1. Introduction
Fermentation has been used to prolong the storage life of vegetables in many cultures, including China. Sichuan-style pickled vegetables are as famous as French cornichons and German sauerkraut [
1]. Sichuan-style pickling relies mainly on the fermentation of lactic acid bacteria to produce high concentrations of lactic acid instead of on the osmotic pressure of salt to inhibit putrefying microorganisms. Low-concentration saline is used to preserve fresh vegetables for Sichuan-style pickling. This is followed by fermentation by lactic acid bacteria. So long as the product is in an airtight environment and the lactic acid reaches a certain concentration, long-term preservation and flavorful taste can be achieved [
2]. Because Sichuan-style pickling involves reusing the salt solution, long-term and repeated fermentation produces a complex microbial system containing abundant bacterial flora, including such known species as
Lactobacillus brevis,
L. plantarum, ethanol-resistant
Fasciococcus,
L. casei,
L. pentosus,
L. sakei, L. alimentarius, and
Leuconostoc mesenteroides [
3,
4,
5]. Because the fermentation conditions of the Sichuan-style pickled vegetables are different from those of other fermented foods, the microorganisms in the Sichuan-style pickled vegetables include special strains with their own characteristics including resistance to acid [
6].
The great numbers of lactic acid bacteria do not only play a key role in the taste and quality of pickled vegetables but also contribute to various biological activities [
7]. Lactic acid bacteria maintain the balance of microbial ecology in human body, improve the digestibility and utilization of food in the gastrointestinal tract, inhibit the growth and reproduction of spoilage bacteria in the intestine, and produce nutrients for the body to use. They also reduce serum cholesterol and the effects of toxin stimulation on body tissue development. Lactic acid bacteria also play a probiotic role in regulating the nutritional status of the body, improving the physiological function of the body, avoiding cell infection, improving the efficacy of drugs, alleviating the effects of toxic substances on the body, promoting immune response, preventing tumorigenesis and slowing down aging [
8,
9,
10,
11,
12]. Reduction of probiotics in the body can lead to abnormalities in the body. Therefore, maintaining the normal level of probiotics in the body plays an important role in human health. Lactic acid bacteria have a good effect on maintenance of normal microbial ecology in the body, so they have been extensively used as probiotics in food, medicine, and the pharmaceutical industry [
13].
The immune system is an important part of how the human body defends itself from foreign pathogens, and it can distinguish invading harmful substances such as bacteria, virus, molds and other pathogenic microorganisms from its own cells and eliminate them [
14]. During the early stage of cancer, which weakens the immune system, cancer cells are only rarely detected and eliminated, which gives them the opportunity to develop into tumors. The immunity of cancer patients also affects the speed of cancer progression and treatment outcomes. Therefore, improving the immunity of cancer patients may increase the success rate of anticancer therapies [
15]. The immunomodulatory effects of lactic acid bacteria on the human body have two main aspects: (1) regulation of non-specific immunity; and (2) regulation of specific immunity. Lactic acid bacteria regulate specific and non-specific immune responses in the body to facilitate maintenance of the normal level of immune functions in the body and play a very important role in nutrition, biological barriers, anti-tumor functions, and other probiotic functions of the body [
16].
Oxidative stress is closely related to the occurrence of disease conditions, such as tumors, inflammation, neurodegeneration, and aging. Under normal circumstances, oxidative metabolism in the bodies of living beings produces a small quantity of free radicals, which can be eliminated by the antioxidant system of the body to maintain redox balance. However, under the influence of some injury factors, accumulation of large quantities of free radicals are induced, thereby forming an imbalance of oxidation and antioxidation, which is known as oxidative stress and is directly related to the onset of cancers [
17].
Tongue cancer is a malignant tumor originating in the anterior part of the tongue and is one of the most common malignant tumors in the oral and maxillofacial region, accounting for 0.8–2.0% of systemic cancer, 5–15.5% of head and neck cancer, and 32.3% of oral cancer; it is ranked first in oral cancer [
18]. A large number of microorganisms are found in the oral cavity. Studies on the inhibitory effects of lactic acid bacteria on tongue cancer through immunomodulation in the oral cavity and in the body are rarely reported. Lactic acid bacteria have not been isolated from Sichuan pickled vegetables by searching references. By comparison with Gene Bank, LF-CQPC08 (sequence: TTAGGCGGTGGCTCCTAAAGGTTACCCCACCGACTTTGGGTGTTAAAACTCTCATGGTGTGACGGGCGGTGTGTACAAGGCCCGGGAACGTATTCACCGCGGCATGCTGATCCGCGATTACTAGCGATTCCGACTTCGTGCAGGCGAGTTGCAGCCTGCAGTCCGAACTGAGAACGGTTTTAAGAGATTTGCTTGCCCTCGCGAGTTCGCGACTCGTTGTACCGTCCATTGTAGCACGTGTGTAGCCCAGGTCATAAGGGGCATGATGATCTGACGTCGTCCCCACCTTCCTCCGGTTTGTCACCGGCAGTCTCACTAGAGTGCCCAACTTAATGCTGGCAACTAGTAACAAGGGTTGCGCTCGTTGCGGGACTTAACCCAACATCTCACGACACGAGCTGACGACGACCATGCACCACCTGTCATTGCGTTCCCGAAGGAAACGCCCTATCTCTAGGGTTGGCGCAAGATGTCAAGACCTGGTAAGGTTCTTCGCGTAGCTTCGAATTAAACCACATGCTCCACCGCTTGTGCGGGCCCCCGTCAATTCCTTTGAGTTTCAACCTTGCGGTCGTACTCCCCAGGCGGAGTGCTTAATGCGTTAGCTCCGGCACTGAAGGGCGGAAACCCTCCAACACCTAGCACTCATCGTTTACGGCATGGACTACCAGGGTATCTAATCCTGTTCGCTACCCATGCTTTCGAGTCTCAGCGTCAGTTGCAGACCAGGTAGCCGCCTTCGCCACTGGTGTTCTTCCATATATCTACGCATTCCACCGCTACACATGGAGTTCCACTACCCTCTTCTGCACTCAAGTTATCCAGTTTCCGATGCACTTCTCCGGTTAAGCCGAAGGCTTTCACATCAGACTTAGAAAACCGCCTGCACTCTCTTTACGCCCAATAAATCCGGATAACGCTTGCCACCTACGTATTACCGCGGCTGCTGGCACGTAGTTAGCCGTGACTTTCTGGTTAAATACCGTCAACGTATGAACAGTTACTCTCATACGTGTTCTTCTTTAACAACAGAGCTTTACGAGCCGAAACCCTTCTTCACTCACGCGGTGTTGCTCCATCAGGCTTGCGCCCATTGTGGAAGATTCCCTACTGCTGCCTCCCGTAGGAGTATGGGCCGTGTCTCAGTCCCATTGTGGCCGATCAGTCTCTCAACTCGGCTATGCATCATCGCCTTGGTAGGCCGTTACCCCACCAACAAGCTAATGCACCGCAGGTCCATCCAGAAGTGATAGCGAGAAGCCATCTTTTAAGCGTTGTTCATGCGAACAACGTTGTTATGCGGTATTAGCATCTGTTTCCAAATGTTGTCCCCCGCTTCTGGGCAGGTTACCTACGTGTTACTCACCCGTCCGCCACTCGTTGGCGACCAAAATCAATCAGGTGCAAGCACCATCAATCAA) was initially considered as a newly discovered lactic acid bacteria. After treatment with LF-CQPC08 for one month by lavage, the IgG serum level was 1.67 times higher than that in mice without LF-CQPC08; the stain showed the immunomodulatory effect. This study evaluated the inhibitory effects of the newly discovered, lactic acid bacteria LF-CQPC08 on tongue cancer mouse model and explored the mechanism underlying the action of LF-CQPC08 on 4-nitroquinoline 1-oxide (4NQO)-induced tongue cancer through immunomodulation. The experimental results of this study may facilitate definition of the functional role of LF-CQPC08 and indicate new areas of tongue cancer inhibition through probiotics.
4. Discussion
Recently, partial modulation of the human immune system against disease has been found to be a way of controlling some diseases. It involves enhancing immunity to prevent cancer cell invasion, suppress cancer, and use as adjuvant cancer therapy [
26]. The splenic index, thymus index, phagocytosis percentage, and phagocytic index reflect the normal state of immune organs to a certain extent and directly reflect the strength of immune functions in the body. A previous study has shown that some active ingredients in food act as immunomodulators to avoid the effects of adverse factors on the immune organs, delay thymic degeneration, and maintain normal immunity [
27]. The effect of lactic acid bacteria on non-specific immunity is mainly through enhancing the activity of mononuclear phagocytic cells (monocytes and macrophages) and polymorphonuclear leukocytes, stimulating the secretion of lysosomes and mononuclear factors, promoting the production of reactive oxygen and reactive nitrogen, and improving the phagocytosis of the mononuclear phagocytic system. Lactic acid bacteria stimulate specific immune responses mainly through the humoral immunity and the cell-mediated immunity [
28]. The humoral immunity is achieved by producing antibodies in the body and increasing IgA, IgM, and IgG levels in mucosa and blood. Cell-mediated immunity is achieved by activating macrophages, B lymphocytes, and natural killer (NK) cells and by promoting the production of cytokines, such as IL and interferon (IFN) [
29]. G-CSF and GM-CSF exert good therapeutic effects on neutropenia caused by cancer. They also have immunomodulatory and other effects on the number and proportion of T lymphocyte subsets in the process of hematopoietic stem/progenitor cells in the peripheral blood [
30]. The experimental results of this study showed that LF-CQPC08 inhibited the effects of carcinogenic compounds on the splenic and thymic indexes in the mouse model. In addition, LF-CQPC08 improved the decline of serum G-CSF, GM-CSF, IgG, and IgM levels caused by oral cancer. The IgG, and IgM serum levels of normal mice in this study were similar to those in previous study [
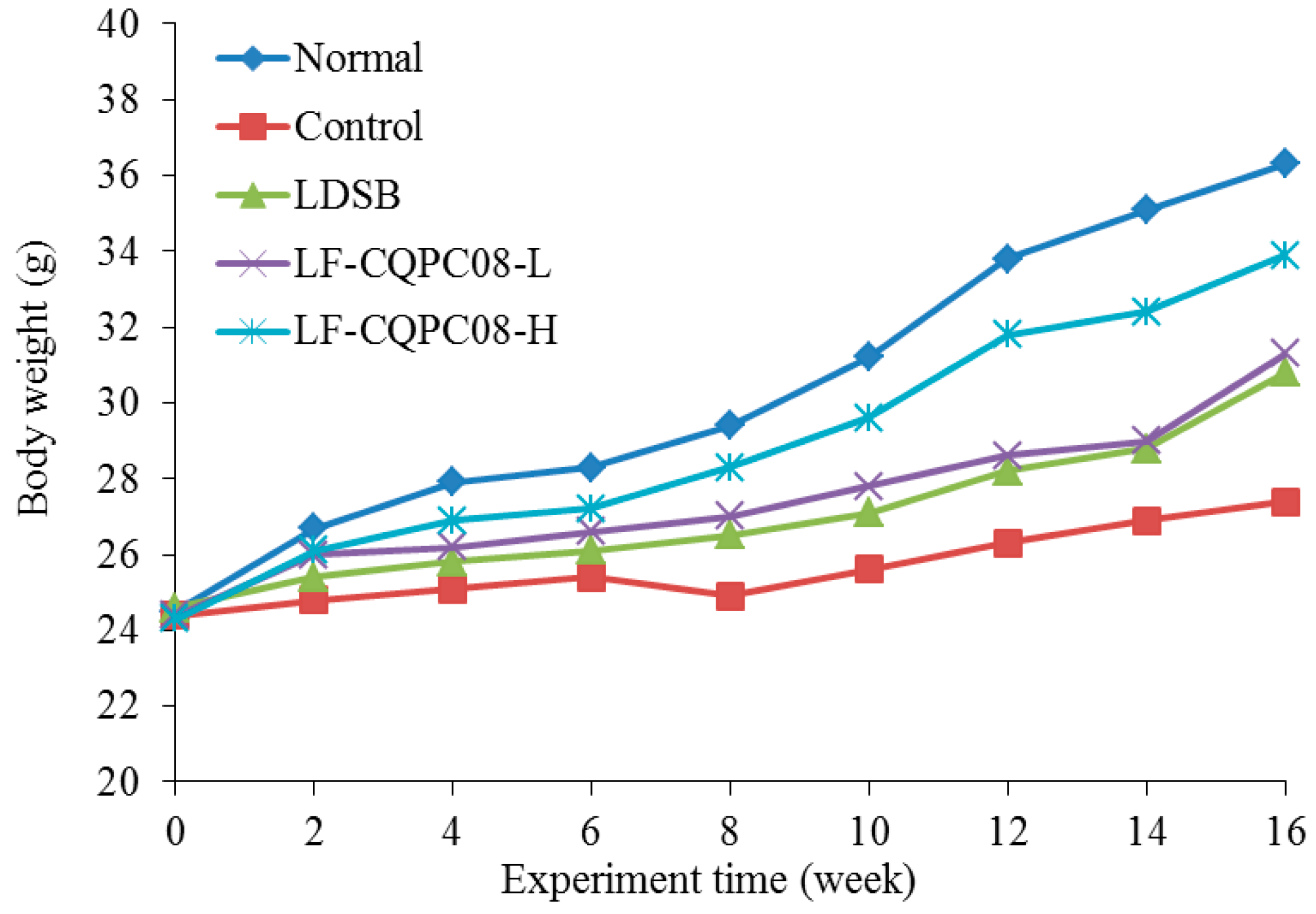
23]. Hence, LF-CQPC08 effectively inhibited the weakened immunity in the mice caused by tongue cancer and improved the immunity of the tumor-bearing mice, with a better effect than the LDSB commonly found on the market.
IL-12 plays an important role in enhancing the cell-mediated immunity and modulation of immune responses. It also stimulates the proliferation of activated T-cells, promotes the differentiation of Th0 cells into Th1 cells, induces cytotoxicity, and promotes the secretion of IFN-γ and GM-CSF of the NK cells, thereby exerting its inhibitory effects on cancer cells [
31]. IL-4 is secreted by activated Th2 cells and promotes the proliferation and activation of B cells. IFN-γ is secreted by Th1 cells, inducing participation and enhancing cell-mediated immunity in the body. The ratio of Th1 and Th2 lymphocyte subsets is relatively constant, which is essential to maintaining the stability of immune functions [
32]. IFN-γ also has antitumor effect and can promote autoimmunity [
33]. TNF-α is mainly secreted by macrophages, and its main function is to regulate immune cells. The endogenous pyrogen TNF-α can cause fever and apoptosis and prevent tumorigenesis [
34]. The inflammatory related cytokine serum levels of normal mice in this study were similar to those in a previous study [
35]. In this study, LF-CQPC08 increased the serum levels of IL-4, IL-12, TNF-α, and IFN-γ in mice with induced tongue cancer, thereby regulating the immune system and inhibiting the tongue cancer in the mouse model.
The presence of large quantities of free radicals in the body promotes cell aging and death, damaging human organs and even DNA in human cells, causing cells to mutate, thereby inducing various diseases, including tumors. Cancer patients have a much lower antioxidant capacity than healthy individuals do, and the antioxidants prevent damage caused by cancer-induced and anticancer-treatment-induced elevation of free radical in normal cells [
36]. Both SOD and GSH-Px are effective antioxidants that enhance the antioxidant capacity in the body, preventing and suppressing cancer [
37]. MDA is one of the most important products of membrane lipid peroxidation. It causes cross-linking polymerization of macromolecules, such as proteins and nucleic acids, and it is cytotoxic, leading to further damage to the body. Thus, controlling the MDA levels in the body also prevents cancer [
38]. In this study, LF-CQPC08 reduced oxidative stress damage in mice with tongue cancer and improved the SOD and GSH-Px activities and reduced the MDA level in the tongue tissues to protect the tongue tissues and lower the damage in the tongue tissue.
P53 plays an important role in the prevention of tumorigenesis. P73 and p63 are newly discovered members of the p53 family, and they have high homology in the sequences. Overexpression of p73 activates p53 and reactive promoter transcription causes cell growth and inhibits and induces apoptosis. One previous study showed that the expression of p73 is positively correlated with tumor metastasis and malignancy [
39]. The structure and functions of p63 are more similar to those of p73 than to those of p53. p63 induces apoptosis in p53-deficient tumor cells. Clinical research has also shown that the positive expression rates of p63 and p53 in oral squamous cell carcinomas are significantly higher than in the normal oral mucosa [
40]. Under normal conditions, p73, p63, and p53 are rarely expressed. In the development of oral cancers, the overexpression of p73, p63, and p53 proteins plays an important role in the pathogenesis of oral squamous cell carcinoma, and they all serve as tumor suppressor proteins. Among them, the interaction between p73 and p63 plays an important role in the development of oral squamous cell carcinomas [
41]. PTEN is a tumor suppressor gene with phosphatase activity, and abnormal expression of PTEN was observed in many malignant tumors. Clinical research has shown that PTEN protein expression in oral squamous cell carcinomas is positively correlated with the degree of tissue differentiation [
42]. In this study, the expression of p73, p63, p53, and PTEN in the normal mice was very weak. With the development of tongue cancer, the abundantly expressed p73, p63, p53, and PTEN exerted their anti-cancer roles. With the action of LF-CQPC08, the malignancy of tongue cancer was reduced, and the expression of p73, p63, p53, and PTEN was also decreased.
Imbalances in reactive oxygen species (ROS) and antioxidant systems lead to oxidative stress, which has been recognized as one of the carcinogenic factors. Nrf2 couples with Keap1 and binds to cytoplasmic actin, which anchors it in the cytoplasm. When a cell is subjected to oxidative stress, Nrf2 is uncoupled from Keap1 and translocated into nuclei. Nrf2 is recognized after binding to Maf protein to form a heterodimer, which combines with antioxidant response elements (ARE) to initiate the gene transcription of phase 2 detoxification enzymes, i.e., GST, NAD(P)H-quinone oxidoreductase 1 (NQO1), SOD, HO-1, and glutamate-cysteine ligase (GCL) and antioxidative stress proteins, thereby improving cells’ ability to resist oxidative stress [
43]. HO-1, which is one of the most widely occurring antioxidant defense enzymes, has antioxidant and anti-inflammatory effects. GST-π is also an important phase 2 detoxification enzyme that inhibits cancer through its antioxidant effects [
44]. In the case of non-overexpression, Nrf2, HO-1, and GST-π exert their antioxidant effects and inhibit oral cancer [
45]. In this study, LF-CQPC08 played a role in regulating the overexpression of Nrf2, HO-1, and GST-π in the tissues of the tongue and inhibited the development of oral cancer, with better therapeutic effect than LDSB.
In mammalian cells, the regulation of mitochondrial extracorporeal membrane permeability occurs mostly on the extracorporeal membrane of mitochondria, or transfers to the extracorporeal membrane of mitochondria after being stimulated by signals. These molecules are divided into two groups, according to their functions: (1) anti-apoptotic proteins, such as Bcl-2, Bcl-xL, and Bcl-w; and (2) pro-apoptotic proteins, such as Bax, Bak, and Noxa [
46]. In oral cancer, Bcl-2 and Bcl-xL expression plays a role in promoting tumor growth, and Bax expression plays a role in suppressing the cancer [
47]. In this study, both LF-CQPC08 and LDSB effectively inhibited the expression of Bcl-2 and Bcl-xL and increased the expression of Bax, thereby exerting their inhibitory effects on the oral cancer in the mice. The inhibitory effect of the LF-CQPC08 was stronger than that of the LDSB.