Characteristics of Instrumental Methods to Describe and Assess the Recrystallization Process in Ice Cream Systems
Abstract
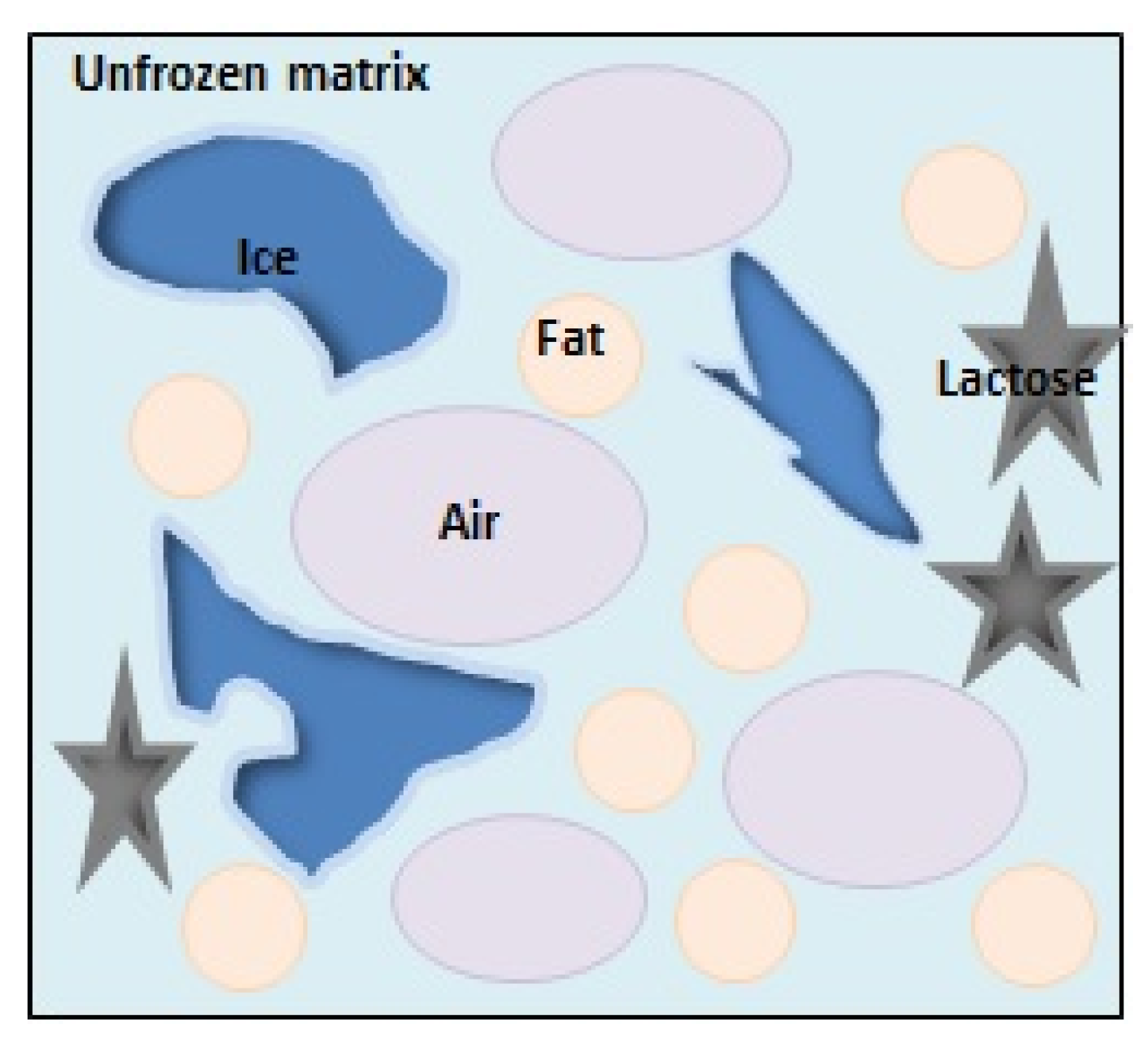
1. Introduction
2. Methods of Testing and Describing the Recrystallization Process
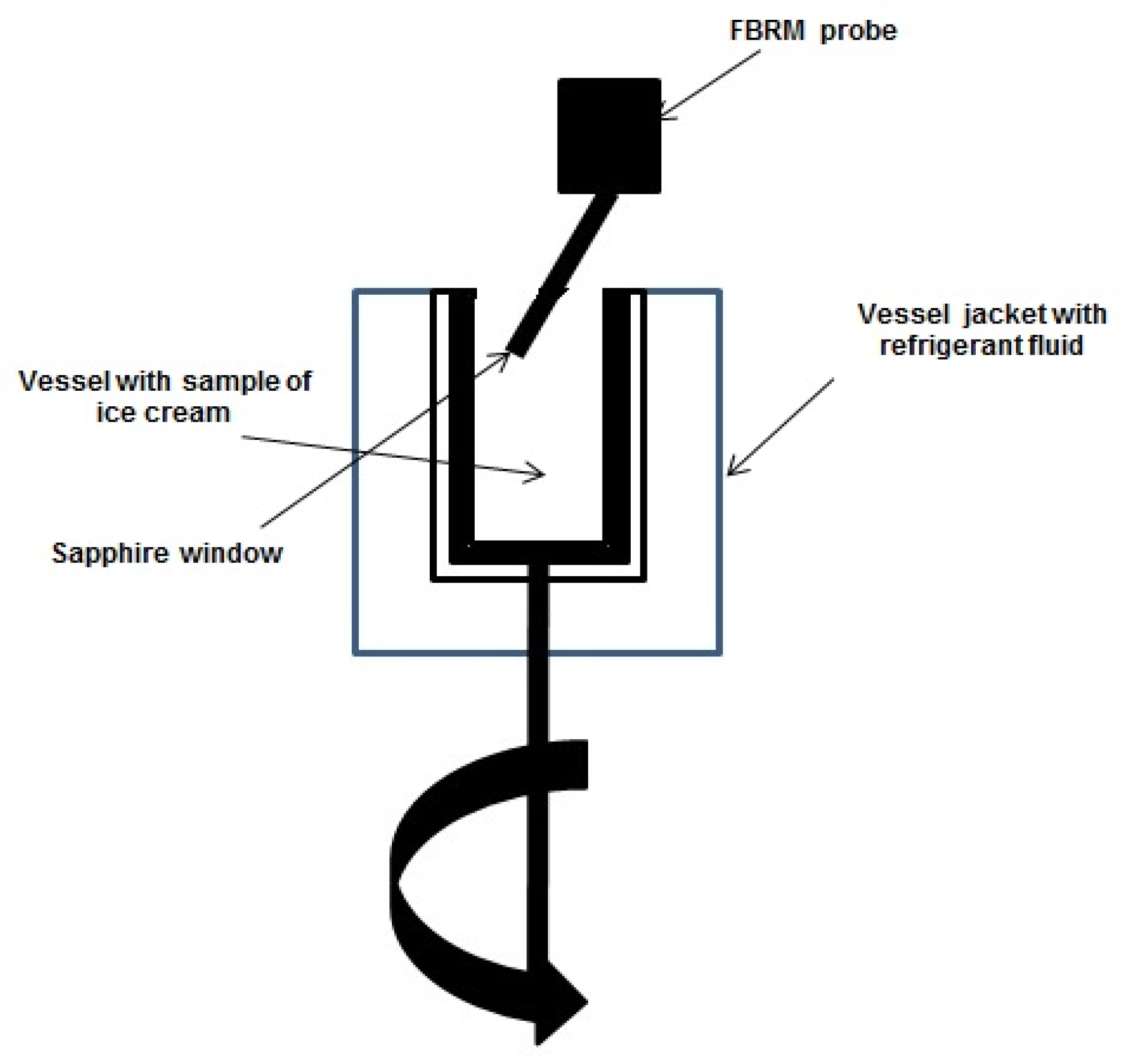
2.1. Focused-Beam Reflectance Measurement (FBRM) Technique
2.2. Oscillatory Thermo-Rheometry Technique (OTR)
2.3. Nuclear Magnetic Resonance (NMR) Technique
2.4. Splat-Cooling Assay
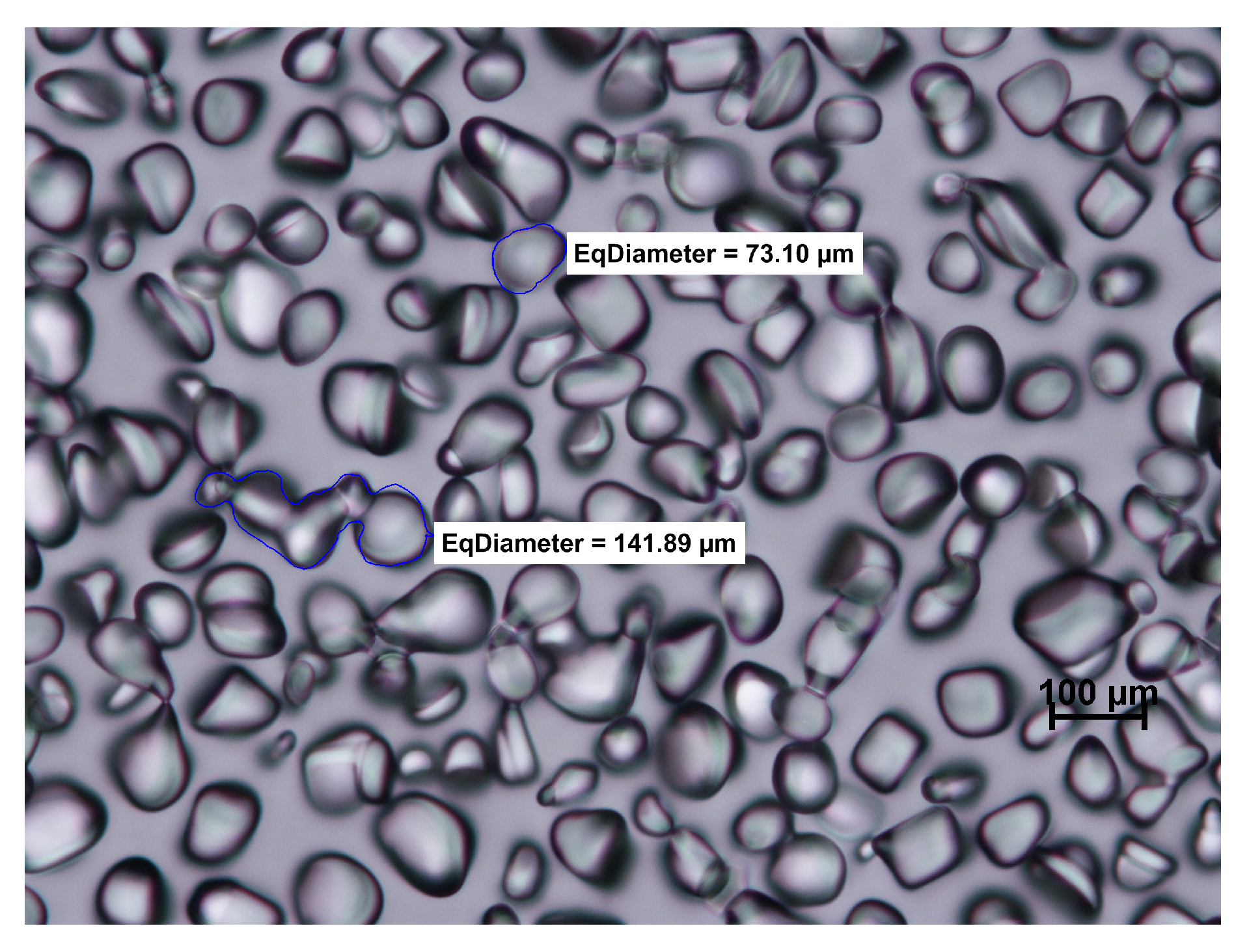
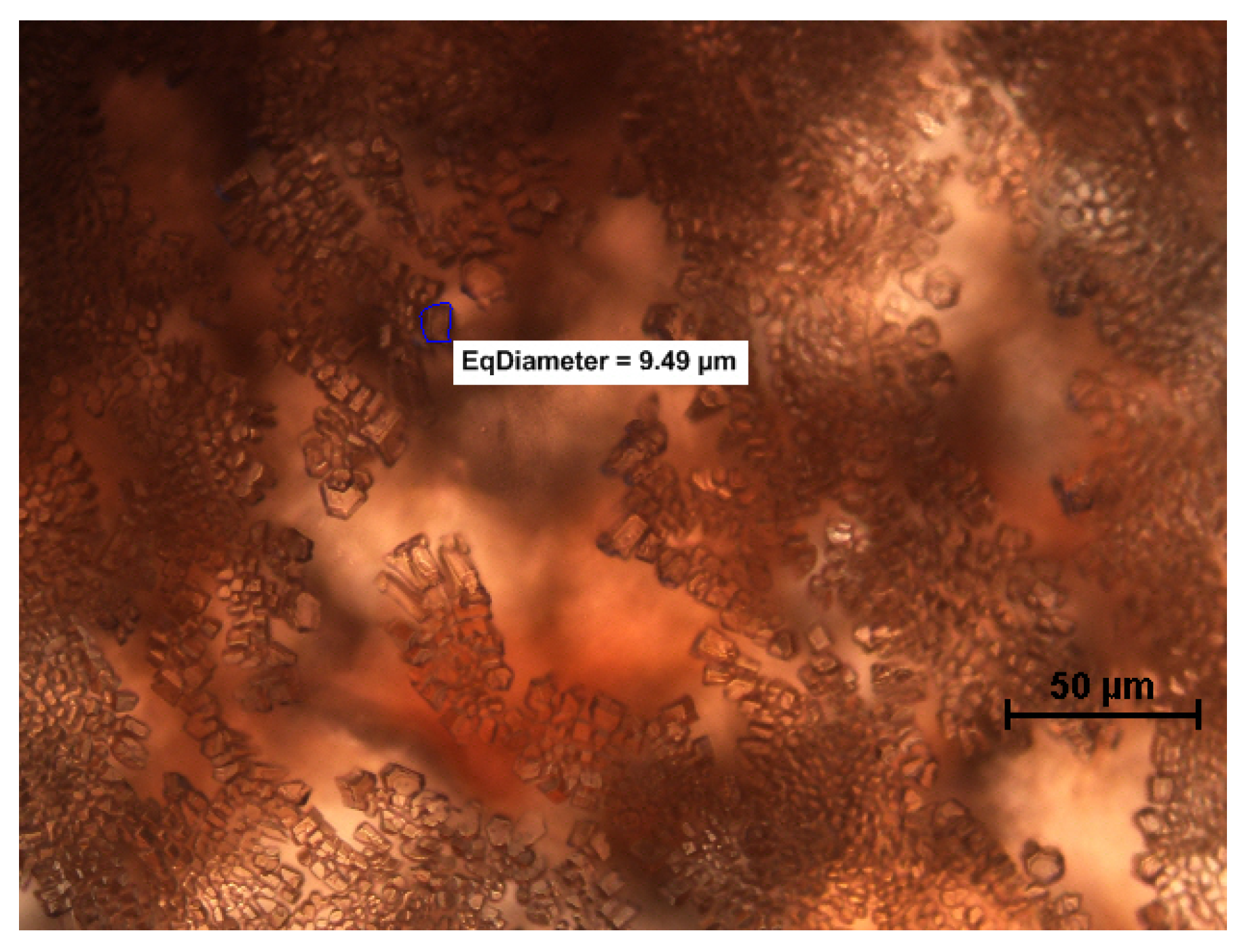
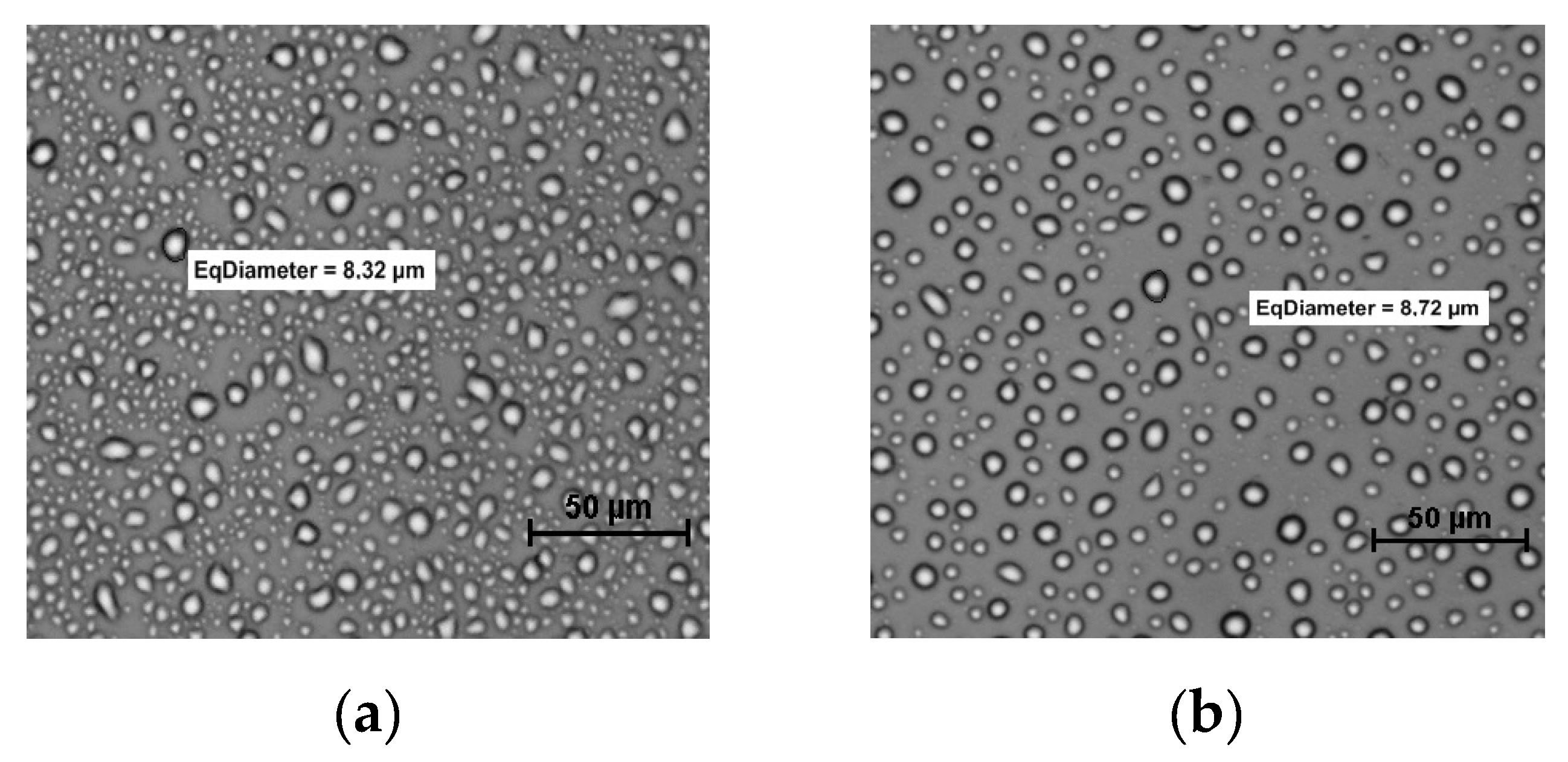
2.5. Microscopy and Image Analysis
2.6. X-ray Microtomography (Micro-CT)
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kamińska-Dwórznicka, A.; Matusiak, M.; Samborska, K.; Witrowa-Rajchert, D.; Gondek, E.; Jakubczyk, E.; Antczak, A. The influence of kappa carrageenan and its hydrolysates on the recrystallization process in sorbet. J. Food Eng. 2015, 167, 162–165. [Google Scholar] [CrossRef]
- Delgado, A.E.; Sun, D.-W. Ultrasound-accelerated freezing. In Handbook of Frozen Food Processing and Packaging; Sun, D.-W., Ed.; CRC Prezz, Taylor & Francis Group: Boca Raton, FL, USA, 2012; pp. 645–666. [Google Scholar]
- Kaale, L.D.; Eikevik, T.M. The development of ice crystals in food products during the superchilling process and following storage, a review. Trends Food Sci. Technol. 2014, 39, 91–103. [Google Scholar] [CrossRef]
- Kamińska-Dwórznicka, A.; Antczak, A.; Samborska, K.; Lenart, A. Acid hydrolysis of kappa-carrageenan as a way of gaining new substances for freezing process modification and protection from excessive recrystallization of ice. Int. J. Food Sci. Technol. 2015, 50, 1799–1806. [Google Scholar] [CrossRef]
- Kamińska-Dwórznicka, A.; Skrzypczak, P.; Gondek, E. Modification of kappa carrageenan by β-galactosidase as a new method to inhibit recrystallization of ice. Food Hydrocoll. 2016, 61, 31–35. [Google Scholar] [CrossRef]
- Amamou, A.H.; Benkhelifa, H.; Alvarez, G.; Flick, D. Study of crystal size evolution by focused-beam reflectance measurement during the freezing of sucrose/water solutions in a scraped-surface heat exchanger. Proc. Biochem. 2010, 45, 1821–1825. [Google Scholar] [CrossRef][Green Version]
- Arellano, M.; Flick, D.; Benkhelifa, H.; Alvarez, G. Rheological characterisation of sorbet using pipe rheometry during the freezing process. J. Food Eng. 2013, 119, 385–394. [Google Scholar] [CrossRef]
- Gaukel, V.; Leiter, A.; Spiess, W.E.L. Synergism of different fish antifreeze proteins and hydrocolloids on recrystallization inhibition of ice in sucrose solutions. J. Food Eng. 2014, 141, 44–50. [Google Scholar] [CrossRef]
- Kamińska, A.; Gaukel, V. Monitoring the growth of crystals in ice cream. Food. Sci. Technol. Qual. 2009, 62, 57–64. (In Polish) [Google Scholar]
- Smith, A.K.; Goff, H.D.; Kakuda, Y. Microstructure and rheological properties of whipped cream as affected by heat treatment and addition of stabilizer. Int. Dairy J. 2000, 10, 295–301. [Google Scholar] [CrossRef]
- Adapa, S.; Schmidt, K.A.; Jeon, I.J.; Herald, T.J.; Flores, R.A. Mechanisms of ice crystallization and recrystallization in ice cream: A review. Food Rev. Int. 2000, 16, 259–271. [Google Scholar] [CrossRef]
- Regand, A.; Goff, H.D. Structure and ice recrystallization in frozen stabilized ice cream model systems. Food Hydrocoll. 2003, 17, 95–102. [Google Scholar] [CrossRef]
- Pinzer, B.R.; Medebach, A.; Limbach, H.J.; Dubois, C.; Stampanoni, M.; Schneebeli, M. 3D—Characterization of three-phase systems using X-ray tomography: Tracking the microstructural evolution in ice cream. Soft Matter 2012, 8, 4584–4594. [Google Scholar] [CrossRef]
- Ndoye, F.T.; Alvarez, G. Characterization of ice recrystallization in ice cream during storage using the focused beam reflectance measurement. J. Food Eng. 2015, 148, 24–34. [Google Scholar] [CrossRef]
- Sutton, R.L.; Wilcox, J.E.A. Recrystallization in ice cream as affected by stabilizers. J. Food Sci. 1998, 63, 104–107. [Google Scholar] [CrossRef]
- Donhowe, D.P.; Hartel, R.W. Recrystallization of ice in ice cream during controlled accelerated storage. Int. Dairy J. 1996, 6, 1191–1208. [Google Scholar] [CrossRef]
- Donhowe, D.P.; Hartel, R.W. Recrystallization of ice during bulk storage of ice cream. Int. Dairy J. 1996, 6, 1209–1221. [Google Scholar] [CrossRef]
- Hartel, R.W. Ice crystallization during the manufacture of ice cream. Trends Food Sci. Technol. 1996, 7, 315–321. [Google Scholar] [CrossRef]
- Cook, K.L.K.; Hartel, R.W. Mechanisms of ice crystallization in ice cream production. Compr. Rev. Food Sci. Food Saf. 2010, 9, 213–222. [Google Scholar] [CrossRef]
- Goff, H.D.; Caldwell, K.B.; Stanley, D.W.; Maurice, T.J. Influence of polysaccharides on the glass transition in frozen sucrose solutions and ice cream. J. Dairy Sci. 1993, 76, 1268–1277. [Google Scholar] [CrossRef]
- Damodaran, S. Inhibition of ice crystal growth in ice cream mix by gelatin hydrolysate. J. Agri. Food Chem. 2007, 55, 10918–10923. [Google Scholar] [CrossRef]
- Regand, A.; Goff, H.D. Effect of biopolymers on structure and ice recrystallization in dynamically frozen ice cream model system. J. Dairy Sci. 2002, 85, 2722–2732. [Google Scholar] [CrossRef]
- Soukoulis, C.; Tzia, C. Impact of the acidification process, hydrocolloids and protein fortifiers on the physical and sensory properties of frozen yogurt. Int. J. Dairy Technol. 2008, 61, 170–177. [Google Scholar] [CrossRef]
- Rochas, C.; Heyraud, A. Acid and enzymic hydrolysis of kappa carrageenan. Polym. Bull. 1981, 5, 81–86. [Google Scholar] [CrossRef]
- Rudolph, B. Seaweed products: Red algae of economic significance. In Marine and Freshwater Products Handbook; Martin, R.E., Carter, E.P., Flick, G.J., Davis, L.M., Eds.; Technomic Publishing Company, Inc.: Lancaster, PA, USA, 2000; pp. 515–529. [Google Scholar]
- Marshall, R.T.; Arbuckle, W.S. Ice Cream, 5th ed.; Chapman and Hall: New York, NY, USA, 1996. [Google Scholar]
- Arellano, M.; Benkhelifa, H.; Flick, D.; Alvarez, G. Online ice crystal size measurements during sorbet freezing by means of the focused beam reflectance measurement (FBRM) technology. Influence of operating conditions. J. Food Eng. 2012, 113, 351–359. [Google Scholar] [CrossRef]
- Greaves, D.; Boxall, J.; Mulligan, J.; Montesi, A.; Creek, J.; Solan, E.D.; Koh, C.A. Measuring the particle size of a known distribution using the focused beam reflectance measurement technique. Chem. Eng. Sci. 2008, 63, 5410–5419. [Google Scholar] [CrossRef]
- Hukkanen, E.J.; Braatz, R.D. Measurement of particle size distribution in suspension polymerization using in situ laser backscattering. Sens. Actuators B Chem. 2003, 96, 451–459. [Google Scholar] [CrossRef]
- Wynn, E.J.W. Relationship between particle-size and chord-length distributions in focused beam reflectance measurement: Stability of direct inversion and weighting. Powder Technol. 2003, 133, 125–133. [Google Scholar] [CrossRef]
- Stanley, D.W.; Goff, H.D.; Smith, A.K. Texture-structure relationship in foamed dairy emulsions. Food Res. Int. 1996, 29, 1–13. [Google Scholar] [CrossRef]
- Wildmoser, H.; Scheiwiller, J.; Windhab, E.J. Impact of disperse microstructure on rheology and quality aspects of ice cream. LWT Food Sci. Technol. 2004, 37, 881–891. [Google Scholar] [CrossRef]
- Eisner, M.D.; Wildmoser, H.; Windhab, E.J. Air cell microstructuring in a high viscous ice cream matrix. Colloids Surf. Physicochem. Eng. Asp. 2005, 263, 390–399. [Google Scholar] [CrossRef]
- Tsevdou, M.; Gogou, E.; Dermesonluoglu, E.; Taoukis, P. Modelling the effect of storage temperature on the viscoelastic properties and quality of ice cream. J. Food Eng. 2015, 148, 35–42. [Google Scholar] [CrossRef]
- Bahram-Parvar, M. A review of modern instrumental techniques for measurements of ice cream characteristics. Food Chem. 2015, 188, 625–631. [Google Scholar] [CrossRef]
- Barbosa, L.L.; Sad, C.M.S.; Morgan, V.G.; Figueiras, P.R.; Castro, E.R.V. Application of low field NMR as an alternative technique to quantification of total acid number and sulphur content in petroleum from Brazilian reservoirs. Fuel 2016, 176, 146–152. [Google Scholar] [CrossRef]
- Schuff, N. In Vivo NMR methods, overview of techniques. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering Encyclopedia of Spectroscopy and Spectrometry, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 211–215. [Google Scholar]
- Lucas, T.; Mariette, F.; Dominiawsyk, S.; Le Ray, D. Water, ice and sucrose behavior in frozen sucrose-protein solutions as studied by 1H NMR. Food Chem. 2004, 84, 77–89. [Google Scholar] [CrossRef]
- Hagiwara, T.; Hartel, R.W.; Matsukawa, S. Relationship between recrystallization rate of ice crystals in sugar solutions and water mobility in freeze-concentrated matrix. Food Biophys. 2006, 1, 74–82. [Google Scholar] [CrossRef]
- Brown, J.R.; Seymour, J.D.; Brox, T.I.; Skidmore, M.L.; Wang, C.; Christner, B.C.; Luo, B.; Codd, S.L. Recrystallization inhibition in ice due to ice binding protein activity detected by nuclear magnetic resonance. Biotechnol. Rep. 2014, 3, 60–64. [Google Scholar] [CrossRef]
- Gabriele, D.; Migliori, M.; Sanzo, R.D.; Rossi, C.O.; Ruffolo, S.A.; de Cindio, B. Characterisation of dairy emulsions by NMR and rheological techniques. Food Hydrocoll. 2009, 23, 619–628. [Google Scholar] [CrossRef]
- Lucas, T.; Ray, D.L.; Barey, P.; Mariette, F. NMR assessment of ice cream: Effect of formulation on liquid and solid fat. Int. Dairy J. 2005, 15, 1225–1233. [Google Scholar] [CrossRef]
- Lucas, T.; Wagener, M.; Barey, P.; Mariette, F. NMR assessment of mix and ice cream. Effect of formulation on liquid water and ice. Int. Dairy J. 2005, 15, 1064–1073. [Google Scholar] [CrossRef]
- Knight, C.A.; Hallett, J.; DeVries, A.L. Solute effects on ice recrystallization: An assessment technique. Cryobiology 2012, 25, 55–60. [Google Scholar] [CrossRef]
- Tomczak, M.M.; Marshall, C.B.; Gilbert, J.A.; Davies, P.L. A facile method for determining ice recrystallization inhibition by antifreeze proteins. Biochem. Biophys. Res. Commun. 2003, 311, 1041–1046. [Google Scholar] [CrossRef]
- Yu, S.O.; Brown, A.; Middleton, A.J.; Tomczak, M.M.; Walker, V.K.; Davies, P.L. Ice restructuring inhibition activities in antifreeze proteins with distinct differences in thermal hysteresis. Cryobiology 2010, 61, 327–334. [Google Scholar] [CrossRef]
- Raymond, J.A.; Knight, C.A. Ice binding, recrystallization inhibition, and cryoprotective properties of ice-active substances associated with Antarctic sea ice diatoms. Cryobiology 2003, 46, 174–181. [Google Scholar] [CrossRef]
- Wharton, D.A.; Wilson, P.W.; Mutch, J.S.; Marshall, C.J.; Lim, M. Recrystallization inhibition assessed by splat cooling and optical recrystallometry. CryoLetters 2007, 28, 61–68. [Google Scholar]
- Arnaud, L.; Gay, M.; Barnola, J.M.; Duval, P. Imaging of firn and bubbly ice in coaxial reflected light: A new technique for the characterization of these porous media. J. Glaciol. 1998, 44, 326–332. [Google Scholar] [CrossRef]
- Faydi, E.; Andrieu, J.; Laurent, P. Experimental study and modeling of the ice crystal morphology of model standard ice cream. Part I: Direct characterization method and experimental data. J. Food Eng. 2001, 48, 238–291. [Google Scholar]
- Caillet, A.; Cogne, C.; Andrieu, J.; Laurent, P.; Rivoire, A. Characterization of ice cream structure by direct optical microscopy. Influence of freezing parameters. LWT Food Sci. Technol. 2003, 36, 743–749. [Google Scholar] [CrossRef]
- Flores, A.A.; Goff, H.D. Ice crystal size distributions in dynamically frozen model solutions and ice cream as affected by stabilizers. J. Diary Sci. 1999, 82, 1399–1407. [Google Scholar] [CrossRef]
- Flores, A.A.; Goff, H.D. Recrystallization in ice cream after constant and cycling temperature storage conditions as affected by stabilizers. J. Dairy Sci. 1999, 82, 1408–1415. [Google Scholar] [CrossRef]
- Raymond, J.A.; DeVries, A.L. Adsorption inhibition, as a mechanisms of freezing resistance in polar fishes. Proc. Natl. Acad. Sci. USA 1977, 74, 2589–2593. [Google Scholar] [CrossRef]
- Barbetta, A.; Bedini, R.; Pecci, R.; Dentini, M. Role of X-ray microtomography in tissue engineering. Ann. Ist. Super. Sanita 2012, 48, 10–18. [Google Scholar]
- Kareh, K.M.; Lee, P.D.; Gourlay, C.M. In situ, time-resolved tomography for validating models of deformation in semi-solid alloys. IOP Conf. Ser. Mater. Sci. Eng. 2012, 33, 012037. [Google Scholar] [CrossRef]
- Guo, E.; Zeng, G.; Kazantsev, D.; Rockett, P.; Bent, J.; Kirkland, M.; Dalen, G.; Eastwood, D.S.; StJohn, D.; Lee, P.D. Synchrotron X-ray tomographic quantification of microstructural evolution in ice cream—A multiphase soft solid. RSC Adv. 2017, 7, 15561–15573. [Google Scholar] [CrossRef]
- Rockett, P.; Karagadde, S.; Guo, E.; Bent, J.; Hazekamp, J.; Kingsley, M.; Vila-Comamala, J.; Lee, P.D. A 4-D dataset for validation of crystal growth in a complex three-phase material, ice cream. IOP Conf. Ser. Mater. Sci. Eng. 2015, 84, 012076. [Google Scholar] [CrossRef]
| Sample | X50 (after 24 h) (µm) | X50 (after 96 h) (µm) |
|---|---|---|
| 30% suc + KK | 6 | 20 |
| 30% suc + 3 h HCL | 7 | 8 |
| 30% suc + 1.5 h H2SO4 | 7 | 12 |
| Sample | X50 (after 24 h) (µm) | X50 (after 96 h) (µm) |
|---|---|---|
| sorbet + KK | 6 | 20 |
| sorbet + 3 h HCL | 7 | 8 |
| sorbet +1.5 h H2SO4 | 7 | 12 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamińska-Dwórznicka, A.; Gondek, E.; Łaba, S.; Jakubczyk, E.; Samborska, K. Characteristics of Instrumental Methods to Describe and Assess the Recrystallization Process in Ice Cream Systems. Foods 2019, 8, 117. https://doi.org/10.3390/foods8040117
Kamińska-Dwórznicka A, Gondek E, Łaba S, Jakubczyk E, Samborska K. Characteristics of Instrumental Methods to Describe and Assess the Recrystallization Process in Ice Cream Systems. Foods. 2019; 8(4):117. https://doi.org/10.3390/foods8040117
Chicago/Turabian StyleKamińska-Dwórznicka, Anna, Ewa Gondek, Sylwia Łaba, Ewa Jakubczyk, and Katarzyna Samborska. 2019. "Characteristics of Instrumental Methods to Describe and Assess the Recrystallization Process in Ice Cream Systems" Foods 8, no. 4: 117. https://doi.org/10.3390/foods8040117
APA StyleKamińska-Dwórznicka, A., Gondek, E., Łaba, S., Jakubczyk, E., & Samborska, K. (2019). Characteristics of Instrumental Methods to Describe and Assess the Recrystallization Process in Ice Cream Systems. Foods, 8(4), 117. https://doi.org/10.3390/foods8040117






