Inclusion of Ethanol Extract of Mesquite Leaves to Enhance the Oxidative Stability of Pork Patties
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Extract Preparation
2.3. Polyphenol Content
2.4. Antioxidant Activity
2.5. Pork Patties’ Preparation and Storage
2.6. Meat Quality Measurements
2.6.1. Proximate Analysis
2.6.2. Measurement of pH
2.6.3. Colour Measurement
2.6.4. Metmyoglobin Formation
2.6.5. Water Holding Capacity
2.6.6. Lipid Oxidation
2.6.7. Total Antioxidant Activity of Meat Extract
2.7. Sensory Evaluation
2.8. Statistical Analysis
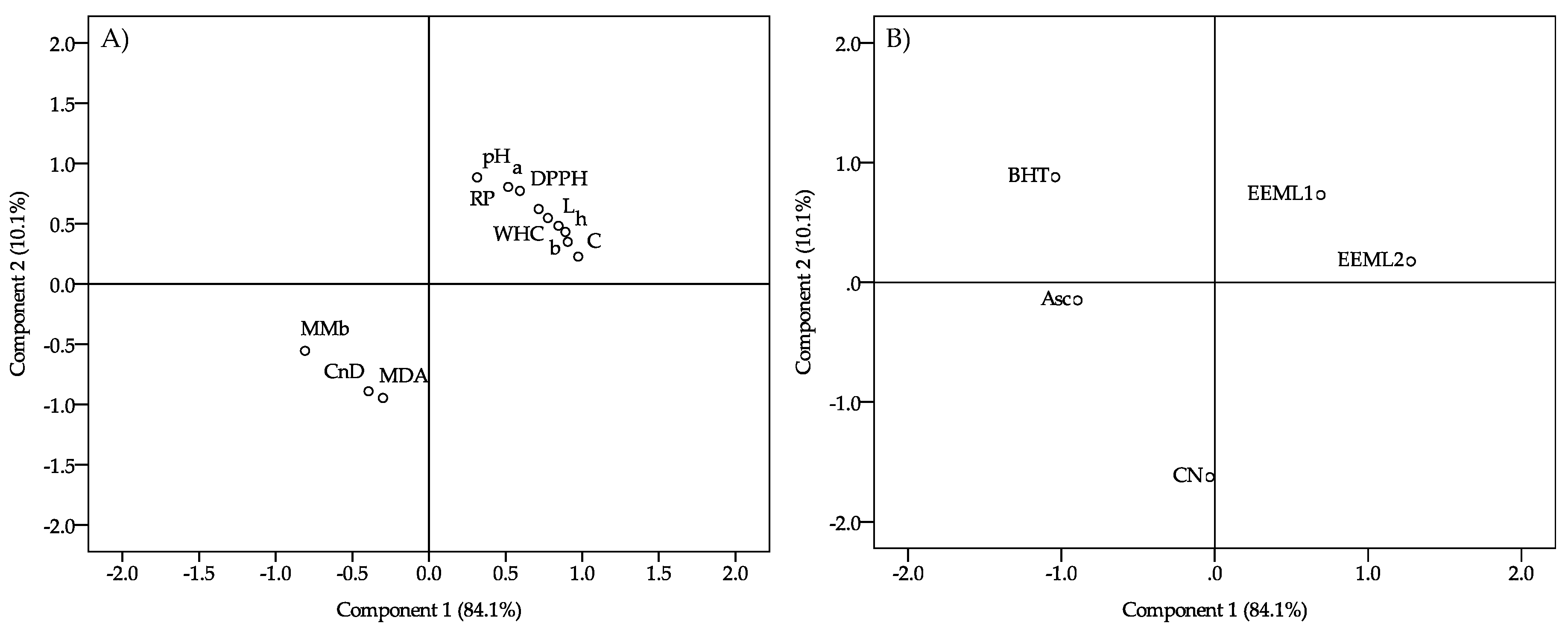
3. Results
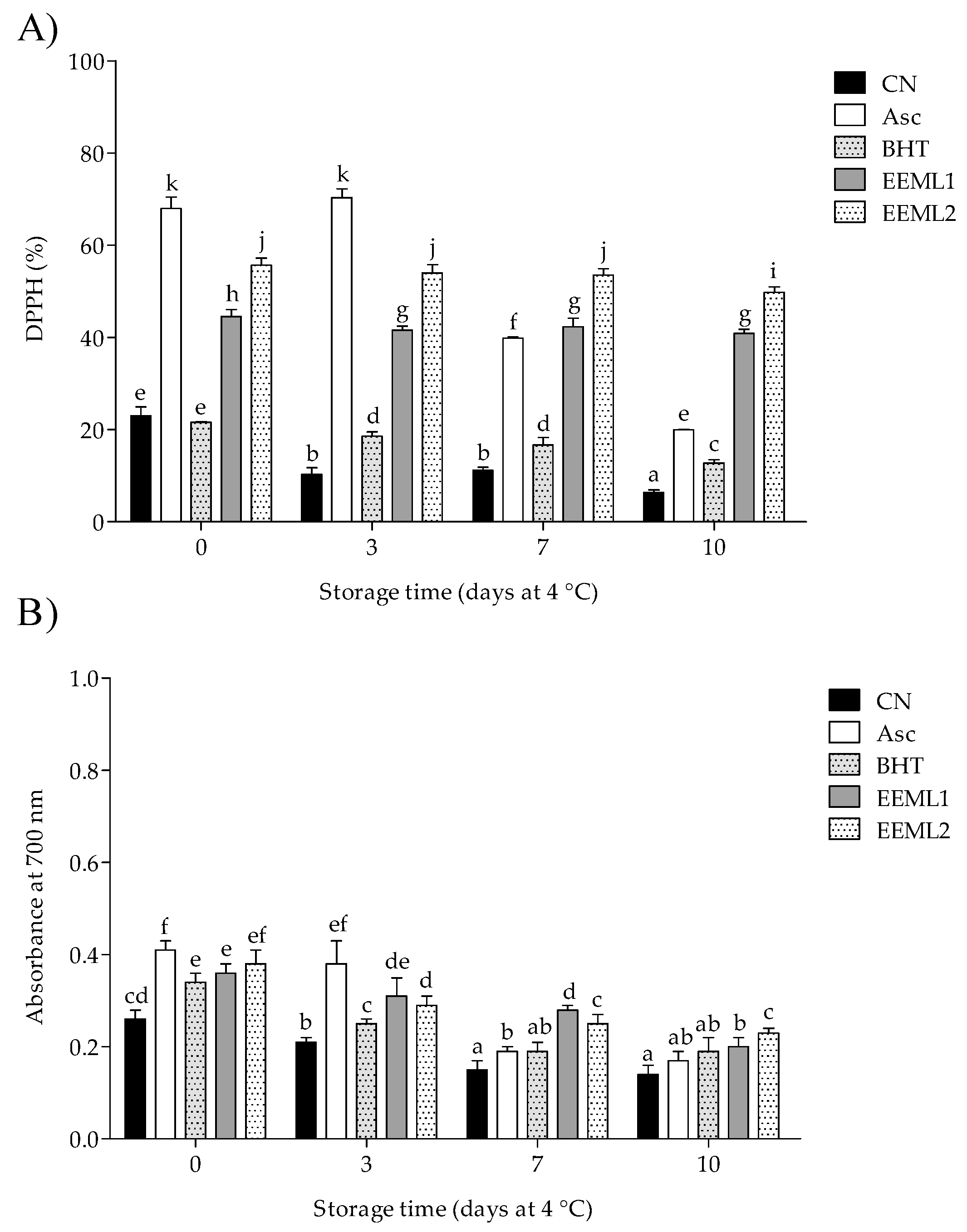
3.1. Polyphenol Content and Antioxidant Activity
3.2. Physicochemical Analysis
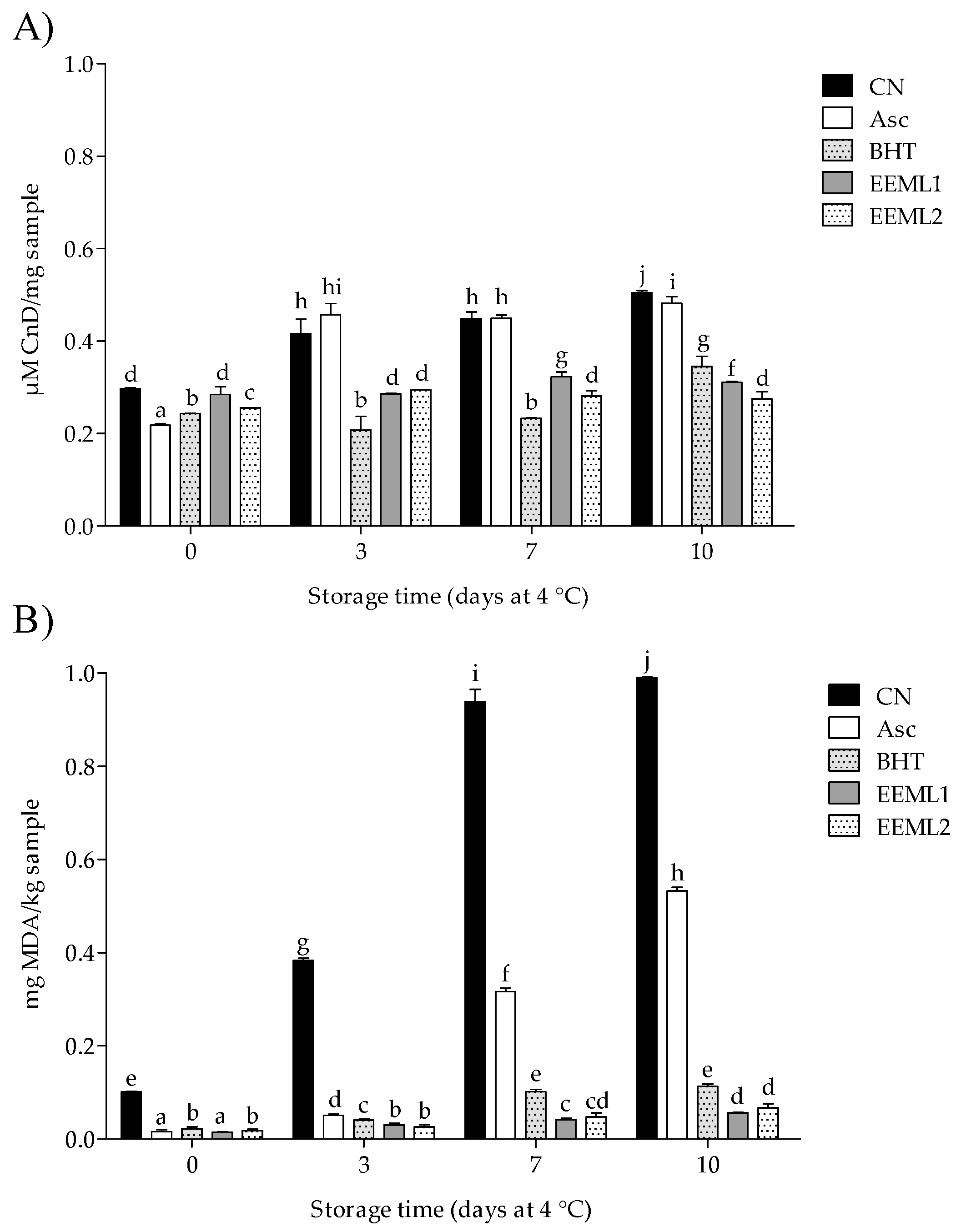
3.3. Lipid Oxidation
3.4. Total Antioxidant Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FIRA Carne De Cerdo 2017. Available online: http://www.ugrpg.org.mx/pdfs/Panorama Agroalimentario Carne de cerdo 2017.pdf (accessed on 1 September 2019).
- Lorenzo, J.M.; Montes, R.; Purriños, L.; Cobas, N.; Franco, D. Fatty acid composition of Celta pig breed as influenced by sex and location of fat in the carcass. J. Sci. Food Agric. 2012, 92, 1311–1317. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.M.D.C.C.; Vicente, A.F.D.R.B. Meat nutritional composition and nutritive role in the human diet. Meat Sci. 2013, 93, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Heck, R.T.; Vendruscolo, R.G.; de Araújo Etchepare, M.; Cichoski, A.J.; de Menezes, C.R.; Barin, J.S.; Lorenzo, J.M.; Wagner, R.; Campagnol, P.C.B. Is it possible to produce a low-fat burger with a healthy n − 6/n − 3 PUFA ratio without affecting the technological and sensory properties? Meat Sci. 2017, 130, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Alves, L.A.A.S.; Lorenzo, J.M.; Gonçalves, C.A.A.; Santos, B.A.; Heck, R.T.; Cichoski, A.J.; Campagnol, P.C.B. Production of healthier bologna type sausages using pork skin and green banana flour as fat replacers. Meat Sci. 2016, 121, 73–78. [Google Scholar] [CrossRef]
- Pateiro, M.; Vargas, F.C.; Chincha, A.A.I.A.; Sant’Ana, A.S.; Strozzi, I.; Rocchetti, G.; Barba, F.J.; Domínguez, R.; Lucini, L.; do Amaral Sobral, P.J.; et al. Guarana seed extracts as a useful strategy to extend the shelf life of pork patties: UHPLC-ESI/QTOF phenolic profile and impact on microbial inactivation, lipid and protein oxidation and antioxidant capacity. Food Res. Int. 2018, 114, 55–63. [Google Scholar] [CrossRef]
- Zamuz, S.; López-Pedrouso, M.; Barba, F.J.; Lorenzo, J.M.; Domínguez, H.; Franco, D. Application of hull, bur and leaf chestnut extracts on the shelf-life of beef patties stored under MAP: Evaluation of their impact on physicochemical properties, lipid oxidation, antioxidant, and antimicrobial potential. Food Res. Int. 2018, 112, 263–273. [Google Scholar] [CrossRef]
- Fernandes, R.D.P.P.; Trindade, M.A.; Lorenzo, J.M.; de Melo, M.P. Assessment of the stability of sheep sausages with the addition of different concentrations of Origanum vulgare extract during storage. Meat Sci. 2018, 137, 244–257. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Vargas, F.C.; Strozzi, I.; Pateiro, M.; Furtado, M.M.; Sant’Ana, A.S.; Rocchetti, G.; Barba, F.J.; Dominguez, R.; Lucini, L.; et al. Influence of pitanga leaf extracts on lipid and protein oxidation of pork burger during shelf-life. Food Res. Int. 2018, 114, 47–54. [Google Scholar] [CrossRef]
- de Carvalho, F.A.L.; Lorenzo, J.M.; Pateiro, M.; Bermúdez, R.; Purriños, L.; Trindade, M.A. Effect of guarana (Paullinia cupana) seed and pitanga (Eugenia uniflora L.) leaf extracts on lamb burgers with fat replacement by chia oil emulsion during shelf life storage at 2 °C. Food Res. Int. 2019, 125, 108554. [Google Scholar] [CrossRef] [PubMed]
- da Silva, S.L.; Amaral, J.T.; Ribeiro, M.; Sebastião, E.E.; Vargas, C.; de Lima Franzen, F.; Schneider, G.; Lorenzo, J.M.; Fries, L.L.M.; Cichoski, A.J.; et al. Fat replacement by oleogel rich in oleic acid and its impact on the technological, nutritional, oxidative, and sensory properties of Bologna-type sausages. Meat Sci. 2019, 149, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M.; Pateiro, M.; Domínguez, R.; Barba, F.J.; Putnik, P.; Kovačević, D.B.; Shpigelman, A.; Granato, D.; Franco, D. Berries extracts as natural antioxidants in meat products: A review. Food Res. Int. 2018, 106, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M.; Munekata, P.E.S.; Gómez, B.; Barba, F.J.; Mora, L.; Pérez-Santaescolástica, C.; Toldrá, F. Bioactive peptides as natural antioxidants in food products—A review. Trends Food Sci. Technol. 2018, 79, 136–147. [Google Scholar] [CrossRef]
- Munekata, P.E.S.E.S.; Domínguez, R.; Franco, D.; Bermúdez, R.; Trindade, M.A.A.; Lorenzo, J.M. Effect of natural antioxidants in Spanish salchichón elaborated with encapsulated n-3 long chain fatty acids in konjac glucomannan matrix. Meat Sci. 2017, 124, 54–60. [Google Scholar] [CrossRef]
- Pateiro, M.; Barba, F.J.F.J.; Domínguez, R.; Sant’Ana, A.S.A.S.; Mousavi Khaneghah, A.; Gavahian, M.; Gómez, B.; Lorenzo, J.M.J.M. Essential oils as natural additives to prevent oxidation reactions in meat and meat products: A review. Food Res. Int. 2018, 113, 156–166. [Google Scholar] [CrossRef]
- Echegaray, N.; Gómez, B.; Barba, F.J.; Franco, D.; Estévez, M.; Carballo, J.; Marszałek, K.; Lorenzo, J.M. Chestnuts and by-products as source of natural antioxidants in meat and meat products: A review. Trends Food Sci. Technol. 2018, 82, 110–121. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Sineiro, J.; Amado, I.R.; Franco, D. Influence of natural extracts on the shelf life of modified atmosphere-packaged pork patties. Meat Sci. 2014, 96, 526–534. [Google Scholar] [CrossRef]
- Cunha, L.C.M.; Monteiro, M.L.G.; Lorenzo, J.M.; Munekata, P.E.S.; Muchenje, V.; de Carvalho, F.A.L.; Conte-Junior, C.A. Natural antioxidants in processing and storage stability of sheep and goat meat products. Food Res. Int. 2018, 111, 379–390. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Domínguez, R.; Campagnol, P.C.B.; Franco, D.; Trindade, M.A.; Lorenzo, J.M. Effect of natural antioxidants on physicochemical properties and lipid stability of pork liver pâté manufactured with healthy oils during refrigerated storage. J. Food Sci. Technol. 2017, 54, 4324–4334. [Google Scholar] [CrossRef]
- Alirezalu, K.; Hesari, J.; Nemati, Z.; Munekata, P.E.S.; Barba, F.J.; Lorenzo, J.M. Combined effect of natural antioxidants and antimicrobial compounds during refrigerated storage of nitrite-free frankfurter-type sausage. Food Res. Int. 2019, 120, 839–850. [Google Scholar] [CrossRef]
- Fernandes, R.P.P.; Trindade, M.A.; Tonin, F.G.; Lima, C.G.; Pugine, S.M.P.; Munekata, P.E.S.; Lorenzo, J.M.; de Melo, M.P. Evaluation of antioxidant capacity of 13 plant extracts by three different methods: Cluster analyses applied for selection of the natural extracts with higher antioxidant capacity to replace synthetic antioxidant in lamb burgers. J. Food Sci. Technol. 2016, 53, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Almanza, S.G.; Moya, E.G. The uses of mesquite (Prosopis spp.) in the highlands of San Luis Potosi, Mexico. For. Ecol. Manag. 1986, 16, 49–56. [Google Scholar] [CrossRef]
- Prabha, D.S.; Dahms, H.U.; Malliga, P. Pharmacological potentials of phenolic compounds from Prosopis spp.—A review. J. Coast. Life Med. 2014, 2, 918–924. [Google Scholar]
- Kaur, R.; Arora, S.; Singh, B. Antioxidant activity of the phenol rich fractions of leaves of Chukrasia tabularis A. Juss. Bioresour. Technol. 2008, 99, 7692–7698. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Popova, M.; Bankova, V.; Butovska, D.; Petkov, V.; Nikolova-Damyanova, B.; Sabatini, A.G.; Marcazzan, G.L.; Bogdanov, S. Validated methods for the quantification of biologically active constituents of poplar-type propolis. Phytochem. Anal. 2004, 15, 235–240. [Google Scholar] [CrossRef]
- Molyneux, P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Geckil, H.; Ates, B.; Durmaz, G.; Erdogan, S.; Yilmaz, I. Antioxidant, free radical scavenging and metal chelating characteristics of propolis. Am. J. Biochem. Biotechnol. 2005, 1, 27–31. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis. In Association of Official Analytical Chemists, 18th ed.; Association of Official Analytical Chemists: Gaitherburg, MD, USA, 2005. [Google Scholar]
- Robertson, A.R.; Lozano, R.D.; Alman, D.H.; Orchard, S.E.; Keitch, J.A.; Connely, R.; Graham, L.A.; Acree, W.L.; John, R.S.; Hoban, R.F.; et al. CIE Recommendations on Uniform Color Spaces, Color-Difference Equations, and Metric Color Terms. Color Res. Appl. 1977, 2, 5–6. [Google Scholar]
- Stewart, M.R.; Zipser, M.W.; Watts, B.M. The use of reflectance spectrophotometry for assay of raw meat pigments. J. Food Sci. 1965, 30, 464–469. [Google Scholar] [CrossRef]
- Sutton, D.S.; Ellis, M.; Lan, Y.; McKeith, F.K.; Wilson, E.R. Influence of slaughter weight and stress gene genotype on the water-holding capacity and protein gel characteristics of three porcine muscles. Meat Sci. 1997, 46, 173–180. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Lipid oxidation: Measurement methods. In Bailey’s Industrial Oil and Fat Products; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Vyncke, W. Evaluation of the direct thiobarbituric acid extraction method for determining oxidative rancidity in mackerel. Fette Seifen Anstrichm 1975, 77, 239–240. [Google Scholar] [CrossRef]
- Huang, B.; He, J.; Ban, X.; Zeng, H.; Yao, X.; Wang, Y. Antioxidant activity of bovine and porcine meat treated with extracts from edible lotus (Nelumbo nucifera) rhizome knot and leaf. Meat Sci. 2011, 87, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M.; Batlle, R.; Gómez, M. Extension of the shelf-life of foal meat with two antioxidant active packaging systems. LWT-Food Sci. Technol. 2014, 59, 181–188. [Google Scholar] [CrossRef]
- García-Andrade, M.; González-Laredo, R.F.; Rocha-Guzmán, N.E.; Gallegos-Infante, J.A.; Rosales-Castro, M.; Medina-Torres, L. Mesquite leaves (Prosopis laevigata), a natural resource with antioxidant capacity and cardioprotection potential. Ind. Crops Prod. 2013, 44, 336–342. [Google Scholar] [CrossRef]
- NOM-003-SAG/GAN-2017 Propóleos, producción y especificaciones para su procesamiento. Available online: https://normateca.agricultura.gob.mx/sites/default/files/normateca/Documentos/norma_oficial_mexicana_nom_003_sag_gan_2017_propoleos_produccion_y_especificaciones_para_su_procesamiento.pdf (accessed on 1 September 2019).
- Serpen, A.; Gökmen, V.; Fogliano, V. Total antioxidant capacities of raw and cooked meats. Meat Sci. 2012, 90, 60–65. [Google Scholar] [CrossRef]
- Berker, K.I.; Güçlü, K.; Tor, İ.; Apak, R. Comparative evaluation of Fe(III) reducing power-based antioxidant capacity assays in the presence of phenanthroline, batho-phenanthroline, tripyridyltriazine (FRAP), and ferricyanide reagents. Talanta 2007, 72, 1157–1165. [Google Scholar] [CrossRef]
- Rodriíguez-Carpena, J.-G.; Morcuende, D.; Andrade, M.-J.; Kylli, P.; Estévez, M. Vocado (Persea americana Mill.) phenolics, in vitro antioxidant and antimicrobial activities, and inhibition of lipid and protein oxidation in porcine patties. J. Agric. Food Chem. 2011, 59, 5625–5635. [Google Scholar] [CrossRef]
- Al-Rimawi, F.; Abu-Lafi, S.; Abbadi, J.; Alamarneh, A.A.; Sawahreh, R.A.; Odeh, I. Analysis of phenolic and flavonoids of wild Ephedra alata plant extracts by LC/PDA and LC/MS and their antioxidant activity. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 130–141. [Google Scholar] [CrossRef]
- Shin, D.J.; Choe, J.; Hwang, K.E.; Kim, C.J.; Jo, C. Antioxidant effects of lotus (Nelumbo nucifera) root and leaf extracts and their application on pork patties as inhibitors of lipid oxidation, alone and in combination. Int. J. Food Prop. 2019, 22, 383–394. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Fernandes, R.D.P.P.; de Melo, M.P.; Trindade, M.A.; Lorenzo, J.M. Influence of peanut skin extract on shelf-life of sheep patties. Asian Pac. J. Trop. Biomed. 2016, 6, 586–596. [Google Scholar] [CrossRef]
- Huff-Lonergan, E.; Baas, T.J.; Malek, M.; Dekkers, J.C.M.; Prusa, K.; Rothschild, M.F. Correlations among selected pork quality traits. J. Anim. Sci. 2002, 80, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Bekhit, A.E.D.; Faustman, C. Metmyoglobin reducing activity. Meat Sci. 2005, 71, 407–439. [Google Scholar] [CrossRef] [PubMed]
- Girolami, A.; Napolitano, F.; Faraone, D.; Braghieri, A. Measurement of meat color using a computer vision system. Meat Sci. 2013, 93, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Tomasevic, I.; Tomovic, V.; Ikonic, P.; Lorenzo Rodriguez, J.M.; Barba, F.J.; Djekic, I.; Nastasijevic, I.; Stajic, S.; Zivkovic, D. Evaluation of poultry meat colour using computer vision system and colourimeter. Br. Food J. 2019, 121, 1078–1087. [Google Scholar] [CrossRef]
- Tomasevic, I.; Tomovic, V.; Milovanovic, B.; Lorenzo, J.; Đorđević, V.; Karabasil, N.; Djekic, I. Comparison of a computer vision system vs. traditional colorimeter for color evaluation of meat products with various physical properties. Meat Sci. 2018, 148, 5–12. [Google Scholar] [CrossRef]
- Brewer, M.; Zhu, L.; Bidner, B.; Meisinger, D.; McKeith, F. Measuring pork color: Effects of bloom time, muscle, pH and relationship to instrumental parameters. Meat Sci. 2001, 57, 169–176. [Google Scholar] [CrossRef]
- Jo, C.; Son, J.H.; Son, C.B.; Byun, M.W. Functional properties of raw and cooked pork patties with added irradiated, freeze-dried green tea leaf extract powder during storage at 4 °C. Meat Sci. 2003, 64, 13–17. [Google Scholar] [CrossRef]
- Greene, B.E.; Hsin, I.M.; Zipser, M.Y.W. Retardation of oxidative color changes in raw ground beef. J. Food Sci. 1971, 36, 940–942. [Google Scholar] [CrossRef]
- Inai, M.; Miura, Y.; Honda, S.; Masuda, A.; Masuda, T. Metmyoglobin reduction by polyphenols and mechanism of the conversion of metmyoglobin to oxymyoglobin by quercetin. J. Agric. Food Chem. 2014, 62, 893–901. [Google Scholar] [CrossRef]
- Mancini, S.; Paci, G.; Fratini, F.; Torracca, B.; Nuvoloni, R.; Dal Bosco, A.; Roscini, V.; Preziuso, G. Improving pork burgers’ quality using Zingiber officinale Roscoe powder (ginger). Meat Sci. 2017, 129, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M. Horsemeat as a source of valuable fatty acids. Eur. J. Lipid Sci. Technol. 2013, 115, 473–474. [Google Scholar] [CrossRef]
- Domínguez, R.; Purriños, L.; Pérez-Santaescolástica, C.; Pateiro, M.; Barba, F.J.; Tomasevic, I.; Campagnol, P.C.B.; Lorenzo, J.M. Characterization of Volatile Compounds of Dry-Cured Meat Products Using HS-SPME-GC/MS Technique. Food Anal. Methods 2019, 12, 1263–1284. [Google Scholar] [CrossRef]
- Gómez, M.; Lorenzo, J.M. Effect of fat level on physicochemical, volatile compounds and sensory characteristics of dry-ripened “chorizo” from Celta pig breed. Meat Sci. 2013, 95, 658–666. [Google Scholar] [CrossRef]
- Pateiro, M.; Franco, D.; Carril, J.A.; Lorenzo, J.M. Changes on physico-chemical properties, lipid oxidation and volatile compounds during the manufacture of celta dry-cured loin. J. Food Sci. Technol. 2014, 52, 4808–4818. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Bedia, M.; Bañón, S. Relationship between flavour deterioration and the volatile compound profile of semi-ripened sausage. Meat Sci. 2013, 93, 614–620. [Google Scholar] [CrossRef]
- Bermúdez, R.; Franco, D.; Carballo, J.; Lorenzo, J.M. Influence of type of muscle on volatile compounds throughout the manufacture of Celta dry-cured ham. Food Sci. Technol. Int. 2015, 21, 581–592. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Gómez, M.; Purriños, L.; Fonseca, S. Effect of commercial starter cultures on volatile compound profile and sensory characteristics of dry-cured foal sausage. J. Sci. Food Agric. 2016, 96, 1194–1201. [Google Scholar] [CrossRef]
- Rodríguez-Carpena, J.G.; Morcuende, D.; Estévez, M. Avocado by-products as inhibitors of color deterioration and lipid and protein oxidation in raw porcine patties subjected to chilled storage. Meat Sci. 2011, 89, 166–173. [Google Scholar] [CrossRef]
| Polyphenol Content | |
| TPC (mg GAE/g) | 278.5 ± 8.5 |
| TFC (mg RE/g) | 226.8 ± 8.3 |
| Antioxidant Activity | |
| DPPH• (%) | |
| 100 µg/mL | 85.3 ± 0.3 b |
| 50 µg/mL | 74.8 ± 4.0 a |
| 25 µg/mL | 68.9 ± 6.6 a |
| Asc (25 µg/mL) | 96.3 ± 2.3 c |
| BHT (50 µg/mL) | 69.3 ± 4.1 a |
| RP (absorbance at 700 nm) | |
| 100 µg/mL | 1.1 ± 0.2 b |
| Asc (25 µg/mL) | 1.4 ± 0.1 b |
| BHT (50 µg/mL) | 0.7 ± 0.2 a |
| Treatment | Moisture | Fat | Ash | Protein |
|---|---|---|---|---|
| CN | 66.98 ± 0.58 | 11.04 ± 0.89 | 1.67 ± 0.11 | 20.54 ± 0.74 |
| Asc | 67.59 ± 0.86 | 11.02 ± 0.50 | 1.68 ± 0.02 | 22.47 ± 0.54 |
| BHT | 67.47 ± 0.70 | 11.10 ± 0.49 | 1.66 ± 0.04 | 23.10 ± 0.45 |
| EEML1 | 67.06 ± 0.14 | 11.29 ± 1.08 | 1.67 ± 0.03 | 22.96 ± 0.13 |
| EEML2 | 66.89 ± 1.26 | 11.05 ± 0.83 | 1.68 ± 0.06 | 23.52 ± 0.87 |
| P-value | 0.765 | 0.992 | 0.995 | 0.246 |
| Item | Treat | Storage Time (days) | |||
|---|---|---|---|---|---|
| 0 | 3 | 7 | 10 | ||
| pH | CN | 5.86 ± 0.02 e | 5.78 ± 0.01 c | 5.76 ± 0.04 bc | 5.53 ± 0.01 a |
| Asc | 5.85 ± 0.05 e | 5.87 ± 0.02 e | 5.82 ± 0.01 d | 5.72 ± 0.03 b | |
| BHT | 5.87 ± 0.01 e | 5.88 ± 0.01 e | 5.86 ± 0.04 e | 5.76 ± 0.01 bc | |
| EEML1 | 5.92 ± 0.01 f | 5.88 ± 0.01 e | 5.88 ± 0.01 e | 5.84 ± 0.02 e | |
| EEML2 | 5.91 ± 0.01 f | 5.88 ± 0.01 e | 5.87 ± 0.02 e | 5.85 ± 0.01 e | |
| L* | CN | 53.37 ± 0.50 a | 53.06 ± 0.35 a | 53.22 ± 0.41 a | 56.60 ± 0.77 b |
| Asc | 53.82 ± 0.88 a | 54.68 ± 0.56 a | 54.19 ± 0.35 a | 54.25 ± 1.22 a | |
| BHT | 54.63 ± 0.79 a | 54.39 ± 1.02 a | 54.35 ± 0.29 a | 53.75 ± 0.50 a | |
| EEML1 | 54.52 ± 0.48 a | 54.31 ± 0.75 a | 53.99 ± 0.65 a | 54.40 ± 0.56 a | |
| EEML2 | 54.39 ± 0.58 a | 53.73 ± 0.41 a | 53.92 ± 0.22 a | 54.03 ± 0.80 a | |
| a* | CN | 15.78 ± 1.08 d | 15.74 ± 1.22 d | 14.03 ± 0.46 cd | 7.23 ± 0.40 a |
| Asc | 15.66 ± 0.44 d | 14.14 ± 0.25 cd | 14.88 ± 0.51 cd | 9.99 ± 0.71 b | |
| BHT | 14.82 ± 1.06 cd | 14.73 ± 0.76 cd | 13.78 ± 0.96 c | 9.05 ± 0.79 b | |
| EEML1 | 12.61 ± 1.25 c | 12.59 ± 1.14 c | 12.79 ± 1.13 c | 10.09 ± 0.76 b | |
| EEML2 | 10.19 ± 1.41 bc | 10.56 ± 0.71 bc | 10.31 ± 0.37 b | 9.18 ± 0.41 b | |
| b* | CN | 15.01 ± 0.99 a | 16.02 ± 1.10 a | 16.77 ± 0.28 a | 17.37 ± 0.15 b |
| Asc | 15.18 ± 0.58 a | 15.02 ± 1.08 a | 15.54 ± 0.32 a | 14.52 ± 0.68 a | |
| BHT | 15.32 ± 1.05 a | 15.90 ± 0.90 a | 15.96 ± 0.78 a | 14.97 ± 0.92 a | |
| EEML1 | 20.75 ± 1.02 c | 21.45 ± 1.10 c | 21.56 ± 0.84 c | 20.14 ± 0.66 c | |
| EEML2 | 25.94 ± 0.10 e | 25.74 ± 0.44 e | 24.40 ± 0.93 de | 22.67 ± 1.11 cd | |
| C* | CN | 21.47 ± 1.13 bc | 19.83 ± 1.69 b | 19.73 ± 0.89 b | 15.37 ± 0.47 a |
| Asc | 21.71 ± 0.61 b | 20.83 ± 2.03 b | 21.87 ± 0.39 b | 16.87 ± 0.89 a | |
| BHT | 20.66 ± 1.57 b | 21.92 ± 1.02 bc | 20.43 ± 2.02 b | 15.73 ± 1.16 a | |
| EEML1 | 23.61 ± 1.61 bc | 23.45 ± 1.07 bc | 23.96 ± 0.58 c | 21.77 ± 1.74 b | |
| EEML2 | 26.53 ± 1.67 d | 26.97 ± 0.60 d | 26.06 ± 0.94 d | 23.61 ± 1.06 bc | |
| H* | CN | 43.57 ± 0.95 a | 44.52 ± 1.24 a | 46.47 ± 1.34 a | 55.69 ± 1.09 b |
| Asc | 43.82 ± 0.53 a | 42.53 ± 1.70 a | 45.50 ± 0.38 a | 53.25 ± 1.10 b | |
| BHT | 44.50 ± 1.15 a | 45.83 ± 1.06 a | 46.40 ± 1.30 a | 56.15 ± 1.60 b | |
| EEML1 | 61.23 ± 2.17 c | 60.19 ± 1.20 c | 60.4+ ± 2.07 c | 54.56 ± 1.19 b | |
| EEML2 | 70.05 ± 1.49 d | 70.50 ± 1.46 d | 69.59 ± 1.90 d | 72.93 ± 1.64 d | |
| MMb | CN | 1.00 ± 0.18 a | 7.90 ± 0.45 b | 71.22 ± 3.10 h | 91.78 ± 2.93 k |
| Asc | 1.70 ± 0.70 a | 14.67 ± 1.30 c | 71.70 ± 1.38 h | 70.12 ± 2.43 h | |
| BHT | 1.40 ± 0.27 a | 8.51 ± 1.98 b | 79.83 ± 2.11 i | 80.97 ± 2.19 j | |
| EEML1 | 1.40 ± 0.18 a | 6.03 ± 0.60 b | 28.75 ± 2.65 e | 41.15 ± 2.63 g | |
| EEML2 | 1.20 ± 0.27 a | 7.73 ± 1.46 b | 17.19 ± 1.26 d | 34.81 ± 2.68 f | |
| WHC | CN | 95.83 ± 0.85 b | 95.08 ± 0.62 b | 95.77 ± 1.00 b | 91.20 ± 0.88 a |
| Asc | 94.26 ± 0.90 b | 94.80 ± 0.91 b | 94.61 ± 1.05 b | 92.20 ± 0.64 a | |
| BHT | 94.92 ± 1.34 b | 95.57 ± 1.01 b | 94.98 ± 0.22 b | 95.42 ± 0.59 b | |
| EEML1 | 95.04 ± 0.80 b | 95.34 ± 1.65 b | 95.68 ± 1.36 b | 95.86 ± 0.47 b | |
| EEML2 | 94.61 ± 0.98 b | 95.25 ± 0.32 b | 95.75 ± 0.27 b | 95.96 ± 0.26 b | |
| Item | Treatments | P-Value | ||
|---|---|---|---|---|
| CN | EEML1 | EEML2 | ||
| Raw patties | ||||
| Colour | 8.8 ± 1.4 c | 5.5 ± 1.2 b | 4.3 ± 1.3 a | 0.013 |
| Appearance | 6.1 ± 1.0 | 5.6 ± 1.0 | 5.4 ± 0.4 | 0.114 |
| Cooked patties | ||||
| Colour | 5.4 ± 1.0 | 5.1 ± 1.1 | 4.7 ± 1.4 | 0.775 |
| Appearance | 5.7 ± 1.1 | 5.4 ± 1.0 | 4.8 ± 1.4 | 0.656 |
| Odour | 5.4 ± 1.3 | 4.7 ± 1.2 | 5.6 ± 1.2 | 0.663 |
| Flavour | 5.5 ± 1.2 | 5.4 ± 1.2 | 4.7 ± 1.2 | 0.689 |
| Juiciness | 4.8 ± 1.2 | 5.6 ± 0.7 | 4.7 ± 1.3 | 0.576 |
| Fat sensation | 5.5 ± 1.5 | 5.5 ± 1.0 | 4.7 ± 1.4 | 0.706 |
| Firmness | 5.3 ± 1.4 | 5.7 ± 1.2 | 5.1 ± 1.3 | 0.852 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Rojo, M.I.; Vargas-Sánchez, R.D.; Torres-Martínez, B.d.M.; Torrescano-Urrutia, G.R.; Lorenzo, J.M.; Sánchez-Escalante, A. Inclusion of Ethanol Extract of Mesquite Leaves to Enhance the Oxidative Stability of Pork Patties. Foods 2019, 8, 631. https://doi.org/10.3390/foods8120631
Ramírez-Rojo MI, Vargas-Sánchez RD, Torres-Martínez BdM, Torrescano-Urrutia GR, Lorenzo JM, Sánchez-Escalante A. Inclusion of Ethanol Extract of Mesquite Leaves to Enhance the Oxidative Stability of Pork Patties. Foods. 2019; 8(12):631. https://doi.org/10.3390/foods8120631
Chicago/Turabian StyleRamírez-Rojo, Margarita Irene, Rey David Vargas-Sánchez, Brisa del Mar Torres-Martínez, Gastón Ramón Torrescano-Urrutia, José Manuel Lorenzo, and Armida Sánchez-Escalante. 2019. "Inclusion of Ethanol Extract of Mesquite Leaves to Enhance the Oxidative Stability of Pork Patties" Foods 8, no. 12: 631. https://doi.org/10.3390/foods8120631
APA StyleRamírez-Rojo, M. I., Vargas-Sánchez, R. D., Torres-Martínez, B. d. M., Torrescano-Urrutia, G. R., Lorenzo, J. M., & Sánchez-Escalante, A. (2019). Inclusion of Ethanol Extract of Mesquite Leaves to Enhance the Oxidative Stability of Pork Patties. Foods, 8(12), 631. https://doi.org/10.3390/foods8120631






