Different Postharvest Responses of Fresh-Cut Sweet Peppers Related to Quality and Antioxidant and Phenylalanine Ammonia Lyase Activities during Exposure to Light-Emitting Diode Treatments
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Light-Emitting Diode (LED) Light Treatment
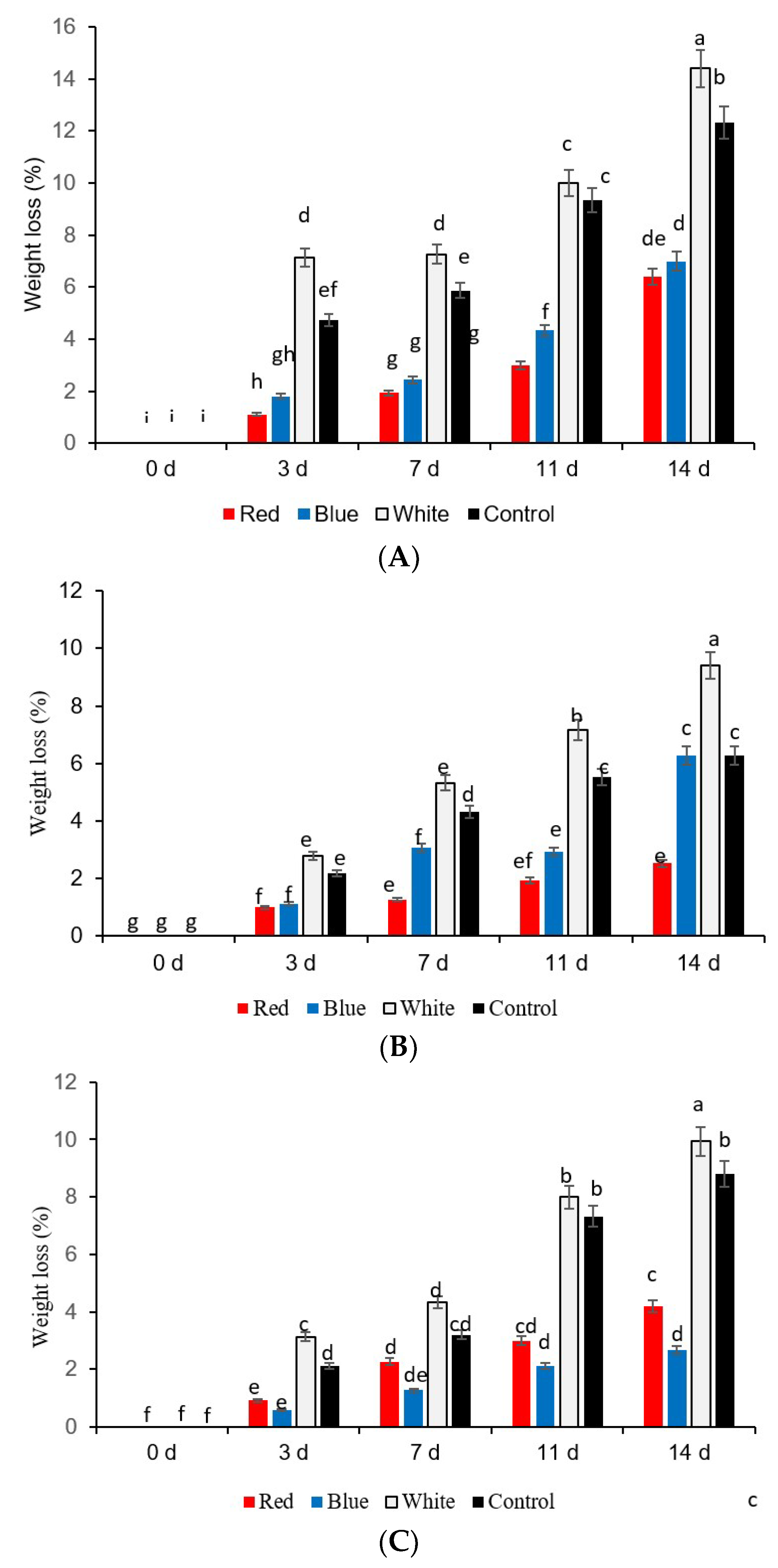
2.2. Weight Loss
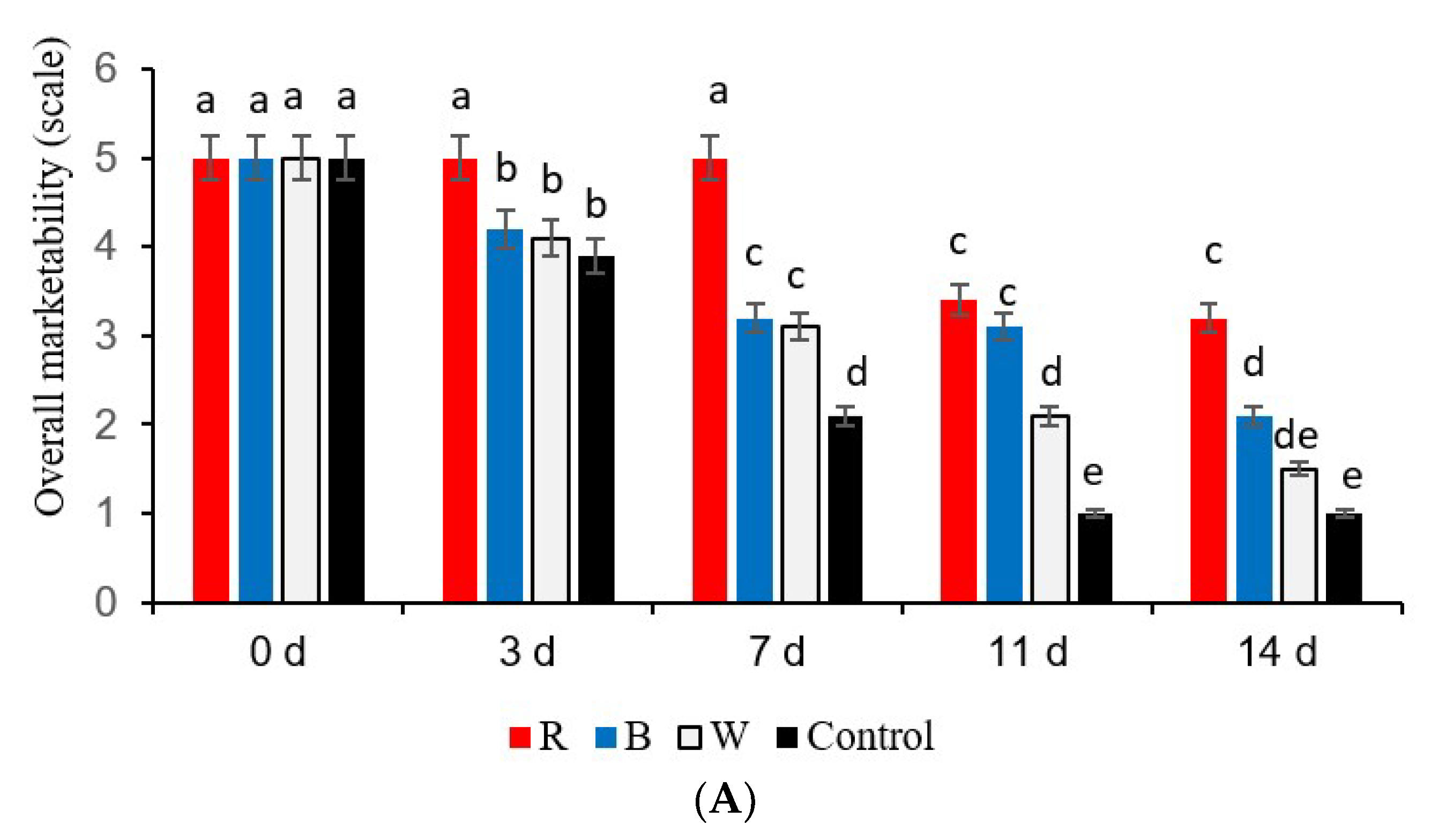
Overall Marketability
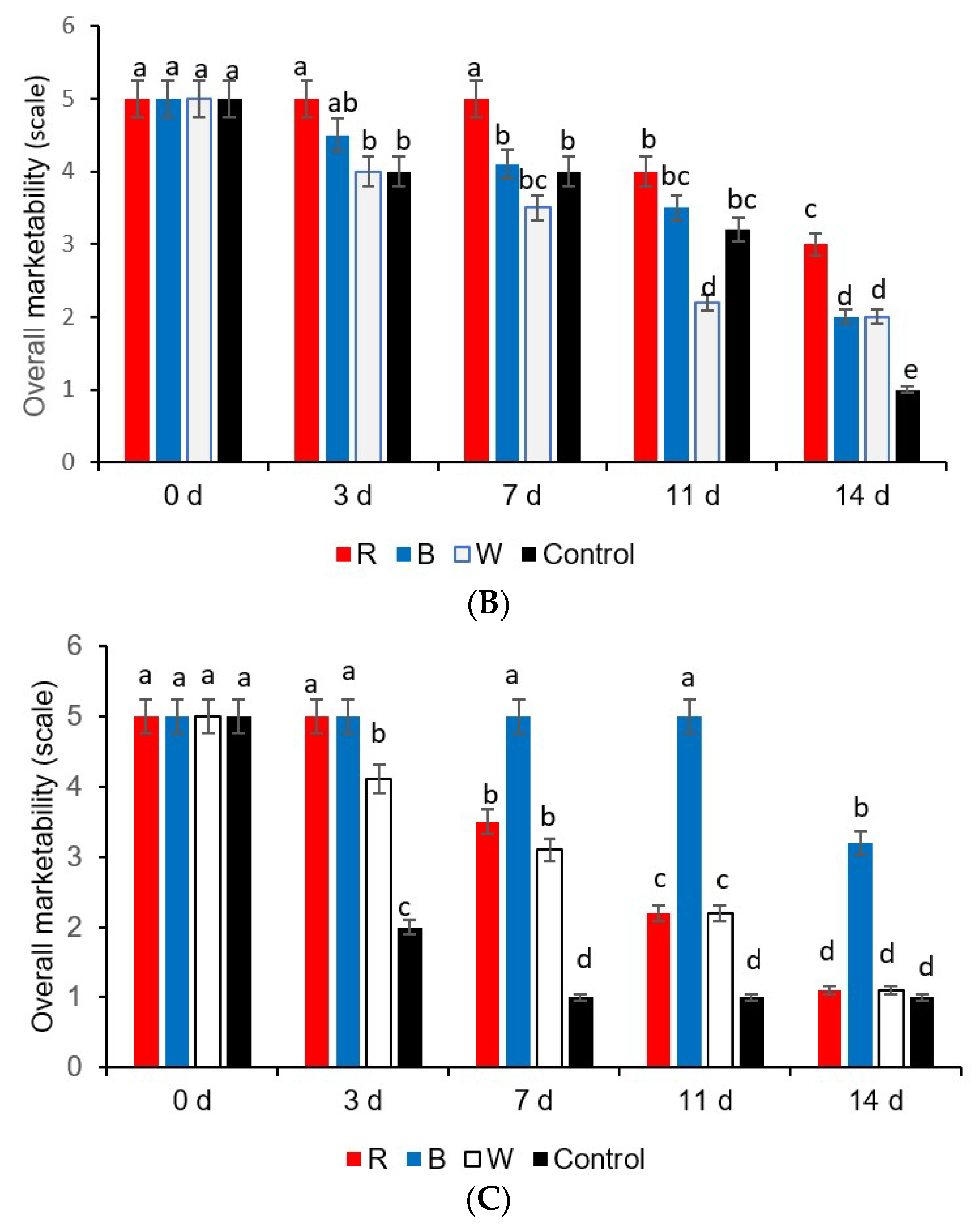
2.3. Color Change (∆E)
2.4. Phytochemical Contents
2.4.1. Ascorbic Acid Content
2.4.2. Chlorophyll Content
2.4.3. Lycopene and β-Carotene Contents
2.4.4. Total Phenolic and Flavonoid Contents
2.4.5. Antioxidant Activity
2.4.6. Phenylalanine Ammonia Lyase (PAL)
2.5. Statistical Analysis
3. Results
3.1. Commercial Value and Overall Marketability
3.2. β-Carotene, Chlorophyll, and Lycopene
3.3. Ascorbic Acid, Total Phenols, Flavonoids, and Antioxidant Activity
3.4. PAL Activity
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Taulavuori, E.; Taulavuori, K.; Holopainen, J.K.; Julkunen-Tiitto, R.; Acar, C.; Dincer, I. Targeted use of LEDs in improvement of production efficiency through phytochemical enrichment. J. Sci. Food Agric. 2017, 97, 5059–5064. [Google Scholar] [CrossRef] [PubMed]
- Bantis, F.; Smirnakou, S.; Ouzounis, T.; Koukounaras, A.; Ntagkas, N.; Radoglou, K. Current status and recent achievements in the field of horticulture with the use of light-emitting diodes (LEDs). Sci. Hortic. 2018, 235, 437–451. [Google Scholar] [CrossRef]
- Nájera, C.; Guil-Guerrero, J.L.; Enríquez, L.J.; Álvaro, J.E.; Urrestarazu, M. LED-enhanced dietary and organoleptic qualities in postharvest tomato fruit. Postharvest Biol. Technol. 2018, 145, 151–156. [Google Scholar] [CrossRef]
- Hasperué, J.H.; Guardianelli, L.; Rodoni, L.M.; Chaves, A.R.; Martínez, G.A. Continuous white-blue LED light exposition delays postharvest senescence of broccoli. LWT-Food Sci. Technol. 2016, 65, 495–502. [Google Scholar] [CrossRef]
- Hasperuéa, J.H.; Rodonia, L.M.; Guardianellia, L.M.; Chavesa, A.R.; Martínez, G.A. Use of LED light for Brussels sprouts postharvest conservation. Sci. Hortic. 2016, 213, 281–286. [Google Scholar] [CrossRef]
- Schmidt-Heydt, M.; Bode, H.; Raupp, F.; Geisen, R. Influence of light on ochratoxin biosynthesis by Penicillium. Mycotoxin Res. 2010, 26, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Castro, S.M.; Saraiva, J.A.; Lopes-da-Silva, J.A.; Delgadillo, I.; Van Loey, A.; Smout, C.; Hendrickx, M. Effect of thermal blanching and of high pressure treatments on sweet green and red bell pepper fruits (Capsicum annuum L.). Food Chem. 2008, 10, 1436–1449. [Google Scholar] [CrossRef]
- Ghasemnehad, M.; Sherafti, M.; Payvast, G.A. Variation in phenolic compounds, ascorbic acid and antioxidant capacity of five coloured bell pepper (Capsicum annum) fruit at two different harvest times. J. Funct. Foods 2011, 3, 44–49. [Google Scholar] [CrossRef]
- Macho, A.; Lucena, C.; Sancho, R.; Daddario, N.; Minassi, A.; Muñoz, E.; Appendino, G. Non-pungent capsaicinoids from sweet pepper synthesis and evaluation of the chemopreventive and anticancer potential. Eur. J. Nutr. 2003, 42, 2–9. [Google Scholar] [CrossRef]
- Manolopoulou, H.; Lambrinos, G.; Xanthopoulos, G. Active Modified Atmosphere Packaging of Fresh-cut Bell Peppers: Effect on Quality Indices. J. Food Res. 2012, 3, 148–158. [Google Scholar] [CrossRef]
- Lister, C.E.; Lancaster, J.E.; Walker, J.R.L. Phenylalanine Ammonia-lyase (PAL) Activity and its Relationship to Anthocyanin and Flavonoid Levels in New Zealand-grown Apple Cultivars. J. Am. Soc. Hortic. Sci. 1996, 121, 281–285. [Google Scholar] [CrossRef]
- Mashabela, M.N.; Selahle, K.M.; Soundy, P.; Crosby, K.M.; Sivakumar, D. Bioactive compounds and fruit quality of green sweet pepper grown under different colored shade netting during postharvest storage. J. Food Sci. 2015, 80, 2612–2618. [Google Scholar] [CrossRef] [PubMed]
- Eyarkai Nambi, V.; Gupta, R.K.; Kumar, S.; Sharma, P.C. Degradation kinetics of bioactive components, antioxidant activity, colour and textural properties of selected vegetables during blanching. J. Food Sci. Technol. 2016, 53, 3073–3082. [Google Scholar] [CrossRef] [PubMed]
- Nagata, M.; Yamashita, I. Simple method for simultaneous determination of chlorophyll and carotenoids in tomato fruit. J. Jpn. Soc. Food Sci. 1992, 39, 925–928. [Google Scholar] [CrossRef]
- Llorach, R.; Tomás-Barberán, F.A.; Ferreres, F. Lettuce and chicory by-products as a source of antioxidant phenolic extracts. J. Agric. Food Chem. 2004, 52, 5109–5116. [Google Scholar] [CrossRef] [PubMed]
- Sellamuthu, P.S.; Sivakumar, D.; Soundy, P.; Korsten, L. Essential oil vapors suppress the development of anthracnose and enhance defense related and antioxidant enzyme activities in avocado fruit. Postharvest Biol. Technol. 2013, 81, 66–72. [Google Scholar] [CrossRef]
- Lee, Y.J.; Ha, J.Y.; Oh, J.E.; Cho, M.S. The effect of LED irradiation on the quality of cabbage stored at a low temperature. Food Sci. Biotechnol. 2014, 23, 1087–1093. [Google Scholar] [CrossRef]
- Nunes, C.N.; Jean-Pierre, D. Relationship between weight loss and visual quality of fruits and vegetables. Proc. Fla. State Hortic. 2007, 120, 235–245. [Google Scholar]
- Zhan, L.; Hub, J.; Li, Y.; Panga, L. Combination of light exposure and low temperature in preserving quality and extending shelf-life of fresh-cut broccoli (Brassica oleracea L.). Postharvest Biol. Technol. 2012, 72, 76–81. [Google Scholar] [CrossRef]
- Huang, F.; Xu, J.Y.; Zhou, W. Effect of LED irradiation on the ripening and nutritional quality of postharvest banana fruit. J. Sci. Food Agric. 2018, 98, 5486–5493. [Google Scholar] [CrossRef]
- Ma, G.; Zhang, L.; Setiawan, C.K.; Yamawaki, K.; Asai, T.; Nishikawa, F.; Maezawa, S.H.; Sato, H.; Kanemitsu, N.; Kato, M. Effect of red and blue LED light irradiation on ascorbate content and expression of genes related to ascorbate metabolism in postharvest broccoli. Postharvest Biol Technol. 2014, 94, 97–103. [Google Scholar] [CrossRef]
- Chen, M.; Chory, J.; Fankhauser, C. Light signal transduction in higher plants. Annu. Rev. Genet. 2004, 38, 87–117. [Google Scholar] [CrossRef] [PubMed]
- Devlin, P.F.; Christie, J.M.; Terry, M.J. Many hands make light work. J. Exp. Bot. 2007, 58, 3071–3077. [Google Scholar] [CrossRef] [PubMed]
- Alba, R.; Cordonnier-Pratt, M.M.; Pratt, L.H. Fruit-localized phytochromes regulate lycopene accumulation independently of ethylene production in tomato. Plant Physiol. 2000, 123, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Simkin, A.J.; Zhu, C.; Kuntz, M.; Sandmann, G. Light-dark regulation of carotenoid biosynthesis in pepper (Capsicum annuum) leaves. J. Plant Physiol. 2003, 160, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Shuai, W.; Xiaodi, W.; Xiangbin, S.; Baoliang, W.; Xiaocui, Z.; Haibo, W.; Fengzhi, L. Red and blue lights significantly affect photosynthetic properties and ultrastructure of mesophyll cells in senescing grape leaves. Hortic. Plant J. 2016, 2, 82–90. [Google Scholar]
- Demotes-Mainard, S.; Péron, T.; Corot, A.; Bertheloot, J.; Le Gourrierec, J.; Pelleschi-Travier, S.; Crespel, L.; Morel, P.; Huché-Thélier, L.; Boumaza, R.; et al. Plant responses to red and far-red lights, applications in horticulture. Environ. Exp. Bot. 2016, 121, 4–21. [Google Scholar] [CrossRef]
- Lin, C. Plant blue-light receptors. Trends Plant Sci. 2000, 5, 337–342. [Google Scholar] [CrossRef]
- Wu, M.C.; YaoHou, C.; Jiang, C.M.; Wang, Y.T.; Wang, C.Y.; Chen, H.H.; Chang, H.M. A novel approach of LED light radiation improves the antioxidant activity of pea seedlings. Food Chem. 2007, 101, 1753–1758. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Ntagkas, N.; Woltering, E.J.; Marcelis, L.F.M. Light regulates ascorbate in plants: An integrated view on physiology and biochemistry. Environ. Exp. Bot. 2018, 147, 271–280. [Google Scholar] [CrossRef]
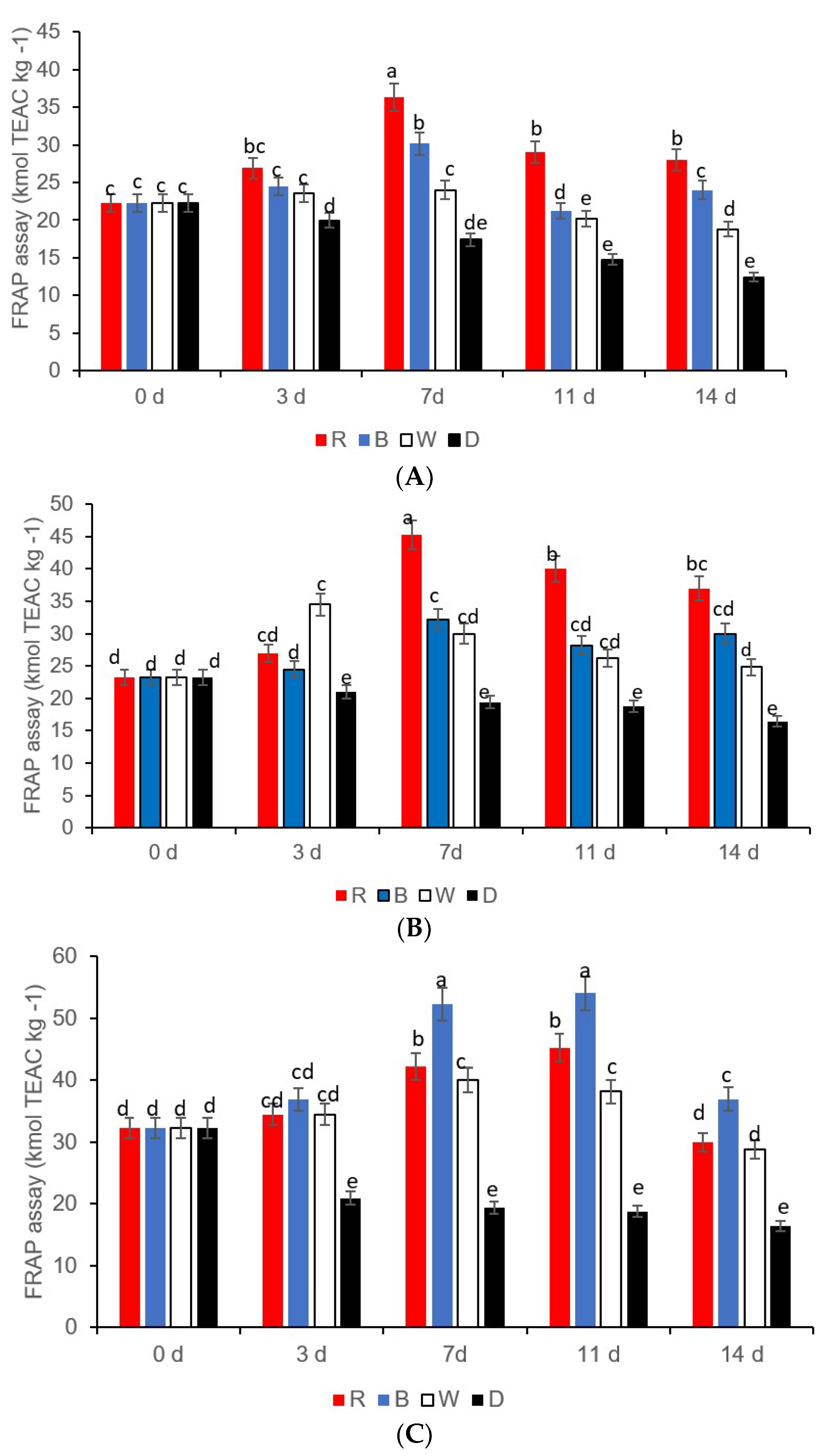
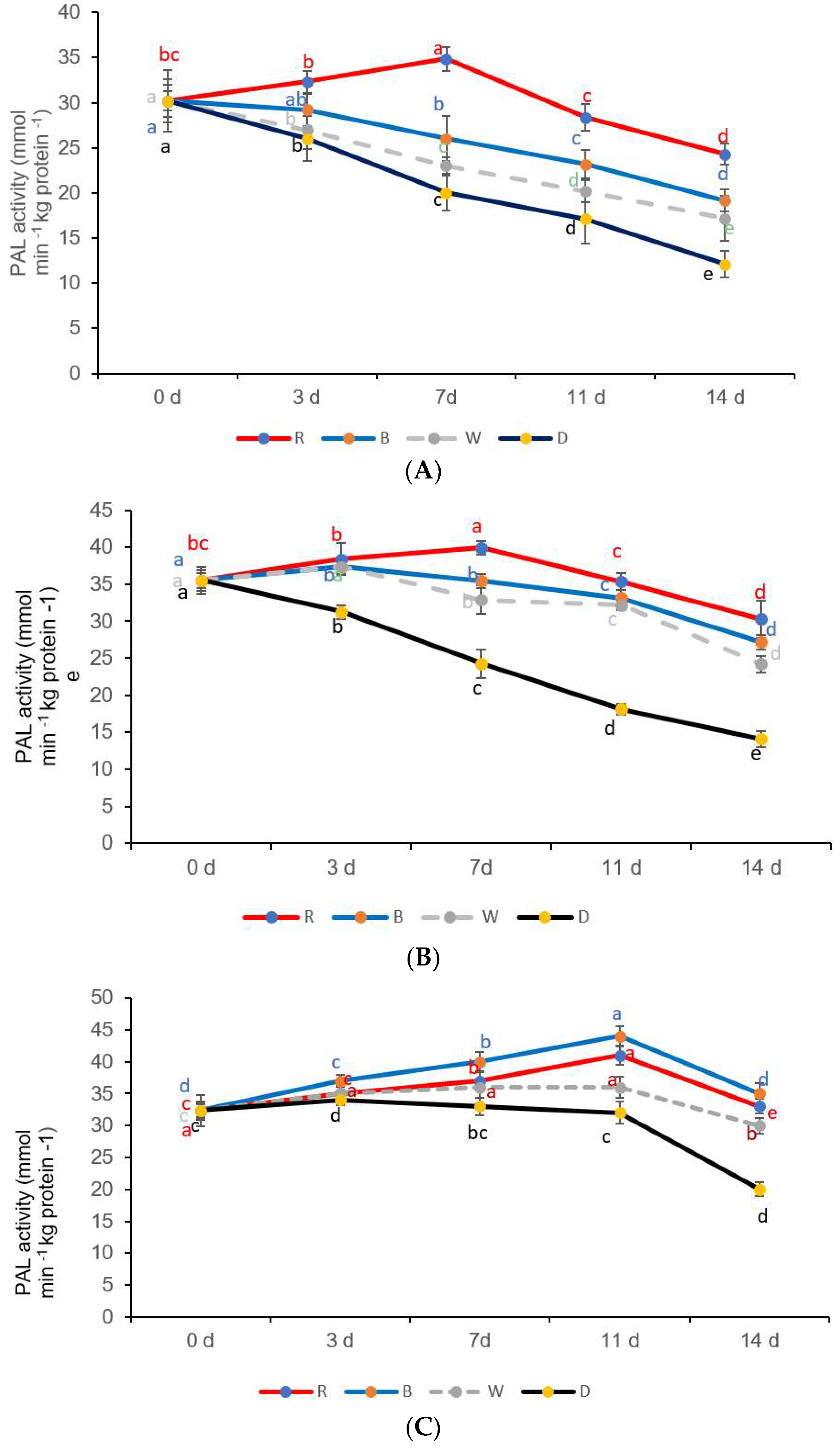
| LED Lights and Storage Time (d) | β-Carotene (g kg−1) | Chlorophyll (g kg−1) | Lycopene (g kg−1) |
|---|---|---|---|
| Red LED light x 0 day | 0.212 a ± 0.01 | 3.19 a ± 0.02 | 0.165 a ± 0.03 |
| Red LED light x 3 days | 0.213 a ± 0.01 | 3.19 a ± 0.05 | 0.167 a ± 0.06 |
| Red LED light x 7 days | 0.214 a ± 0.02 | 3.00 a ± 0.03 | 0.168 a ± 0.01 |
| Red LED light x 11 days | 0.190 b ± 0.01 | 2.7 b ± 0.04 | 0.166 a ± 0.02 |
| Red LED light x 14 days | 0.181 b ± 0.04 | 1.75 c ± 0.08 | 0.160 ab ± 0.03 |
| Blue LED light x 0 days | 0.212 a ± 0.01 | 3.19 a ± 0.01 | 0.165 a ± 0.02 |
| Blue LED light x 3 days | 0.187 b ± 0.05 | 3.12 a ± 0.05 | 0.165 a ± 0.01 |
| Blue LED light x 7 days | 0.175 b ± 0.10 | 2.70 b ± 0.02 | 0.162 a ± 0.02 |
| Blue LED light x 11 days | 0.143 c ± 0.08 | 2.70 b ± 0.02 | 0.140 b ± 0.10 |
| Blue LED light x 14 days | 0.141 c ± 0.03 | 1.87 c ± 0.10 | 0.138 b ± 0.08 |
| White light x 0 day | 0.212 a ± 0.02 | 3.19 a ± 0.02 | 0.165 a ± 0.01 |
| White light x 3 days | 0.175 b ± 0.06 | 3.07 a ± 0.07 | 0.165 a ± 0.07 |
| White light x 7 days | 0.158 b ± 0.03 | 2.43 b ± 0.01 | 0.120 b ± 0.12c |
| White light x 11 days | 0.141 c ± 0.02 | 1.86 c ± 0.04 | 0.115 c ± 0.07 |
| White light x 14 days | 0.089 d ± 0.02 | 1.70 c ± 0.08 | 0.114 c ± 0.05 |
| Darkness x 0 days | 0.212 a ± 0.02 | 3.19 a ± 0.01 | 0.165 a ± 0.03 |
| Darkness x 3 days | 0.184 b ± 0.03 | 3.07 a ± 0.06 | 0.113 c ± 0.14 |
| Darkness x 7 days | 0.140 c ± 0.07 | 1.75 c ± 0.05 | 0.106 cd ± 0.05 |
| Darkness x 11 days | 0.137 c ± 0.04 | 1.74 c ± 0.03 | 0.099 d ± 0.02 |
| Darkness x 14 days | 0.082 d ± 0.03 | 1.68 c ± 0.09 | 0.093 d ± 0.01 |
| Fresh-Cuts | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| LED Lights and Storage Time (d) | Yellow Sweet Pepper | Green Sweet Pepper | Red Sweet Pepper | ||||||
| Ascorbic Acid Content (g kg−1) | Total Phenols (g kg−1) | Total Flavonoid (Quercetin Content) (g kg−1) | Ascorbic Acid Content (g kg−1) | Total Phenols (g kg−1) | Total Flavonoid (Quercetin Content) (g kg−1) | Ascorbic Acid Content (g kg−1) | Total Phenols (g kg−1) | Total Flavonoid (Quercetin Content) (g kg–1)] | |
| Red LED light x 0 day | 1.58 a+0.03 | 0.96 bc ± 0.09 | 0.028 c ± 0.03 | 1.98 a ± 0.06 | 0.72 bc ± 0.02 | 0.020 c ± 0.02 | 1.75 a ± 0.05 | 1.02 c ± 0.04 | 0.034 c ± 0.09 |
| Red LED light x 3 days | 1.57 a ± 0.09 | 1.01 b ± 0.03 | 0.043 a ± 0.12 | 1.96 a ± 0.04 | 1.02 a ± 0.04 | 0.030 b ± 0.06 | 1.73 a ± 0.02 | 1.26 b ± 0.09 | 0.036 c ± 0.02 |
| Red LED light x 7 days | 1.56 a ± 0.04 | 1.28 a ± 0.05 | 0.045 a ± 0.15 | 1.95 a ± 0.19 | 1.07 a ± 0.18 | 0.035 a ± 0.03 | 1.72 a ± 0.09 | 1.29 b ± 0.03 | 0.042 bc ± 0.11 |
| Red LED light x 11 days | 1.34 b ± 0.12 | 1.10 b ± 0.01 | 0.035 b ± 0.09 | 1.70 b ± 0.02 | 0.80 b ± 0.12 | 0.027 b ± 0.05 | 1.50 b ± 0.13 | 1.30 b ± 0.01 | 0.038 c ± 0.07 |
| Red LED light x 14 days | 1.26 bc ± 0.04 | 0.87 bc ± 0.11 | 0.032 bc ± 0.06 | 1.45 c ± 0.12 | 0.78 bc ± 0.02 | 0.029 b ± 0.03 | 1.23 c ± 0.01 | 1.01 c ± 0.21 | 0.035 c ± 0.14 |
| Blue LED light x 0 days | 1.58 a ± 0.03 | 0.96 bc ± 0.09 | 0.028 c ± 0.04 | 1.98 a ± 0.6 | 0.76 bc ± 0.02 | 0.021 c ± 0.04 | 1.75 a ± 0.05 | 1.02 c ± 0.04 | 0.034 c ± 0.01 |
| Blue LED light x 3 days | 1.33 b ± 0.15 | 0.94 bc ± 0.17 | 0.032 bc ± 0.25 | 1.94 a ± 0.16 | 0.98 ab ± 0.04 | 0.025 bc ± 0.01 | 1.73 a ± 0.19 | 1.55 a ± 0.010 | 0.050 b ± 0.05 |
| Blue LED light x 7 days | 1.32 b ± 0.02 | 0.84 bc ± 0.03 | 0.034 bc ± 0.02 | 1.69 b ± 0.08 | 0.80 b ± 0.15 | 0.028 b ± 0.09 | 1.75 a ± 0.12 | 1.60 a ± 0.08 | 0.064 a ± 0.12 |
| Blue LED light x 11 days | 1.21 c ± 0.13 | 0.67 c ± 0.12 | 0.035 b ± 0.04 | 1.44 c ± 0.01 | 0.79 b ± 0.16 | 0.027 b ± 0.01 | 1.70 a ± 0.14 | 1.63 a ± 0.04 | 0.067 a ± 0.16 |
| Blue LED light x 14 days | 1.20 c ± 0.095 | 0.56 cd ± 0.06 | 0.032 bc ± 0.01 | 1.30 cd ± 02 | 0.78 b ± 0.07 | 0.026 b ± 0.04 | 1.45 b ± 0.09 | 1.30 b ± 0.02 | 0.049 b ± 0.02 |
| White light x 0 days | 1.58 a ± 0.03 | 0.96 bc ± 0.09 | 0.028 c ± 0.01 | 1.98 a ± 0.06 | 0.72 bc ± 0.02 | 0.020 c ± 0.07 | 1.75 a ± 0.05 | 1.02 c ± 0.04 | 0.034 c ± 09 |
| White light x 3 days | 1.31 b ± 0.08 | 0.91 a ± 0.06 | 0.030 c ± 0.03 | 1.95 a ± 0.04 | 0.73 bc ± 0.03 | 0.021 c ± 0.02 | 1.72 a ± 0.18 | 1.24 b ± 0.08 | 0.033 c ± 0.14 |
| White light x 7 days | 1.26 c ± 0.02 | 0.75 c ± 0.11 | 0.030 c ± 0.04 | 1.43 c ± 0.08 | 0.74 bc ± 0.15 | 0.025 bc ± 0.06 | 1.24 c ± 0.12 | 1.26 b ± 0.01 | 0.034 c ± 0.09 |
| White light x 11 days | 1.20 c ± 0.01 | 0.52 d ± 0.05 | 0.030 c ± 0.17 | 1.38 cd ± 0.15 | 0.72 bc ± 0.08 | 0.025 bc ± 0.03 | 1.23 c ± 0.07 | 1.29 b ± 0.21 | 0.033 c ± 0.12 |
| White light x 14 days | 1.17 c ± 0.12 | 0.50 d ± 0.13 | 0.030 c ± 0.03 | 1.18 d ± 0.04 | 0.70 bc ± 0.02 | 0.021 c ± 0.08 | 1.10 cd ± 0.04 | 0.98 c ± 0.13 | 0.032 c ± 0.17 |
| Darkness x 0 days | 1.58 a ± 0.03 | 1.30 cd ± 02 | 0.028 c ± 0.02 | 1.98 a ± 0.06 | 0.72 bc ± 0.02 | 0.020 c ± 0.11 | 1.75 a ± 0.05 | 1.02 c ± 0.04 | 0.034 c ± 09 |
| Darkness x 3 days | 1.32 b ± 0.09 | 0.84 bc ± 0.02 | 0.029 c ± 0.19 | 1.92 a ± 0.03 | 0.65 c ± 0.03 | 0.020 c ± 0.03 | 1.23 c ± 0.10 | 0.98 c ± 0.17 | 0.032 c ± 0.01 |
| Darkness x 7 days | 1.16 c ± 0.15 | 0.52 d ± 0.17 | 0.030 c ± 0.03 | 1.31 cd ± 0.18 | 0.64 c ± 0.18 | 0.021 c ± 0.07 | 1.11 cd ± 0.17 | 0.96 c ± 0.04 | 0.030 cd ± 0.15 |
| Darkness x 11 days | 0.81 d ± 0.06 | 0.50 d ± 0.12 | 0.030 c ± 0.12 | 1.30 cd ± 0.01 | 0.42 d ± 0.04 | 0.025 bc ± 0.04 | 0.96 d ± 0.02 | 0.96 c ± 0.02 | 0.021 d ± 0.06 |
| Darkness x 14 days | 0.78 d ± 0.02 | 0.29 e ± 0.01 | 0.029 c ± 0.04 | 1.14 d ± 0.12 | 0.41 d ± 0.01 | 0.021 c ± 0.02 | 0.97 d ± 0.12 | 0.71 d ± 0.15 | 0.020 d ± 0.08 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maroga, G.M.; Soundy, P.; Sivakumar, D. Different Postharvest Responses of Fresh-Cut Sweet Peppers Related to Quality and Antioxidant and Phenylalanine Ammonia Lyase Activities during Exposure to Light-Emitting Diode Treatments. Foods 2019, 8, 359. https://doi.org/10.3390/foods8090359
Maroga GM, Soundy P, Sivakumar D. Different Postharvest Responses of Fresh-Cut Sweet Peppers Related to Quality and Antioxidant and Phenylalanine Ammonia Lyase Activities during Exposure to Light-Emitting Diode Treatments. Foods. 2019; 8(9):359. https://doi.org/10.3390/foods8090359
Chicago/Turabian StyleMaroga, Gludia M., Puffy Soundy, and Dharini Sivakumar. 2019. "Different Postharvest Responses of Fresh-Cut Sweet Peppers Related to Quality and Antioxidant and Phenylalanine Ammonia Lyase Activities during Exposure to Light-Emitting Diode Treatments" Foods 8, no. 9: 359. https://doi.org/10.3390/foods8090359
APA StyleMaroga, G. M., Soundy, P., & Sivakumar, D. (2019). Different Postharvest Responses of Fresh-Cut Sweet Peppers Related to Quality and Antioxidant and Phenylalanine Ammonia Lyase Activities during Exposure to Light-Emitting Diode Treatments. Foods, 8(9), 359. https://doi.org/10.3390/foods8090359





