Phenolic and Antioxidant Analysis of Olive Leaves Extracts (Olea europaea L.) Obtained by High Voltage Electrical Discharges (HVED)
Abstract
1. Introduction
“Green extraction is based on the discovery and design of extraction processes which will reduce energy consumption, allows use of alternative solvents and renewable natural products, and ensure a safe and high-quality extract/product”[4].
2. Materials and Methods
2.1. Plant Material
2.2. Conventional and HVED Assisted Extraction
Experimental Design and Statistical Analysis
2.3. Determination of Total Phenolic Content (TPC)
2.4. Determination of Antioxidant Capacity (AOC)
2.4.1. DPPH (2,2-Diphenyl-2-Picrylhydrazyl) Free Radical Assay
2.4.2. Ferric Reducing Antioxidant Power (FRAP) Assay
2.5. NIR
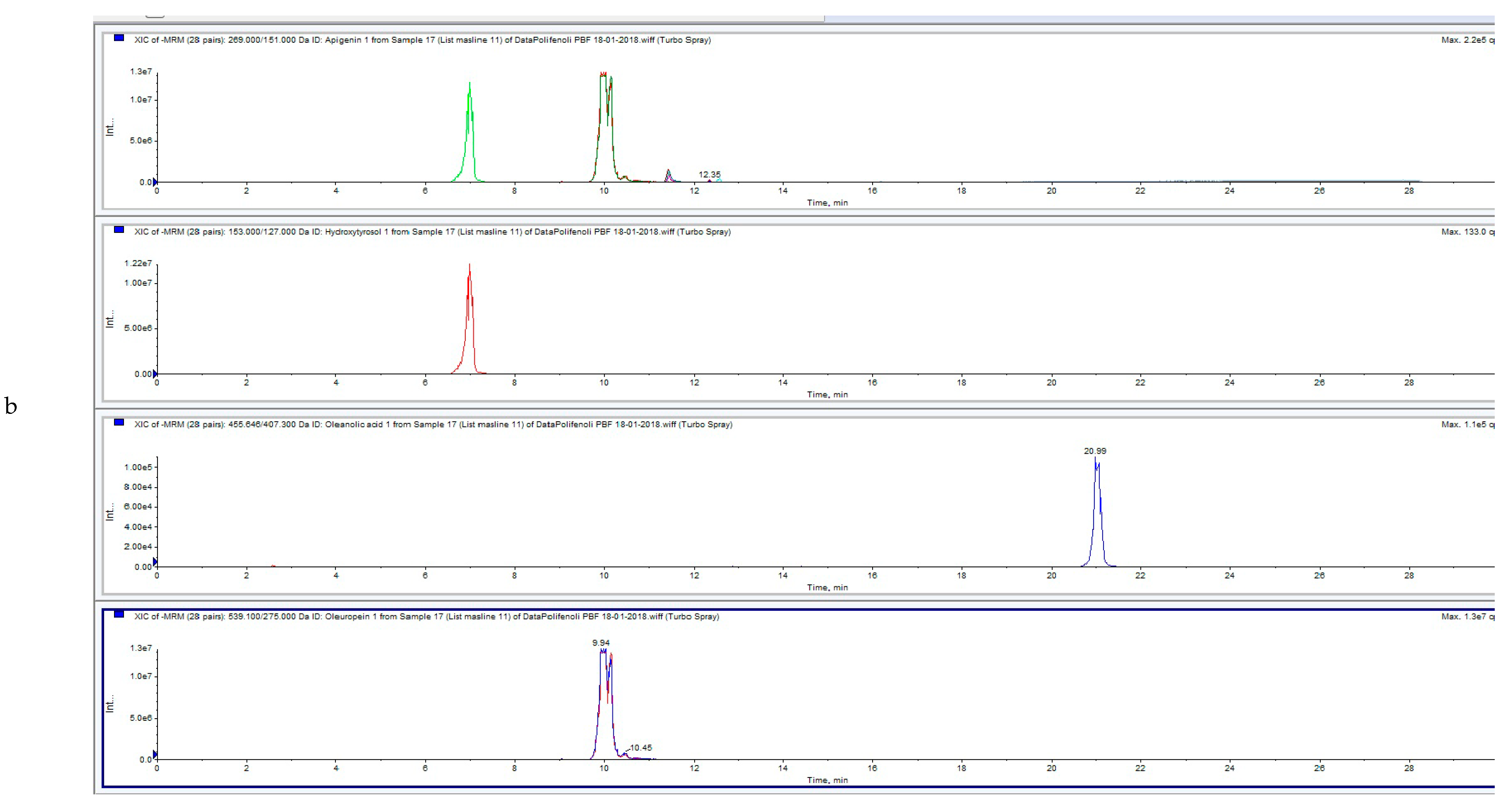
2.6. UPLC-MS/MS
2.7. Colorimetric Evaluation of OLEs
2.8. Electrophoretic Light Scattering (ELS)
2.9. Dynamic Light Scattering (DLS)
2.10. Determination of Pesticides and Metals in OLs
3. Results and Discussion
3.1. Influence of HVED Treatment on Physical Parameters of OLEs
3.2. Influence of HVED Treatment on TPC and Antioxidant Activity of OLEs
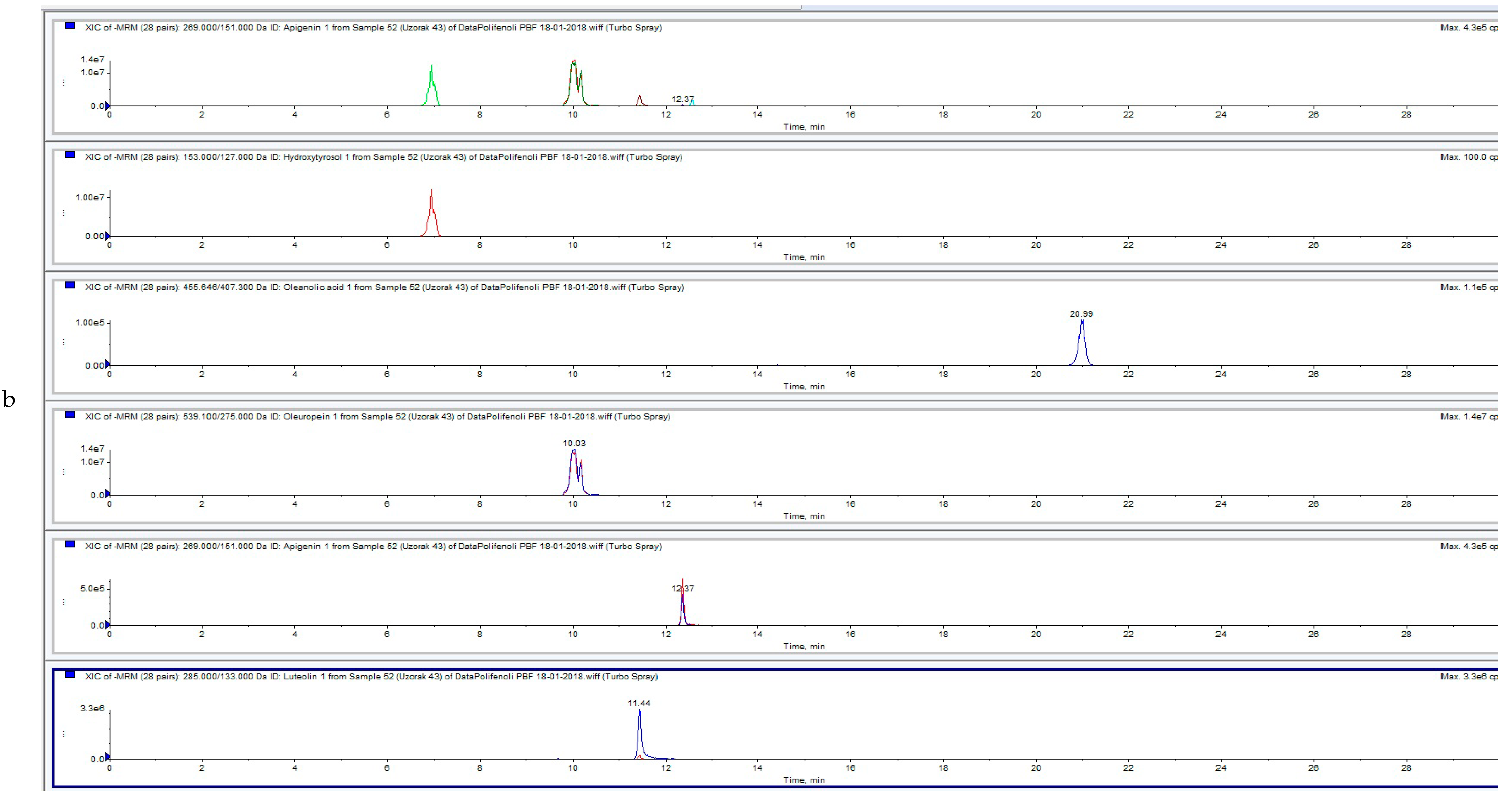
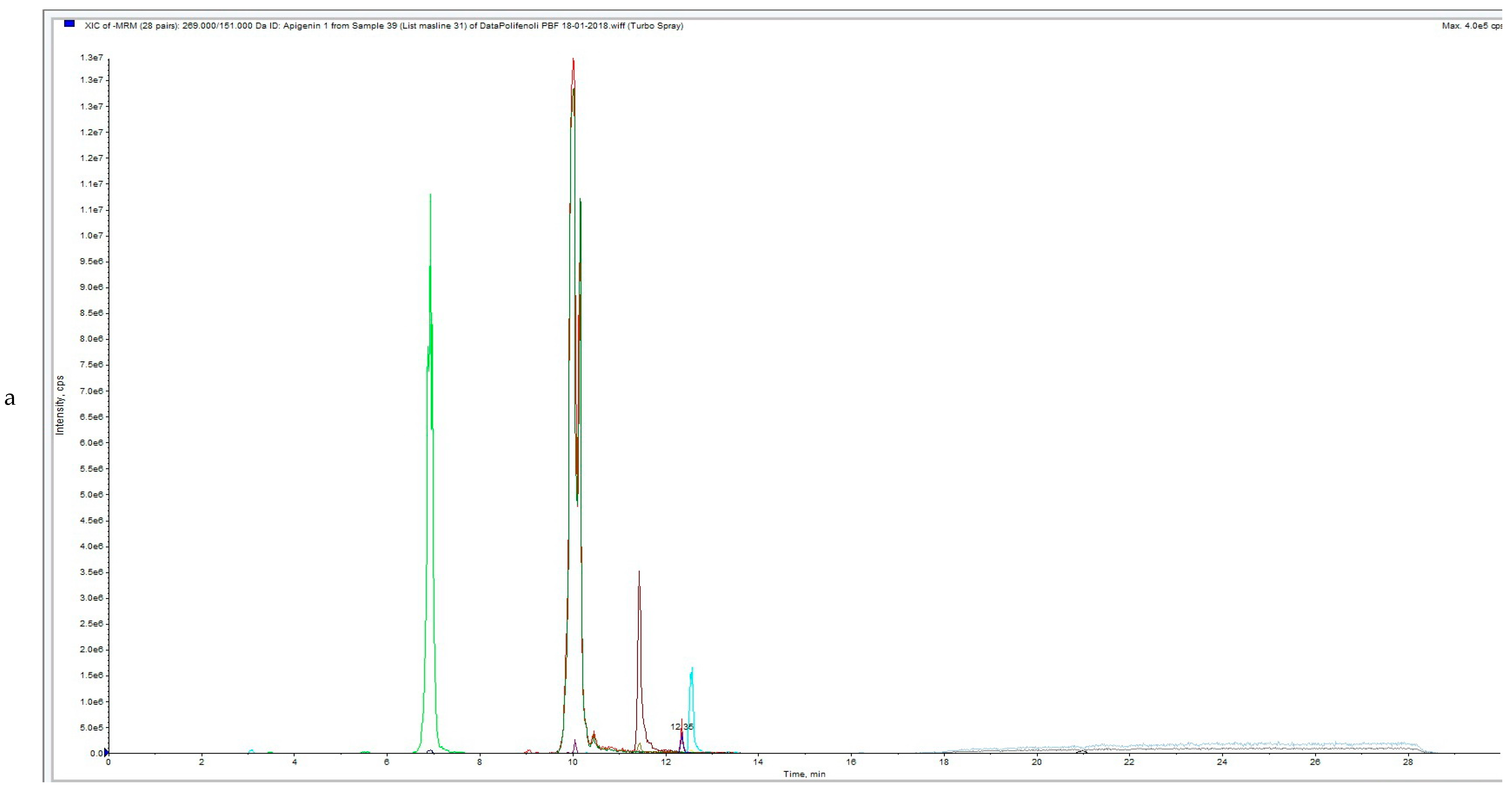
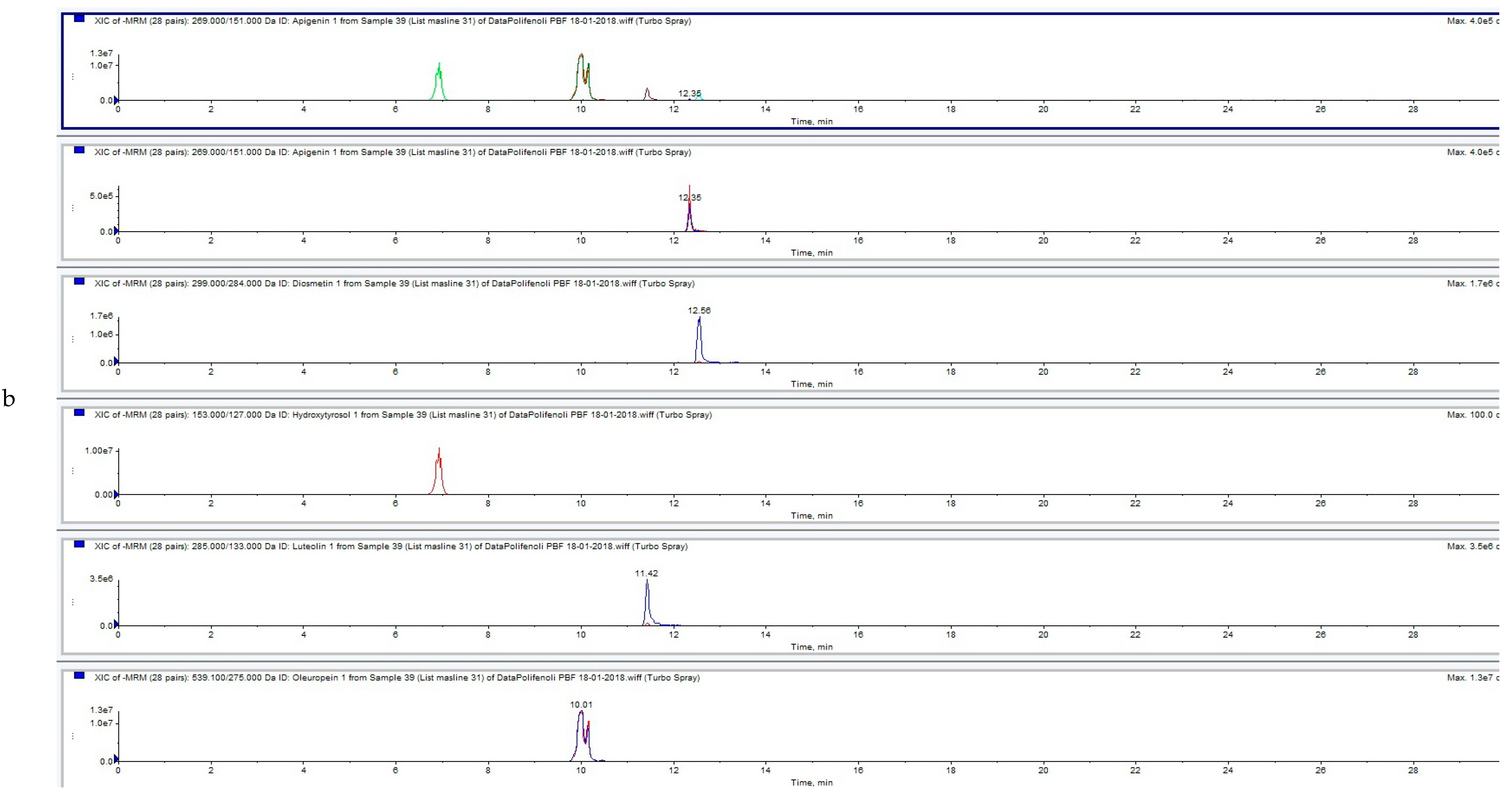
3.3. Influence of HVED Treatment on Soluble Phenolic Compounds in OLEs Analyzed by UPLC-MS/MS
3.4. Influence of HVED Treatment on Color Parameters of OLEs
3.5. Influence of HVED Treatment and Electroporation Phenomena on Particle Size and Zeta Potential of OLEs
3.6. Statistical Analysis of HVED Treatment Parameters and Ethanol Influence in Extraction of Bioactive Compound Form OLEs
| Source | Main Effects | Interactions | |||||
|---|---|---|---|---|---|---|---|
| A: Treatment Time | B: Voltage | C: Ethanol Content | AB | AC | BC | ||
| p-value | pH | 0.1751 | 0.4805 | 0.0037 | 0.2276 | 0.5162 | 0.7081 |
| Conductivity | 0.0431 | 0.5992 | 0.0017 | 0.2722 | 0.3039 | 0.8873 | |
| Temperature difference | 0.0867 | 0.0588 | 0.0564 | 0.1082 | 0.1968 | 0.6541 | |
| Power | 0.2380 | 0.0099 | 0.2097 | 0.3828 | 0.8125 | 0.2500 | |
| DLS | 0.6704 | 0.7696 | 0.3177 | 0.3569 | 0.6945 | 0.8880 | |
| PI | 0.4634 | 0.6328 | 0.2114 | 0.9126 | 0.7919 | 0.9259 | |
| ELS | 0.0703 | 0.1162 | 0.0163 | 0.2681 | 0.4337 | 0.1984 | |
| L* | 0.3500 | 0.5867 | 0.3631 | 0.5392 | 0.4922 | 0.6159 | |
| a* | 0.2793 | 0.6684 | 0.0474 | 0.5779 | 0.5319 | 0.7388 | |
| b* | 0.1225 | 0.8636 | 0.1252 | 0.8647 | 0.5679 | 0.3522 | |
| TPC | 0.1972 | 0.4714 | 0.1067 | 0.6437 | 0.6604 | 0.9387 | |
| FRAP | 0.6781 | 0.9773 | 0.1791 | 0.4250 | 0.8373 | 0.7394 | |
| DPPH | 0.7467 | 0.8024 | 0.2450 | 0.2872 | 0.6684 | 0.4903 | |
| Yield of extraction | 0.1982 | 0.4710 | 0.1072 | 0.6450 | 0.6633 | 0.9392 | |
| Source | Main Effects | Interactions | |||||
|---|---|---|---|---|---|---|---|
| A: Treatment Time | B: Voltage | C: Ethanol Content | AB | AC | BC | ||
| p value | pH | 0.1610 | 0.1516 | 0.0255 | 0.1209 | 0.2870 | 0.2384 |
| Conductivity | 0.3678 | 0.7244 | 0.0128 | 0.4040 | 0.8518 | 0.9879 | |
| Temperature Difference | 0.1782 | 0.1677 | 0.0829 | 0.1081 | 0.2583 | 0.1489 | |
| Power | 0.5736 | 0.0046 | 0.4091 | 0.1835 | 0.1957 | 0.1552 | |
| DLS | 0.4132 | 0.3820 | 0.0277 | 0.3044 | 0.1810 | 0.0969 | |
| PI | 0.2785 | 0.4608 | 0.2353 | 0.7913 | 0.3141 | 0.5596 | |
| ELS | 0.2004 | 0.3594 | 0.0295 | 0.2676 | 0.1189 | 0.4675 | |
| L* | 0.6960 | 0.4382 | 0.2718 | 0.9382 | 0.6644 | 0.4497 | |
| a* | 0.4285 | 0.4968 | 0.0706 | 0.9572 | 0.5046 | 0.4027 | |
| b* | 0.4367 | 0.5694 | 0.2359 | 0.9952 | 0.4904 | 0.5976 | |
| TPC | 0.7034 | 0.8342 | 0.0652 | 0.7084 | 0.5300 | 0.5030 | |
| FRAP | 0.5573 | 0.2974 | 0.3888 | 0.6255 | 0.3050 | 0.6206 | |
| DPPH | 0.1798 | 0.8050 | 0.1974 | 0.1946 | 0.6913 | 0.4720 | |
| Extraction Yield | 0.7026 | 0.8341 | 0.0650 | 0.7089 | 0.5312 | 0.5062 | |
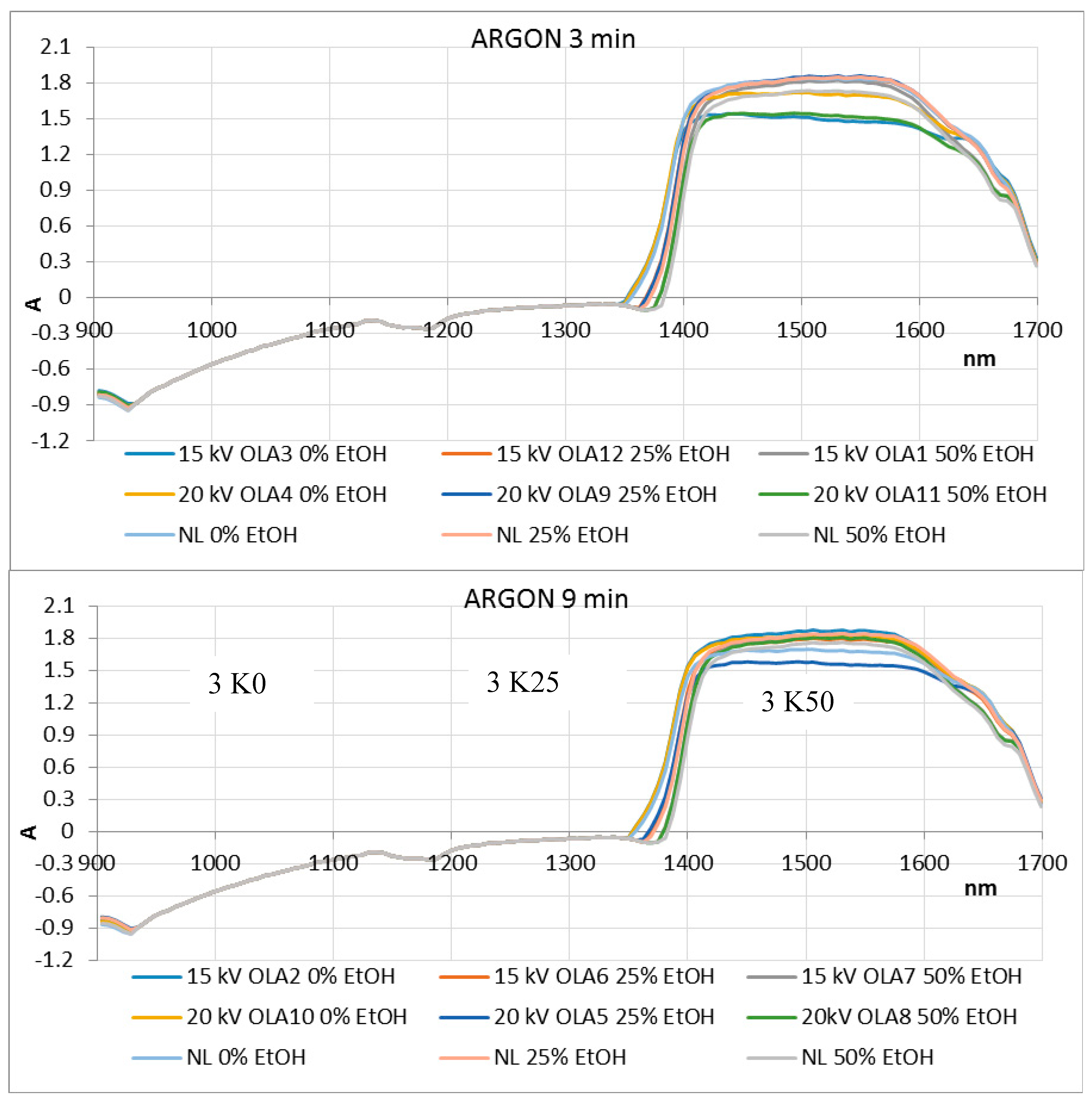
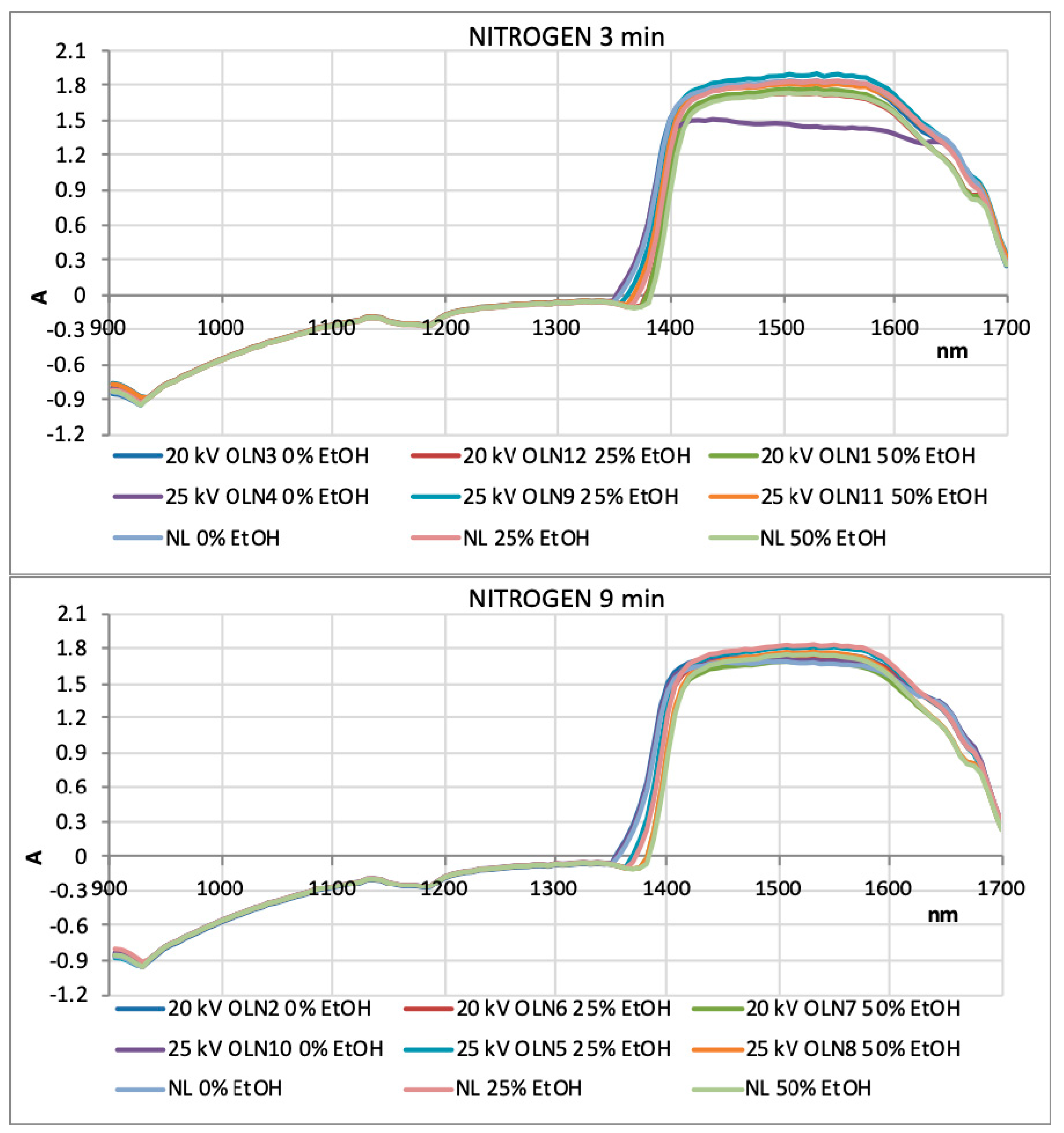
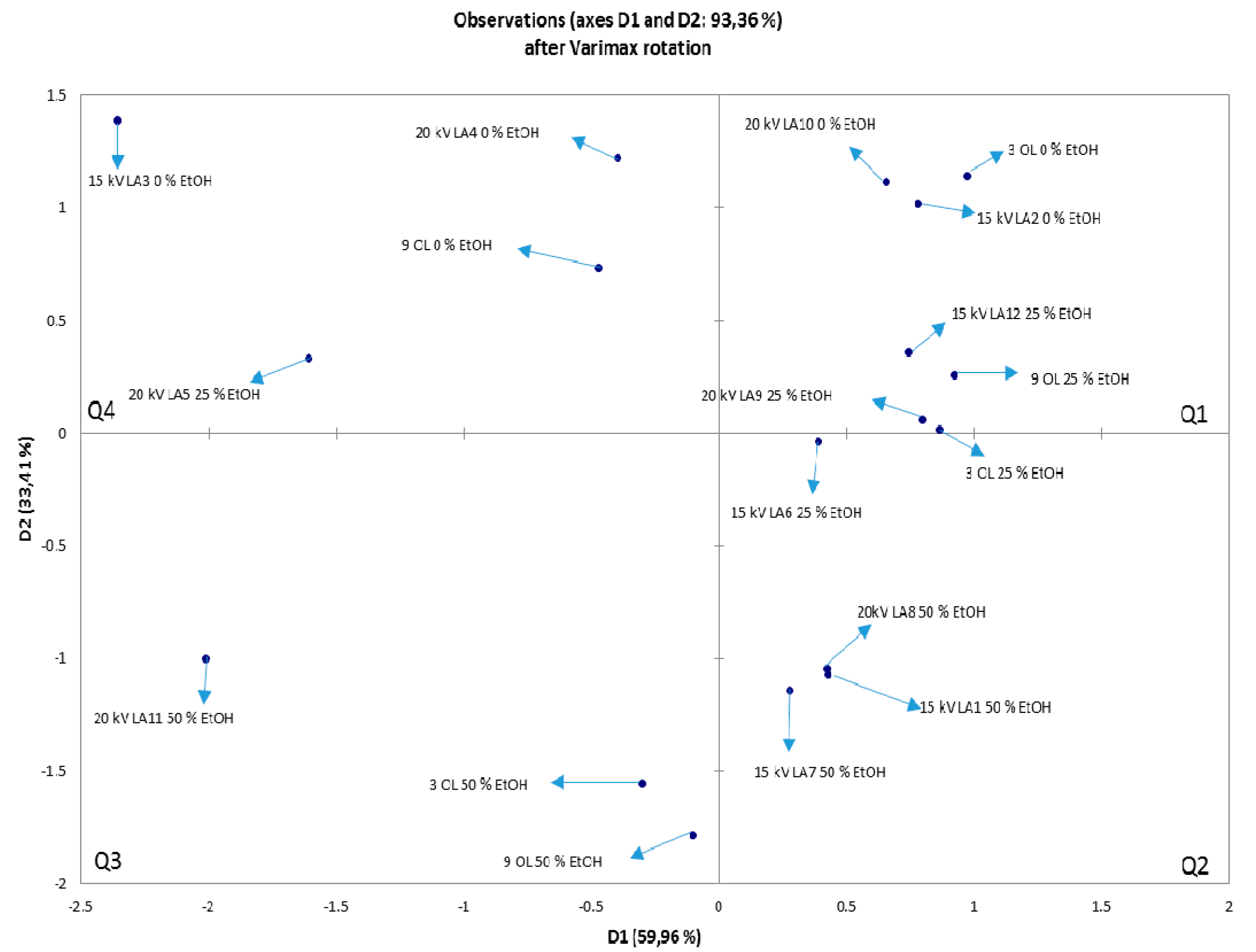
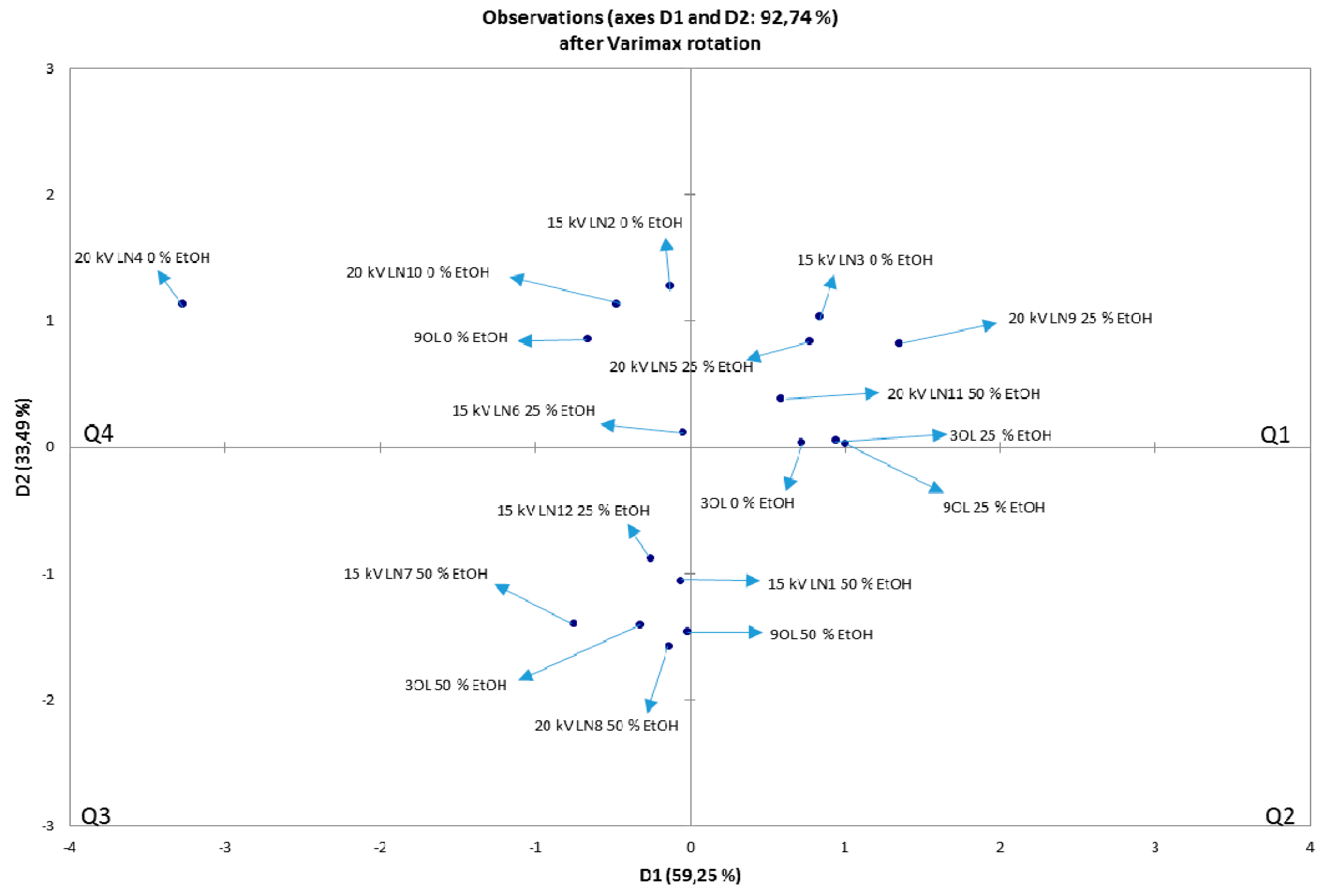
3.7. NIR and PCA Analysis of HVED Treated OLEs
3.8. Analysis of Pesticides and Metals in OL Samples
3.9. Critical Consideration on Costs of Equipment and Application of HVED for Extraction
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Putnik, P.; Lorenzo, J.; Barba, F.; Roohinejad, S.; Režek Jambrak, A.; Granato, D.; Montesano, D.; Bursać Kovačević, D. Novel food processing and extraction technologies of high-added value compounds from plant materials. Foods 2018, 7, 106. [Google Scholar] [CrossRef] [PubMed]
- Giacometti, J.; Bursać Kovačević, D.; Putnik, P.; Gabrić, D.; Bilušić, T.; Krešić, G.; Stulić, V.; Barba, F.J.; Chemat, F.; Barbosa-Cánovas, G.; et al. Extraction of bioactive compounds and essential oils from mediterranean herbs by conventional and green innovative techniques: A review. Food Res. Int. 2018, 113, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Şahin, S.; Bilgin, M. Olive tree (olea europaea L.) leaf as a waste by-product of table olive and olive oil industry: A review. J. Sci. Food Agric. 2018, 98, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [PubMed]
- Watson, W.J.W. How do the fine chemical, pharmaceutical, and related industries approach green chemistry and sustainability? Green Chem. 2012, 14, 251–259. [Google Scholar] [CrossRef]
- Soni, M.G.; Burdock, G.A.; Christian, M.S.; Bitler, C.M.; Crea, R. Safety assessment of aqueous olive pulp extract as an antioxidant or antimicrobial agent in foods. Food Chem. Toxicol. 2006, 44, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Japón-Luján, R.; Luque-Rodríguez, J.M.; Luque de Castro, M.D. Dynamic ultrasound-assisted extraction of oleuropein and related biophenols from olive leaves. J. Chromatogr. A 2006, 1108, 76–82. [Google Scholar] [CrossRef]
- Benavente-García, O.; Castillo, J.; Lorente, J.; Ortuño, A.; Del Rio, J.A. Antioxidant activity of phenolics extracted from olea europaea l. Leaves. Food Chem. 2000, 68, 457–462. [Google Scholar] [CrossRef]
- Ferreira, I.C.F.R.; Barros, L.; Soares, M.E.; Bastos, M.L.; Pereira, J.A. Antioxidant activity and phenolic contents of Olea europaea l. leaves sprayed with different copper formulations. Food Chem. 2007, 103, 188–195. [Google Scholar] [CrossRef]
- Lockyer, S.; Rowland, I.; Spencer, J.P.E.; Yaqoob, P.; Stonehouse, W. Impact of phenolic-rich olive leaf extract on blood pressure, plasma lipids and inflammatory markers: A randomised controlled trial. Eur. J. Nutr. 2016, 56, 1421–1432. [Google Scholar] [CrossRef]
- Wainstein, J.; Ganz, T.; Boaz, M.; Bar Dayan, Y.; Dolev, E.; Kerem, Z.; Madar, Z. Olive leaf extract as a hypoglycemic agent in both human diabetic subjects and in rats. J. Med. Food 2012, 15, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Vizza, D.; Lupinacci, S.; Toteda, G.; Puoci, F.; Ortensia, I.P.; De Bartolo, A.; Lofaro, D.; Scrivano, L.; Bonofiglio, R.; Russa, A.L.; et al. An olive leaf extract rich in polyphenols promotes apoptosis in cervical cancer cells by upregulating p21cip/waf1 gene expression. Nutr. Cancer 2019, 71, 320–333. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.-C.; Kim, H.W.; Min, B.K.; Cho, J.Y.; Son, H.J.; Lee, J.Y.; Kim, J.-Y.; Kwon, S.-B.; Li, Q.; Lee, H.-W. Inhibitory effect of olive leaf extract on obesity in high-fat diet-induced mice. In Vivo 2019, 33, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Sano, M.; Seno, K.; Oogaki, Y.; Takahashi, H.; Ohkuchi, A.; Yokozawa, M.; Yamauchi, K.; Iwata, H.; Kuwayama, T.; et al. Olive leaf extract (Olea Vita) suppresses inflammatory cytokine production and NLRP3 inflammasomes in human placenta. Nutrients 2019, 11, 970. [Google Scholar] [CrossRef] [PubMed]
- Sarbishegi, M. Antioxidant effects of olive leaf extract in prevention of Alzheimer’s disease and Parkinson’s disease. Gene. Cell Tissue 2018, 5, e79847. [Google Scholar] [CrossRef]
- Ganjalikhan Hakemi, S.; Sharififar, F.; Haghpanah, T.; Babaee, A.; Eftekhar-Vaghefi, S.H. The effects of olive leaf extract on the testis, sperm quality and testicular germ cell apoptosis in male rats exposed to busulfan. Int. J. Fertil. Steril. 2019, 13, 57–65. [Google Scholar] [PubMed]
- Vidičević, S.; Tošić, J.; Stanojević, Ž.; Isaković, A.; Mitić, D.; Ristić, D.; Dekanski, D. Standardized Olea europaea L. leaf extract exhibits protective activity in carbon tetrachloride-induced acute liver injury in rats: The insight into potential mechanisms. Arch. Physiol. Biochem. 2019, 11, 1–9. [Google Scholar]
- Ranieri, M.; Di Mise, A.; Difonzo, G.; Centrone, M.; Venneri, M.; Pellegrino, T.; Russo, A.; Mastrodonato, M.; Caponio, F.; Valenti, G.; et al. Green olive leaf extract (OLE) provides cytoprotection in renal cells exposed to low doses of cadmium. PLoS ONE 2019, 14, e0214159. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, S.; Cui, W.; He, J.; Wang, Z.; Yang, X. Olive leaf extract inhibits lead poisoning-induced brain injury. Neural. Regen. Res. 2013, 8, 2021–2029. [Google Scholar]
- Caponio, F.; Difonzo, G.; Calasso, M.; Cosmai, L.; De Angelis, M. Effects of olive leaf extract addition on fermentative and oxidative processes of table olives and their nutritional properties. Food Res. Int. 2019, 116, 1306–1317. [Google Scholar] [CrossRef] [PubMed]
- Martínez, L.; Castillo, J.; Ros, G.; Nieto, G. Antioxidant and antimicrobial activity of rosemary, pomegranate and olive extracts in fish patties. Antioxidants 2019, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Khemakhem, I.; Fuentes, A.; Lerma-García, M.J.; Ayadi, M.A.; Bouaziz, M.; Barat, J.M. Olive leaf extracts for shelf life extension of salmon burgers. Food Sci. Technol. Int. 2018, 25, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Putnik, P.; Barba, F.J.; Španić, I.; Zorić, Z.; Dragović-Uzelac, V.; Bursać Kovačević, D. Green extraction approach for the recovery of polyphenols from Croatian olive leaves (Olea europea). Food Bioprod. Process. 2017, 106, 19–28. [Google Scholar] [CrossRef]
- Žugčić, T.; Abdelkebir, R.; Alcantara, C.; Collado, M.C.; García-Pérez, J.V.; Meléndez-Martínez, A.J.; Režek Jambrak, A.; Lorenzo, J.M.; Barba, F.J. From extraction of valuable compounds to health promoting benefits of olive leaves through bioaccessibility, bioavailability and impact on gut microbiota. Trends Food Sci. Technol. 2019, 83, 63–77. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Meullemiestre, A.; Turk, M.; Perino, S.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Review of green food processing techniques. Preservation, transformation, and extraction. Innov. Food Sci. Emerg. 2017, 41, 357–377. [Google Scholar] [CrossRef]
- Madej, K.; Kalenik, T.K.; Piekoszewski, W. Sample preparation and determination of pesticides in fat-containing foods. Food Chem. 2018, 269, 527–541. [Google Scholar] [CrossRef] [PubMed]
- Zaanouni, N.; Gharssallaoui, M.; Eloussaief, M.; Gabsi, S. Heavy metals transfer in the olive tree and assessment of food contamination risk. Environ. Sci. Pollut. Res. 2018, 25, 18320–18331. [Google Scholar] [CrossRef]
- Alcázar Román, R.; Amorós, J.A.; Pérez de los Reyes, C.; García Navarro, F.J.; Bravo, S. Major and trace element content of olive leaves. OLIVAE 2014, 119, 1–7. [Google Scholar]
- Batista Napotnik, T.; Miklavčič, D. In vitro electroporation detection methods—An overview. Bioelectrochemistry 2018, 120, 166–182. [Google Scholar] [CrossRef]
- Režek Jambrak, A.; Donsì, F.; Paniwnyk, L.; Djekic, I. Impact of novel nonthermal processing on food quality: Sustainability, modelling, and negative aspects. J. Food Qual. 2019, 2019, 2. [Google Scholar] [CrossRef]
- Režek Jambrak, A.; Vukušić, T.; Donsi, F.; Paniwnyk, L.; Djekic, I. Three pillars of novel nonthermal food technologies: Food safety, quality, and environment. J. Food Qual. 2018, 2018, 18. [Google Scholar] [CrossRef]
- Chemat, F.; Zill, E.H.; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef] [PubMed]
- Attri, P.; Arora, B.; Choi, E.H. Retracted article: Utility of plasma: A new road from physics to chemistry. Rsc Adv. 2013, 3, 12540–12567. [Google Scholar] [CrossRef]
- Li, Z.; Fan, Y.; Xi, J. Recent advances in high voltage electric discharge extraction of bioactive ingredients from plant materials. Food Chem. 2019, 277, 246–260. [Google Scholar] [CrossRef] [PubMed]
- Shortle, E.; O’Grady, M.N.; Gilroy, D.; Furey, A.; Quinn, N.; Kerry, J.P. Influence of extraction technique on the anti-oxidative potential of hawthorn (Crataegus monogyna) extracts in bovine muscle homogenates. Meat. Sci. 2014, 98, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Achat, S.; Tomao, V.; Madani, K.; Chibane, M.; Elmaataoui, M.; Dangles, O.; Chemat, F. Direct enrichment of olive oil in oleuropein by ultrasound-assisted maceration at laboratory and pilot plant scale. Ultrason Sonochem. 2012, 19, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.J.; Geladi, P.; Britz, T.J.; Manley, M. Near-infrared (NIR) hyperspectral imaging and multivariate image analysis to study growth characteristics and differences between species and strains of members of the genus Fusarium. Anal. Bioanal. Chem. 2012, 404, 1759–1769. [Google Scholar] [CrossRef]
- Bursać Kovačević, D.; Putnik, P.; Dragović-Uzelac, V.; Pedisić, S.; Režek Jambrak, A.; Herceg, Z. Effects of cold atmospheric gas phase plasma on anthocyanins and color in pomegranate juice. Food Chem. 2016, 190, 317–323. [Google Scholar] [CrossRef]
- Xi, J.; He, L.; Yan, L.-G. Continuous extraction of phenolic compounds from pomegranate peel using high voltage electrical discharge. Food Chem. 2017, 230, 354–361. [Google Scholar] [CrossRef]
- Garcia-Castello, E.M.; Rodriguez-Lopez, A.D.; Mayor, L.; Ballesteros, R.; Conidi, C.; Cassano, A. Optimization of conventional and ultrasound assisted extraction of flavonoids from grapefruit (Citrus paradisi L.) solid wastes. LWT-Food Sci. Technol. 2015, 64, 1114–1122. [Google Scholar] [CrossRef]
- Bellumori, M.; Innocenti, M.; Binello, A.; Boffa, L.; Mulinacci, N.; Cravotto, G. Selective recovery of rosmarinic and carnosic acids from rosemary leaves under ultrasound- and microwave-assisted extraction procedures. C. R. Chim. 2016, 19, 699–706. [Google Scholar] [CrossRef]
- Boussetta, N.; Vorobiev, E. Extraction of valuable biocompounds assisted by high voltage electrical discharges: A review. C. R. Chim. 2014, 17, 197–203. [Google Scholar] [CrossRef]
- El Kantar, S.; Boussetta, N.; Rajha, H.N.; Maroun, R.G.; Louka, N.; Vorobiev, E. High voltage electrical discharges combined with enzymatic hydrolysis for extraction of polyphenols and fermentable sugars from orange peels. Food Res. Int. 2018, 107, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Barba, F.J.; Brianceau, S.; Turk, M.; Boussetta, N.; Vorobiev, E. Effect of alternative physical treatments (ultrasounds, pulsed electric fields, and high-voltage electrical discharges) on selective recovery of bio-compounds from fermented grape pomace. Food Bioprocess. Technol. 2015, 8, 1139–1148. [Google Scholar] [CrossRef]
- Almohammed, F.; Koubaa, M.; Khelfa, A.; Nakaya, M.; Mhemdi, H.; Vorobiev, E. Pectin recovery from sugar beet pulp enhanced by high-voltage electrical discharges. Food Bioprod. Process. 2017, 103, 95–103. [Google Scholar] [CrossRef]
- Roselló-Soto, E.; Barba, F.J.; Parniakov, O.; Galanakis, C.M.; Lebovka, N.; Grimi, N.; Vorobiev, E. High voltage electrical discharges, pulsed electric field, and ultrasound assisted extraction of protein and phenolic compounds from olive kernel. Food Bioprocess. Technol. 2015, 8, 885–894. [Google Scholar] [CrossRef]
- Sarkis, J.R.; Boussetta, N.; Tessaro, I.C.; Marczak, L.D.F.; Vorobiev, E. Application of pulsed electric fields and high voltage electrical discharges for oil extraction from sesame seeds. J. Food Eng. 2015, 153, 20–27. [Google Scholar] [CrossRef]
- Nyström, M.; Aimar, P.; Luque, S.; Kulovaara, M.; Metsämuuronen, S. Fractionation of model proteins using their physiochemical properties. Colloids Surf. A Phys. Eng. Asp. 1998, 138, 185–205. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
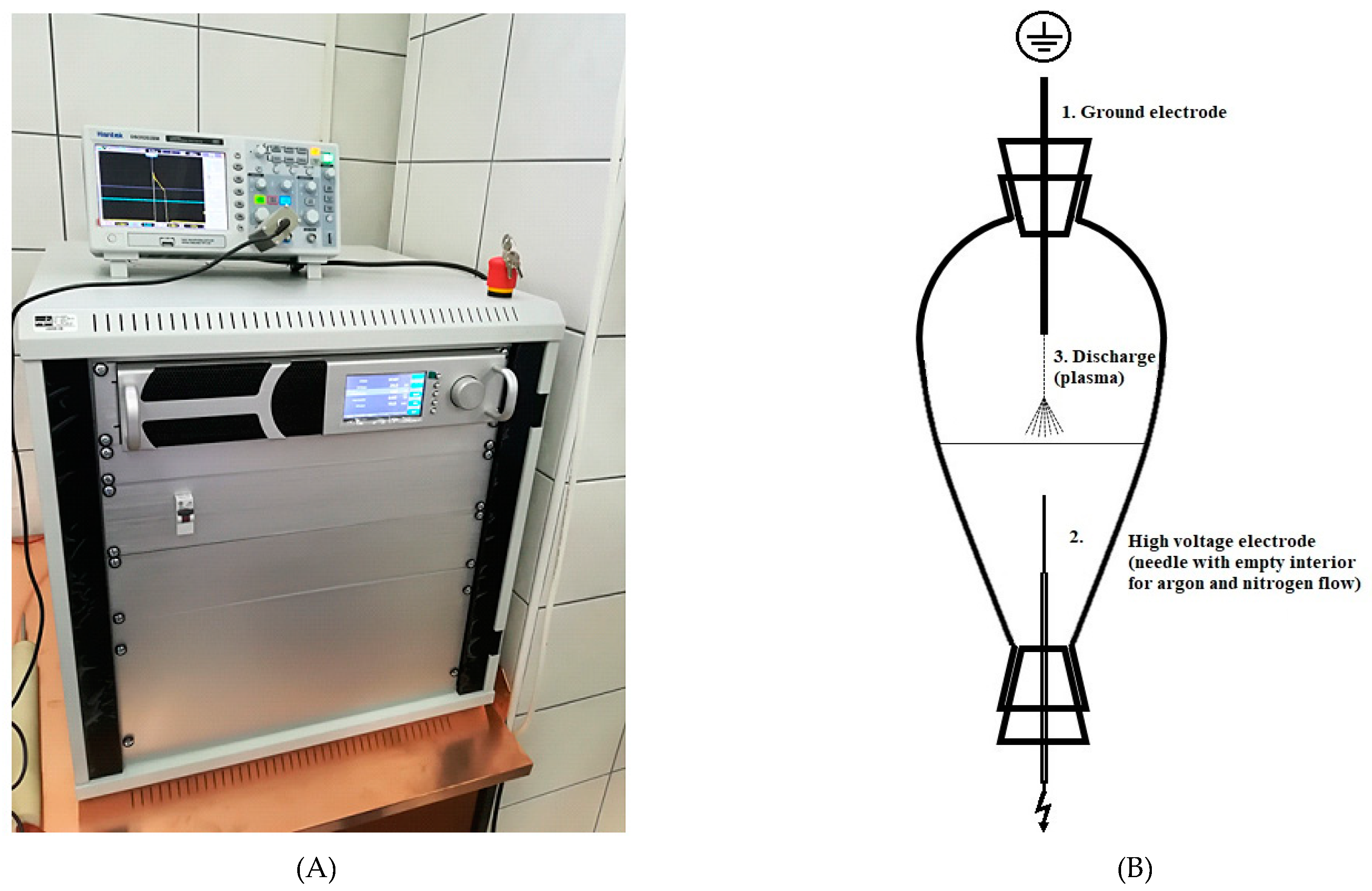
| ID | Sample | Treatment Time (min) | Voltage (kV) | Ethanol Content (%) | Stirring (min) | Extraction Type |
|---|---|---|---|---|---|---|
| 1 | 3 OL0 | 0 | 0 | 0 | 3 | CE |
| 2 | 9 OL0 | 0 | 0 | 0 | 9 | |
| 3 | 3 OL25 | 0 | 0 | 25 | 3 | |
| 4 | 9 OL25 | 0 | 0 | 25 | 9 | |
| 5 | 3 OL50 | 0 | 0 | 50 | 3 | |
| 6 | 9 OL50 | 0 | 0 | 50 | 9 | |
| 7 | OLN1 | 3 | 20 | 50 | / | HVED |
| 8 | OLN2 | 9 | 20 | 0 | / | |
| 9 | OLN3 | 3 | 20 | 0 | / | |
| 10 | OLN4 | 3 | 25 | 0 | / | |
| 11 | OLN5 | 9 | 25 | 25 | / | |
| 12 | OLN6 | 9 | 20 | 25 | / | |
| 13 | OLN7 | 9 | 20 | 50 | / | |
| 14 | OLN8 | 9 | 25 | 50 | / | |
| 15 | OLN9 | 3 | 25 | 25 | / | |
| 16 | OLN10 | 9 | 25 | 0 | / | |
| 17 | OLN11 | 3 | 25 | 50 | / | |
| 18 | OLN12 | 3 | 20 | 25 | / | |
| 19 | OLA1 | 3 | 15 | 50 | / | |
| 20 | OLA2 | 9 | 15 | 0 | / | |
| 21 | OLA3 | 3 | 15 | 0 | / | |
| 22 | OLA4 | 3 | 20 | 0 | / | |
| 23 | OLA5 | 9 | 20 | 25 | / | |
| 24 | OLA6 | 9 | 15 | 25 | / | |
| 25 | OLA7 | 9 | 15 | 50 | / | |
| 26 | OLA8 | 9 | 20 | 50 | / | |
| 27 | OLA9 | 3 | 20 | 25 | / | |
| 28 | OLA10 | 9 | 20 | 0 | / | |
| 29 | OLA11 | 3 | 20 | 50 | / | |
| 30 | OLA12 | 3 | 15 | 25 | / |
| ID | Sample | pH | Conductivity (μS/cm) | Starting Temperature (°C) | Final Temperature (°C) | Power (kW) | Extraction Type |
|---|---|---|---|---|---|---|---|
| 1 | 3 OL0 | 5.54 ± 0.21 | 516.0 ± 4 | 20.8 ± 0.9 | 20.8 ± 0.5 | / | CE |
| 2 | 9 OL0 | 5.45 ± 0.11 | 349.0 ± 3 | 19.7 ± 0.4 | 19.7 ± 0.4 | / | |
| 3 | 3 OL25 | 6.07 ± 0.09 | 139.2 ± 2.3 | 20.1 ± 0.2 | 20.1 ± 0.6 | / | |
| 4 | 9 OL25 | 6.21 ± 0.15 | 141.6 ± 3.2 | 20.0 ± 0.5 | 20.0 ± 0.6 | / | |
| 5 | 3 OL50 | 6.88 ± 0.12 | 36.6 ± 1.1 | 21.0 ± 0.4 | 21.0 ± 0.4 | / | |
| 6 | 9 OL50 | 6.51 ± 0.11 | 41.3 ± 1.2 | 21.0 ± 0.7 | 21.0 ± 0.2 | / | |
| 7 | OLN1 | 6.46 ± 0.22 | 51.4 ± 1.2 | 26.4 ± 0.8 | 26.7 ± 0.8 | 12 ± 1 | HVED |
| 8 | OLN2 | 5.80 ± 0.16 | 242.4 ± 3.3 | 24.2 ± 0.6 | 24.9 ± 0.3 | 12 ± 1 | |
| 9 | OLN3 | 5.87 ± 0.14 | 272.1 ± 2.3 | 25.2 ± 0.5 | 25.6 ± 0.6 | 12 ± 1 | |
| 10 | OLN4 | 5.65 ± 0.21 | 224.3 ± 3.2 | 24.8 ± 0.6 | 26.2 ± 0.6 | 19 ± 1 | |
| 11 | OLN5 | 5.93 ± 0.19 | 124.7 ± 1.2 | 23.4 ± 0.3 | 23.6 ± 0.5 | 17 ± 0 | |
| 12 | OLN6 | 5.85 ± 0.18 | 126.5 ± 2.5 | 24.3 ± 0.4 | 23.8 ± 0.4 | 12 ± 1 | |
| 13 | OLN7 | 6.11 ± 0.17 | 63.4 ± 1.3 | 23.5 ± 0.5 | 23.3 ± 0.6 | 10 ± 1 | |
| 14 | OLN8 | 6.09 ± 0.21 | 57.9 ± 1.1 | 22.7 ± 0.7 | 23.3 ± 0.3 | 20 ± 1 | |
| 15 | OLN9 | 6.00 ± 0.15 | 93.4 ± 1.2 | 25.5 ± 0.3 | 25.7 ± 0.4 | 19 ± 1 | |
| 16 | OLN10 | 5.78 ± 0.11 | 275.6 ± 3.1 | 23.3 ± 0.2 | 28.4 ± 0.6 | 22 ± 1 | |
| 17 | OLN11 | 6.10 ± 0.12 | 51.9 ± 1.1 | 28.2 ± 0.5 | 26.7 ± 0.7 | 19 ± 0 | |
| 18 | OLN12 | 5.99 ± 0.17 | 104.6 ± 1.2 | 25.9 ± 0.4 | 26.1 ± 0.6 | 14 ± 1 | |
| 19 | OLA1 | 6.45 ± 0.16 | 53.2 ± 1.1 | 23.7 ± 0.6 | 23.8 ± 0.6 | 7 ± 1 | |
| 20 | OLA2 | 5.30 ± 0.15 | 268.3 ± 3.4 | 23.6 ± 0.7 | 25.6 ± 0.6 | 9 ± 1 | |
| 21 | OLA3 | 5.21 ± 0.12 | 250.6 ± 4.5 | 23.6 ± 0.7 | 24.7 ± 0.5 | 10 ± 1 | |
| 22 | OLA4 | 5.28 ± 0.19 | 233.3 ± 3.2 | 23.7 ± 0.5 | 25.1 ± 0.4 | 14 ± 1 | |
| 23 | OLA5 | 5.77 ± 0.12 | 126.4 ± 2.3 | 24.3 ± 0.3 | 26.1 ± 0.3 | 16 ± 0 | |
| 24 | OLA6 | 5.88 ± 0.11 | 127.5 ± 2.3 | 24.0 ± 0.4 | 24.2 ± 0.6 | 7 ± 0 | |
| 25 | OLA7 | 6.32 ± 0.12 | 61.9 ± 1.6 | 23.9 ± 0.6 | 23.1 ± 0.6 | 9 ± 1 | |
| 26 | OLA8 | 6.25 ± 0.13 | 59.0 ± 1 | 23.2 ± 0.4 | 24.4 ± 0.5 | 13 ± 1 | |
| 27 | OLA9 | 6.00 ± 0.15 | 94.6 ± 2.0 | 23.8 ± 0.6 | 24.5 ± 0.6 | 14 ± 1 | |
| 28 | OLA10 | 5.18 ± 0.16 | 285.7 ± 3.8 | 24.5 ± 0.7 | 29.9 ± 0.4 | 16 ± 1 | |
| 29 | OLA11 | 6.36 ± 0.17 | 49.6 ± 1.3 | 24.5 ± 0.6 | 24.6 ± 0.7 | 12 ± 0 | |
| 30 | OLA12 | 5.88 ± 0.19 | 105.9 ± 2.8 | 24.2 ± 0.5 | 24.0 ± 0.6 | 7 ± 1 |
| ID | Sample | TPC (mg GAE/g) | DPPH (µmol TAE/g) | FRAP (µmol FE/g) | Yield (%) | Extraction Type |
|---|---|---|---|---|---|---|
| 1 | 3 OL0 | 5.32 ± 0.02 | 27.06 ± 0.01 | 63.36 ± 2.24 | 0.53 ± 0.00 | CE |
| 2 | 9 OL0 | 15.85 ± 0.11 | 31.10 ± 0.08 | 146.93 ± 3.54 | 1.58 ± 0.01 | |
| 3 | 3 OL25 | 13.35 ± 0.08 | 24.78 ± 0.05 | 150.50 ± 4.57 | 1.33 ± 0.01 | |
| 4 | 9 OL25 | 16.04 ± 0.10 | 30.46 ± 0.07 | 179.07 ± 6.09 | 1.60 ± 0.01 | |
| 5 | 3 OL50 | 14.06 ± 0.09 | 32.71 ± 0.06 | 159.79 ± 5.42 | 1.41 ± 0.01 | |
| 6 | 9 OL50 | 20.61 ± 0.14 | 33.31 ± 0.08 | 221.93 ± 6.99 | 2.06 ± 0.01 | |
| 7 | OLN1 | 49.21 ± 0.10 | 31.49 ± 0.07 | 266.21 ± 7.53 | 4.92 ± 0.01 | HVED |
| 8 | OLN2 | 29.82 ± 0.07 | 28.60 ± 0.05 | 208.36 ± 4.28 | 2.98 ± 0.01 | |
| 9 | OLN3 | 18.08 ± 0.08 | 25.96 ± 0.06 | 197.64 ± 2.00 | 1.81 ± 0.01 | |
| 10 | OLN4 | 33.12 ± 0.12 | 27.46 ± 0.08 | 199.79 ± 5.22 | 3.31 ± 0.01 | |
| 11 | OLN5 | 24.17 ± 0.05 | 31.31 ± 0.04 | 374.79 ± 6.74 | 2.42 ± 0.01 | |
| 12 | OLN6 | 26.95 ± 0.08 | 30.14 ± 0.09 | 308.36 ± 4.45 | 2.69 ± 0.01 | |
| 13 | OLN7 | 45.47 ± 0.06 | 30.49 ± 0.10 | 209.79 ± 2.01 | 4.55 ± 0.01 | |
| 14 | OLN8 | 47.21 ± 0.09 | 31.81 ± 0.08 | 229.79 ± 5.82 | 4.72 ± 0.01 | |
| 15 | OLN9 | 29.99 ± 0.07 | 28.35 ± 0.01 | 301.21 ± 4.68 | 3.00 ± 0.01 | |
| 16 | OLN10 | 28.95 ± 0.11 | 29.99 ± 0.08 | 256.21 ± 5.74 | 2.89 ± 0.01 | |
| 17 | OLN11 | 45.82 ± 0.16 | 27.96 ± 0.07 | 561.93 ± 9.11 | 4.58 ± 0.02 | |
| 18 | OLN12 | 35.03 ± 0.10 | 31.53 ± 0.02 | 284.07 ± 3.21 | 3.50 ± 0.01 | |
| 19 | OLA1 | 39.56 ± 0.08 | 30.78 ± 0.15 | 234.07 ± 2.66 | 3.96 ± 0.01 | |
| 20 | OLA2 | 32.69 ± 0.05 | 26.53 ± 0.08 | 145.50 ± 2.87 | 3.27 ± 0.00 | |
| 21 | OLA3 | 9.82 ± 0.04 | 29.71 ± 0.11 | 97.64 ± 1.15 | 0.98 ± 0.00 | |
| 22 | OLA4 | 26.69 ± 0.02 | 25.53 ± 0.08 | 169.07 ± 5.92 | 2.67 ± 0.00 | |
| 23 | OLA5 | 36.17 ± 0.04 | 29.60 ± 0.17 | 315.50 ± 4.00 | 3.62 ± 0.00 | |
| 24 | OLA6 | 31.21 ± 0.09 | 30.64 ± 0.02 | 321.21 ± 6.38 | 3.12 ± 0.01 | |
| 25 | OLA7 | 53.64 ± 0.14 | 29.85 ± 0.12 | 443.36 ± 5.21 | 5.36 ± 0.01 | |
| 26 | OLA8 | 65.99 ± 0.06 | 31.53 ± 0.01 | 237.64 ± 3.73 | 6.60 ± 0.01 | |
| 27 | OLA9 | 30.77 ± 0.05 | 26.81 ± 0.08 | 326.21 ± 2.16 | 3.08 ± 0.01 | |
| 28 | OLA10 | 21.21 ± 0.04 | 29.67 ± 0.05 | 196.21 ± 2.97 | 2.12 ± 0.00 | |
| 29 | OLA11 | 42.60 ± 0.11 | 32.53 ± 0.10 | 343.36 ± 3.01 | 4.26 ± 0.01 | |
| 30 | OLA12 | 26.43 ± 0.01 | 30.03 ± 0.08 | 354.07 ± 5.36 | 2.64 ± 0.00 |
| ID | Sample | Apigenin | Diosmetin | Hydroxytyrosol | Luteolin | Oleanolic Acid | Oleuropein | Quercentin | Extraction Type |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 OL0 | 156 | 124 | 9.17 | 2356.6 | 4.45 | 50.4 | N/A | CE |
| 2 | 9 OL0 | 172 | 121 | 11.6 | 71.5 | 2.12 | 56.2 | 0.054 | |
| 3 | 3 OL25 | 125 | 112 | 494 | 550 | 0.66 | 3959 | 0.140 | |
| 4 | 9 OL25 | 144 | 132 | 125 | 580 | 0.79 | 825 | 0.124 | |
| 5 | 3 OL50 | 31.9 | 14.5 | 4120 | 154 | 3918 | 23485 | 247 | |
| 6 | 9 OL50 | 193 | 154 | 13.0 | 80.8 | 8.37 | 108 | 3.01 | |
| 7 | OLN1 | 58.6 | 47.0 | 3016 | 353 | 3386 | 17646 | 9.07 | HVED |
| 8 | OLN2 | 157 | 118 | 86.6 | 138 | 8.84 | 99.3 | N/A | |
| 9 | OLN3 | 109 | 101 | 97.5 | 96.3 | 3.65 | 76.1 | 0.048 | |
| 10 | OLN4 | 127 | 98.1 | 96.5 | 122 | N/A | 74.2 | 0.042 | |
| 11 | OLN5 | 116 | 119 | 80.4 | 361 | 1.83 | 163 | 0.109 | |
| 12 | OLN6 | 134 | 127 | 67.5 | 424 | 2.92 | 165 | 0.115 | |
| 13 | OLN7 | 38.9 | 30.0 | 3090 | 255 | 2803 | 17311 | 7.17 | |
| 14 | OLN8 | 44.3 | 33.0 | 3294 | 265 | 2705 | 18413 | 9.0 | |
| 15 | OLN9 | 149 | 159 | 108 | 375 | 8.57 | 168 | 0.179 | |
| 16 | OLN10 | 111 | 94.2 | 192 | 252 | 3.76 | 178 | 0.103 | |
| 17 | OLN11 | 106 | 106 | 97.5 | 403 | 2.00 | 424 | 0.121 | |
| 18 | OLN12 | 56.8 | 49.2 | 3426 | 386 | 2851 | 17388 | 8.54 | |
| 19 | OLA1 | 53.6 | 59.9 | 2964 | 357 | 2004 | 16103 | 5.27 | |
| 20 | OLA2 | 198 | 76.6 | 10.1 | 29.8 | 5.74 | 57.4 | 0.043 | |
| 21 | OLA3 | 124 | 130 | 112 | 215 | 2.41 | 97.9 | 0.032 | |
| 22 | OLA4 | 113 | 100 | 64.0 | 113 | N/A | 52.5 | 0.021 | |
| 23 | OLA5 | 110 | 123 | 128 | 487 | N/A | 247 | 0.146 | |
| 24 | OLA6 | 115 | 122 | 145 | 457 | N/A | 315 | 0.218 | |
| 25 | OLA7 | 60.1 | 60.6 | 3660 | 431 | 1844 | 17127 | 6.43 | |
| 26 | OLA8 | 48.2 | 41.5 | 3682 | 334 | 2043 | 17895 | 15.6 | |
| 27 | OLA9 | 111 | 116 | 149 | 480 | 6.22 | 465 | 0.189 | |
| 28 | OLA10 | 94.2 | 85.3 | 139 | 166 | N/A | 102 | 0.061 | |
| 29 | OLA11 | 51.4 | 51.0 | 2967 | 372 | 2193 | 16695 | 5.79 | |
| 30 | OLA12 | 142 | 141 | 77.2 | 459 | 7.51 | 166 | 0.191 |
| ID | Sample | L* | a* | b* | C | H | ∆C | ∆H | ∆E | Extraction Type |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 OL0 | 81.86 | 4.96 | 42.70 | 42.99 | 1.46 | / | / | / | CE |
| 2 | 9 OL0 | 82.06 | 6.59 | 46.76 | 47.22 | 1.43 | / | / | / | |
| 3 | 3 OL25 | 89.56 | 0.06 | 30.53 | 30.53 | 1.57 | / | / | / | |
| 4 | 9 OL25 | 91.02 | −0.37 | 31.26 | 31.26 | −1.56 | / | / | / | |
| 5 | 3 OL50 | 94.12 | −3.82 | 27.89 | 28.15 | −1.43 | / | / | / | |
| 6 | 9 OL50 | 91.03 | −3.82 | 38.68 | 38.87 | −1.47 | / | / | / | |
| 7 | OLN1 | 80.34 | −2.69 | 45.25 | 45.33 | −1.51 | 17.18 | 2.74 | 22.19 | HVED |
| 8 | OLN2 | 79.75 | 7.78 | 44.90 | 45.57 | 1.40 | −1.65 | 1.46 | 3.20 | |
| 9 | OLN3 | 81.28 | 6.06 | 42.60 | 43.03 | 1.43 | 0.04 | 1.10 | 1.25 | |
| 10 | OLN4 | 80.38 | 4.01 | 40.60 | 40.80 | 1.47 | −2.19 | 0.72 | 2.74 | |
| 11 | OLN5 | 82.35 | 1.17 | 29.06 | 29.08 | 1.53 | −2.18 | 1.57 | 9.08 | |
| 12 | OLN6 | 81.25 | −2.59 | 45.68 | 45.75 | −1.51 | 14.49 | 1.69 | 17.56 | |
| 13 | OLN7 | 84.44 | 2.87 | 36.30 | 36.41 | 1.49 | −2.45 | 6.66 | 9.69 | |
| 14 | OLN8 | 86.03 | −3.34 | 42.78 | 42.91 | −1.49 | 4.04 | 0.84 | 6.48 | |
| 15 | OLN9 | 83.26 | 0.59 | 26.29 | 26.30 | 1.55 | −4.23 | 0.58 | 7.61 | |
| 16 | OLN10 | 80.90 | 6.73 | 47.19 | 47.67 | 1.43 | 0.45 | 0.08 | 1.25 | |
| 17 | OLN11 | 88.01 | −3.70 | 40.73 | 40.90 | −1.48 | 12.75 | 1.54 | 14.22 | |
| 18 | OLN12 | 85.42 | 0.55 | 27.78 | 27.79 | 1.55 | −2.74 | 0.52 | 4.99 | |
| 19 | OLA1 | 84.82 | −2.01 | 41.69 | 41.74 | −1.52 | 13.59 | 3.01 | 16.74 | |
| 20 | OLA2 | 78.28 | 10.44 | 52.28 | 53.31 | 1.37 | 6.09 | 2.86 | 7.72 | |
| 21 | OLA3 | 86.90 | 3.20 | 41.42 | 41.54 | 1.49 | −1.44 | 1.63 | 5.49 | |
| 22 | OLA4 | 80.40 | 8.10 | 48.30 | 48.97 | 1.40 | 5.99 | 2.32 | 6.58 | |
| 23 | OLA5 | 80.45 | 4.78 | 47.39 | 47.63 | 1.47 | 16.37 | 4.33 | 19.96 | |
| 24 | OLA6 | 83.55 | 2.67 | 41.24 | 41.33 | 1.51 | 10.06 | 2.75 | 12.83 | |
| 25 | OLA7 | 83.12 | −1.37 | 43.09 | 43.11 | −1.54 | 4.24 | 2.73 | 9.38 | |
| 26 | OLA8 | 85.95 | −2.71 | 38.36 | 38.46 | −1.50 | −0.41 | 1.08 | 5.21 | |
| 27 | OLA9 | 82.62 | 2.21 | 37.57 | 37.63 | 1.51 | 7.10 | 1.92 | 10.12 | |
| 28 | OLA10 | 78.98 | 9.06 | 50.87 | 51.67 | 1.39 | 4.45 | 1.79 | 5.70 | |
| 29 | OLA11 | 84.57 | −2.25 | 36.37 | 36.44 | −1.51 | 8.29 | 2.38 | 12.87 | |
| 30 | OLA12 | 82.27 | 2.50 | 36.80 | 36.88 | 1.50 | 6.35 | 2.21 | 9.92 |
| ID | Sample | Z Average Mean (d·nm) | PI | Zeta potential (mV) | Extraction Type |
|---|---|---|---|---|---|
| 1 | 3 OL0 | 541.55 ± 6.38 | 0.62 ± 0.01 | −9.31 ± 0.47 | CE |
| 2 | 9 OL0 | 370.60 ± 4.57 | 0.69 ± 0.06 | −7.96 ± 0.25 | |
| 3 | 3 OL25 | 270.45 ± 3.65 | 0.63 ± 0.11 | −11.30 ± 2.44 | |
| 4 | 9 OL25 | 303.25 ± 4.56 | 0.68 ± 0.08 | −8.98 ± 1.12 | |
| 5 | 3 OL50 | 333.10 ± 4.58 | 0.54 ± 0.00 | −10.43 ± 0.81 | |
| 6 | 9 OL50 | 166.50 ± 2.92 | 0.56 ± 0.06 | −18.07 ± 0.12 | |
| 7 | OLN1 | 394.60 ± 3.81 | 0.41 ± 0.03 | −27.13 ± 1.46 | HVED |
| 8 | OLN2 | 672.00 ± 7.83 | 0.48 ± 0.06 | −15.77 ± 0.30 | |
| 9 | OLN3 | 751.35 ± 9.64 | 0.73 ± 0.04 | −17.79 ± 0.66 | |
| 10 | OLN4 | 1545.50 ± 15.01 | 0.65 ± 0.04 | −18.87 ± 1.45 | |
| 11 | OLN5 | 139.30 ± 2.45 | 0.32 ± 0.04 | −53.77 ± 0.27 | |
| 12 | OLN6 | 203.70 ± 3.65 | 0.53 ± 0.05 | −45.99 ± 5.52 | |
| 13 | OLN7 | 653.15 ± 6.83 | 0.95 ± 0.14 | −22.21 ± 0.37 | |
| 14 | OLN8 | 378.15 ± 3.63 | 0.59 ± 0.07 | −33.25 ± 1.46 | |
| 15 | OLN9 | 279.15 ± 3.72 | 0.28 ± 0.01 | −28.09 ± 0.88 | |
| 16 | OLN10 | 952.40 ± 9.43 | 0.71 ± 0.10 | −16.43 ± 0.10 | |
| 17 | OLN11 | 193.50 ± 1.23 | 0.36 ± 0.08 | −31.94 ± 2.48 | |
| 18 | OLN12 | 304.05 ± 4.72 | 0.32 ± 0.04 | −36.44 ± 2.44 | |
| 19 | OLA1 | 452.90 ± 6.74 | 0.60 ± 0.06 | −23.81 ± 0.75 | |
| 20 | OLA2 | 1551.50 ± 16.63 | 0.85 ± 0.13 | −19.76 ± 0.37 | |
| 21 | OLA3 | 318.20 ± 3.74 | 0.42 ± 0.05 | −16.96 ± 0.40 | |
| 22 | OLA4 | 846.95 ± 8.72 | 0.71 ± 0.15 | −17.53 ± 0.23 | |
| 23 | OLA5 | 177.25 ± 1.53 | 0.43 ± 0.04 | −54.14 ± 2.72 | |
| 24 | OLA6 | 183.25 ± 1.74 | 0.26 ± 0.00 | −36.95 ± 0.26 | |
| 25 | OLA7 | 447.40 ± 2.82 | 0.50 ± 0.11 | −29.91 ± 0.03 | |
| 26 | OLA8 | 369.40 ± 2.76 | 0.63 ± 0.06 | −35.07 ± 0.73 | |
| 27 | OLA9 | 198.45 ± 1.34 | 0.31 ± 0.03 | −41.08 ± 4.01 | |
| 28 | OLA10 | 506.95 ± 5.45 | 0.69 ± 0.06 | −19.41 ± 0.10 | |
| 29 | OLA11 | 522.55 ± 6.28 | 0.48 ± 0.10 | −23.29 ± 1.53 | |
| 30 | OLA12 | 164.70 ± 1.28 | 0.46 ± 0.00 | −35.08 ± 5.12 |
| Pesticides | MRL mg/kg | Content mg/kg |
|---|---|---|
| Alachlor | 0.02 | <0.005 |
| Aldrin and Dieldrin (Aldrin and dieldrin combined expressed as dieldrin) | 0.01 | <0.002 |
| Captan (Sum of captan and THPI, expressed as captan) | 0.06 | <0.020 |
| DDT (sum of p,p´-DDT, o,p´-DDT, p-p´-DDE and p,p´-TDE (DDD) expressed as DDT) | 0.05 | <0.004 |
| Endosulphan (sum of alpha- and beta-isomers and endosulphan-sulphate expresses as endosulphan) | 0.05 | <0.002 |
| Endrin | 0.01 | <0.004 |
| Heptachlor (sum of heptachlor and heptachlor epoxide expressed as heptachlor) | 0.01 | <0.002 |
| Hexachlorobenzene | 0.01 | <0.002 |
| Hexachlorocyclohexane (HCH), alpha-isomer | 0.01 | <0.002 |
| Hexachlorocyclohexane (HCH), beta-isomer | 0.01 | <0.002 |
| Iprodione | 20 | <0.010 |
| Lindane (Gamma-isomer of hexachlorocyclohexane (HCH)) | 0.01 | <0.002 |
| Methoxychlor | 0.01 | <0.010 |
| Tolylphluanid (Sum of tolylphluanid and dimethylaminosulphotoluidide expressed as tolylphluanid) | 0.05 | <0.020 |
| Vinclozolin | 0.02 | <0.002 |
| Metals | MRL mg/kg | Content mg/kg |
|---|---|---|
| Lead (Pb) | 3.00 | <0.050 |
| Cadmium (Cd) | 1.00 | 0.011 |
| Mercury (Hg) | 0.50 | 0.023 |
| Arsenic (As) | - | <0.005 |
| Chromium (Cr) | - | 0.240 |
| Nickel (Ni) | - | 1.82 |
| Manganese (Mn) | - | 52.0 |
| Iron (Fe) | - | 101 |
| Copper (Cu) | - | 9.70 |
| Zinc (Zn) | - | 15.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Žuntar, I.; Putnik, P.; Bursać Kovačević, D.; Nutrizio, M.; Šupljika, F.; Poljanec, A.; Dubrović, I.; Barba, F.J.; Režek Jambrak, A. Phenolic and Antioxidant Analysis of Olive Leaves Extracts (Olea europaea L.) Obtained by High Voltage Electrical Discharges (HVED). Foods 2019, 8, 248. https://doi.org/10.3390/foods8070248
Žuntar I, Putnik P, Bursać Kovačević D, Nutrizio M, Šupljika F, Poljanec A, Dubrović I, Barba FJ, Režek Jambrak A. Phenolic and Antioxidant Analysis of Olive Leaves Extracts (Olea europaea L.) Obtained by High Voltage Electrical Discharges (HVED). Foods. 2019; 8(7):248. https://doi.org/10.3390/foods8070248
Chicago/Turabian StyleŽuntar, Irena, Predrag Putnik, Danijela Bursać Kovačević, Marinela Nutrizio, Filip Šupljika, Andreja Poljanec, Igor Dubrović, Francisco J. Barba, and Anet Režek Jambrak. 2019. "Phenolic and Antioxidant Analysis of Olive Leaves Extracts (Olea europaea L.) Obtained by High Voltage Electrical Discharges (HVED)" Foods 8, no. 7: 248. https://doi.org/10.3390/foods8070248
APA StyleŽuntar, I., Putnik, P., Bursać Kovačević, D., Nutrizio, M., Šupljika, F., Poljanec, A., Dubrović, I., Barba, F. J., & Režek Jambrak, A. (2019). Phenolic and Antioxidant Analysis of Olive Leaves Extracts (Olea europaea L.) Obtained by High Voltage Electrical Discharges (HVED). Foods, 8(7), 248. https://doi.org/10.3390/foods8070248









