Cistus incanus L. as an Innovative Functional Additive to Wheat Bread
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Chemical Composition of Raw Material
2.3. Baking Procedure of Bread
2.4. Physical Properties of Bread
2.5. Color Measurements
2.6. Textural Properties of Bread Crumbs
2.7. Total Phenolic Content
2.8. Antioxidant Activity
2.9. Sensory Evaluation of Bread
2.10. Statistical Analysis
3. Results and Discussion
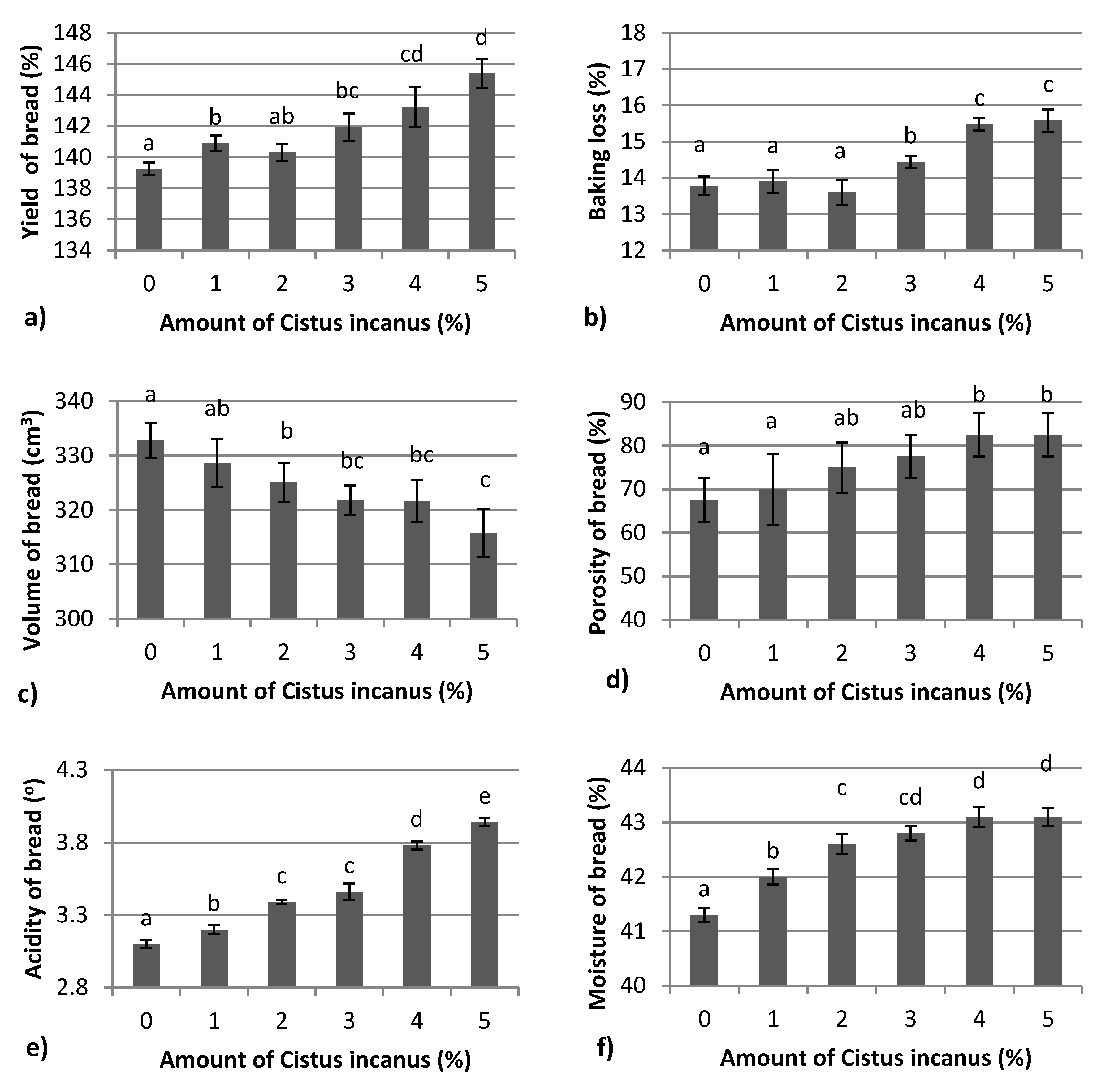
3.1. Physical Properties of Bread Fortified with Cistus incanus
3.2. Color and Texture of Bread Crumbs Modified with CI
3.3. Total Phenolic Content and Antioxidant Activity of Bread Incorporated with Cistus incanus L.
3.4. Sensory Evaluation of Bread Enriched with Cistus incanus L. Herb
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tomás-Menor, L.; Morales-Soto, A.; Barrajón-Catalán, E.; Roldán-Segura, C.; Segura-Carretero, A.; Micol, V. Correlation between the antibacterial activity and the composition of extracts derived from various Spanish Cistus species. Food. Chem. Toxicol. 2013, 55, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Attaguile, G.; Russo, A.; Campisi, A.; Savoca, F.; Acquaviva, R.; Ragusa, N.; Vanella, A. Antioxidant activity and protective effect on DNA cleavage of extracts from Cistus incanus, L. and Cistus monspeliensis, L. Cell Biol. Toxicol. 2000, 16, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Gori, A.; Ferrini, F.; Marzano, M.C.; Tattini, M.; Centritto, M.; Baratto, M.C.; Pogni, R.; Brunetti, C. Characterisation and antioxidant activity of crude extract and polyphenolic rich fractions from C. Incanus Leaves. Int. J. Mol. Sci. 2016, 17, 1344. [Google Scholar] [CrossRef] [PubMed]
- Küpeli, E.; Yesilada, E. Flavonoids with anti-inflammatory and antinociceptive activity from Cistus laurifolius L. leaves through bioassay-guided procedures. J. Ethnopharmacol. 2007, 112, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Ehrhardt, C.; Hrincius, E.R.; Korte, V.; Mazur, I.; Droebner, K.; Poetter, A.; Ludwig, S. A polyphenol rich plant extract, CYSTUS052, exerts anti influenza virus activity in cell culture without toxic side effects or the tendency to induce viral resistance. Antiviral Res. 2007, 76, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Loizzo, M.R.; Ben Jemia, M.; Senatore, F.; Bruno, M.; Menichini, F.; Tundis, R. Chemistry and functional properties in prevention of neurodegenerative disorders of five Cistus species essential oils. Food Chem. Toxicol. 2013, 59, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Jeszka-Skowron, M.; Zgoła-Grześkowiak, A.; Frankowski, R. Cistus incanus a promising herbal tea rich in bioactive compounds: LC–MS/MS Determination of catechins, flavonols, phenolic acids and alkaloids—A comparison with Camellia sinensis, Rooibos and Hoan ngoc herbal tea. J. Food Compos. Anal. 2018, 74, 71–81. [Google Scholar] [CrossRef]
- Wittpahl, G.; Kölling-Speer, I.; Basche, S.; Herrmann, E.; Hannig, M.; Speer, K.; Hannig, C. The polyphenolic composition of Cistus incanus herbal tea and its antibacterial and anti-adherent activity against Streptococcus mutans. Planta Med. 2015, 81, 1727–1735. [Google Scholar] [CrossRef] [PubMed]
- Kalli, V.; Kollia, E.; Roidaki, A.; Proestos, C.; Markaki, P. Cistus incanus, L. extract inhibits aflatoxin B 1 production by Aspergillus parasiticus in Macadamia nuts. Ind. Crops Prod. 2018, 111, 63–68. [Google Scholar] [CrossRef]
- Roidaki, A.; Kollia, E.; Panagopoulou, E.; Chiou, A.; Varzakas, T.; Markaki, P.; Proestos, C. Super foods and super herbs: Antioxidant and antifungal activity. Curr. Res. Nutr. Food Sci. J. 2016, 14 (Suppl. 2), 138–145. [Google Scholar] [CrossRef]
- Riehle, P.; Vollmer, M.; Rohn, S. Phenolic compounds in Cistus incanus herbal infusions—Antioxidant capacity and thermal stability during the brewing process. Food Res. Int. 2013, 53, 891–899. [Google Scholar] [CrossRef]
- Kalus, U.; Grigorov, A.; Kadecki, O.; Jansen, J.P.; Kiesewetter, H.; Radtke, H. Cistus incanus (CYSTUS052) for treating patients with infection of the upper respiratory tract. A prospective, randomised, placebo-controlled clinical study. Antiviral Res. 2009, 84, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Dimcheva, V.; Karsheva, M. Cistus incanus from Strandja mountain as a source of bioactive antioxidants. Plants 2018, 26, 8. [Google Scholar] [CrossRef] [PubMed]
- Riehle, P.; Rusche, N.; Saake, B.; Rohn, S. Influence of the leaf content and herbal particle size on the presence and extractability of quantitated phenolic compounds in Cistus incanus herbal teas. J. Agric. Food Chem. 2014, 62, 10978–10988. [Google Scholar] [CrossRef] [PubMed]
- Dziki, D.; Różyło, R.; Gawlik-Dziki, U.; Świeca, M. Current trends in the enhancement of antioxidant activity of wheat bread by the addition of plant materials rich in phenolic compounds. Trends Food Sci. Technol. 2014, 40, 48–61. [Google Scholar] [CrossRef]
- Al-Dmoor, H.M. Flat bread: Ingredients and fortification. quality assurance and safety of crops and foods. Quality Assur. Safety Crop. Foods 2012. [Google Scholar] [CrossRef]
- Psodorov, D.; Šimurina, O.; Runjajić-Antić, D.; Arsić, I.; Filipčev, B.; Bodroža-Solarov, M. New bakery products with the addition of medicinal herbs. In Proceedings of the 3rd International Congress FLOUR-BREAD 2005—5th Croatian Congress of Cereal Technologists, Osijek, Croatia, 26–29 October 2005. [Google Scholar]
- Kumar, D. Herbal Bioactives and Food Fortification, Exstraction and Formulation; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Zhumaliyeva, G.; Chomanov, U.; Tultabayeva, T.; Kenenbay, G.; Shoman, A.; Nurynbetova, G. Mineral composition study of complex additives of bakery products withanti-diabetic appointment. Int. J. Pharm. Technol. 2016, 11, 33–37. [Google Scholar]
- Singh, N.; Jha, A.; Chaudhary, A.; Upadhyay, A. Enhancement of the functionality of bread by incorporation of Shatavari (Asparagus racemosus). J. Food Sci. Technol. 2014, 51, 2038–2045. [Google Scholar] [CrossRef] [PubMed]
- Seidel, C.; Boehm, V.; Vogelsang, H.; Wagner, A.; Persin, C.; Glei, M.; Pool-Zobel, B.L.; Jahreis, G. Influence of prebiotics and antioxidants in bread on the immune system, antioxidative status and antioxidative capacity in male smokers and non-smokers. Br. J. Nutr. 2007, 97, 349–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhillon, G.K.; Ahluwalia, P.; Kaur, A.K. Effect of Oregano herb on dough rheology and bread quality. Int. J. Food Sci. Nutr. Diet. 2013, 2, 40–44. [Google Scholar] [CrossRef]
- Skąpska, S.; Marszałek, K.; Woźniak, Ł.; Zawada, K.; Wawer, I. Aronia dietary drinks fortified with selected herbal extracts preserved by thermal pasteurization and high pressure carbon dioxide. LWT Food Sci. Technol. 2017, 85, 423–426. [Google Scholar] [CrossRef]
- Lisiecka, K.; Wójtowicz, A.; Dziki, D.; Gawlik-Dziki, U. The influence of Cistus incanus L. leaves on wheat pasta quality. J. Food Sci. Techol. 2019, 1–12. [Google Scholar] [CrossRef]
- The International Organization for Standardization. Cereals and Pulses—Determination of the Nitrogen Content and Calculation of the Crude Protein Content—Kjeldahl Method; Technical Report ISO 20483:2013; The International Organization for Standardization: Geneva, Switzerland, 2013. [Google Scholar]
- The International Organization for Standardization. Wheat and Wheat Flour—Gluten Content—Part 2: Determination of Wet Gluten and Gluten Index by Mechanical Means; Techincal Report ISO 21415-2:2015; The International Organization for Standardization: Geneva, Switzerland, 2015. [Google Scholar]
- The International Organization for Standardization. Wheat, Rye and Their Flours, Durum Wheat and Durum Wheat Semolina—Determination of the Falling Number According to Hagberg-Perten; Technical Report ISO 3093:2009; The International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- International Association for Cereal Science and Technology. Cereals and Cereal Products—Determination of Total Fat Content; Technical Report No. 133; International Association for Cereal Science and Technology: Vienna, Austria, 1984. [Google Scholar]
- The International Organization for Standardization. Cereals, Pulses and By-Products—Determination of Ash Yield by Incineration; Technical Report ISO 2171:2007; The International Organization for Standardization: Geneva, Switzerland, 2007. [Google Scholar]
- International Association for Cereal Science and Technology. Determination of the Moisture Content of Cereals and Cereal Products; Technical report No. 109; International Association for Cereal Science and Technology: Vienna, Austria, 1960. [Google Scholar]
- Różyło, R.; Dziki, D.; Laskowski, J. Changes in the physical and the sensorial properties of wheat bread caused by interruption and slowing of the fermentation of yeast-based leaven. J. Cereal Sci. 2014, 59, 88–94. [Google Scholar] [CrossRef]
- Romankiewicz, D.; Hassoon, W.H.; Cacak-Pietrzak, G.; Sobczyk, M.B.; Wirkowska-Wojdyła, M.; Ceglińska, A.; Dziki, D. The effect of chia seeds (Salvia hispanica, L.) addition on quality and nutritional value of wheat bread. J. Food Qual. 2017. [Google Scholar] [CrossRef]
- Belyaev, A.G.; Kovaleva, A.E.; Pyanikova, E.A. The influence of fireweed powder on the quality of wheat bread. Proc. Vor. State Univ. Eng. Technol. 2019, 80, 254–258. (In Russian) [Google Scholar] [CrossRef]
- Różyło, R.; Wójcik, M.; Dziki, D.; Biernacka, B.; Cacak-Pietrzak, G.; Gawłowski, S.; Zdybel, A. Freeze-dried Elderberry and Chokeberry as natural colorants for gluten-free wafer sheets. Int. Agrophysics 2019, 33, 217–225. [Google Scholar] [CrossRef]
- Armero, E.; Collar, C. Texture properties of formulated wheat doughs: Relationships with dough and bread technological quality. Zeitschrift für Lebensmitteluntersuchung und-Forschung A 1997, 204, 136–145. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J. Colorimetry of total phenolics with phosphomolybdic. Am. J. Enol. Viticulture 1965, 6, 144–158. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Guo, J.T.; Lee, H.L.; Chiang, S.H.; Lin, F.I.; Chang, C.Y. Antioxidant properties of the extracts from different parts of broccoli in Taiwan. J. Food Drug Anal. 2001, 9, 96–101. [Google Scholar]
- Su, X.Y.; Wang, Z.Y.; Liu, J.R. In vitro and in vivo antioxidant activity of Pinus koraiensis seed extract containing phenolic compounds. Food Chem. 2009, 4, 681–686. [Google Scholar] [CrossRef]
- Lim, H.S.; Park, S.H.; Ghafoor, K.; Hwang, S.Y.; Park, J. Quality and antioxidant properties of bread containing turmeric (Curcuma longa, L.) cultivated in South Korea. Food Chem. 2011, 4, 1577–1582. [Google Scholar] [CrossRef]
- Różyło, R. Effect of process modifications in two cycles of dough mixing on physical properties of wheat bread baked from weak flour. Food Bioprocess Technol. 2014, 7, 774–783. [Google Scholar] [CrossRef]
- Ćurić, D.; Karlović, D.; Tušak, D.; Petrović, B.; Dugum, J. Gluten as a standard of wheat flour quality. Food Technol. Biotechnol. 2001, 39, 353–361. [Google Scholar]
- Ning, J.; Hou, G.G.; Sun, J.; Wan, X.; Dubat, A. Effect of green tea powder on the quality attributes and antioxidant activity of whole-wheat flour pan bread. LWT Food Sci. Technol. 2017, 79, 342–348. [Google Scholar] [CrossRef]
- Wang, R.; Zhou, W.; Isabelle, M. Comparison study of the effect of green tea extract (GTE) on the quality of bread by instrumental analysis and sensory evaluation. Food Res. Int. 2007, 40, 470–479. [Google Scholar] [CrossRef]
- Pasrija, D.; Ezhilarasi, P.N.; Indrani, D.; Anandharamakrishnan, C. Microencapsulation of green tea polyphenols and its effect on incorporated bread quality. LWT Food Sci. Technol. 2015, 64, 289–296. [Google Scholar] [CrossRef]
- Kutsenkova, V.; Nepovinnykh, N.; Lymina, N.; Senchikhin, V. Recipe development and medical and biological evaluation of bakery products fortified with non-traditional vegetable raw materials. Food Process. Tech. Technol. 2019, 49, 23–31. [Google Scholar] [CrossRef]
- Moore, M.M.; Dal Bello, F.; Arendt, E.K. Sourdough fermented by Lactobacillus plantarum FST 1.7 improves the quality and shelf life of gluten-free bread. Eur. Food Res. Technol. 2008, 226, 1309–1316. [Google Scholar] [CrossRef]
- Katina, K. Sourdough: A tool for the improved flavour, texture and shelf-life of wheat bread. VTT Publ. 2005, 569, 3–92. [Google Scholar]
- Varone, L.; Gratani, L. Physiological response of eight Mediterranean maquis species to low air temperatures during winter. Photosynthetica 2007, 45, 385–391. [Google Scholar] [CrossRef]
- Bourekoua, H.; Różyło, R.; Gawlik-Dziki, U.; Benatallah, L.; Zidoune, M.N.; Dziki, D. Evaluation of physical, sensorial, and antioxidant properties of gluten-free bread enriched with Moringa oleifera leaf powder. Eur. Food Res. Technol. 2018, 244, 189–195. [Google Scholar] [CrossRef]
- Zhu, F.; Sakulnak, R.; Wang, S. Effect of black tea on antioxidant, textural, and sensory properties of Chinese steamed bread. Food Chem. 2016, 194, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Bourekoua, H.; Różyło, R.; Gawlik-Dziki, U.; Benatallah, L.; Zidoune, M.N.; Dziki, D. Pomegranate seed powder as a functional component of gluten-free bread (physical, sensorial and antioxidant evaluation). Int. J. Food Sci. Technol. 2018, 53, 1906–1913. [Google Scholar] [CrossRef]
- Abdelghafor, R.F.; Mustafa, A.I.; Ibrahim, A.M.H.; Krishnan, P.G. Quality of bread from composite flour of sorghum and hard white winter wheat. Adv. J. Food Sci. Technol. 2011, 3, 9–15. [Google Scholar]
- Mansoor, K.A.; Matalka, K.Z.; Qadan, F.S.; Awad, R.; Schmidt, M. Two new proanthocyanidin trimers isolated from Cistus incanus L. demonstrate potent antiinflammatory activity and selectivity to cyclooxygenase isoenzymes inhibition. Nat. Prod. Res. 2016, 30, 1919–1926. [Google Scholar] [CrossRef]
- Bourekoua, H.; Różyło, R.; Benatallah, L.; Wójtowicz, A.; Łysiak, G.; Zidoune, M.N.; Sujak, A. characteristics of gluten-free bread: Quality improvement by the addition of starches/hydrocolloids and their combinations using a definitive screening design. Eur. Food Res. Technol. 2018, 244, 345–354. [Google Scholar] [CrossRef]
- Dziki, D.; Cacak-Pietrzak, G.; Gawlik-Dziki, U.; Sułek, A.; Kocira, S.; Biernacka, B. Effect of Moldavian dragonhead (Dracocephalum moldavica L.) leaves on the baking properties of wheat flour and quality of bread. Cy Ta J. Food. 2019, 17, 536–543. [Google Scholar] [CrossRef]
- Adams, A.; Kruma, Z.; Verhé, R.; De Kimpe, N.; Kreicbergs, V. Volatile profiles of rapeseed oil flavored with basil, oregano, and thyme as a function of flavoring conditions. J. Am. Oil Chem. Soc. 2011, 88, 201–212. [Google Scholar] [CrossRef]

| Sample | Ash Content (%) | L* | a* | b* | ΔE | |
|---|---|---|---|---|---|---|
| CI herbs | 5.01 c ± 0.211 | 54.93 e ± 0.75 | 0.68 b ± 0.03 | 20.04 d ± 0.44 | - | |
| Proportion of CI added | 0% | 0.82 a ± 0.018 | 82.44 a ± 0.176 | 0.09 a ± 0.003 | 10.35 a ± 0.062 | - |
| 1% | 0.90 b ± 0.037 | 75.71 b ± 0.217 | 2.39 c ± 0.038 | 10.65 a ± 0.094 | 7.20 | |
| 2% | 1.00 b ± 0.041 | 72.89 c ± 0.267 | 2.38 c ± 0.065 | 12.67 b ± 0.206 | 9.99 | |
| 3% | 1.01 b ± 0.086 | 72.64 c ± 0.356 | 2.39 c ± 0.037 | 12.69 b ± 0.235 | 10.12 | |
| 4% | 1.06 b ± 0.027 | 70.90 d ± 0.206 | 2.91 d ± 0.063 | 14.45 c ± 0.184 | 12.21 | |
| 5% | 1.10 b ± 0.064 | 69.98 d ± 0.330 | 2.88 d ± 0.065 | 14.59 c ± 0.227 | 13.09 | |
| Sample | Hardness (N) | Springiness (-) | Cohesiveness (-) | Chewiness (N) | |
|---|---|---|---|---|---|
| Proportion of CI added | 0% | 12.9 a ± 0.59 | 0.91 a ± 0.031 | 0.61 a ± 0.041 | 7.2 ab ± 0.53 |
| 1% | 12.7 a ± 0.78 | 0.88 ab ± 0.037 | 0.57 a ± 0.038 | 6.4 b ± 0.41 | |
| 2% | 12.8 a ± 0.68 | 0.89 a ± 0.028 | 0.56 a ± 0.031 | 6.5 b ± 0.36 | |
| 3% | 12.9 a ± 0.94 | 0.88 ab ± 0.045 | 0.55 a ± 0.018 | 6.6 ab ± 0.40 | |
| 4% | 13.7 ab ± 0.63 | 0.86 b ± 0.019 | 0.56 a ± 0.011 | 6.7 ab ± 0.35 | |
| 5% | 14.3 b ± 0.46 | 0.89 a ± 0.026 | 0.57 a ± 0.026 | 7.4 a ± 0.41 | |
| Sample | (mg GAE∙g d.w.−1) | EC50 (mg d.w.∙mL−1) | |||
|---|---|---|---|---|---|
| TPC | ABTS | CHEL | OH∙ | ||
| Amount of CI added | 0% | 4.8 a ± 0.06 | 156.9 a ± 9.83 | 65.3 a ± 0.36 | 39.9 a ± 2.11 |
| 1% | 5.6 b ± 0.06 | 77.5 b ± 0.92 | 43.8 b ± 2.35 | 35.0 b ± 1.10 | |
| 2% | 6.7 c ± 0.10 | 48.6 cd ± 1.25 | 40.7 bc ± 0.58 | 26.9 c ± 1.21 | |
| 3% | 7.9 d ± 0.25 | 38.3 de ± 1.55 | 38.1 cd ± 0.32 | 25.7 c ± 0.83 | |
| 4% | 8.3 e ± 0.12 | 33.1 e ± 1.15 | 36.7 d ± 0.56 | 24.8 cd ± 0.95 | |
| 5% | 10.1 f ± 0.06 | 24.8 e ± 0.70 | 33.9 e ± 0.83 | 22.5 d ± 0.89 | |
| Amount of CI Added | Sensory Attributes | ||||
|---|---|---|---|---|---|
| Appearance | Taste | Aroma | Texture | Overall Acceptability | |
| 0% | 6.3 a ± 0.62 | 6.7 a ± 0.76 | 6.8a ± 0.93 | 7.0 a ± 0.87 | 6.7 a ± 0.84 |
| 1% | 6.4 a ± 0.87 | 6.6 a ± 0.79 | 6.9a ± 0.85 | 6.6 ab ± 0.73 | 6.5 a ± 0.73 |
| 2% | 6.7 a ± 0.91 | 6.1ab ± 0.59 | 6.5 ab ± 0.74 | 6.4 ab ± 0.81 | 6.5 a ± 0.82 |
| 3% | 6.8 a ± 0.83 | 5.6 ab ± 0.63 | 5.8 ab ± 0.62 | 6.3 ab ± 0.75 | 6.4 a ± 0.81 |
| 4% | 6.0 a ± 0.59 | 5.1 b ± 0.71 | 5.3 b ± 0.63 | 6.0 ab ± 0.79 | 5.2 b ± 0.37 |
| 5% | 5.5 a ± 0.64 | 5.0 b ± 0.65 | 5.2 b ± 0.59 | 4.9 b ± 0.56 | 5.1 b ± 0.44 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cacak-Pietrzak, G.; Różyło, R.; Dziki, D.; Gawlik-Dziki, U.; Sułek, A.; Biernacka, B. Cistus incanus L. as an Innovative Functional Additive to Wheat Bread. Foods 2019, 8, 349. https://doi.org/10.3390/foods8080349
Cacak-Pietrzak G, Różyło R, Dziki D, Gawlik-Dziki U, Sułek A, Biernacka B. Cistus incanus L. as an Innovative Functional Additive to Wheat Bread. Foods. 2019; 8(8):349. https://doi.org/10.3390/foods8080349
Chicago/Turabian StyleCacak-Pietrzak, Grażyna, Renata Różyło, Dariusz Dziki, Urszula Gawlik-Dziki, Alicja Sułek, and Beata Biernacka. 2019. "Cistus incanus L. as an Innovative Functional Additive to Wheat Bread" Foods 8, no. 8: 349. https://doi.org/10.3390/foods8080349
APA StyleCacak-Pietrzak, G., Różyło, R., Dziki, D., Gawlik-Dziki, U., Sułek, A., & Biernacka, B. (2019). Cistus incanus L. as an Innovative Functional Additive to Wheat Bread. Foods, 8(8), 349. https://doi.org/10.3390/foods8080349









