Y.; Choi, I.; Kim, G. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef]
- Ciccoritti, R.; Taddei, F.; Nicoletti, I.; Gazza, L.; Corradini, D.; D’Egidio, M.G.; Martini, D. Use of bran fractions and debranned kernels for the development of pasta with high nutritional and healthy potential. Food Chem. 2017, 225, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Fares, C.; Platani, C.; Baiano, A.; Menga, V. Effect of processing and cooking on phenolic acid profile and antioxidant capacity of durum wheat pasta enriched with debranning fractions of wheat. Food Chem. 2010, 119, 1023–1029. [Google Scholar] [CrossRef]
- Zielinski, H.; Kozlowska, H.; Lewczuk, B. Bioactive compounds in the cereal grains before and after hydrothermal processing. Innov. Food Sci. Emerg. Technol. 2001, 2, 159–169. [Google Scholar] [CrossRef]
- Dordević, T.M.; Šiler-Marinković, S.S.; Dimitrijević-Branković, S.I. Effect of fermentation on antioxidant properties of some cereals and pseudo cereals. Food Chem. 2010, 119, 957–963. [Google Scholar] [CrossRef]
- Gobbetti, M.; Rizzello, C.G.; Di Cagno, R.; De Angelis, M. How the sourdough may affect the functional features of leavened baked goods. Food Microbiol. 2014, 37, 30–40. [Google Scholar] [CrossRef]
- Resano, H.; Sanjuán, A.I.; Albisu, L.M. Consumers’ acceptability of cured ham in Spain and the influence of information. Food Qual. Prefer. 2007, 18, 1064–1076. [Google Scholar] [CrossRef]
- Laureati, M.; Conte, A.; Padalino, L.; Del Nobile, M.A.; Pagliarini, E. Effect of fiber information on consumer’s expectation and liking of wheat bran enriched pasta. J. Sens. Stud. 2016, 31, 348–359. [Google Scholar] [CrossRef]
| Ashes | Moisture | Protein Content | Dietary Fiber | pH | TTA | L | a | b | ΔE76 | |
|---|---|---|---|---|---|---|---|---|---|---|
| g/100 g DM 1 | g/100 g | g/100 g DM | g/100 g DM | mL NaOH 0.1N | ||||||
| fermentation | ns | ns | ns | ns | *** | *** | ||||
| cooking | *** | *** | ns | *** | *** | |||||
| fermentation*cooking | ns | ns | ** | *** | *** | |||||
| CP raw | 0.97 a | 30.51 b | 13.75 b | 8.01 | 6.50 a | 2.48 b | 63.96 a | 2.45 a | 14.16 a | |
| CP cooked | 0.70 b | 58.24 a | 14.64 a | 6.65 a | 1.25 c | 4.26 | ||||
| SP raw | 0.97 a | 31.01 b | 14.32 b | 8.16 | 5.11 c | 5.98 a | 68.01 b | 2.50 a | 15.46 b | |
| SP cooked | 0.61 b | 58.39 a | 13.72 b | 5.67 b | 2.45 b |
| Cooked Samples | Cooking Loss * (%) | Swelling Index ** (g∙g−1) | Protein Loss in Water *** (%) | Firmness (N) | Protein Digestibility (%) | Protein Availability (%) |
|---|---|---|---|---|---|---|
| CP | 4.61 a | 1.34 a | 0.36 a | 6.88 b | 86.6 b | 12.6 b |
| SP | 5.27 b | 1.36 a | 0.45 b | 5.64 a | 82.9 a | 11.9 a |
| Aminoacids | Fermentation | Cooking | Fermentation*Cooking | CP | SP | ||
|---|---|---|---|---|---|---|---|
| Raw | Cooked | Raw | Cooked | ||||
| Aspartic acid | ** | *** | ns | 23.34 | 24.32 | 33.27 | 29.70 |
| Glutamic acid | *** | ** | ns | 14.86 | 15.19 | 29.48 | 26.36 |
| Asparagine | *** | *** | * | 21.63 | 20.62 | 14.86 | 13.41 |
| Glutamine | *** | *** | *** | 4.84 | 5.24 | 1.24 | 1.34 |
| Serine | *** | *** | * | 3.03 | 2.77 | 5.62 | 5.41 |
| Histidine | * | ** | ns | 2.22 | 2.13 | 2.84 | 2.46 |
| Glycine | ns | *** | ns | 3.81 | 4.10 | 4.39 | 4.52 |
| Threonine | *** | *** | ns | 2.20 | 2.27 | 3.75 | 3.48 |
| Arginine | *** | ** | ns | 10.86 | 8.55 | 17.56 | 13.58 |
| Alanine | ns | *** | ns | 11.15 | 11.43 | 12.11 | 11.52 |
| GABA | *** | *** | ns | 17.88 | 17.19 | 13.44 | 12.40 |
| Tyrosine | * | ** | ns | 5.06 | 4.37 | 6.59 | 5.14 |
| Valine | ** | *** | ns | 7.78 | 7.39 | 10.86 | 9.82 |
| Methionine | *** | ** | ns | 0.68 | 0.70 | 1.31 | 1.23 |
| Tryptophan | ns | ** | ns | 14.60 | 11.37 | 13.97 | 10.02 |
| Phenylalanine | *** | ** | ns | 3.36 | 2.96 | 6.26 | 4.81 |
| Isoleucine | ** | *** | ** | 1.91 | 1.83 | 1.83 | 1.28 |
| Leucine | *** | ** | ns | 3.09 | 3.04 | 9.24 | 7.70 |
| Lysine | ns | ns | ns | 3.52 | 2.86 | 3.86 | 2.95 |
| Proline | ns | *** | ns | 3.81 | 5.05 | 4.92 | 4.80 |
| FAA 1 | * | *** | ns | 159.65 | 153.39 | 197.41 | 171.91 |
| EAA 2 | ** | ** | ns | 39.38 | 34.55 | 53.92 | 43.74 |
| Samples | Total Phenolic Content 1 (mg/100 g DM) | Antioxidant Activity 2 (%) | Phytic Acid (g/100 g DM) |
|---|---|---|---|
| fermentation | *** | *** | ** |
| cooking | *** | *** | ** |
| fermentation*cooking | *** | ** | ns |
| CP raw | 23.53 c | 3.57 c | 0.26 a |
| CP cooked | 36.13 b | 6.53 b | 0.40 b |
| SP raw | 37.02 b | 6.33 b | 0.19 c |
| SP cooked | 104.87 a | 7.83 a | 0.25 d |
| Attributes | Significance | CP | SP Blind | SP Informed |
|---|---|---|---|---|
| wholesome | *** | 0.29 a | 0.42 a | 0.90 b |
| balanced diet | *** | 0.29 a | 0.42 a | 0.81 b |
| satiating | *** | 0.46 a | 0.54 a | 0.77 b |
| distinctive odor | *** | 0.29 a | 0.79 b | 0.98 b |
| home made | *** | 0.37 a | 0.79 b | 0.98 b |
| hard to chew | *** | 0.79 b | 0.21 a | 0.21 a |
| tasteful | *** | 0.21 a | 0.81 b | 1.00 b |
| unusual taste | *** | 0.04 a | 0.29 b | 0.034 a |
| “al dente” | *** | 0.19 a | 0.50 b | 1.00 c |
| adsorbs sauce | *** | 0.56 a | 0.77 b | 0.92 b |
| sticky | *** | 0.33 b | 0.19 a,b | 0.00 a |
| gently sour | *** | 0.00 a | 0.48 b | 0.71 c |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fois, S.; Campus, M.; Piu, P.P.; Siliani, S.; Sanna, M.; Roggio, T.; Catzeddu, P. Fresh Pasta Manufactured with Fermented Whole Wheat Semolina: Physicochemical, Sensorial, and Nutritional Properties. Foods 2019, 8, 422. https://doi.org/10.3390/foods8090422
Fois S, Campus M, Piu PP, Siliani S, Sanna M, Roggio T, Catzeddu P. Fresh Pasta Manufactured with Fermented Whole Wheat Semolina: Physicochemical, Sensorial, and Nutritional Properties. Foods. 2019; 8(9):422. https://doi.org/10.3390/foods8090422
Chicago/Turabian StyleFois, Simonetta, Marco Campus, Piero Pasqualino Piu, Silvia Siliani, Manuela Sanna, Tonina Roggio, and Pasquale Catzeddu. 2019. "Fresh Pasta Manufactured with Fermented Whole Wheat Semolina: Physicochemical, Sensorial, and Nutritional Properties" Foods 8, no. 9: 422. https://doi.org/10.3390/foods8090422
APA StyleFois, S., Campus, M., Piu, P. P., Siliani, S., Sanna, M., Roggio, T., & Catzeddu, P. (2019). Fresh Pasta Manufactured with Fermented Whole Wheat Semolina: Physicochemical, Sensorial, and Nutritional Properties. Foods, 8(9), 422. https://doi.org/10.3390/foods8090422





