Study of the Chronology of Expression of Ten Extracellular Matrix Molecules during the Myogenesis in Cattle to Better Understand Sensory Properties of Meat
Abstract
1. Introduction
2. Materials and Methods
2.1. Muscle Samples
2.2. Transverse Sections Preparation
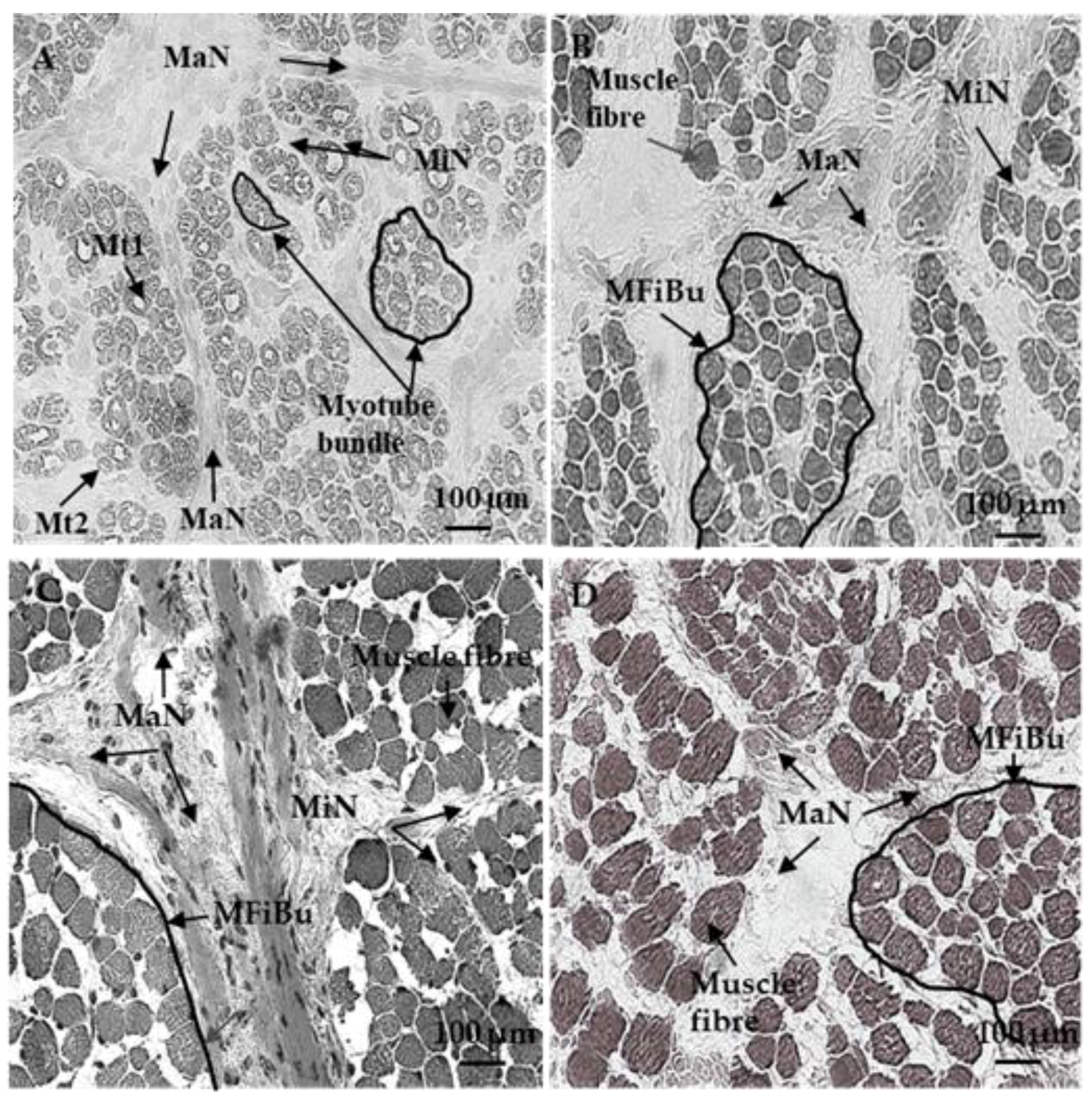
2.3. Azorubine Staining
2.4. Antibodies
2.5. Immunohistochemistry
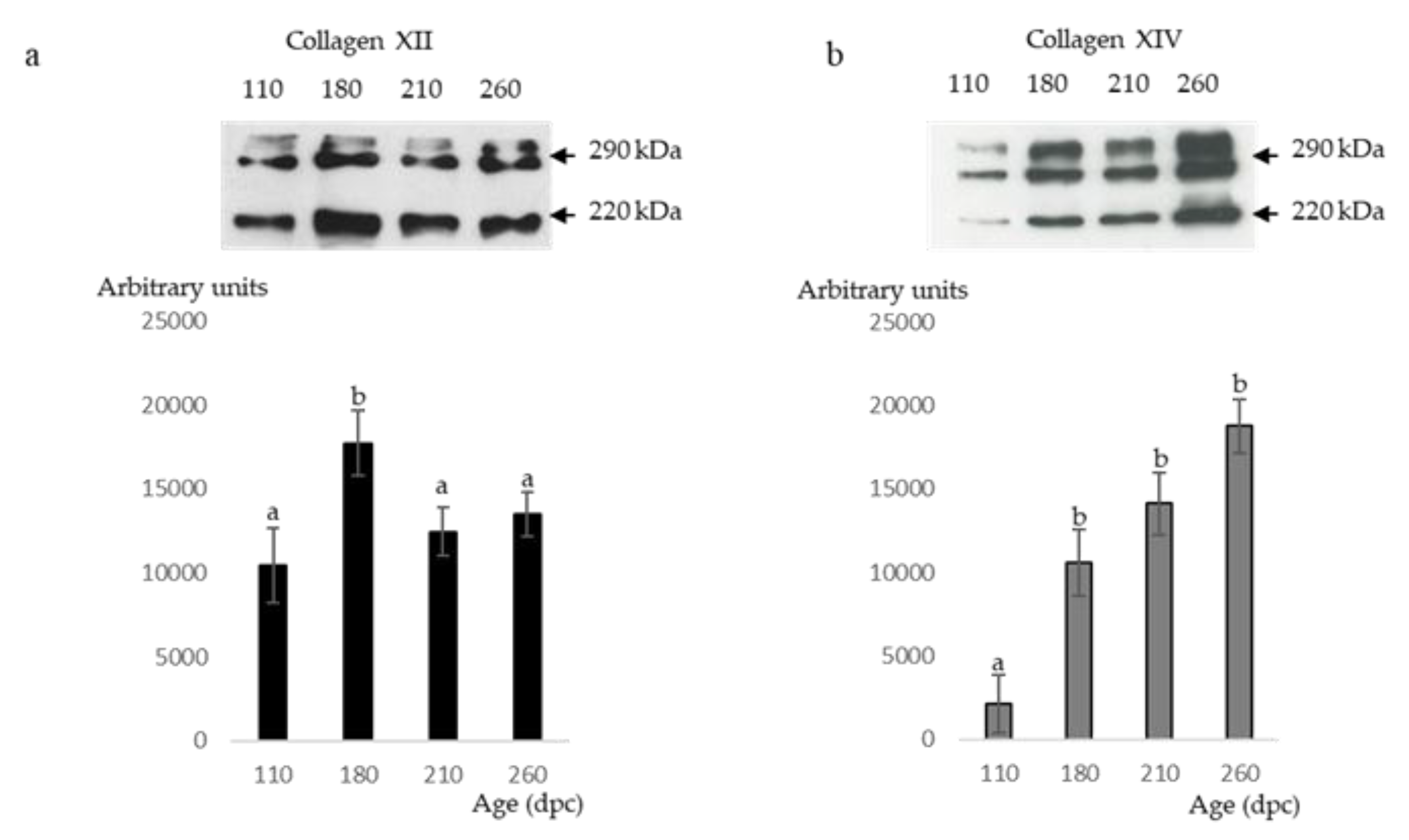
2.6. Western Blot Analyses of Type XII and XIV Collagens
2.7. Image Acquisition and Analysis
2.8. Statistical Analysis
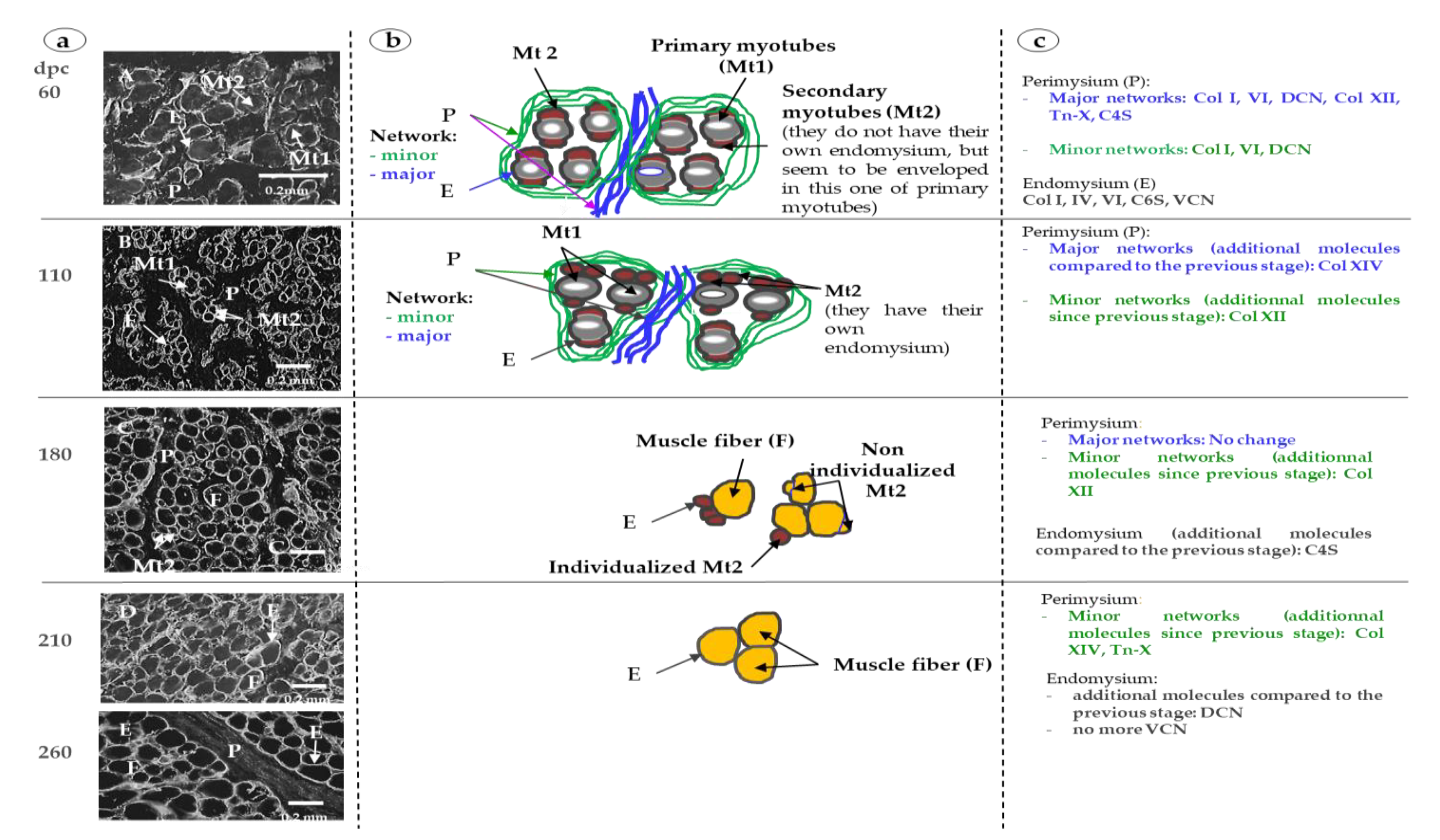
3. Results
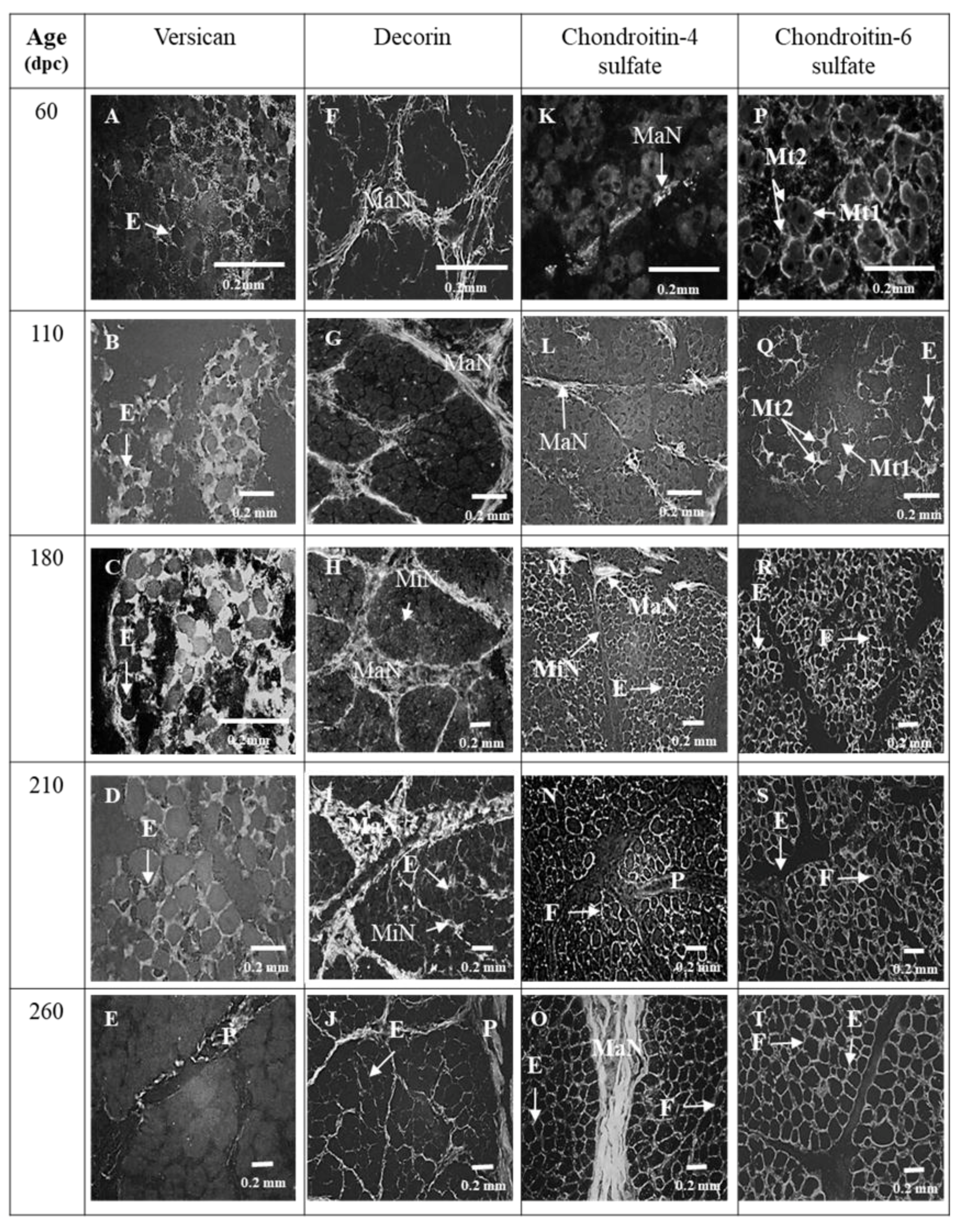
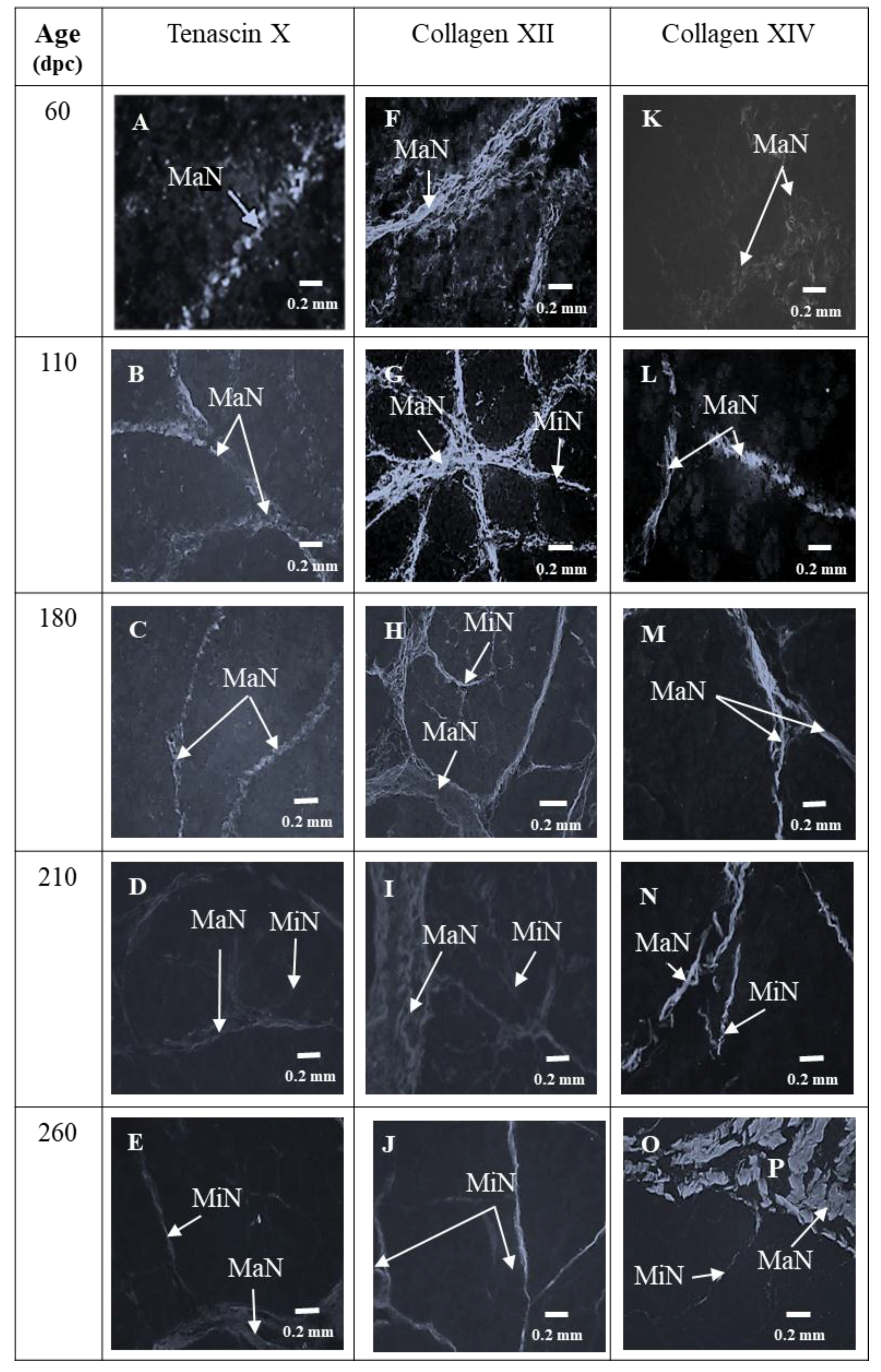
3.1. Sixty and 110 dpc
3.1.1. Perimysium
3.1.2. Endomysium
3.2. 180 dpc to 260 dpc
3.2.1. ECM Molecules Whose Location Changed
3.2.2. ECM Molecules Whose Location Did not Change
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Listrat, A.; Lebret, B.; Louveau, I.; Astruc, T.; Bonnet, M.; Lefaucheur, L.; Picard, B.; Bugeon, J. How muscle structure and composition influence meat and flesh quality. Sci. World J. 2016, 2016, 3182746. [Google Scholar] [CrossRef] [PubMed]
- Chriki, S.; Gardner, G.; Jurie, C.; Picard, B.; Micol, D.; Brun, J.-P.; Journaux, L.; Hocquette, J.-F. Cluster analysis application identifies muscle characteristics of importance for beef tenderness. BMC Biochem. 2012, 13, 29. [Google Scholar] [CrossRef] [PubMed]
- Chriki, S.; Picard, B.; Jurie, C.; Reichstadt, M.; Micol, D.; Brun, J.-P.; Journaux, L.; Hocquette, J.-F. Meta-analysis of the comparison of the metabolic and contractile characteristics of two bovine muscles: Longissimus thoracis and Semitendinosus. Meat Sci. 2012, 91, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Picard, B.; Gagaoua, M.; Micol, D.; Cassar-Malek, I.; Hocquette, J.F.; Terlouw, C.E. Inverse relationships between biomarkers and beef tenderness according to contractile and metabolic properties of the muscle. J. Agric. Food Chem. 2014, 62, 9808–9818. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T. The role of intramuscular connective tissue in meat texture. Anim. Sci. J. 2010, 81, 21–27. [Google Scholar] [CrossRef]
- Lepetit, J. A theoretical approach of the relationships between collagen content, collagen cross-links and meat tenderness. Meat Sci. 2007, 76, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Brandan, E.; Gutierrez, J. Role of skeletal muscle proteoglycans during myogenesis. Matrix Biol. 2013, 32, 289–297. [Google Scholar] [CrossRef]
- Kresse, H.; Hausser, H.; Schonherr, E. Small proteoglycans. Experientia 1993, 49, 403–416. [Google Scholar] [CrossRef]
- Lethias, C.; Carisey, A.; Comte, J.; Cluzel, C.; Exposito, J.Y. A model of tenascin-X integration within the collagenous network. FEBS Lett. 2006, 580, 6281–6285. [Google Scholar] [CrossRef]
- Ehnis, T.; Dieterich, W.; Bauer, M.; Kresse, H.; Schuppan, D. Localization of a binding site for the proteoglycan decorin on collagen XIV (undulin). J. Biol. Chem. 1997, 272, 20414–20419. [Google Scholar] [CrossRef]
- Elefteriou, F.; Exposito, J.Y.; Garrone, R.; Lethias, C. Binding of tenascin-X to decorin. FEBS Lett. 2001, 495, 44–47. [Google Scholar] [CrossRef]
- Font, B.; Eichenberger, D.; Rosenberg, L.M.; Van der Rest, M. Characterization of the interactions of type XII collagen with two small proteoglycans from fetal bovine tendon, decorin and fibromodulin. Matrix Biol. 1996, 15, 341–348. [Google Scholar] [CrossRef]
- Margaron, Y.; Bostan, L.; Exposito, J.Y.; Malbouyres, M.; Trunfio-Sfarghiu, A.M.; Berthier, Y.; Lethias, C. Tenascin-X increases the stiffness of collagen gels without affecting fibrillogenesis. Biophys. Chem. 2010, 147, 87–91. [Google Scholar] [CrossRef]
- Nishiyama, T.; McDonough, A.M.; Bruns, R.R.; Burgeson, R.E. Type XII and type XIV collagens mediate interactions between banded collagen fibers in vitro and may modulate extracellular matrix deformability. J. Biol. Chem. 1994, 269, 28193–28199. [Google Scholar] [PubMed]
- Bailey, A.J.; Light, N.D. The effect of ante- and post-mortem factors on meat connective tissue: Growth rate, handling and conditioning. In Connective Tissue in Meat and Meat Products; Elsevier Applied Science: London, UK, 1989; pp. 195–224. [Google Scholar]
- Dubost, A.; Micol, D.; Lethias, C.; Listrat, A. New insight of some extracellular matrix molecules in beef muscles. Relationships with sensory qualities. Animal 2016, 10, 821–828. [Google Scholar] [CrossRef]
- Picard, B.; Lefaucheur, L.; Berri, C.; Duclos, M. Muscle fibre ontogenesis in farm animal species. Reprod. Nutr. Dev. 2002, 42, 415–431. [Google Scholar] [CrossRef]
- Chal, J.; Pourquié, O. Making muscle: Skeletal myogenesis in vivo and in vitro. Development 2017, 144, 2104–2122. [Google Scholar] [CrossRef] [PubMed]
- Jackson, H.E.; Ingham, P.W. Control of muscle fibre-type diversity during embryonic development: The zebrafish paradigm. Mech. Dev. 2013, 130, 447–457. [Google Scholar] [CrossRef]
- Jiwlawat, N.; Lynch, E.; Jeffrey, J.; Van Dyke, J.M.; Suzuki, M. Current Progress and challenges for skeletal muscle differentiation from human pluripotent stem cells using transgene-free approaches. Stem Cells Int. 2018, 2018, 6241681. [Google Scholar] [CrossRef]
- Listrat, A.; Lethias, C.; Hocquette, J.F.; Renand, G.; Menissier, F.; Geay, Y.; Picard, B. Age-related changes and location of types I, III, XII and XIV collagen during development of skeletal muscles from genetically different animals. Histochem. J. 2000, 32, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Listrat, A.; Picard, B.; Geay, Y. Age-related changes and location of type I, III and IV collagens during skeletal muscle development of double-muscled and normal bovine foetuses. J. Muscle Res. Cell Motil. 1998, 19, 1–14. [Google Scholar] [CrossRef]
- Listrat, A.; Picard, B.; Geay, Y. Age-related changes and location of type I, III, IV, V and VI collagens during development of four foetal skeletal muscles of double-muscled and normal bovine animals. Tissue Cell 1999, 31, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Robelin, J.; Lacourt, A.; Béchet, D.; Ferrara, M.; Briand, Y.; Geay, Y. Muscle differentiation in the bovine fetus: A histological and histochemical approach. Growth Dev. Aging 1991, 55, 151–160. [Google Scholar] [PubMed]
- Berthod, F.; Germain, L.; Guignard, R.; Lethias, C.; Garrone, R.; Damour, O.; Van der Rest, M.; Auger, F.A. Differential expression of collagens XII and XIV in human skin and in reconstructed skin. J. Investig. Dermatol. 1997, 108, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Elefteriou, F.; Exposito, J.Y.; Garrone, R.; Lethias, C. Characterization of the bovine tenascin-X. J. Biol. Chem. 1997, 272, 22866–22874. [Google Scholar] [CrossRef]
- Hayes, A.J.; Hall, A.; Brown, L.; Tubo, R.; Caterson, B. Macromolecular organization and in vitro growth characteristics of scaffold-free neocartilage grafts. J. Histochem. Cytochem. 2007, 55, 853–866. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for quantification of microgram quantities of protein utilizing principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Meunier, B.; Picard, B.; Astruc, T.; Labas, R. Development of image analysis tool for the classification of muscle fibre type using immunohistochemical staining. Histochem. Cell Biol. 2010, 134, 307–317. [Google Scholar] [CrossRef]
- Pöschl, E.; Schlötzer-Schrehardt, U.; Brachvogel, B.; Saito, K.; Ninomiya, Y.; Mayer, U. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development 2004, 131, 1619–1628. [Google Scholar] [CrossRef]
- Cassar-Malek, I.; Ueda, Y.; Bernard, C.; Jurie, C.; Sudre, K.; Listrat, A.; Barnola, I.; Gentes, G.; Leroux, C.; Renand, G. Molecular and biochemical muscle characteristics of Charolais bulls divergently selected for muscle growth. Indic. Milk Beef Qual. 2005, 112, 371–377. [Google Scholar]
- Nusgens, B.; Delain, D.; Senechal, H.; Winand, R.; Lapierre, C.M.; Wahrmann, J.P. Metabolic changes in the extracellular matrix during differentiation of myoblasts of the L6 line and of a Myo- non-fusing mutant. Exp. Cell Res. 1986, 162, 51–62. [Google Scholar] [CrossRef]
- Nandan, D.; Clarke, E.P.; Ball, E.H.; Sanwal, B.D. Ethyl-3,4-dihydroxybenzoate inhibits myoblast differentiation: Evidence for an essential role of collagen. J. Cell Biol. 1990, 110, 1673–1679. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, V.; Dye, D.E.; Kinnear, B.F.; van Kuppevelt, T.H.; Grounds, M.D.; Coombe, D.R. Interactions between Skeletal Muscle Myoblasts and their Extracellular Matrix Revealed by a Serum Free Culture System. PLoS ONE 2015, 10, e0127675. [Google Scholar] [CrossRef] [PubMed]
- Carrino, D.A.; Oron, U.; Pechak, D.G.; Caplan, A.I. Reinitiation of chondroitin sulphate proteoglycan synthesis in regenerating skeletal muscle. Development 1988, 103, 641–656. [Google Scholar]
- Carrino, D.A.; Sorrell, J.M.; Caplan, A.I. Dynamic expression of proteoglycans during chicken skeletal muscle development and maturation. Poult. Sci. 1999, 78, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Gagnière, H.; Picard, B.; Geay, Y. Contractile differentiation of foetal cattle muscles: Intermuscular variability. Reprod. Nutr. Dev. 1999, 39, 637–655. [Google Scholar] [CrossRef]
- Stupka, N.; Kintakas, C.; White, J.D.; Fraser, F.W.; Hanciu, M.; Aramaki-Hattori, N.; Martin, S.; Coles, C.; Collier, F.; Ward, A.C.; et al. Versican processing by a disintegrin-like and metalloproteinase domain with thrombospondin-1 repeats proteinases-5 and -15 facilitates myoblast fusion. J. Biol. Chem. 2013, 288, 1907–1917. [Google Scholar] [CrossRef]
- Miura, T.; Kishioka, Y.; Wakamatsu, J.-I.; Hattori, A.; Nishimura, T. Interaction between myostatin and extracellular matrix components. Anim. Sci. J. 2010, 81, 102–107. [Google Scholar] [CrossRef]
- Hennebry, A.; Berry, C.; Siriett, V.; O’Callaghan, P.; Chau, L.; Watson, T.; Sharma, M.; Kambadur, R. Myostatin regulates fiber-type composition of skeletal muscle by regulating MEF2 and MyoD gene expression. Am. J. Physiol.-Cell Physiol. 2009, 296, C525–C534. [Google Scholar] [CrossRef]
- Kishioka, Y.; Thomas, M.; Wakamatsu, J.; Hattori, A.; Sharma, M.; Kambadur, R.; Nishimura, T. Decorin enhances the proliferation and differentiation of myogenic cells through suppressing myostatin activity. J. Cell Physiol. 2008, 215, 856–867. [Google Scholar] [CrossRef]
- Wu, H.; Naya, F.J.; McKinsey, T.A.; Mercer, B.; Shelton, J.M.; Chin, E.R.; Simard, A.R.; Michel, R.N.; Bassel-Duby, R.; Olson, E.N.; et al. MEF2 responds to multiple calcium-regulated signals in the control of skeletal muscle fiber type. EMBO J. 2000, 19, 1963–1973. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.Q.; Zwolanek, D.; Izu, Y.Y.; Gandhy, S.; Schreiber, G.; Brockmann, K.; Devoto, M.; Tian, Z.Z.; Hu, Y.; Veit, G.; et al. Recessive and dominant mutations in COL12A1 cause a novel EDS/myopathy overlap syndrome in humans and mice. Hum. Mol. Genet. 2014, 23, 2339–2352. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Tong, J.; Zhao, J.; Underwood, K.R.; Zhu, M.; Ford, S.P.; Nataniellz, P.W. Fetal programming of skeletal muscle development in ruminant animals. J. Anim. Sci. 2010, 88, E51–E60. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhao, J.-X.; Yan, X.; Zhu, M.-J.; Long, N.M.; McCormick, R.J.; Ford, S.P.; Nataniellz, P.W.; Du, M. Maternal Obesity Enhances Collagen Accumulation and Cross-Linking in Skeletal Muscle of Ovine Offspring. PLoS ONE 2012, 7, e31691. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Listrat, A.; Gagaoua, M.; Picard, B. Study of the Chronology of Expression of Ten Extracellular Matrix Molecules during the Myogenesis in Cattle to Better Understand Sensory Properties of Meat. Foods 2019, 8, 97. https://doi.org/10.3390/foods8030097
Listrat A, Gagaoua M, Picard B. Study of the Chronology of Expression of Ten Extracellular Matrix Molecules during the Myogenesis in Cattle to Better Understand Sensory Properties of Meat. Foods. 2019; 8(3):97. https://doi.org/10.3390/foods8030097
Chicago/Turabian StyleListrat, Anne, Mohammed Gagaoua, and Brigitte Picard. 2019. "Study of the Chronology of Expression of Ten Extracellular Matrix Molecules during the Myogenesis in Cattle to Better Understand Sensory Properties of Meat" Foods 8, no. 3: 97. https://doi.org/10.3390/foods8030097
APA StyleListrat, A., Gagaoua, M., & Picard, B. (2019). Study of the Chronology of Expression of Ten Extracellular Matrix Molecules during the Myogenesis in Cattle to Better Understand Sensory Properties of Meat. Foods, 8(3), 97. https://doi.org/10.3390/foods8030097





