Brosimum alicastrum Sw. (Ramón): An Alternative to Improve the Nutritional Properties and Functional Potential of the Wheat Flour Tortilla
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Tortilla Development
2.3. Proximate Composition, pH, Activity Water (Aw) and Titratable Acidity
2.4. Extraction and Quantification of Minerals
2.5. Extraction and Quantification of Carotenoids
2.6. Extraction and Quantification of Ascorbic Acid
2.7. Sensory Characterization
2.8. Consumer Acceptance Test
2.9. Rheological Measurements
2.10. Content of Total Phenolic Compounds and Flavonoids
2.11. Antioxidant Capacity
2.12. Identification of Individual Phenolic Compounds in Ramón Seed Flour (RSF) by Ultra-Performance Liquid Chromatography Quadrupole Time of Flight Mass Spectrometry (UPLC-QTOF-MS)
2.13. Statistical Analysis
3. Results and Discussion
3.1. Tortilla Preparation and Food Safety
3.2. Physicochemical and Micronutrient Characterization
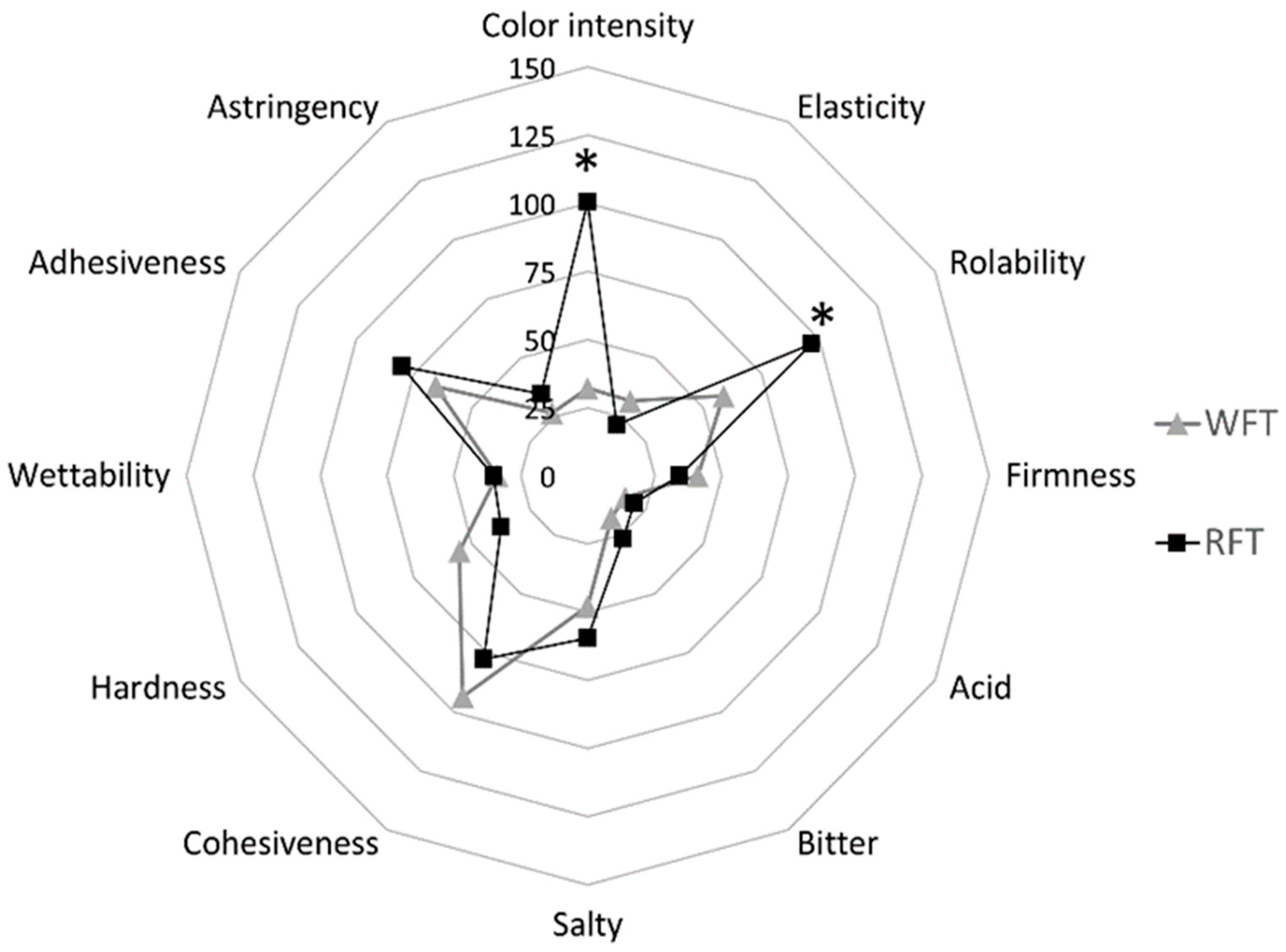
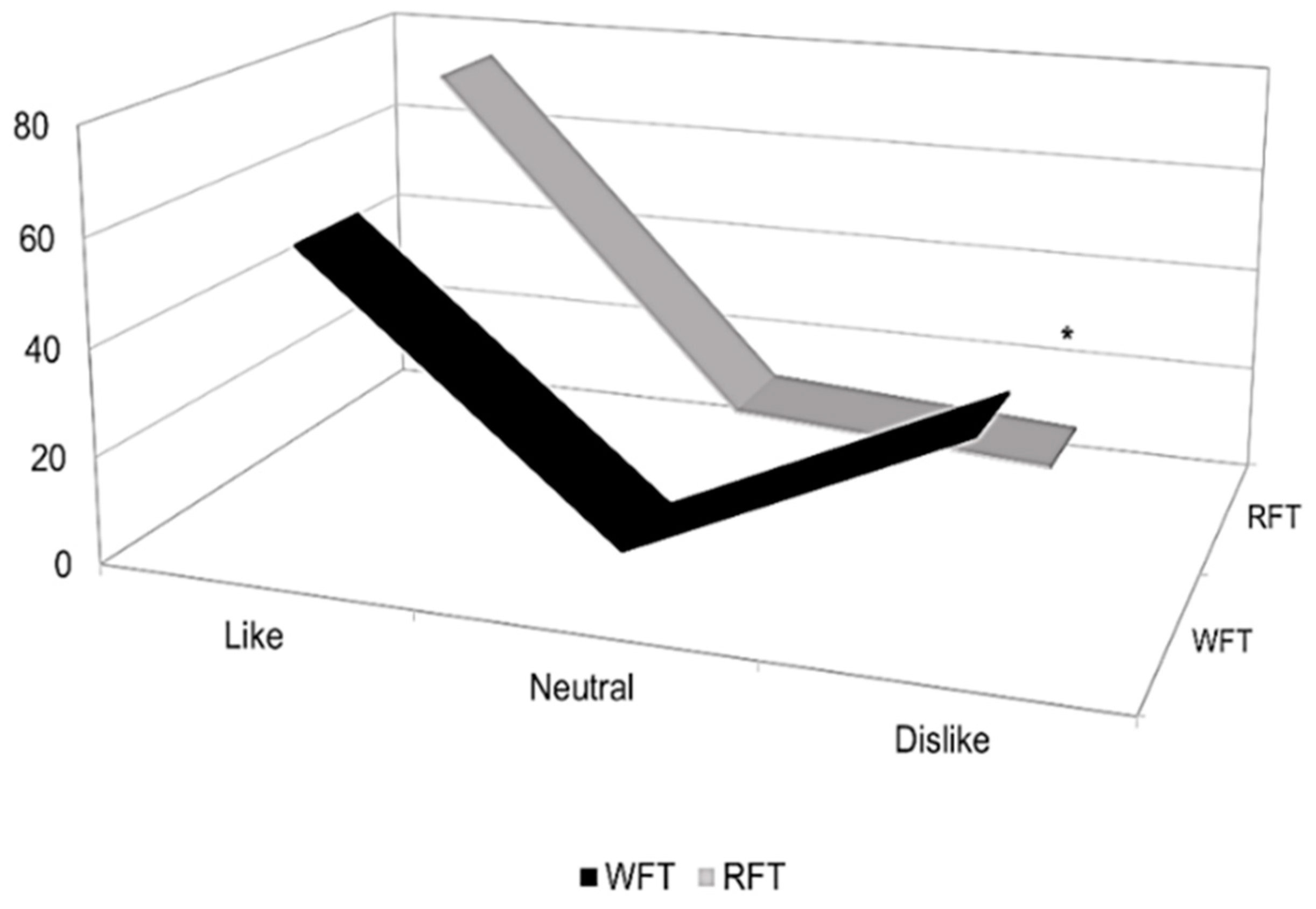
3.3. Sensory Attributes and Consumer Acceptance
3.4. Rheological Characterization
3.5. Polyphenolic Quantification and Antioxidant Capacity
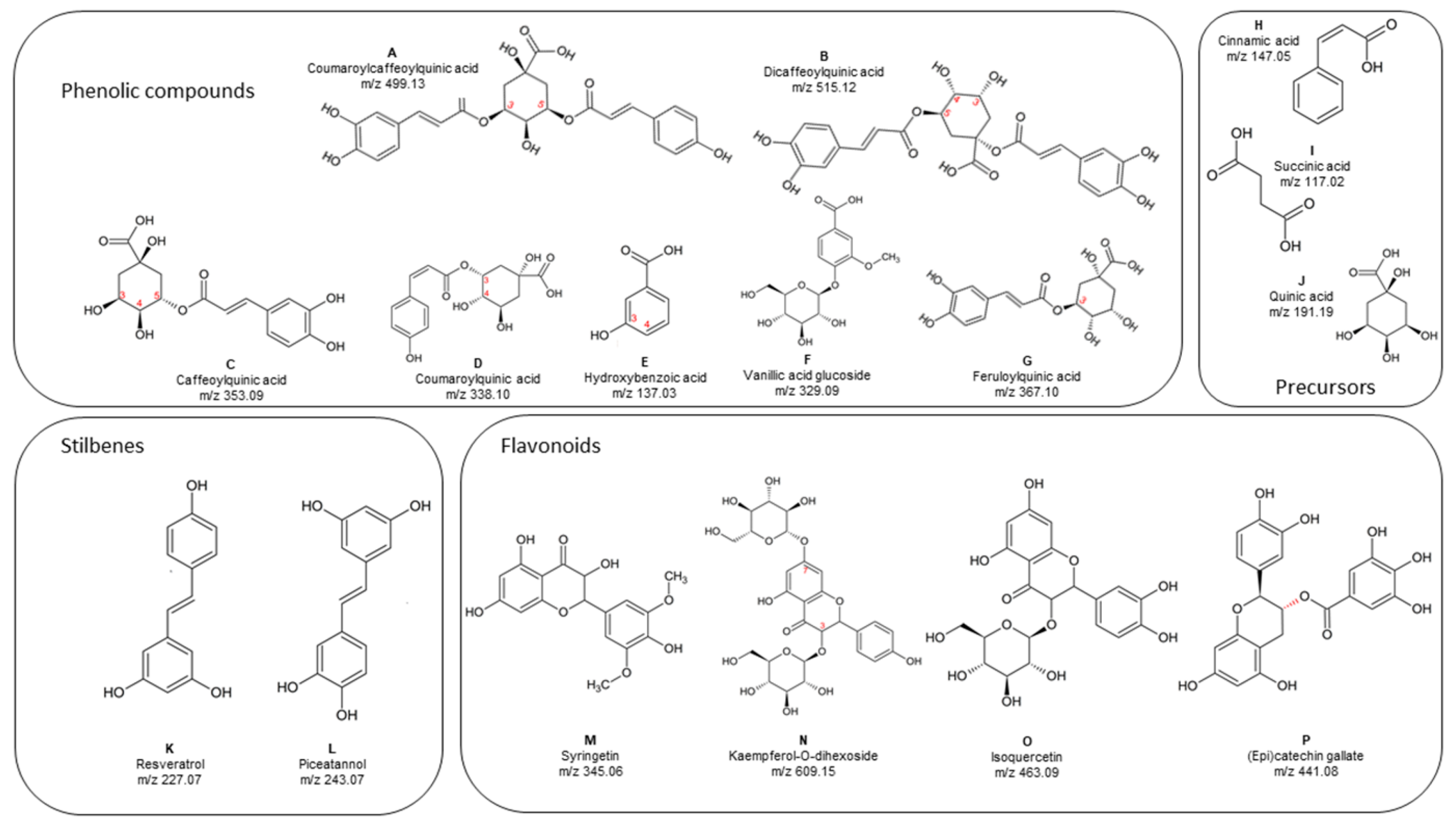
3.6. Identification of Individual Phenolic Compounds in RSF by UPLC-QTOF-MS
4. Conclusions
5. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Solano, R. GRUMA: Inicio de Cobertura. Available online: https://www.monex.com.mx/portal/download/reportes/Inicio%20de%20Cobertura%20de%20Gruma%20(Septiembre%202018).pdf (accessed on 12 December 2018).
- CEDRSSA Consumo. Distribución y Producción de Alimentos: El Caso Del Complejo Maíz-Tortilla; Centro de Estudios para el Desarrollo Rural Sustentable y la Soberanía Alimentaria: Ciudad de México, Mexico, 2014.
- Calleja, M.; Basilia, M. La tortilla como identidad culinaria y producto de consumo global. Región Soc. 2016, 28, 161–194. [Google Scholar] [CrossRef]
- USDA. Full Report (All Nutrients) 12005, Seeds, Breadnut Tree Seeds, Dried; U.S. Department of Agriculture: Washington, DC, USA, 2016.
- Rivera, J.Á. Deficiencias de micronutrimentos en México: Un problema invisible de salud pública. Salud Pública Mex. 2012, 54, 101–102. [Google Scholar]
- Levy, T.; Amaya, M.A.; Cuevas, L. Desnutrición y obesidad: Doble carga en México. Rev. Digit. Univ. 2015, 16, 1–17. [Google Scholar]
- Peláez, R.B. Desnutrición y enfermedad. Nutr. Hosp. 2013, 6, 10–23. [Google Scholar]
- Carter, C.T. Chemical and Functional Properties of Brosimum alicastrum Seed Powder (Maya Nut, Ramón Nut). Master’s Thesis, Clemson University, Clemson, SC, USA, 2015. [Google Scholar]
- Larqué, A. El sector forestal en apoyo a la “Cruzada contra el Hambre” La inclusión de Brosimum alicastrum (Ramón) como estudio de caso. For. XXI 2014, 11, 11–12. [Google Scholar]
- Waniska, R.D. Processing of flour tortillas. In Tortillas Wheat Flour and Corn Products; Rooney, L.W., Serna-Saldivar, S.O., Eds.; AACC International: Washington, DC, USA, 2016; pp. 125–145. [Google Scholar]
- Secretaria de Salud Norma Oficial Mexicana NOM-247-SSA1-2008, Productos y Servicios. Cereales y Sus Productos. Cereales, Harinas de Cereales, Sémolas o Semolinas. Alimentos a Base de: Cereales, Semillas Comestibles, de Harinas, Sémolas o Semolinas o Sus Mezclas; Gobierno Federal: Ciudad de México, Mexico, 2008.
- AOAC. Official Methods of Analysis of AOAC International, 17th ed.; Horwitz, W., Ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2000.
- Moreno-Escamilla, J.O.; Alvarez-Parrilla, E.; De La Rosa, L.A.; Núñez-Gastélum, J.A.; González-Aguilar, G.A.; Rodrigo-García, J. Effect of Different Elicitors and Preharvest Day Application on the Content of Phytochemicals and Antioxidant Activity of Butterhead Lettuce (Lactuca sativa var. capitata) Produced under Hydroponic Conditions. J. Agric. Food Chem. 2017, 65, 5244–5254. [Google Scholar] [CrossRef]
- Alvarez-Parrilla, E.; de la Rosa, L.A.; Amarowicz, R.; Shahidi, F. Antioxidant Activity of Fresh and Processed Jalapeño and Serrano Peppers. J. Agric. Food Chem. 2011, 59, 163–173. [Google Scholar] [CrossRef]
- Meilgaard, M.; Civille, G.V.; Carr, T.B. Sensory Evaluation Techniques, 5th ed.; CRC Press: New York, NY, USA, 2015. [Google Scholar]
- Lawless, H.; Heymann, H. Sensory Evaluation of Food Principles and Practices, 2nd ed.; Springer: New York, NY, USA, 2010. [Google Scholar]
- Reyes-Vega, M.L.; Peralta-Rodríguez, R.D.; Anzaldúa-Morales, A.; Figueroa-Cárdenas, J.D.; Martínez-Bustos, F. Relating Sensory Textural Attributes of Corn Tortilla to Some Instrumental Measurements. J. Texture Stud. 1998, 29, 361–373. [Google Scholar] [CrossRef]
- Flores-Farías, R.; Martínez-Bustos, F.; Salinas-Moreno, Y.; Chang, Y.K.; Hernández, J.G.; Ríos, E. Physicochemical and rheological characteristics of commercial nixtamalised Mexican maize flours for tortillas. J. Sci. Food Agric. 2000, 80, 657–664. [Google Scholar] [CrossRef]
- Kelekei, N.N.; Pascut, S.; Waniska, R.D. The effects of storage temperature on the staling of wheat flour tortillas. J. Cereal Sci. 2003, 37, 377–380. [Google Scholar] [CrossRef]
- Suhendro, E.L. Tortilla bending technique: An objective method for corn tortilla texture measurement. Cereal Chem. 1998, 75, 854–858. [Google Scholar] [CrossRef]
- de la Rosa, L.A.; Alvarez-Parrilla, E.; Shahidi, F. Phenolic compounds and antioxidant activity of kernels and shells of Mexican pecan (Carya illinoinensis). J. Agric. Food Chem. 2011, 59, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Torres-Aguirre, G.A.; Muñoz-Bernal, Ó.A.; Álvarez-Parrilla, E.; Núñez-Gastélum, J.A.; Wall-Medrano, A.; Sáyago-Ayerdi, S.G.; de la Rosa, L.A. Optimización de la extracción e identificación de compuestos polifenólicos en anís (Pimpinella anisum), clavo (Syzygium aromaticum) y cilantro (Coriandrum sativum) mediante HPLC acoplado a espectrometría de masas. TIP Rev. Espec. Cienc. Químico-Biol. 2018, 21, 103–115. [Google Scholar]
- Ramírez-Sánchez, S.; Ibáñez-Vázquez, D.; Gutiérrez-Peña, M.; Ortega-Fuentes, M.S.; García-Ponce, L.L.; Larqué-Saavedra, A. El ramón (Brosimum alicastrum Swartz) una alternativa para la seguridad alimentaria en México. AGRO Product. 2017, 10, 80–83. [Google Scholar]
- Raza, H.; Pasha, I.; Shoaib, M.; Zaaboul, F.; Niazi, S.; Aboshora, W. Review on functional and rheological attributes of kafirin for utilization in gluten free baking industry. J. Food Sci. Nutr. Res. 2017, 4, 150–157. [Google Scholar]
- Remondetto, G.; Añón, M.C.; González, R.J. Hydration Properties of Soybean Protein Isolates. Braz. Arch. Biol. Technol. 2001, 44, 425–431. [Google Scholar] [CrossRef]
- Bullermann, L.B.; Bianchini, A. The microbiology of Cereal and Cereal Products. Food Qual. 2011, 18, 26–29. [Google Scholar]
- Cravioto, R.; Massieu, G.; Guzman, G.G.; Calvo, D.J. Valor nutritivo de plantas alimenticias de Yucatán. Boletín. Sanit. Panam. Nutr. 1952, 32, 328–339. [Google Scholar]
- FDA Title 21 Food and Drugs-Food Labeling. Code of Federal Regulations. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=101.54 (accessed on 16 November 2019).
- Cruz-Góngora, V.; De Villalpando, S.; Mundo-Rosas, V.; Shamah-Levy, T. Prevalencia de anemia en niños y adolescentes mexicanos: Comparativo de tres encuestas nacionales. Salud Publica Mex. 2013, 55, 180–189. [Google Scholar]
- Frei, B.; Higdon, J.V. Antioxidant activity of tea polyphenols in vivo: Evidence from animal studies. J. Nutr. 2003, 133, 3275–3284. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.M. Potassium and Health. Am. Soc. Nutr. 2013, 4, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Ringer, J.; Bartlett, Y. The significance of potassium. Pharm. J. 2007, 278, 497–500. [Google Scholar]
- USDA. Food Data Central; U.S. Department of Agriculture: Washington, DC, USA, 2018.
- Guevara-Arauza, J.C.; Órnelas Paz, J.J.; Rosales Mendoza, S.; Soria Guerra, R.E.; Paz Maldonado, L.M.T.; Pimentel González, D.J. Biofunctional activity of tortillas and bars enhanced with nopal. Preliminary assessment of functional effect after intake on the oxidative status in healthy volunteers. Chem. Cent. J. 2011, 5, 1–10. [Google Scholar] [CrossRef]
- Escudero, E.; González, P. La fibra dietética. Nutr. Hosp. 2006, 21, 61–72. [Google Scholar]
- García-Estévez, I.; Alcalde-Eon, C.; Puente, V.; Escribano-Bailón, M.T. Enological tannin effect on red wine color and pigment composition and relevance of the yeast fermentation products. Molecules 2017, 22, 2046. [Google Scholar] [CrossRef]
- Miranda, G.; Ventura, J.; Suárez, S.; Fuertes, C. Actividad citotóxica y antioxidante de los productos de la reacción de Maillard de los sistemas modelo D-glucosa-glicina y D-glucosa-L-lisina. Rev. Soc. Quím. Perú. 2007, 4, 215–225. [Google Scholar]
- Ribeiro, F.A. Wheat Flour Tortilla: Quality Prediction and Study of Physical and Textural Changes during Storage. Master’s Thesis, Texas A&M University, Calgary, TX, USA, 2009. [Google Scholar]
- Martínez-Vázquez, J.I.; Pérez-Carrera, S.N.; Quiroz-Ramírez, M.A.; Espitia-Orozco, F.J. Mejoramiento de la calidad proteica de toretillas hechas a base de maíz adicionadas con soya y amaranto. Investig. Desarro. Cienc. Tecnol. Aliment. 2017, 2, 312–316. [Google Scholar]
- Domínguez-Zarate, P.A.; García-Martínez, I.; Güemes-Vera, N.; Totosaus, A. Textura, color y aceptación sensorial de tortillas y pan producidos con harina de ramón (Brosimum alicastrum) para incrementar la fibra dietética total. Cienc. Tecnol. Agropecu. 2019, 20, 699–719. [Google Scholar] [CrossRef]
- Bourne, M. Food Texture and Viscosity: Concept and Measurement, 2nd ed.; Taylor, S.L., Ed.; Academic Press: New York, NY, USA, 2002. [Google Scholar]
- Vázquez, J.A. Desarrollo de Tortillas de Maíz Fortificadas Con Fuentes de Proteína y Fibra y Su Efecto Biológico en Un Modelo Animal. Ph.D. Thesis, Universidad Autónoma de Nuevo León, Autónoma de Nuevo León, Mexico, 2013. [Google Scholar]
- Rodríguez, E.; Fernández, A.; Ayala, A. Reología y textura de masas: Aplicaciones en trigo y maíz. Rev. Ing. Investig. 2005, 57, 72–75. [Google Scholar]
- Pérez-Pacheco, E.; Moo-Huchin, V.M.; Estrada-León, R.J.; Ortiz-Fernández, A.; May-Hernández, L.H.; Ríos-Soberanis, C.R.; Betancur-Ancona, D. Isolation and characterization of starch obtained from Brosimum alicastrum Swarts Seeds. Carbohydr. Polym. 2014, 101, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Hleap, J.I.; Velasco, V.A. Análisis de las propieades de textura durante el almacenamiento de salchcihas elaboradas a partir de tilapia roja (Oreochromis sp.). Biotecnol. Sect. Agropecu. Agroind. 2010, 8, 46–56. [Google Scholar]
- de la Vega, G. Proteínas de la harina de trigo: Clasificación y propiedades funcionales. Temas Cienc. Tecnol. 2009, 13, 27–32. [Google Scholar]
- Edwards, W.P. The Science of Bakery Products; The Royal Society of Chemistry: Cambridge, UK, 2007. [Google Scholar]
- Mesas, J.M.; Alegre, M.T. El pan y su proceso de elaboración. Cienc. Tecnol. Aliment. 2002, 3, 307–313. [Google Scholar] [CrossRef][Green Version]
- Wannerberger, L.; Eliasson, A.; Sindberg, A. Interfacial behaviour of secalin and rye flour-milling streams in comparison with gliadin. J. Cereal Sci. 1997, 25, 243–252. [Google Scholar] [CrossRef]
- Badui, S. Química de los Alimentos, 5th ed.; López Ballesteros, G., Ed.; Pearson Educación: Naucalpan de Juárez, Estado de México, Mexico, 2012. [Google Scholar]
- Moo-Huchin, V.M.; Cabrera-Sierra, M.J.; Estrada-León, R.J.; Ríos-Soberanis, C.R.; Betancur-Ancona, D.; Chel-Guerrero, L.; Ortiz-Fernández, A.; Estrada-Mota, I.A.; Pérez-Pacheco, E. Determination of some physicochemical and rheological characteristics of starch obtained from Brosimum alicastrum swartz seeds. Food Hydrocoll. 2015, 45, 48–54. [Google Scholar] [CrossRef]
- Tokpunar, H.K. Chemical Composition and Antioxidant Properties of Maya Nut (Brosimum alicastrum). Master’s Thesis, Clemson University, Clemson, SC, USA, 2010. [Google Scholar]
- Chen, O.; Blumberg, J. Phytochemical composition of nuts. Asia Pac. J. Clin. Nutr. 2008, 17, 329–332. [Google Scholar]
- Marinova, D.; Ribarova, F.; Atanassova, M. Total phenolics and flavonoids in Bulgarian fruits and vegetables. J. Univ. Chem. Technol. Metall. 2005, 40, 255–260. [Google Scholar]
- Sariburun, E.; Sahin, S.; Demir, C.; Türkben, C.; Uylaser, V. Phenolic content and antioxidant activity of raspberry and blackberry cultivars. J. Food Sci. 2010, 75, 328–335. [Google Scholar] [CrossRef]
- Alasalvar, C.; Shahidi, F. Natural antioxidants in tree nuts. Eur. J. Lipid Sci. Technol. 2009, 111, 1056–1062. [Google Scholar] [CrossRef]
- Pellegrini, N.; Serafini, M.; Salvatore, S.; Del Rio, D.; Bianchi, M.; Brighenti, F. Total antioxidant capacity of spices, dried fruits, nuts, pulses, cereals and sweets consumed in Italy assessed by three different in vitro assays. Mol. Nutr. Food Res. 2006, 50, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Kuskoski, E.M.; Asuero, A.G.; Troncoso, A.M.; Mancini-Filho, J.; Fett, R. Aplicación de diversos métodos químicos para determinar actividad antioxidante en pulpa de frutos. Ciência Tecnol. Aliment. 2005, 25, 726–732. [Google Scholar] [CrossRef]
- Clifford, M.N.; Jaganath, I.B.; Ludwig, I.A.; Crozier, A. Chlorogenic acids and the acyl-quinic acids: Discovery, biosynthesis, bioavailability and bioactivity. Nat. Prod. Rep. 2017, 34, 1391–1421. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.I.; Sousa, M.J.; Alves, R.C.; Ferreira, I.C.F.R. Exploring plant tissue culture to improve the production of phenolic compounds: A review. Ind. Crops Prod. 2016, 82, 9–22. [Google Scholar] [CrossRef]
- Ozer, H.K. Phenolic compositions and antioxidant activities of Maya nut (Brosimum alicastrum): Comparison with commercial nuts. Int. J. Food Prop. 2017, 20, 2772–2781. [Google Scholar] [CrossRef]
- Vazquez-Flores, A.A.; Wong-Paz, J.E.; Lerma-Herrera, M.A.; Martinez-Gonzalez, A.I.; Olivas-Aguirre, F.J.; Aguilar, C.N.; Wall-Medrano, A.; Gonzalez-Aguilar, G.A.; Alvarez-Parrilla, E.; de la Rosa, L.A. Proanthocyanidins from the kernel and shell of pecan (Carya illinoinensis): Average degree of polymerization and effects on carbohydrate, lipid, and peptide hydrolysis in a simulated human digestive system. J. Funct. Foods 2017, 28, 227–234. [Google Scholar] [CrossRef]
- Choi, J.Y.; Lee, J.W.; Jang, H.; Kim, J.G.; Lee, M.K.; Jong, J.T.; Lee, M.S.; Hwang, B.Y. Quinic acid esters from Erycibe obtusifolia with antioxidant and tyrosinase inhibitory activities. Nat. Prod. Res. 2019. [Google Scholar] [CrossRef]
- Dybkowska, E.; Sadowska, A.; Świderski, F.; Rakowska, R.; Wysocka, K. The occurrence of resveratrol in foodstuffs and its potential for supporting cancer prevention and treatment. A review. Rocz. Panstw. Zakl. Hig. 2018, 69, 5–14. [Google Scholar]

| Parameter | RSF | WF | RFT | WFT |
|---|---|---|---|---|
| Calories (Kcal) | 336 | 349 | 350 | 357 |
| Water (%) | 13.3 ± 0.14 a | 12.9 ± 0.02 b | 25.0 ± 0.07 a | 22.1 ± 0.10 b |
| Protein (%) | 11.5 ± 0.39 a | 9.6 ± 0.16 b | 7.3 ± 0.11 a | 7.6 ± 0.10 a |
| Fat (%) | 0.6 ± 0.00 a | 0.6 ± 0.01 a | 9.6 ± 0.08 a | 8.9 ± 0.02 b |
| Ashes (%) | 3.4 ± 0.11 a | 0.6 ± 0.02 b | 3.4 ± 0.06 a | 3.1 ± 0.02 b |
| Total Carbohydrates (%) | 71.2 ± 0.56 b | 76.3 ± 0.17 a | 54.6 ± 0.16 b | 58.3 ± 0.15 a |
| Crude Fiber (%) | 3.4 ± 0.13 a | 0.4 ± 0.00 b | 0.9 ± 0.15 a | 0.2 ± 0.01 b |
| Dietary Fiber (%) | 13.0 ± 0.21 a | 1.6 ± 0.00 b | 3.6 ± 0.20 a | 0.8 ± 0.01 b |
| pH | 5.5 ± 0.01 a | 5.9 ± 0.01 b | 6.3 ± 0.01 b | 6.6 ± 0.02 a |
| Activity water (Aw) | 0.3 ± 0.02 a | 0.2 ± 0.01 b | 0.9 ± 0.02 a | 0.9 ± 0.01 a |
| Titratable acidity (% CAE) * | 0.004 ± 0.00 a | 0.001 ± 0.00 b | 0.002 ± 0.00 a | 0.001 ± 0.00 b |
| Cupper (mg/100 g) | 0.5 ± 0.10 a | 0.2 ± 0.00 b | 0.3 ± 0.00 a | 0.2 ± 0.00 b |
| Potassium (mg/100 g) | 1256.0 ± 12.00 a | 159.0 ± 5.00 b | 367.6 ± 13.00 a | 210.0 ± 10.00 b |
| Iron (mg/100 g) | 4.0 ± 0.70 a | 5.0 ± 0.20 a | 3.9 ± 0.20 a | 4.1 ± 1.20 a |
| Zinc (mg/100 g) | 1.0 ± 0.10 b | 6.0 ± 0.10 a | 4.5 ± 1.60 a | 4.9 ± 0.00 a |
| Sodium (mg/100 g) | 47.0 ± 0.10 a | 20.0 ± 0.10 b | 369.2 ± 0.40 b | 378.0 ± 0.60 a |
| Vitamin C mg (Ascorbic acid/100g) | 2.3 ± 0.01 a | 0.6 ± 0.06 b | 0.9 ± 0.09 a | 0.7 ± 0.03 a |
| Carotenoids (mg β-carotenoids/100 g) | 1.2 ± 0.10 a | 0.9 ± 0.10 a | 1.0 ± 0.00 a | 0.9 ± 0.00 a |
| Characteristic | RSF Dough | WF Dough |
|---|---|---|
| Gumminess (N) | 10.2 ± 3.2 a | 39.1 ± 1.0 b |
| Hardness (N) | 34.1 ± 10.0 a | 60.5 ± 14.0 a |
| Adhesiveness (J) | 0.03 ± 0.0 a | 0.003 ± 0.0 b |
| Elasticity (mm) | 1.8 ± 0.0 a | 1.6 ± 0.1 b |
| Chewiness (Nm) | 1.9 ± 0.6 a | 6.3 ± 1.5 b |
| Cohesiveness (Nm) | 3.5 ± 0.1 a | 4.0 ± 0.1 b |
| Characteristic | RFT | WFT |
|---|---|---|
| Cutting force (N) | −0.8 ± 0.1 a | 2.6 ± 0.3 b |
| Cutting work (J) | 0.04 ± 0.0 a | 0.15 ± 0.0 b |
| Elongation force (N) | 0.3 ± 0.1 a | 3.1 ±0 0.0 b |
| Elongation distance (mm) | 3.9 ± 0.4 a | 4.1 ± 0.5 a |
| Extensibility max force (N) | 0.8 ± 0.0 a | 1.5 ± 0.2 b |
| Rupture distance (mm) | 3.3 ± 0.3 a | 3.2 ± 1.0 a |
| Cohesiveness (N) | 3.6 ± 0.2 a | 4.0 ± 0.0 b |
| Work to max extension (J) | 0.002 ± 0.0 a | 0.004 ± 0.0 b |
| Antioxidant Capacity | |||||
|---|---|---|---|---|---|
| Samples | TPC | TF | DPPH | ABTS | FRAP |
| mg GAE/g | mg CE/g | mmol TEAC/100 g | mmol TEAC/100 g | mmol TEAC/100 g | |
| RSF | 65.8 ± 2.26 a | 4.4 ± 0.18 a | 0.9 ± 0.09 a | 14.3 ± 0.10 a | 0.41 ± 0.04 a |
| WF | 0.9 ± 0.02 b | 0.1 ± 0.01 b | 0.0 ± 0.01 b | 0.3 ± 0.09 b | 0.04 ± 0.00 b |
| RFT | 21.1 ± 1.50 a | 0.7 ± 0.10 a | 0.3 ± 0.01 a | 0.4 ± 0.01 a | 0.04 ± 0.00 a |
| WFT | 1.8 ± 0.02 b | 0.5 ± 0.10 a | 0.2 ± 0.01 b | 0.2 ± 0.00 b | 0.04 ± 0.01 a |
| Compound | Tentative Identification | Formula | Rt (min) | m/z [M − H]− | Measured Mass | Exact Mass | Δm ppm | Abundance |
|---|---|---|---|---|---|---|---|---|
| 1 | Vanillic acid glucoside | C14H18O9 | 0.47 | 329.0886 | 330.0961 | 330.0951 | 3.02 | 5170 |
| 2 | Succinic acid | C4H6O4 | 0.47 | 117.0195 | 118.0265 | 118.0266 | −0.84 | 2542 |
| 3 | Caffeoylquinic acid | C16H18O9 | 0.53 | 353.0885 | 354.0960 | 354.0951 | 2.54 | 86,163 |
| 4 | Catechin gallate | C22H18O10 | 0.59 | 441.0813 | 442.0886 | 442.0902 | −3.16 | 1111 |
| 5 | Chlorogenic acid * | C16H18O9 | 0.94 | 353.0884 | 354.0958 | 354.0951 | 1.97 | 122,874 |
| 6 | m−Hydroxybenzoic acid | C7H6O3 | 1.03 | 137.0244 | 138.0318 | 138.0317 | 0.72 | 11,067 |
| 7 | Quinic acid | C7H12O6 | 1.52 | 191.0563 | 192.0632 | 192.0634 | −1.04 | 13,344 |
| 8 | Caffeoylquinic acid | C16H18O9 | 1.54 | 353.0882 | 354.0956 | 354.0951 | 1.41 | 51,125 |
| 9 | p−coumaroylquinic acid | C16H18O8 | 1.66 | 337.0936 | 338.1006 | 338.1002 | 1.18 | 8509 |
| 10 | 3−O−Feruloyl quinic acid | C17H20O9 | 2.40 | 367.1036 | 368.1109 | 368.1107 | 0.54 | 17,141 |
| 11 | Syringetin | C17H14O8 | 2.54 | 345.0628 | 346.0703 | 346.0689 | 4.04 | 5800 |
| 12 | p−coumaroylquinic acid | C16H18O8 | 2.91 | 337.0928 | 338.1001 | 338.1002 | −0.29 | 8910 |
| 13 | Cinnamic acid | C9H8O2 | 2.97 | 147.0448 | 148.0519 | 148.0524 | −3.37 | 4009 |
| 14 | Kaempferol−O−dihexoside | C27H30O16 | 3.73 | 609.1457 | 610.1544 | 610.1534 | 1.63 | 3113 |
| 15 | Isoquercetin | C21H20O12 | 3.86 | 463.0894 | 464.0968 | 464.0955 | 2.80 | 2519 |
| 16 | Dicaffeoylquinic acid | C25H24O12 | 4.16 | 515.1204 | 516.1282 | 516.1268 | 2.71 | 51,207 |
| 17 | Dicaffeoylquinic acid | C25H24O12 | 4.29 | 515.1206 | 516.1276 | 516.1268 | 1.55 | 82,208 |
| 18 | p−Coumaroyl−caffeoylquinic acid | C25H24O11 | 4.96 | 499.1259 | 500.1329 | 500.1319 | 1.99 | 3648 |
| 19 | Piceatannol | C14H12O4 | 5.58 | 243.0673 | 244.0748 | 244.0736 | 1.63 | 1112 |
| 20 | Resveratrol | C14H12O3 | 7.06 | 227.0723 | 228.0784 | 228.0786 | −0.87 | 1669 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subiria-Cueto, R.; Larqué-Saavedra, A.; Reyes-Vega, M.L.; de la Rosa, L.A.; Santana-Contreras, L.E.; Gaytán-Martínez, M.; Vázquez-Flores, A.A.; Rodrigo-García, J.; Corral-Avitia, A.Y.; Núñez-Gastélum, J.A.; et al. Brosimum alicastrum Sw. (Ramón): An Alternative to Improve the Nutritional Properties and Functional Potential of the Wheat Flour Tortilla. Foods 2019, 8, 613. https://doi.org/10.3390/foods8120613
Subiria-Cueto R, Larqué-Saavedra A, Reyes-Vega ML, de la Rosa LA, Santana-Contreras LE, Gaytán-Martínez M, Vázquez-Flores AA, Rodrigo-García J, Corral-Avitia AY, Núñez-Gastélum JA, et al. Brosimum alicastrum Sw. (Ramón): An Alternative to Improve the Nutritional Properties and Functional Potential of the Wheat Flour Tortilla. Foods. 2019; 8(12):613. https://doi.org/10.3390/foods8120613
Chicago/Turabian StyleSubiria-Cueto, Rodrigo, Alfonso Larqué-Saavedra, María L. Reyes-Vega, Laura A. de la Rosa, Laura E. Santana-Contreras, Marcela Gaytán-Martínez, Alma A. Vázquez-Flores, Joaquín Rodrigo-García, Alba Y. Corral-Avitia, José A. Núñez-Gastélum, and et al. 2019. "Brosimum alicastrum Sw. (Ramón): An Alternative to Improve the Nutritional Properties and Functional Potential of the Wheat Flour Tortilla" Foods 8, no. 12: 613. https://doi.org/10.3390/foods8120613
APA StyleSubiria-Cueto, R., Larqué-Saavedra, A., Reyes-Vega, M. L., de la Rosa, L. A., Santana-Contreras, L. E., Gaytán-Martínez, M., Vázquez-Flores, A. A., Rodrigo-García, J., Corral-Avitia, A. Y., Núñez-Gastélum, J. A., & Martínez-Ruiz, N. R. (2019). Brosimum alicastrum Sw. (Ramón): An Alternative to Improve the Nutritional Properties and Functional Potential of the Wheat Flour Tortilla. Foods, 8(12), 613. https://doi.org/10.3390/foods8120613





