Characterization and Differentiation of Spanish Vinegars from Jerez and Condado de Huelva Protected Designations of Origin
Abstract
1. Introduction
2. Materials and Methods
2.1. Vinegar Samples
2.2. Spectrophotometric L*a*b* Measurements
2.3. Determination of Polyphenols and Furanic Compounds
2.4. Determination of Volatile Compounds
2.5. Statistical Analysis
3. Results and Discussion
3.1. Spectrophotometric L*a*b* measurements
3.2. Furanic and Polyphenolic Compounds
3.3. Volatile Compounds
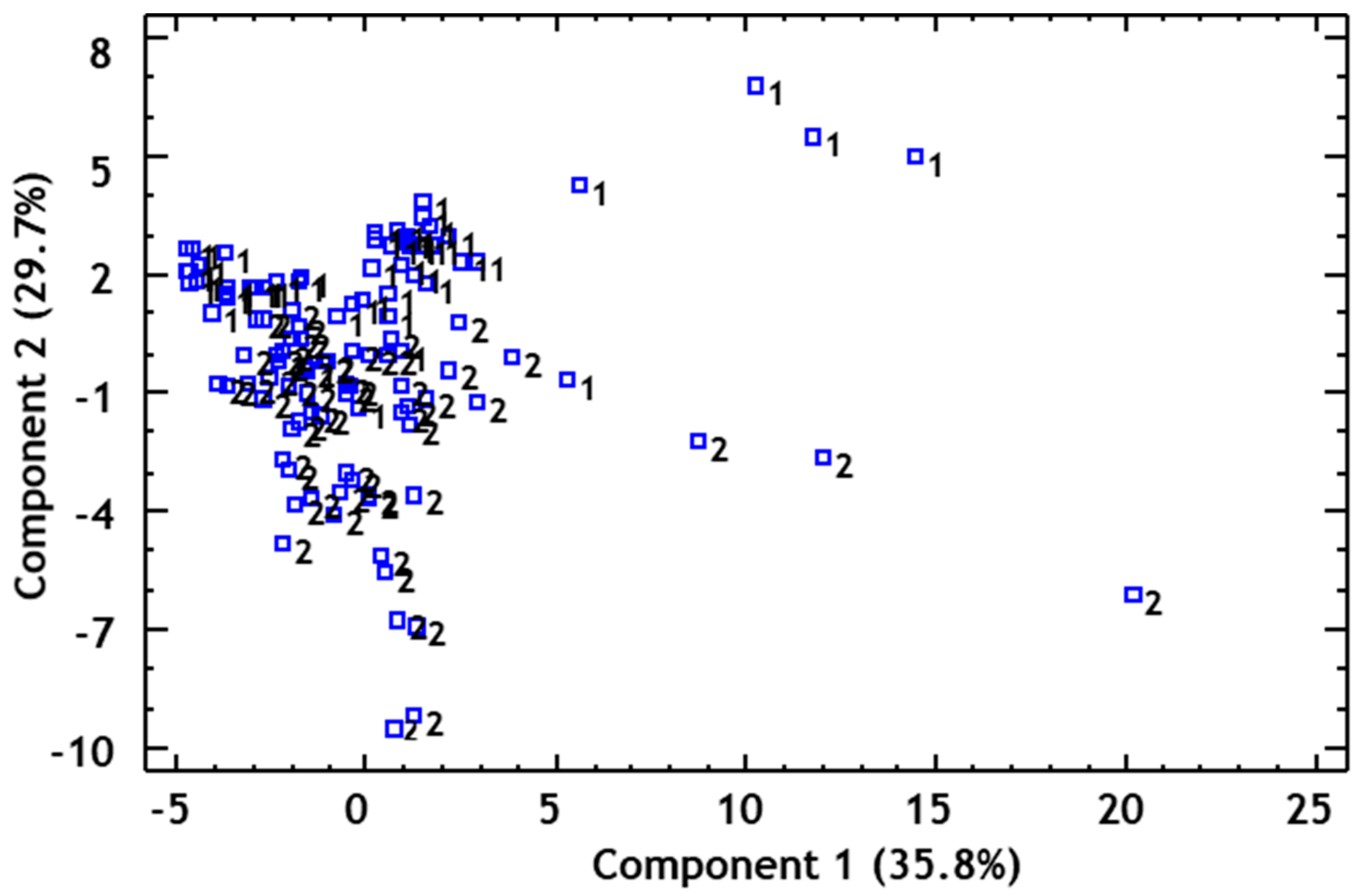
3.4. Joint Analysis of Polyphenolic, Furanic, and Volatile Constituents
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Solieri, L.; Giudici, P. Vinegars of the World. In Vinegars of the World; Springer Milan: Milano, Italy, 2009. [Google Scholar]
- Chinnici, F.; Guerrero, E.D.; Sonni, F.; Natali, N.; Marín, R.N.; Riponi, C. Gas chromatography-mass spectrometry (GC-MS) characterization of volatile compounds in quality vinegars with protected European geographical indication. J. Agric. Food Chem. 2009, 57, 4784–4792. [Google Scholar] [CrossRef]
- Junta de Andalucía. Inscripción de la Denominación de Origen Protegida “Vinagre de Jerez”. Boletín Oficial de la Junta de Andalucía. 2008, pp. 29–35. Available online: https://www.juntadeandalucia.es/boja/2008/184/d15.pdf (accessed on 16 July 2019).
- Junta de Andalucía. Inscripción de la Denominación de Origen Protegida “Vinagre del Condado de Huelva”. In Boletín Oficial de la Junta de Andalucía; Junta de Andalucía: Sevillla, Spain, 2008; pp. 35–40. [Google Scholar]
- Guerrero, E.D.; Castro Mejías, R.; Marín, R.N.; Barroso, C.G. Optimization of stir bar sorptive extraction applied to the determination of pesticides in vinegars. J. Chromatogr. A. 2007, 1165, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Callejón, R.M.; González, A.G.; Troncoso, A.M.; Morales, M.L. Optimization and validation of headspace sorptive extraction for the analysis of volatile compounds in wine vinegars. J. Chromatogr. A. 2008, 1204, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, E.D.; Chinnici, F.; Natali, N.; Marín, R.N.; Riponi, C. Solid-phase extraction method for determination of volatile compounds in traditional balsamic vinegar. J. Sep. Sci. 2008, 31, 3030–3036. [Google Scholar] [CrossRef] [PubMed]
- Dávalos, A.; Bartolomé, B.; Gómez-Cordovés, C. Antioxidant properties of commercial grape juices and vinegars. Food Chem. 2005, 93, 325–330. [Google Scholar] [CrossRef]
- Garcia-Parrilla, C.; Heredia, F.J.; Troncoso, A.M. Phenols HPLC Analysis by Direct Injection of Sherry Wine Vinegar. J. Liq. Chromatogr. Relat. Technol. 1996, 19, 247–258. [Google Scholar] [CrossRef]
- Cejudo Bastante, M.J.; Durán Guerrero, E.; Castro Mejías, R.; Natera Marín, R.; Rodríguez Dodero, M.C.; Barroso, C.G. Study of the polyphenolic composition and antioxidant activity of new sherry vinegar-derived products by maceration with fruits. J. Agric. Food Chem. 2010, 58, 11814–11820. [Google Scholar] [CrossRef] [PubMed]
- Marrufo-Curtido, A.; Cejudo-Bastante, M.J.; Rodríguez-Dodero, M.C.; Natera-Marín, R.; Castro-Mejías, R.; García-Barroso, C.; Durán-Guerrero, E. Novel vinegar-derived product enriched with dietary fiber: Effect on polyphenolic profile, volatile composition and sensory analysis. J. Food Sci. Technol. 2015, 52, 7608–7624. [Google Scholar] [CrossRef] [PubMed]
- Casale, M.; Armanino, C.; Casolino, C.; Oliveros, C.C.; Forina, M. A Chemometrical Approach for Vinegar Classification by Headspace Mass Spectrometry of Volatile Compounds. Food Sci. Technol. Res. 2006, 12, 223–230. [Google Scholar] [CrossRef]
- Pizarro, C.; Esteban-Díez, I.; Sáenz-González, C.; González-Sáiz, J.M. Vinegar classification based on feature extraction and selection from headspace solid-phase microextraction/gas chromatography volatile analyses: A feasibility study. Anal. Chim. Acta 2008, 608, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Natera, R.; Castro, R.; De Valme García-Moreno, M.; Hernández, M.J.; García-Barroso, C. Chemometric studies of vinegars from different raw materials and processes of production. J. Agric. Food Chem. 2003, 51, 3345–3351. [Google Scholar] [CrossRef] [PubMed]
- Cejudo-Bastante, M.J.; Durán-Guerrero, E.; Natera-Marín, R.; Castro-Mejías, R.; García-Barroso, C. Characterisation of commercial aromatised vinegars: Phenolic compounds, volatile composition and antioxidant activity. J. Sci. Food Agric. 2013, 93, 1284–1302. [Google Scholar] [CrossRef] [PubMed]
- Marrufo-Curtido, A.; Cejudo-Bastante, M.J.; Durán-Guerrero, E.; Castro-Mejías, R.; Natera-Marín, R.; Chinnici, F.; García-Barroso, C. Characterization and differentiation of high quality vinegars by stir sorptive extraction coupled to gas chromatography-mass spectrometry (SBSE-GC-MS). Lwt-Food Sci. Technol. 2012, 47, 332–341. [Google Scholar] [CrossRef]
- Ríos-Reina, R.; Segura-Borrego, M.P.; García-González, D.L.; Morales, M.L.; Callejón, R.M. A comparative study of the volatile profile of wine vinegars with protected designation of origin by headspace stir bar sorptive extraction. Food Res. Int. 2019, 123, 298–310. [Google Scholar] [CrossRef] [PubMed]
- García-Parrilla, M.C.; González, G.A.; Heredia, F.J.; Troncoso, A.M. Differentiation of Wine Vinegars Based on Phenolic Composition. J. Agric. Food Chem. 1997, 45, 3487–3492. [Google Scholar] [CrossRef]
- Guerrero, E.D.; Mejías, R.C.; Marín, R.N.; Lovillo, M.P.; Barroso, C.G. A new FT-IR method combined with multivariate analysis for the classification of vinegars from different raw materials and production processes. J. Sci. Food Agric. 2010, 90, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Reina, R.; Callejón, R.M.; Oliver-Pozo, C.; Amigo, J.M.; García-González, D.L. ATR-FTIR as a potential tool for controlling high quality vinegar categories. Food Control. 2017, 78, 230–237. [Google Scholar] [CrossRef]
- Ríos-Reina, R.; García-González, D.L.; Callejón, R.M.; Amigo, J.M. NIR spectroscopy and chemometrics for the typification of Spanish wine vinegars with a protected designation of origin. Food Control. 2018, 89, 108–116. [Google Scholar] [CrossRef]
- Ríos-Reina, R.; Elcoroaristizabal, S.; Ocaña-González, J.A.; García-González, D.L.; Amigo, J.M.; Callejón, R.M. Characterization and authentication of Spanish PDO wine vinegars using multidimensional fluorescence and chemometrics. Food Chem. 2017, 230, 108–116. [Google Scholar] [CrossRef]
- Úbeda, C.; del Barrio-Galán, R.; Peña-Neira, Á.; Medel-Marabolí, M.; Durán-Guerrero, E. Location effects on the aromatic composition of monovarietal cv. Carignan wines. Am. J. Enol. Vitic. 2017, 68, 390–399. [Google Scholar] [CrossRef]
- C.I.E. (Commission Internationale de L’Eclairage). Publication C.I.E. No.15.2. In Colorimetry; C.I.E.: Vienna, Austria, 1986. [Google Scholar]
- Schwarz, M.; Rodríguez, M.C.; Guillén, D.A.; Barroso, C.G. Development and validation of UPLC for the determination of phenolic compounds and furanic derivatives in Brandy de Jerez. J. Sep. Sci. 2009, 32, 1782–1790. [Google Scholar] [CrossRef]
- Guerrero, E.D.; Marín, R.N.; Mejías, R.C.; Barroso, C.G. Optimisation of stir bar sorptive extraction applied to the determination of volatile compounds in vinegars. J. Chromatogr. A 2006, 1104, 47–53. [Google Scholar] [CrossRef]
- Kaiser, H.F. The Application of Electronic Computers to Factor Analysis. Educ. Psychol. Meas. 1960, 20, 141–151. [Google Scholar] [CrossRef]
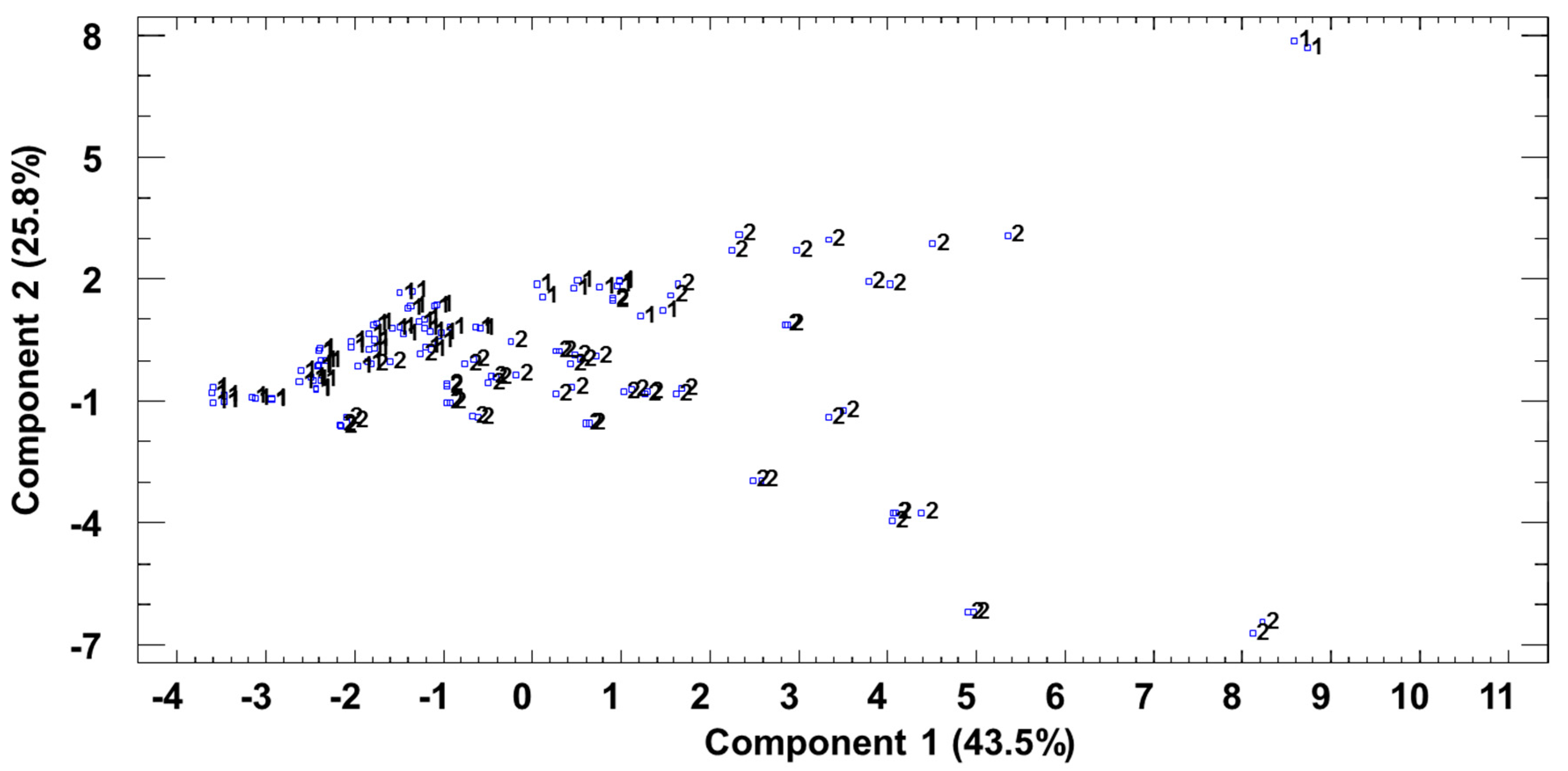
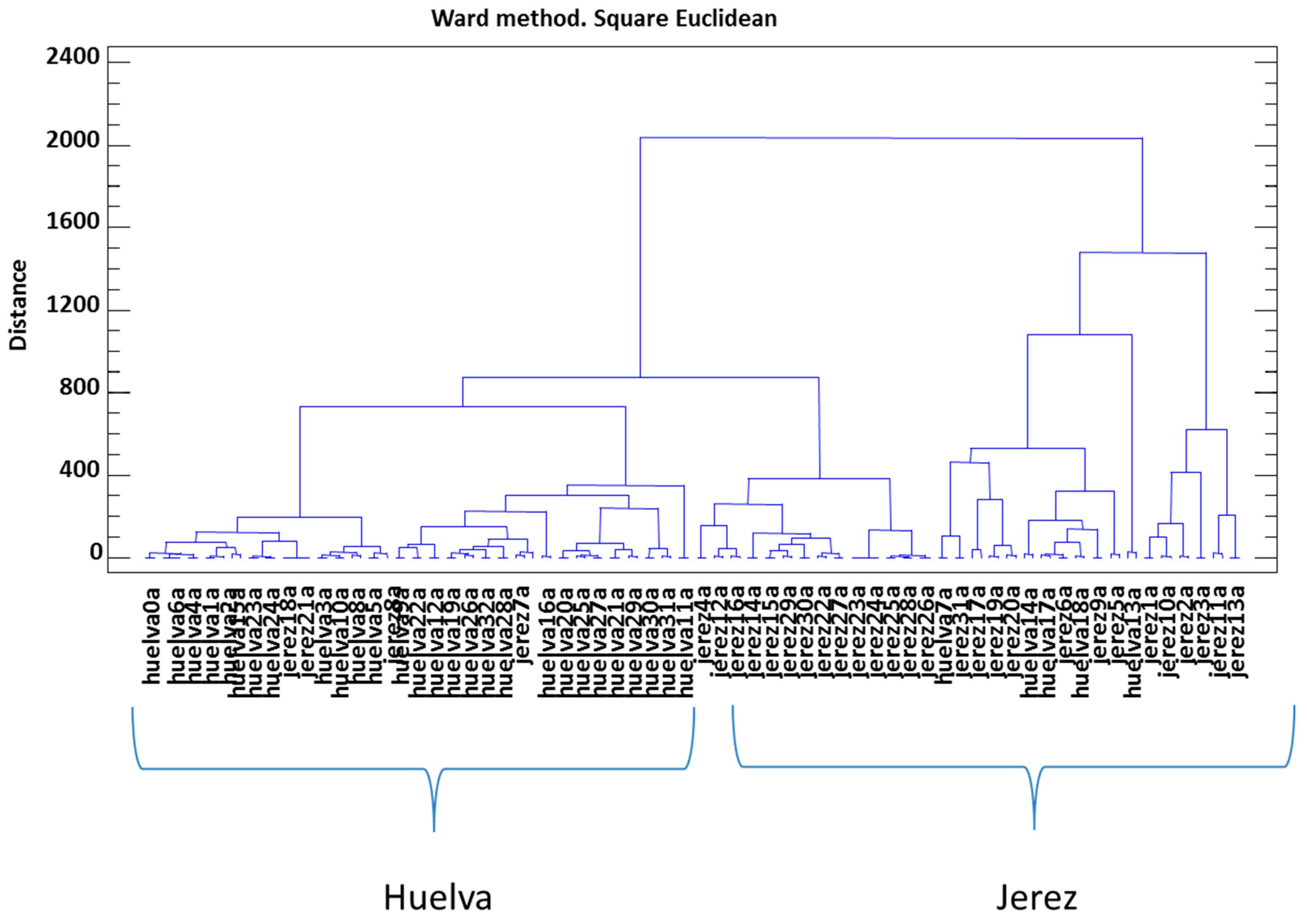
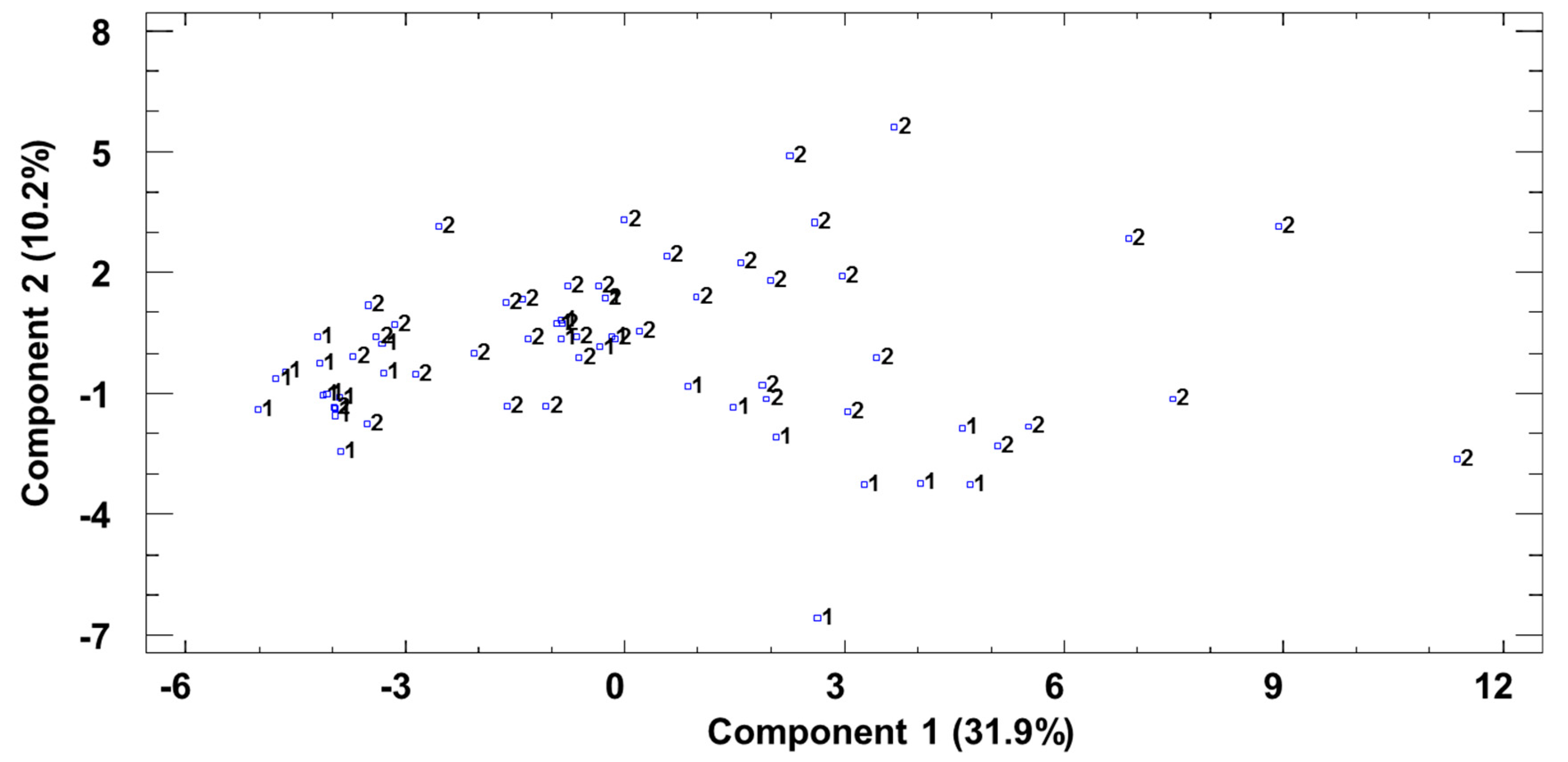
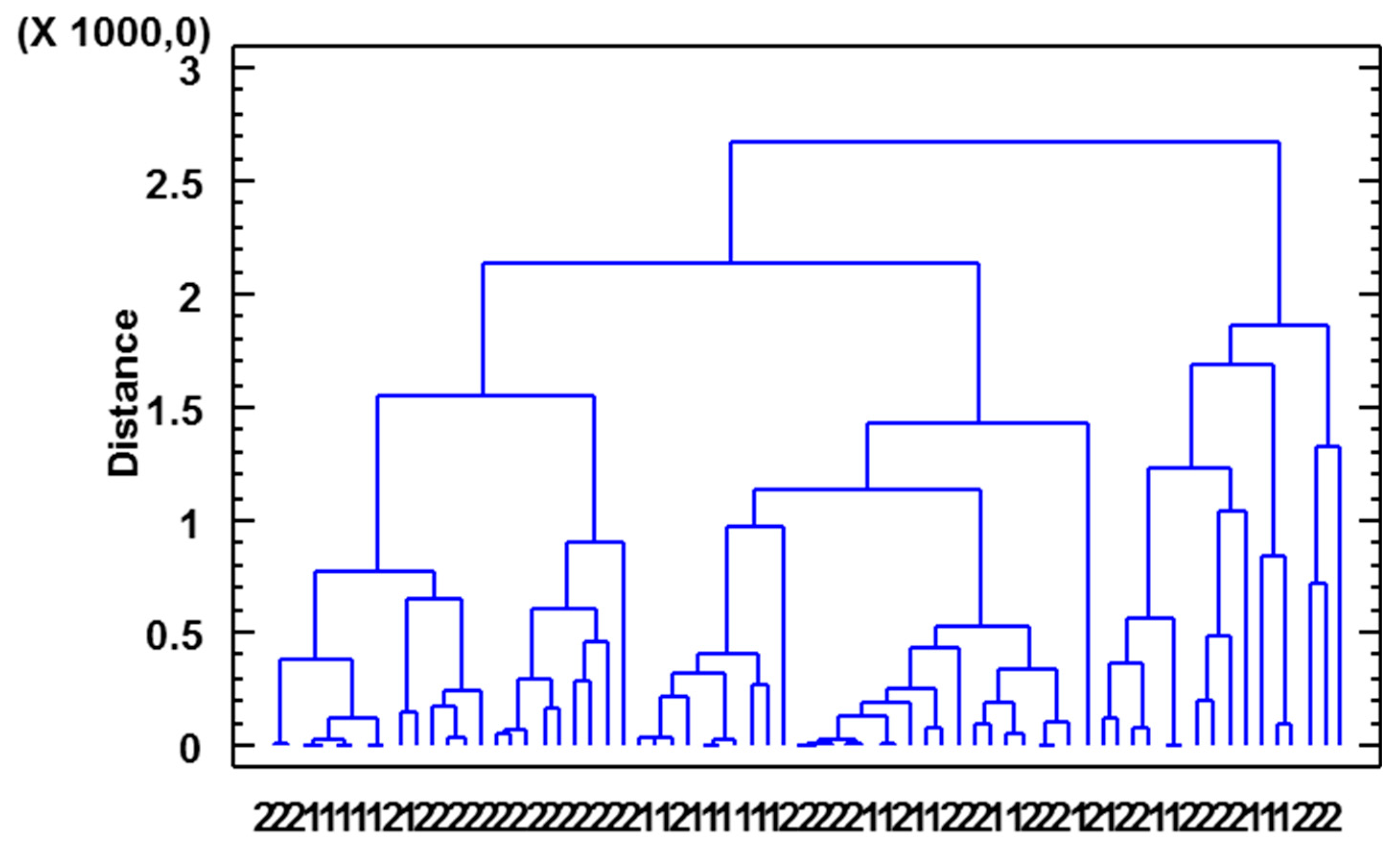
| Jerez | Huelva | F-Ratio | p-Value | |
|---|---|---|---|---|
| L* + | 92.09 ± 3.24 | 89.44 ± 6.57 | 4.09 | 0.0476 |
| a* | 0.82 ± 1.17 | 1.73 ± 2.45 | 3.49 | 0.0664 |
| b* | 14.56 ± 7.54 | 19.46 ± 13.83 | 3.03 | 0.0866 |
| Polyphenolic Compounds | Jerez (mg/L) | Huelva (mg/L) | F-Ratio | p-Value |
|---|---|---|---|---|
| Gallic acid * | 50.7 | 36.7 | 7.78 | 0.0061 |
| 5-Hydroxymethyl-2-furaldehyde | 70.9 | 66.9 | 0.04 | 0.84 |
| Furfural | 56 | 77.9 | 6.62 | 0.0112 |
| Tyrosol * | 20.8 | 15.6 | 14.25 | 0.0002 |
| Syringic acid * | 11.7 | 6.5 | 25.96 | 0 |
| Protocatechuic acid | 58.2 | 67.8 | 1.94 | 0.1661 |
| Furoic acid * | 64.5 | 172.6 | 34.36 | 0 |
| Caftaric acid * | 14.3 | 3.2 | 42.34 | 0 |
| Coutaric acid * | 13.5 | 3.4 | 73.59 | 0 |
| Caffeic acid * | 6.6 | 2.2 | 43.83 | 0 |
| p-Coumaric acid * | 8.1 | 1.7 | 71.36 | 0 |
| Syringaldehyde * | 7.3 | 3.6 | 50.84 | 0 |
| Protocatechuic aldehyde | 1.8 | 1.6 | 0.51 | 0.4785 |
| Ethyl caffeate * | 1.5 | 0.5 | 20.62 | 0 |
| Ethyl coumarate * | 1.2 | 0.1 | 20.62 | 0 |
| Vanilline * | 4.3 | 3 | 13.34 | 0.0004 |
| Volatile Compounds | Jerez (RA) | Huelva (RA) | F-Ratio | p-Value |
|---|---|---|---|---|
| Eugenol | 0.0121 | 0.0135 | 0.52 | 0.4735 |
| Ethyl pentanoate | 0.049 | 0.0047 | 67 | 0.4144 |
| Ethyl lactate | 1.9712 | 3.8071 | 0 | 0.9513 |
| n-Butyl acetate | 2.1283 | 1.1981 | 3.76 | 0.0566 |
| trans-2-Hexen-1-ol | 0.0074 | 0.0318 | 2.43 | 0.124 |
| Furfural | 0.0283 | 0.0282 | 2.15 | 0.147 |
| Isobutyric acid | 0.1154 | 0.4554 | 0.16 | 0.6948 |
| 2-Acetyl-5-methylfurfural | 0.0171 | 0.013 | 0.02 | 0.8935 |
| 4-Ethylguaiacol * | 0.1262 | 0.0141 | 48.24 | 0 |
| 4-Ethylphenol * | 0.2041 | 0.0279 | 31.79 | 0 |
| 5-Acetoxymethyl-2-furaldehyde * | 0.0311 | 0.0056 | 8.67 | 0.0044 |
| Octanoic acid * | 1.1339 | 0.6532 | 21.45 | 0 |
| Decanoic acid * | 1.7839 | 1.1319 | 12.4 | 0.0008 |
| Ethyl isobutyrate | 0.3935 | 0.4827 | 0.12 | 0.7349 |
| Propyl acetate | 0.0389 | 1.8364 | 1.8 | 0.1837 |
| Isobutyl acetate | 2.2364 | 4.7232 | 1.99 | 0.1626 |
| Ethyl 2-methylbutyrate | 0.1942 | 0.2033 | 0.87 | 0.3545 |
| Ethyl isovalerate | 1.853 | 1.7505 | 2.37 | 0.1281 |
| Hexanal | 0.0293 | 0.1588 | 0.64 | 0.4247 |
| Isobutanol ** | 0.0155 | 0.5006 | 5.67 | 0.02 |
| Isopentyl acetate * | 7.8289 | 3.309 | 9.34 | 0.0032 |
| 2-Methyl-1-butanol | 0.402 | 0.2769 | 1.07 | 0.3052 |
| 3-Methyl-1-butanol | 0.5129 | 0.3514 | 0.45 | 0.5066 |
| Ethyl hexanoate | 0.2035 | 0.1724 | 0.24 | 0.6227 |
| Hexyl acetate | 0.0271 | 0.0262 | 0.03 | 0.8537 |
| 3-Hydroxy-2-butanone ** | 0.0279 | 0.0486 | 5.85 | 0.0183 |
| cis-3-Hexenylacetate | 0.0318 | 0.1708 | 3.51 | 0.0653 |
| Hexanol | 0.01 | 0.011 | 0.67 | 0.4175 |
| Ethyl octanoate | 0.2668 | 0.1358 | 0.89 | 0.3497 |
| Benzaldehyde | 0.1167 | 0.065 | 0 | 0.9476 |
| Isovaleric acid * | 1.1198 | 0.4681 | 11.69 | 0.0011 |
| Diethyl succinate | 0.1328 | 0.0899 | 0.52 | 0.4713 |
| α-Terpineol | 0.0025 | 0.0015 | 0.31 | 0.3341 |
| Benzyl acetate | 0.1321 | 0.0426 | 2.65 | 0.1081 |
| Ethylphenyl acetate | 1.4938 | 1.0218 | 3.77 | 0.0563 |
| Hexanoic acid ** | 0.3401 | 0.1818 | 6.67 | 0.012 |
| Phenylethanol * | 1.5502 | 0.5302 | 8.91 | 0.0039 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durán-Guerrero, E.; Schwarz, M.; Fernández-Recamales, M.Á.; Barroso, C.G.; Castro, R. Characterization and Differentiation of Spanish Vinegars from Jerez and Condado de Huelva Protected Designations of Origin. Foods 2019, 8, 341. https://doi.org/10.3390/foods8080341
Durán-Guerrero E, Schwarz M, Fernández-Recamales MÁ, Barroso CG, Castro R. Characterization and Differentiation of Spanish Vinegars from Jerez and Condado de Huelva Protected Designations of Origin. Foods. 2019; 8(8):341. https://doi.org/10.3390/foods8080341
Chicago/Turabian StyleDurán-Guerrero, Enrique, Mónica Schwarz, M. Ángeles Fernández-Recamales, Carmelo G. Barroso, and Remedios Castro. 2019. "Characterization and Differentiation of Spanish Vinegars from Jerez and Condado de Huelva Protected Designations of Origin" Foods 8, no. 8: 341. https://doi.org/10.3390/foods8080341
APA StyleDurán-Guerrero, E., Schwarz, M., Fernández-Recamales, M. Á., Barroso, C. G., & Castro, R. (2019). Characterization and Differentiation of Spanish Vinegars from Jerez and Condado de Huelva Protected Designations of Origin. Foods, 8(8), 341. https://doi.org/10.3390/foods8080341






