Classification of Hen Eggs by HPLC-UV Fingerprinting and Chemometric Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Standard Solutions
2.2. Instrumentation
2.3. Samples and Sample Treatment
2.4. Data Analysis
3. Results and Discussion
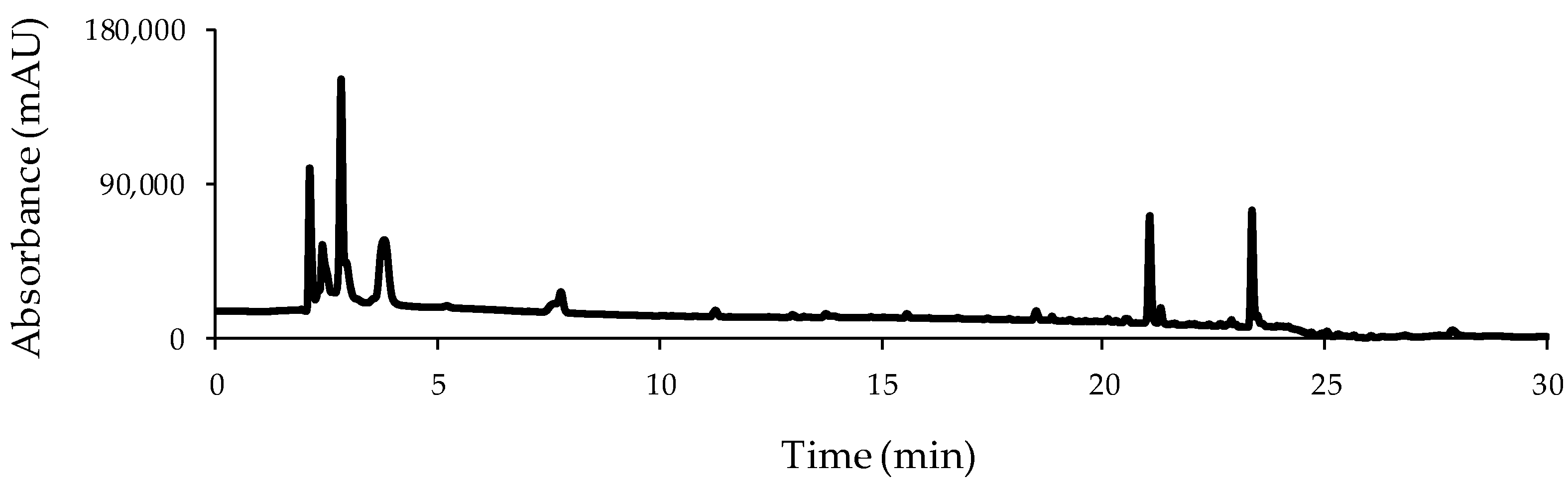
3.1. HPLC-UV Chromatographic Separation
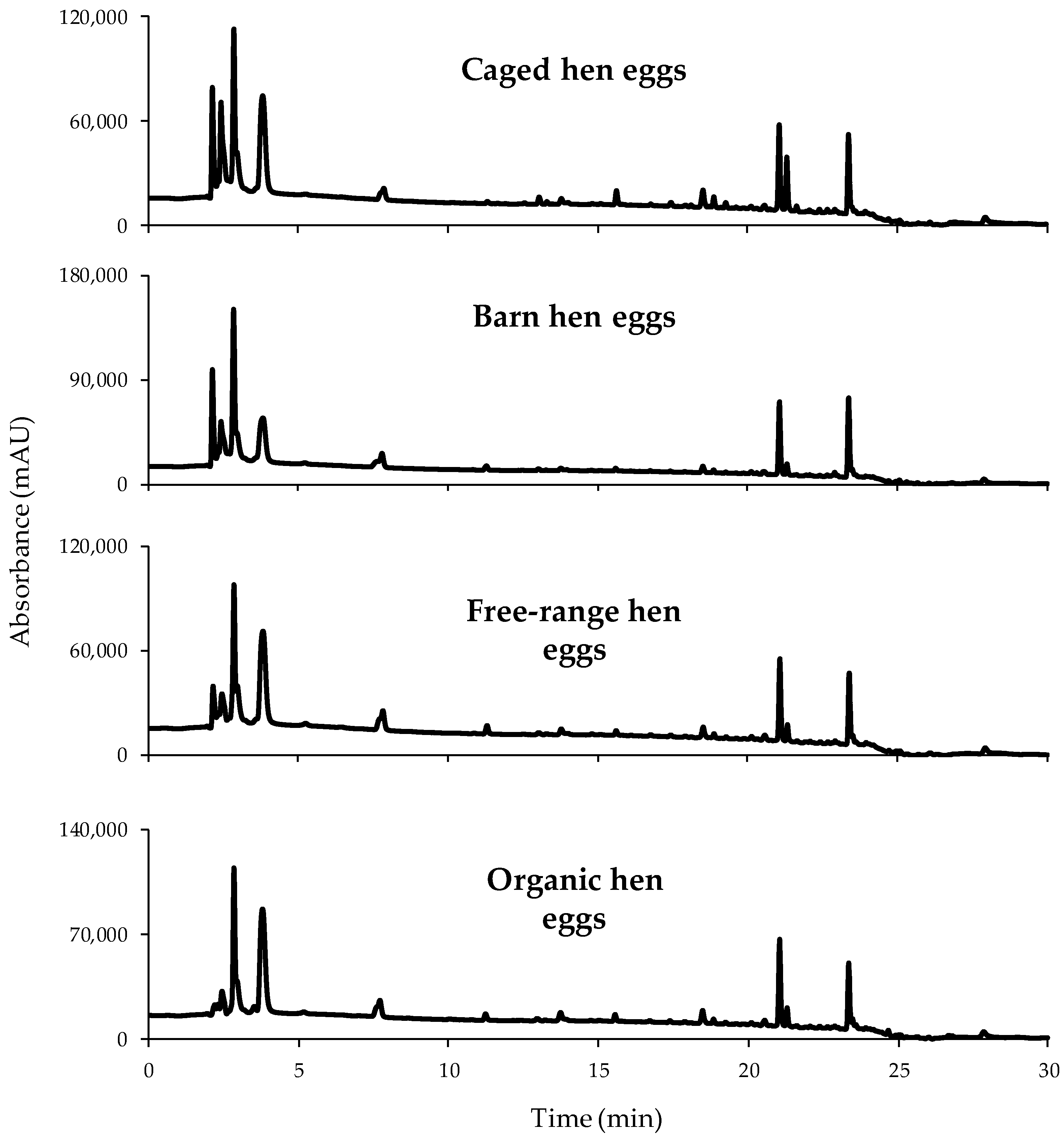
3.2. HPLC-UV Fingerprints
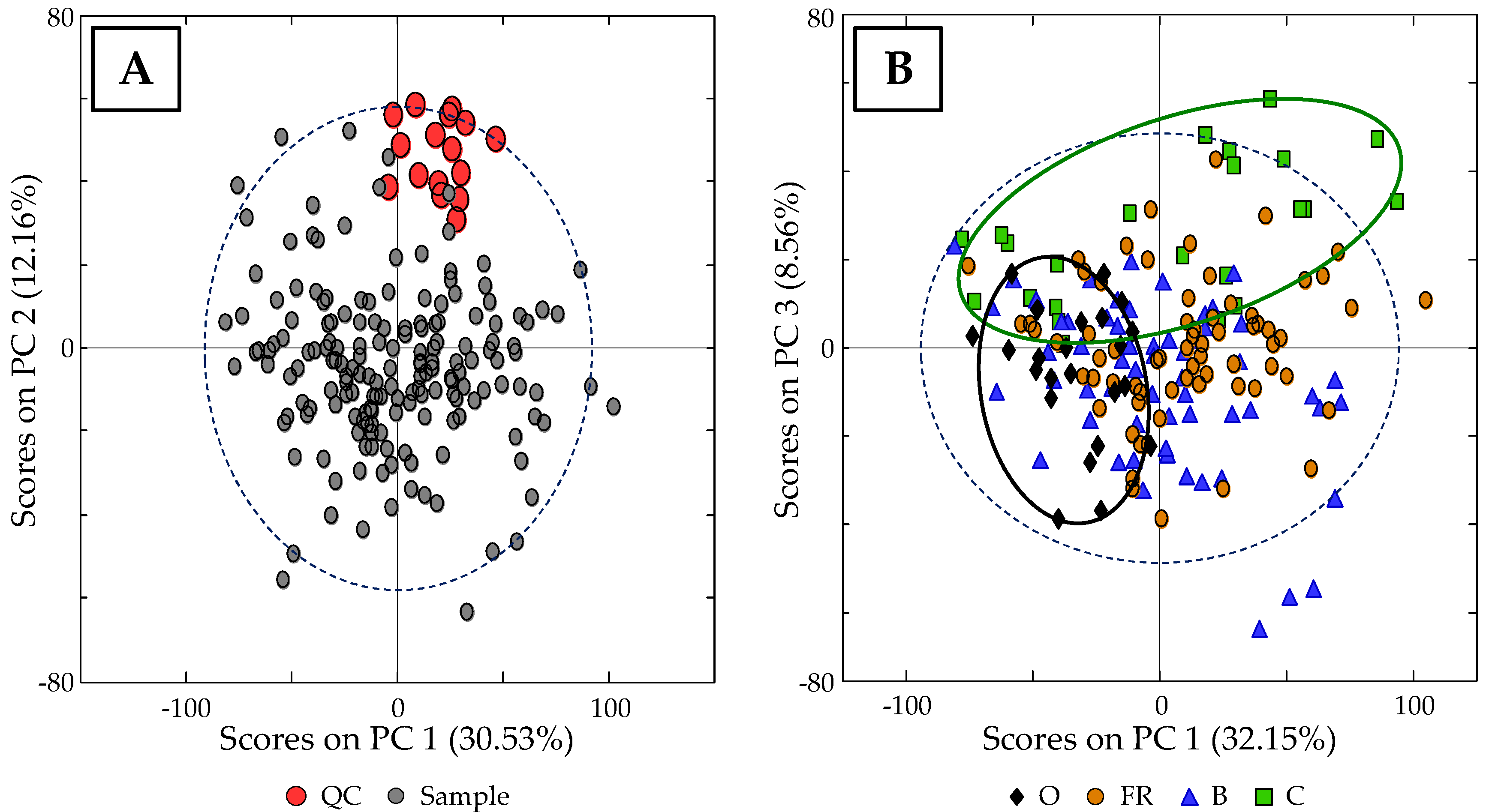
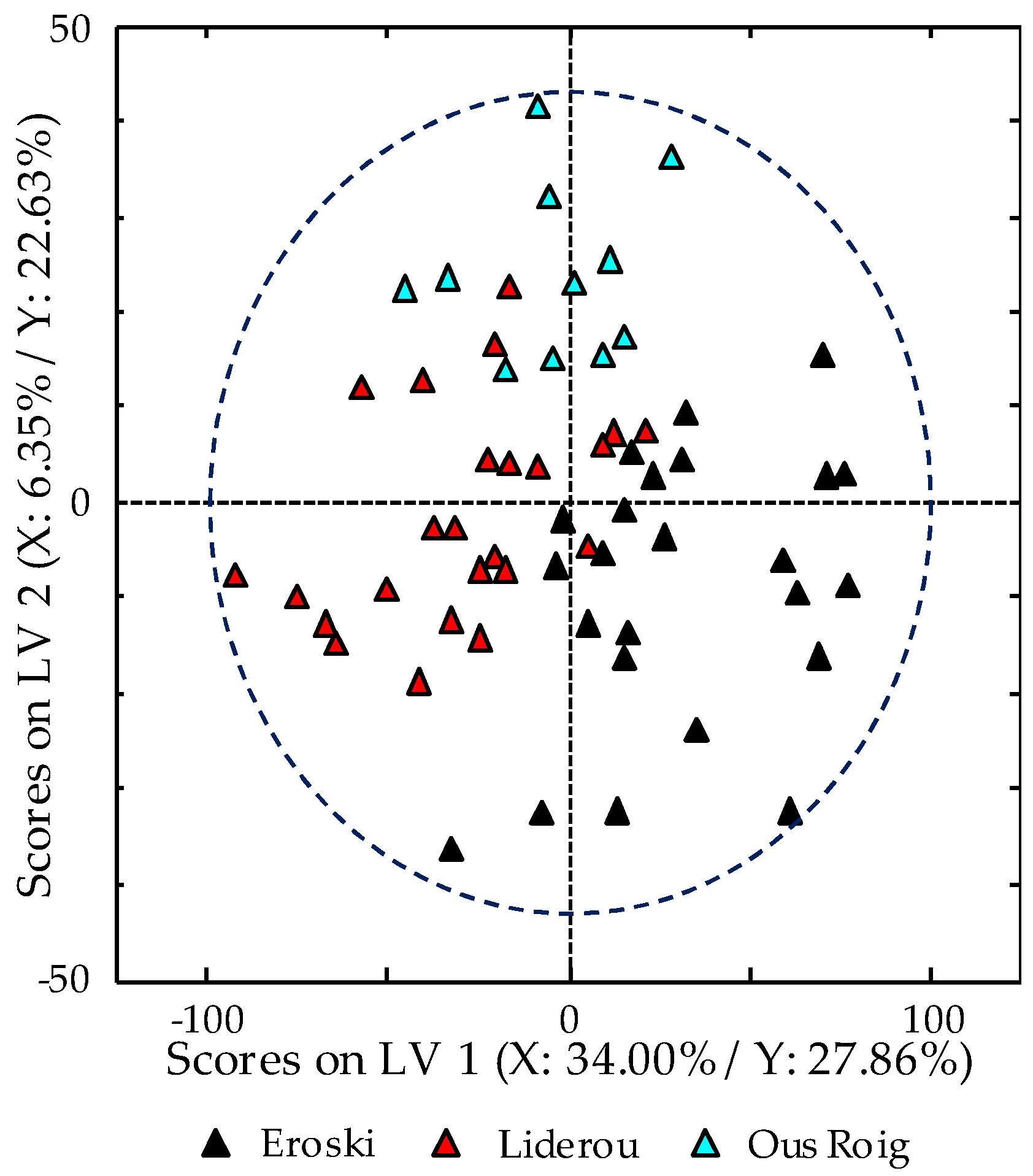
3.3. Classification of Samples According to Egg Type: PCA Study
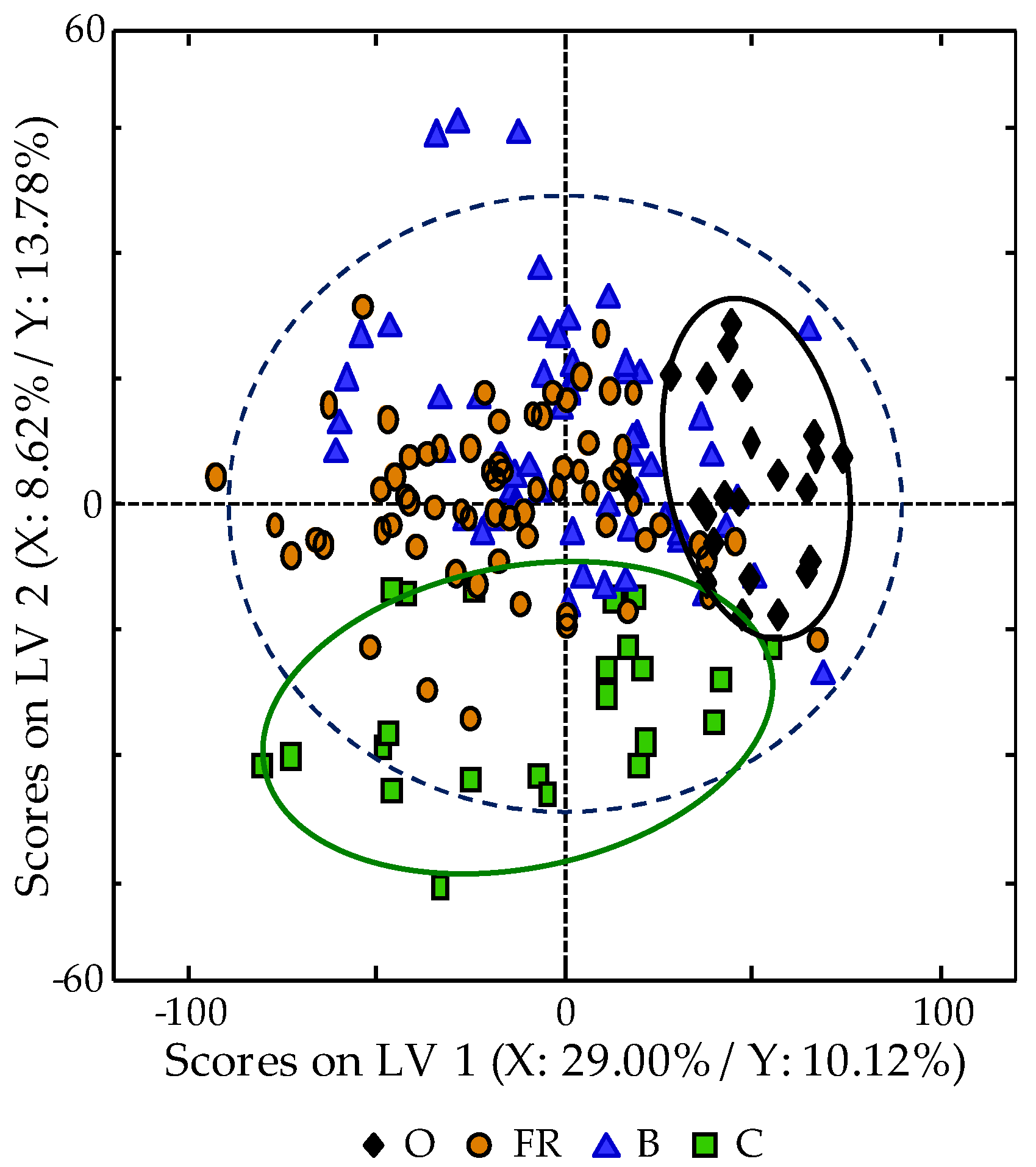
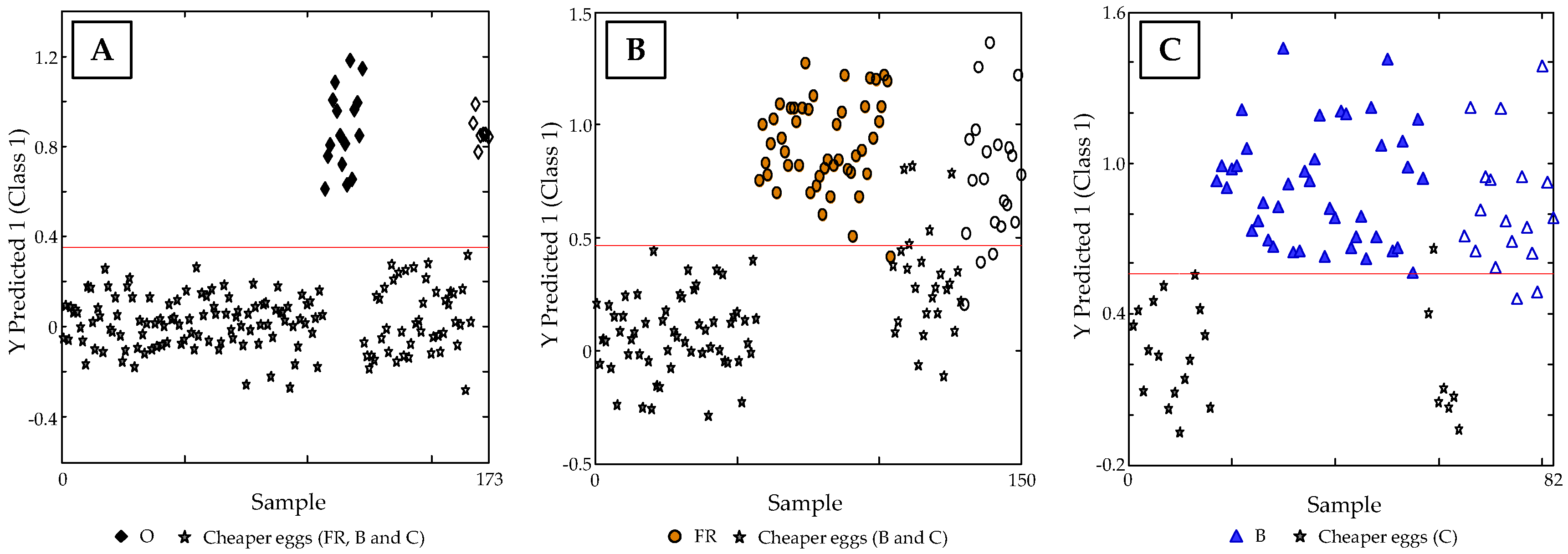
3.4. Classification of Samples According to Egg Type: PLS-DA Study
3.5. Supervised PLS-DA Method Validation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cherian, G.; Holsonbake, T.B.; Goeger, M.P. Fatty acid composition and egg components of specialty eggs. Poult. Sci. 2002, 81, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson, S.; Elwinger, K.; Pickova, J. Fatty acid and carotenoid composition of egg yolk as an effect of microalgae addition to feed formula for laying hens. Food Chem. 2006, 99, 530–537. [Google Scholar] [CrossRef]
- European Comission. Agriculture and Rural Development. Available online: https://www.ec.europa.eu/agriculture/eggs_en (accessed on 18 June 2019).
- European Union. Council Directive 1999/74/EC of 19 July 1999 laying down minimum standards for the protection of laying hens. Off. J. Eur. Communities 1999, L203, 53–57. [Google Scholar]
- European Union. Commission Directive 2002/4/EC of 30 January 2002 on the registration of establishments keeping laying hens, covered by Council Directive 1999/74/EC. Off. J. Eur. Communities 2002, L30, 44–46. [Google Scholar]
- European Union. Commission Regulation (EC) No 589/2008 of 23 June 2008 laying down detailed rules for implementing Council Regulation (EC) No 1234/2007 as regards marketing standards for eggs. Off. J. Eur. Union 2008, L163, 6–23. [Google Scholar]
- The European Parliament and the Council of the European Union. Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific hygiene rules for food business operators on the hygiene of foodstuffs. Off. J. Eur. Union 2004, L139, 151. [Google Scholar]
- Johnson, A.E.; Sidwick, K.L.; Pirgozliev, V.R.; Edge, A.; Thompson, D.F. Metabonomic Profiling of Chicken Eggs during Storage Using High-Performance Liquid Chromatography-Quadrupole Time-of-Flight Mass Spectrometry. Anal. Chem. 2018, 90, 7489–7494. [Google Scholar] [CrossRef] [PubMed]
- Rogers, K.M.; Van Ruth, S.; Alewijn, M.; Philips, A.; Rogers, P. Verification of Egg Farming Systems from the Netherlands and New Zealand Using Stable Isotopes. J. Agric. Food Chem. 2015, 63, 8372–8380. [Google Scholar] [CrossRef] [PubMed]
- Tres, A.; O’Neill, R.; Van Ruth, S.M. Fingerprinting of fatty acid composition for the verification of the identity of organic eggs. Lipid Technol. 2011, 23, 40–42. [Google Scholar] [CrossRef]
- Van Ruth, S.; Alewijn, M.; Rogers, K.; Newton-Smith, E.; Tena, N.; Bollen, M.; Koot, A. Authentication of organic and conventional eggs by carotenoid profiling. Food Chem. 2011, 126, 1299–1305. [Google Scholar] [CrossRef]
- Barbosa, R.M.; Nacano, L.R.; Freitas, R.; Batista, B.L.; Barbosa, F. The Use of Decision Trees and Naïve Bayes Algorithms and Trace Element Patterns for Controlling the Authenticity of Free-Range-Pastured Hens’ Eggs. J. Food Sci. 2014, 79, C1672–C1677. [Google Scholar] [CrossRef] [PubMed]
- Borges, E.M.; Volmer, D.A.; Gallimberti, M.; Ferreira De Souza, D.; Luiz De Souza, E.; Barbosa, F. Evaluation of macro- and microelement levels for verifying the authenticity of organic eggs by using chemometric techniques. Anal. Methods 2015, 7, 2577–2584. [Google Scholar] [CrossRef]
- Bandoniene, D.; Walkner, C.; Zettl, D.; Meisel, T. Rare Earth Element Labeling as a Tool for Assuring the Origin of Eggs and Poultry Products. J. Agric. Food Chem. 2018, 66, 11729–11738. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, X.; Dediu, L.; Victor, C. Review of the current application of fingerprinting allowing detection of food adulteration and fraud in China. Food Control 2011, 22, 1126–1135. [Google Scholar] [CrossRef]
- Riedl, J.; Esslinger, S.; Fauhl-Hassek, C. Review of validation and reporting of non-targeted fingerprinting approaches for food authentication. Anal. Chim. Acta 2015, 885, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Cuadros-Rodríguez, L.; Ruiz-Samblás, C.; Valverde-Som, L.; Pérez-Castaño, E.; González-Casado, A. Chromatographic fingerprinting: An innovative approach for food “identitation” and food authentication—A tutorial. Anal. Chim. Acta 2016, 909, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Sobolev, A.P.; Circi, S.; Capitani, D.; Ingallina, C.; Mannina, L. Molecular fingerprinting of food authenticity. Curr. Opin. Food Sci. 2017, 16, 59–66. [Google Scholar] [CrossRef]
- Esteki, M.; Shahsavari, Z.; Simal-Gandara, J. Use of spectroscopic methods in combination with linear discriminant analysis for authentication of food products. Food Control 2018, 91, 100–112. [Google Scholar] [CrossRef]
- Chen, H.; Tan, C.; Lin, Z. Non-destructive identification of native egg by near-infrared spectroscopy and data driven-based class-modeling. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 206, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Cavanna, D.; Catellani, D.; Dall’Asta, C.; Suman, M. Egg product freshness evaluation: A metabolomic approach. J. Mass Spectrom. 2018, 53, 849–861. [Google Scholar] [CrossRef] [PubMed]
- Eigenvector Research Incorporated. Powerful Resources for Intelligent Data Analysis. Available online: http://www.eigenvector.com/software/solo.htm (accessed on 15 January 2019).
- Massart, D.L.; Vandeginste, B.G.M.; Buydens, L.M.C.; de Jong, S.; Lewi, P.J.; Smeyers-Verbeke, J. Handbook of Chemometrics and Qualimetrics; Elsevier: Amsterdam, The Netherlands, 1997. [Google Scholar]
| Egg Type | Manufacturer | Egg Size | Number of Samples |
|---|---|---|---|
| Organic hen eggs (O) | ViuBi | M/L | 23 |
| Free-range hen eggs (FR) | Vall de Mestral | - | 23 |
| Ous Roig (Ebre) | - | 23 | |
| Ous Roig | L/XL | 22 | |
| Barn hen eggs (B) | Liderou | M | 24 |
| Eroski | L | 24 | |
| Ous Roig | L | 11 | |
| Caged hen eggs (C) | Eroski | M | 12 |
| Eroski | L | 11 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campmajó, G.; Cayero, L.; Saurina, J.; Núñez, O. Classification of Hen Eggs by HPLC-UV Fingerprinting and Chemometric Methods. Foods 2019, 8, 310. https://doi.org/10.3390/foods8080310
Campmajó G, Cayero L, Saurina J, Núñez O. Classification of Hen Eggs by HPLC-UV Fingerprinting and Chemometric Methods. Foods. 2019; 8(8):310. https://doi.org/10.3390/foods8080310
Chicago/Turabian StyleCampmajó, Guillem, Laura Cayero, Javier Saurina, and Oscar Núñez. 2019. "Classification of Hen Eggs by HPLC-UV Fingerprinting and Chemometric Methods" Foods 8, no. 8: 310. https://doi.org/10.3390/foods8080310
APA StyleCampmajó, G., Cayero, L., Saurina, J., & Núñez, O. (2019). Classification of Hen Eggs by HPLC-UV Fingerprinting and Chemometric Methods. Foods, 8(8), 310. https://doi.org/10.3390/foods8080310







