Antioxidant and Antimicrobial Potential of Phenolic Metabolites from Traditionally Used Mediterranean Herbs and Spices
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
2.2. Plant Material and Extract Preparation
2.3. Phenolic Composition
2.3.1. Total Phenolics, Non-Flavonoids, Flavonoids, Flavanol Monomers, and Proanthocyanidins
2.3.2. Individual Phenolic Compounds
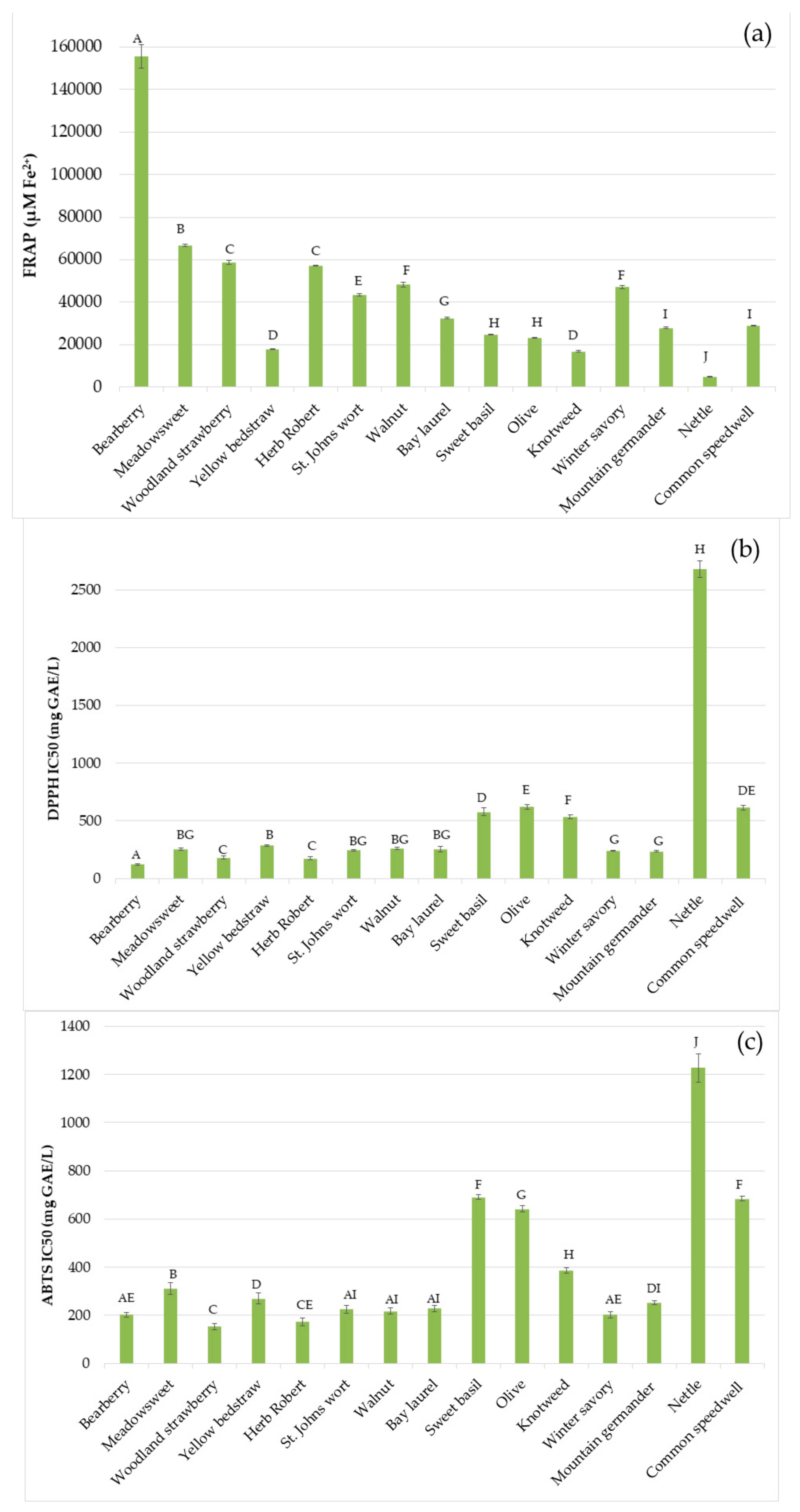
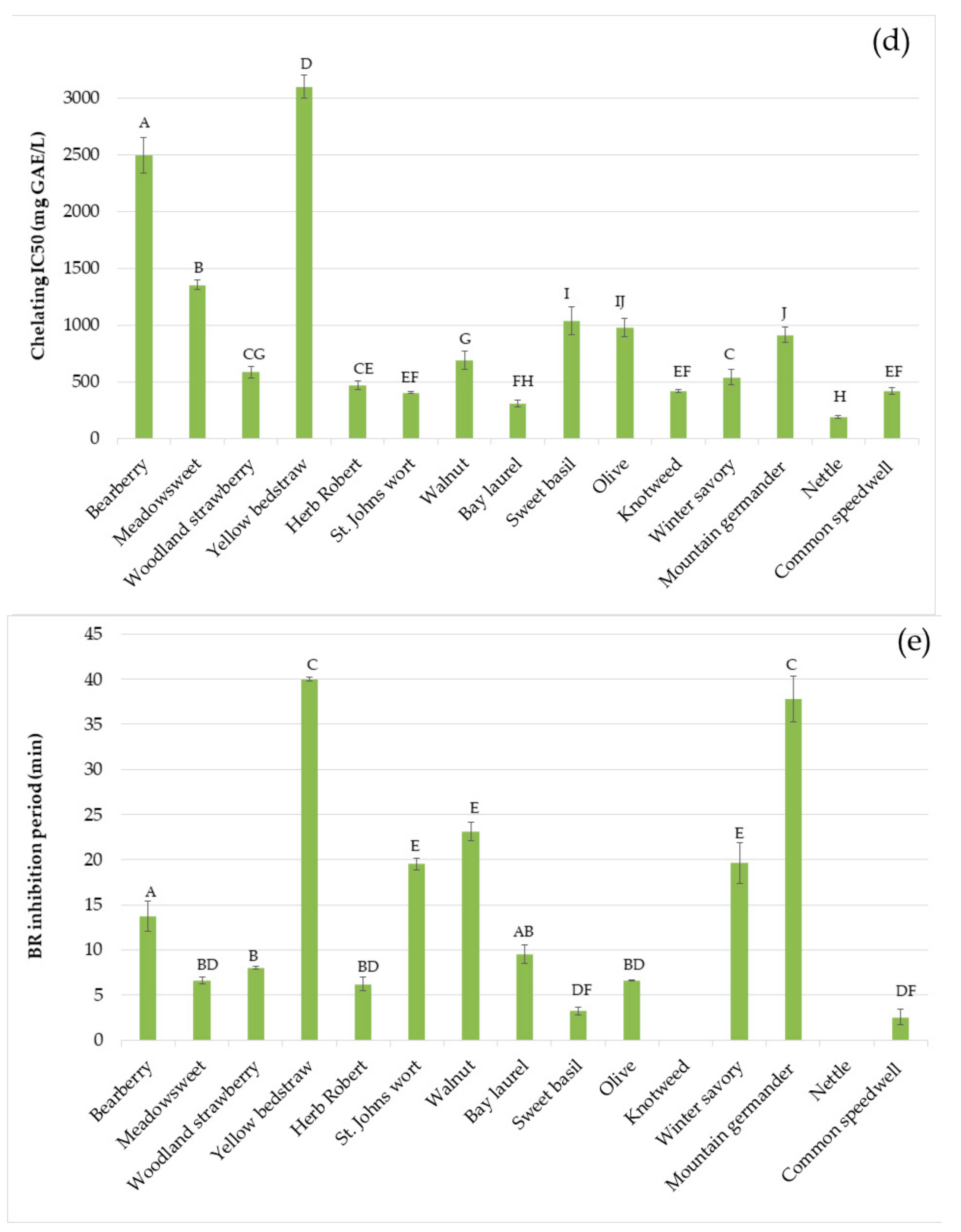
2.4. Antioxidant Activity
2.5. Antimicrobial Activity
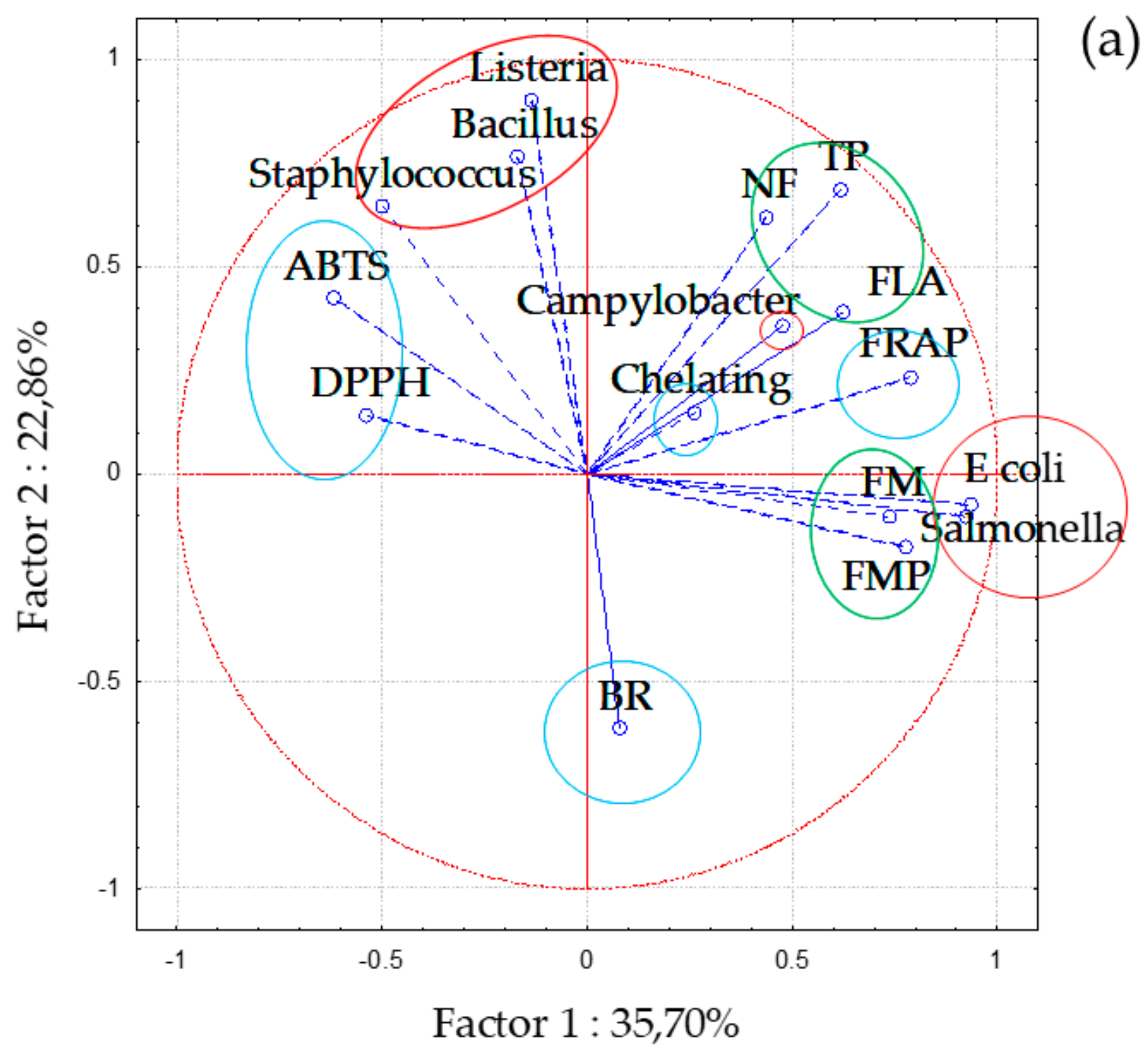
2.6. Statistical Analysis
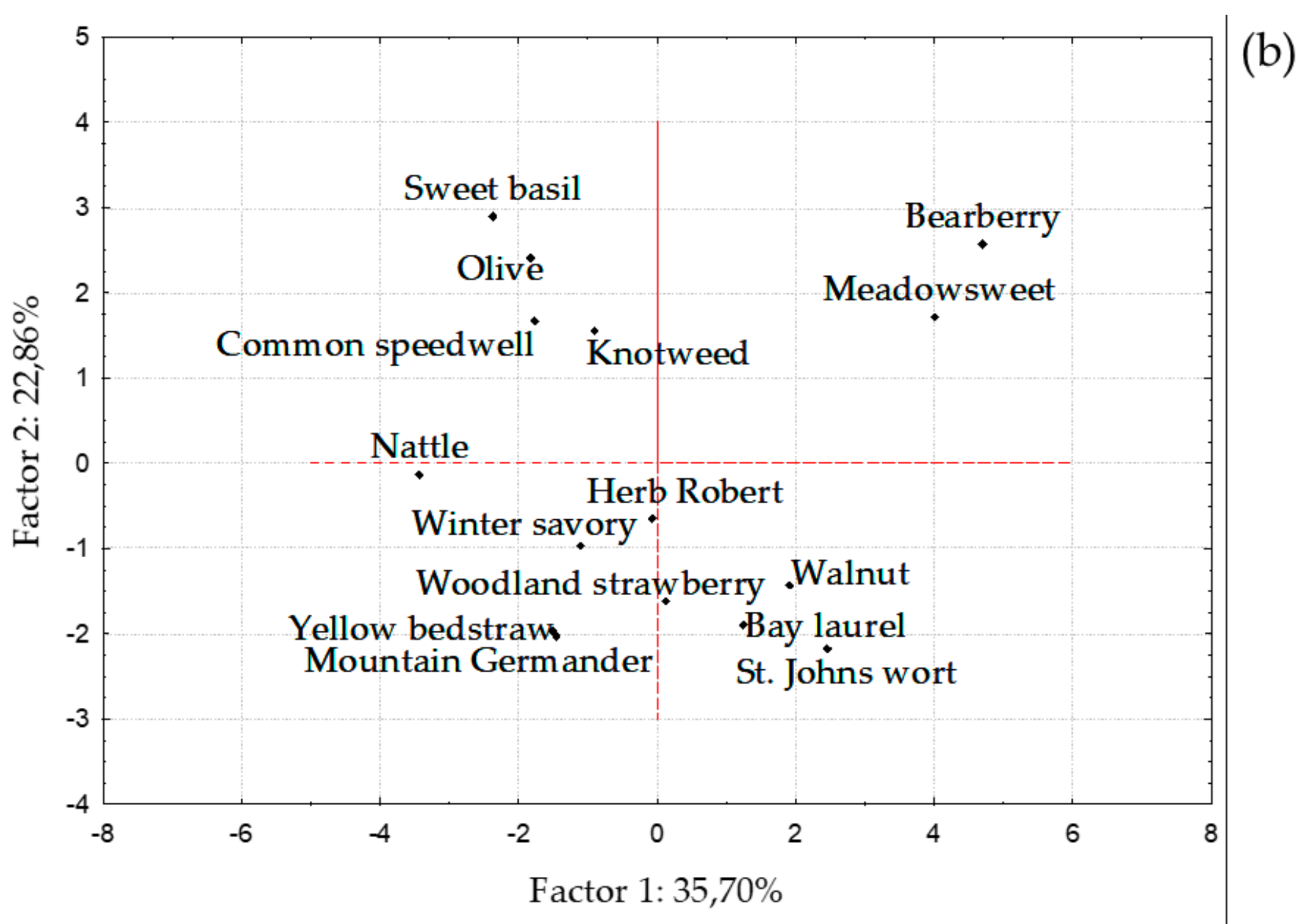
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. Fact Sheet N°134, Geneva: World Health Organization. 2008. Available online: https://apps.who.int/gb/ebwha/pdf_files/EB134/B134_24-en.pdf (accessed on 5 June 2019).
- Gunjan, M.; Naing, T.W.; Saini, R.S.; bin Ahmad, A.; Naidu, J.R.; Kumar, I. Marketing trends & future prospects of herbal medicine in the treatment of various disease. World J. Pharm. Res. 2015, 4, 132–155. [Google Scholar]
- Wangkheirakpam, S. Traditional and folk medicine as a target for drug discovery. In Natural Products and Drug Discovery; Mandal, S.C., Mandal, V., Konishi, T., Eds.; Elsevier: Oxford, UK, 2018; pp. 29–56. [Google Scholar] [CrossRef]
- Guldiken, B.; Ozkan, G.; Catalkaya, G.; Ceylan, F.D.; Yalcinkaya, I.E.; Capanoglu, E. Phytochemicals of herbs and spices: Health versus toxicological effects. Food Chem. Toxicol. 2018, 119, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Vermerris, W.; Nicholson, R. Phenolic Compounds and their Effects on Human Health. In Phenolic Compound Biochemistry; Springer: Dordrecht, The Netherlands, 2008; pp. 235–255. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly) phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed]
- Cetin-Karaca, H. Evaluation of Natural Antimicrobial Phenolic Compounds Against Foodborne Pathogens. Master’s Theses, University of Kentucky, Lexington, KY, USA, 2011. [Google Scholar]
- Skroza, D.; Šimat, V.; Smole Možina, S.; Katalinić, V.; Boban, N.; Generalić Mekinić, I. Interactions of resveratrol with other phenolics and activity against food-borne pathogens. Food Sci. Nutr. 2019, 7, 2312–2318. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Graciá, C.; Gonzalez-Bermudez, C.A.; Cabellero-Valcarcel, A.M.; Santaella-Pascual, M.; Frontela-Saseta, C. Use of herbs and spices for food preservation: Advantages and limitations. Food Sci. 2015, 6, 38–43. [Google Scholar] [CrossRef]
- Martillanes, S.; Rocha-Pimienta, J.; Cabrera-Bañegil, M.; Martín-Vertedor, D.; Delgado-Adámez, J. Application of phenolic compounds for food preservation: Food additive and active packaging. In Phenolic Compounds-Biological Activity; Soto-Hernandez, M., Palma-Tenango, M., del Rosario Garcia-Mateos, M., Eds.; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Campêlo, M.C.S.; Medeiros, J.M.S.; Silva, J.B.A. Natural products in food preservation. Int. Food Res. J. 2019, 26, 41–46. [Google Scholar] [CrossRef]
- Dolina, K.; Łuczaj, Ł. Wild food plants used on the Dubrovnik coast (south-eastern Croatia). Acta Soc. Bot. Pol. 2014, 83, 175–181. [Google Scholar] [CrossRef]
- Generalić Mekinić, I.; Skroza, D.; Ljubenkov, I.; Šimat, V.; Smole Možina, S.; Katalinić, V. In vitro antioxidant and antibacterial activity of Lamiaceae phenolic extracts: A correlation study. Food Technol. Biotechnol. 2014, 52, 119–127. [Google Scholar]
- González-Rodríguez, J.; Pérez-Juan, P.; Luque de Castro, M.D. Method for the simultaneous determination of total polyphenol and anthocyan indexes in red wines using a flow injection approach. Talanta 2002, 56, 53–59. [Google Scholar] [CrossRef]
- Amerine, M.A.; Ough, C.S. Methods for Analysis of Musts and Wines, 1st ed.; John Wiley and Sons: New York, NY, USA, 1980; pp. 181–185. [Google Scholar]
- Kramling, T.A.; Singleton, V.L. An estimate of the nonflavonoid phenols in wines. Am. J. Enol. Vitic. 1969, 20, 86–92. [Google Scholar]
- Arnous, A.; Makris, D.P.; Kefalas, P. Correlation of pigment and flavanol content with antioxidant properties in selected aged regional wines from Greece. J. Food Compos. Anal. 2002, 15, 655–665. [Google Scholar] [CrossRef]
- Katalinić, V.; Smole Možina, S.; Skroza, D.; Generalić, I.; Abramovič, H.; Miloš, M.; Ljubenkov, I.; Piskernik, S.; Pezo, I.; Terpinc, P.; et al. Polyphenolic profile, antioxidant properties and antimicrobial activity of grape skin extracts of 14 Vitis vinifera varieties grown in Dalmatia (Croatia). Food Chem. 2010, 119, 715–723. [Google Scholar] [CrossRef]
- Generalić, I.; Skroza, D.; Šurjak, J.; Možina, S.S.; Ljubenkov, I.; Katalinić, A.; Šimat, V.; Katalinić, V. Seasonal variations of phenolic compounds and biological properties in sage (Salvia officinalis L.). Chem. Biodivers. 2012, 9, 441–457. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as measurement of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Von Gadow, A.; Joubert, E.; Hansmann, C.F. Comparison of antioxidant activity of aspalathin with that of other plant phenols of Rooibosd tea (Aspalathon linearis), a-tocopherol, BHT, and BHA. J. Agric. Food Chem. 1997, 45, 632–638. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Generalić, I.; Skroza, D.; Ljubenkov, I.; Katalinić, A.; Burčul, F.; Katalinić, V. Influence of the phenophase on the phenolic profile and antioxidant properties of Dalmatian sage. Food Chem. 2011, 127, 427–433. [Google Scholar] [CrossRef]
- Yen, G.-C.; Duh, P.-D.; Chuang, D.-Y. Antioxidant activity of anthraquinones and anthrone. Food Chem. 2000, 70, 437–441. [Google Scholar] [CrossRef]
- Klančnik, A.; Piskernik, S.; Jeršek, B.; Možina, S.S. Evaluation of diffusion and dilution methods to determine the antibacterial activity of plant extracts. J. Microbiol. Methods 2010, 81, 121–126. [Google Scholar] [CrossRef]
- Katalinić, V.; Miloš, M.; Kulišić, T.; Jukić, M. Screening of 70 medicinal plant extracts for antioxidant capacity and total phenols. Food Chem. 2006, 94, 550–557. [Google Scholar] [CrossRef]
- Komes, D.; Belščak-Cvitanović, A.; Horžić, D.; Rusak, G.; Likić, S.; Berendika, M. Phenolic composition and antioxidant properties of some traditionally used medicinal plants affected by the extraction time and hydrolysis. Phytochem. Anal. 2011, 22, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Kivilompolo, M.; Hyötyläinen, T. Comprehensive two-dimensional liquid chromatography in analysis of Lamiaceae herbs: Characterisation and quantification of antioxidant phenolic acids. J. Chromatogr. 2007, 1145, 115–164. [Google Scholar] [CrossRef] [PubMed]
- Fecka, I.; Turek, S. Determination of polyphenolic compounds in commercial herbal drugs and spices from Lamiaceae: Thyme, wild thyme and sweet marjoram by chromatographic techniques. Food Chem. 2008, 108, 1039–1053. [Google Scholar] [CrossRef] [PubMed]
- Tzima, K.; Brunton, N.P.; Rai, D.K. Qualitative and quantitative analysis of polyphenols in Lamiaceae plants—A review. Plants 2018, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Wojdyło, A.; Ozimiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Lee, O.-H.; Lee, B.-Y.; Lee, J.; Lee, H.-B.; Son, J.-Y.; Park, C.-S.; Shetty, K.; Kim, Y.-C. Assessment of phenolics-enriched extract and fractions of olive leaves and their antioxidant activities. Bioresour. Technol. 2009, 100, 6107–6113. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N.; Kashchenko, N.I.; Chirikova, N.K. Meadowsweet teas as new functional beverages: Comparative analysis of nutrients, phytochemicals and biological effects of four Filipendula species. Molecules 2017, 22, 16. [Google Scholar] [CrossRef]
- Danila, A.O.; Gatea, F.; Radu, G.L. Polyphenol composition and antioxidant activity of selected medicinal herbs. Chem. Nat. Comp. 2011, 47, 22–26. [Google Scholar] [CrossRef]
- Harbourne, N.; Jacquier, J.C.; O’Riordan, D. Optimisation of the aqueous extraction conditions of phenols from meadowsweet (Filipendula ulmaria L.) for incorporation into beverages. Food Chem. 2009, 116, 722–727. [Google Scholar] [CrossRef]
- Generalić Mekinić, I.; Skroza, D.; Ljubenkov, I.; Krstulović, L.; Smole Možina, S.; Katalinić, V. Phenolic acids profile, antioxidant and antibacterial activity of chamomile, common yarrow and immortelle (Asteraceae). Nat. Prod. Commun. 2014, 9, 1745–1748. [Google Scholar]
- Abramovič, H.; Abram, V.; Čuk, A.; Čeh, B.; Smole Možina, S.; Vidmar, M.; Pavlovič, M.; Poklar Ulrih, N. Antioxidative and antibacterial properties of organically grown thyme (Thymus sp.) and basil (Ocimum basilicum L.). Turk. J. Agric. For. 2018, 42, 185–194. [Google Scholar] [CrossRef]
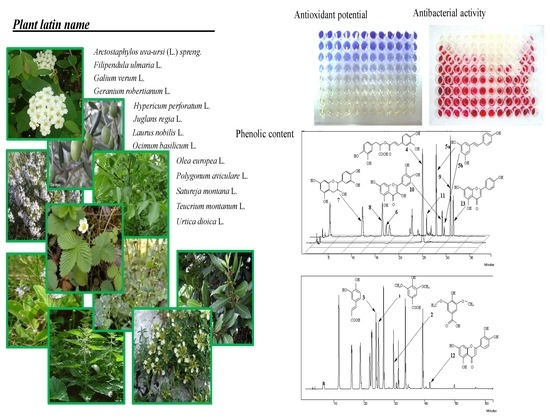
| Common Name | Pharmacopeial Name | Latin Name | Family | Plant Part |
|---|---|---|---|---|
| Bearberry | Uvae ursi folium | Arctostaphylos uva-ursi (L.) spreng. | Ericaceae | leaves |
| Meadowsweet | Filipendulae ulmariae herba | Filipendula ulmaria L. | Rosaceae | herb |
| Woodland strawberry | Fragariae folium | Fragaria vesca L. | Rosaceae | leaves |
| Yellow bedstraw | Galii veri herba | Galium verum L. | Rubiaceae | herb |
| Herb Robert | Geranii robertiani herba | Geranium robertianum L. | Geraniaceae | herb |
| St. Johns wort | Hyperici herba | Hypericum perforatum L. | Hypericiceae | herb |
| Walnut | Juglandis folium | Juglans regia L. | Juglandaceae | leaves |
| Bay laurel | Lauri folium | Laurus nobilis L. | Laureaceae | leaves |
| Sweet basil | Basilici herba | Ocimum basilicum L. | Lamiaceae | herb |
| Olive | Oleae folium | Olea europea L. | Oleaceae | leaves |
| Knotweed | Polygonum herba | Polygonum aviculare L. | Polygonaceae | herb |
| Winter savory | Satureja montana herba | Satureja montana L. | Lamiaceae | herb |
| Mountain germander | Teucrii montani herba | Teucrium montanum L. | Lamiaceae | herb |
| Nettle | Urticae folium | Urtica dioica L. | Urticaceae | leaves |
| Common speedwell | Veronicae herba | Veronica officinalis L. | Plantaginaceae | herb |
| Plant Extract | TP (mg GAE/g) | FLA (mg GAE/g) | NF (mg GAE/g) | FM (mg EE/g) | FMP (mg CE/g) |
|---|---|---|---|---|---|
| Bearberry | 607.5 ± 2.1 A | 97.0 ± 1.4 A | 511.7 ± 1.1 A | 38.6 ± 0.9 A | 5.8 ± 0.3 A |
| Meadowsweet | 347.8 ± 3.6 B | 214.0 ± 3.4 B | 133.8 ± 0.3B | 39.6 ± 0.4 B | 11.4 ± 0.3 B |
| Woodland strawberry | 134.1 ± 1.3 C | 45.1 ± 1.2 C | 89.0 ± 0.2 C | 5.4 ± 0.6 C | 3.1 ± 0.1 C |
| Yellow bedstraw | 71.2 ± 1.2 D | 8.9 ± 0.8 D | 62.2 ± 0.5 D | 16.7 ± 0.2 D | 0.0 ± 0.0 D |
| Herb Robert | 119.6 ± 0.5 E | 26.7 ± 0.7 E | 92.6 ± 0.8 E | 1.1 ± 0.2 EH | 0.2 ± 0.0 D |
| St. Johns wort | 145.3 ± 0.7 F | 109.4 ± 0.4 F | 35.8 ± 0.3 F | 56.2 ± 1.0 F | 12.8 ± 0.0 E |
| Walnut | 169.0 ± 0.5 G | 109.7 ± 0.3 F | 59.3 ± 0.3 G | 43.0 ± 0.6 G | 6.5 ± 0.0 F |
| Bay laurel | 148.3 ± 0.6 H | 110.5 ± 0.1 F | 37.7 ± 0.7 F | 39.5 ± 0.7 AB | 12.4 ± 0.1 G |
| Sweet basil | 212.4 ± 1.1 I | 94.0 ± 0.9 A | 118.4 ± 0.6 H | 1.9 ± 0.1 E | 0.0 ± 0.0 D |
| Olive | 223.0 ± 2.0 J | 77.6 ± 4.4 G | 143.4 ± 2.4 I | 1.1 ± 0.1 EH | 0.0 ± 0.0 D |
| Knotweed | 207.0 ± 1.2 K | 54.7 ± 4.1 H | 150.4 ± 3.1 J | 46.2 ± 1.2 I | 5.4 ± 0.2 H |
| Winter savory | 109.8 ± 0.7 L | 52.2 ± 0.5 H | 57.6 ± 0.2 G | 0.7 ± 0.1 H | 0.0 ± 0.0 D |
| Mountain Germander | 89.4 ± 2.0 M | 8.5 ± 2.6 D | 80.9 ± 3.3 K | 8.8 ± 0.2 J | 0.0 ± 0.0 D |
| Nettle | 150.6 ± 0.2 H | 152.4 ± 0.6 H | 98.2 ± 0.7 L | 1.0 ± 0.0 EH | 0.1 ± 0.0 D |
| Common speedwell | 227.9 ± 0.9 N | 96.6 ± 1.1 A | 129.3 ± 0.2 M | 10.2 ± 0.3 K | 0.1 ± 0.0 D |
| Plant Extract | Hydroxybenzoic Acid Derivates | Hydroxycinnamic Acid Derivates | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gallic Acid | Protocatechuic Acid | p-hydroxyben. Acid | m-hydroxyben. Acid | Vanillic Acid | Syringic Acid | Caffeic Acid | p-coumaric Acid | o-coumaric Acid | t-ferulic Acid | Cinnamic Acid | Rosmarinic Acid | |
| Bearberry | 0.98 ± 0.01A | n.d. | 0.89 ± 0.10 A | n.d. | n.d. | 24.69 ± 0.08 A | n.d. | n.d. | n.d. | n.d. | 0.08 ± 0.00 A | n.d. |
| Meadowsweet | 1.71 ± 0.07 B | 0.34 ± 0.02 A | 1.22 ± 0.07 B | 1.69 ± 0.46 A | n.d. | n.d. | n.d. | 0.35 ± 0.00 A | n.d. | n.d. | 0.18 ± 0.08 B | n.d. |
| Woodland strawberry | 1.03 ± 0.03 C | n.d. | 1.24 ± 0.03 B | 1.02 ± 0.03 B | 12.60 ± 0.04 A | 0.42 ± 0.00 B | 1.72 ± 0.04 A | 0.63 ± 0.01 B | 0.76 ± 0.01 A | 0.57 ± 0.13 A | 1.69 ± 0.01 C | n.d. |
| Yellow bedstraw | 0.02 ± 0.00 D | 0.11 ± 0.00 B | 0.30 ± 0.02 C | n.d. | n.d. | 0.06 ± 0.00 C | 0.08 ± 0.00 B | 0.04 ± 0.00 C | n.d. | 0.04 ± 0.00 E | 0.04 ± 0.00 D | n.d. |
| Herb Robert | 0.20 ± 0.01 E | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| St. Johns wort | 0.02 ± 0.00 D | 0.52 ± 0.01 C | 3.66 ± 0.07 D | n.d. | n.d. | 0.08 ± 0.01 C | 2.41 ± 0.20 C | n.d. | n.d. | 0.18 ± 0.00 BD | 0.14 ± 0.00 EF | n.d. |
| Walnut | 0.02 ± 0.00 D | 0.37 ± 0.01 A | n.d. | n.d. | n.d. | 0.63 ± 0.02 D | 0.21 ± 0.01 D | 0.19 ± 0.01 D | n.d. | 0.13 ± 0.01 BC | 0.69 ± 0.01 | n.d. |
| Bay laurel | 0.20 ± 0.08 E | 0.34 ± 0.18 A | 0.43 ± 0.19 E | n.d. | n.d. | n.d. | n.d. | 0.11 ± 0.05 E | n.d. | 0.19 ± 0.10 BD | 0.07 ± 0.04 A | n.d. |
| Sweet basil | 0.02 ± 0.00 D | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 18.38 ± 0.44 A |
| Olive | 0.04 ± 0.00 D | n.d. | n.d. | n.d. | n.d. | 0.13 ± 0.00 E | 0.35 ± 0.01 E | 0.42 ± 0.01 F | n.d. | 0.06 ± 0.01 CE | 0.12 ± 0.00 E | n.d. |
| Knotweed | n.d. | n.d. | n.d. | n.d. | 0.54 ± 0.02 B | 0.61 ± 0.00 D | n.d. | n.d. | 0.10 ± 0.00 B | 0.52 ± 0.02 A | 0.16 ± 0.01 BF | n.d. |
| Winter savory | 0.02 ± 0.00 D | 0.04 ± 0.01 B | 0.18 ± 0.01 F | n.d. | 0.82 ± 0.01 C | 1.20 ± 0.02 F | 0.33 ± 0.3 EF | n.d. | 0.15 ± 0.01 C | 0.04 ± 0.01 E | n.d. | 25.15 ± 0.47 B |
| Mountain Germander | n.d. | 0.06 ± 0.01 B | 0.24 ± 0.00 CF | n.d. | 1.05 ± 0.02 D | 0.13 ± 0.01 E | n.d. | 1.88 ± 0.00 G | n.d. | 0.23 ± 0.0 D | n.d. | 26.78 ± 0.38 C |
| Nettle | 0.02 ± 0.00 D | n.d. | n.d. | 0.22 ± 0.02 C | n.d. | n.d. | 1.64 ± 0.01 A | 0.03 ± 0.01 C | n.d. | 0.05 ± 0.00 E | n.d. | n.d. |
| Common speedwell | 0.18 ± 0.00 E | 0.20 ± 0.00 D | n.d. | n.d. | 0.93 ± 0.01 E | n.d. | 0.25 ± 0.02 DF | 0.03 ± 0.00 C | 0.13 ± 0.01 D | 0.43 ± 0.01 F | 0.03 ± 0.00 D | n.d. |
| Plant Extract | Catechin | Epicatechin | Quercetine | Quercetin-4-glucoside | Rutin | Kaempferol | Luteolin | Apigenin |
|---|---|---|---|---|---|---|---|---|
| Bearberry | 1.81 ± 0.08 A | 0.49 ± 0.04 A | n.d. | n.d. | 1.58 ± 0.05 A | n.d. | n.d. | n.d. |
| Meadowsweet | 0.70 ± 0.03 B | n.d. | n.d. | 4.74 ± 0.04 A | 0.68 ± 0.08 B | n.d. | n.d. | n.d. |
| Woodland strawberry | 0.20 ± 0.00 C | n.d. | n.d. | n.d. | 2.67 ± 0.01 C | n.d. | n.d. | n.d. |
| Yellow bedstraw | 1.41 ± 0.11 D | 0.11 ± 0.01 B | 0.32 ± 0,01 A | n.d. | 2.49 ± 0.04 D | 0.13 ± 0.00 A | n.d. | n.d. |
| Herb Robert | n.d. | 0.45 ± 0.04 C | n.d. | 0.81 ± 0.08 B | 2.13 ± 0.10 E | n.d. | n.d. | n.d. |
| St. Johns wort | 1.37 ± 0.05 D | 0.28 ± 0.00 D | 0.29 ± 0.01 B | n.d. | 2.39 ± 0.01 F | 0.13 ± 0.00 A | n.d. | 0.02 ± 0.00 A |
| Walnut | 0.64 ± 0.01 B | 0.10 ± 0.01 B | 0.20 ± 0.00 C | n.d. | 0.36 ± 0.04 G | n.d. | 0.30 ± 0.01 A | 0.04 ± 0.00 B |
| Bay laurel | n.d. | 0.26 ± 0.01 D | n.d. | n.d. | 1.05 ± 0.09 H | n.d. | n.d. | n.d. |
| Sweet basil | n.d. | n.d. | 0.04 ± 0.00 D | 0.94 ± 0.03 C | 0.70 ± 0.03 B | n.d. | n.d. | n.d. |
| Olive | n.d. | n.d. | n.d. | n.d. | 0.30 ± 0.00 GI | n.d. | n.d. | n.d. |
| Knotweed | n.d. | n.d. | n.d. | 1.59 ± 0.04 D | n.d. | n.d. | n.d. | n.d. |
| Winter savory | n.d. | 0.22 ± 0.02 E | n.d. | n.d. | 0.10 ± 0.01 J | 0.02 ± 0.00 B | 0.57 ± 0.02 B | 0.12 ± 0.01 C |
| Mountain Germander | 0.05 ± 0.00 E | n.d. | n.d. | 0.81 ± 0.08 B | n.d. | n.d. | n.d. | n.d. |
| Nettle | 0.86 ± 0.04 F | n.d. | n.d. | n.d. | 0.27 ± 0.02 I | n.d. | 0.15 ± 0.02 C | 0.05 ± 0.00 B |
| Common speedwell | n.d. | n.d. | n.d. | n.d. | n.d. | 0.02 ± 0.00 B | n.d. | n.d. |
| Plant Extract | MIC (mg/mL) | |||||
|---|---|---|---|---|---|---|
| Escherichia coli | Salmonella Infantis | Campylobacter coli | Staphylococcus aureus | Bacillus cereus | Listeria monocytogenes | |
| Bearberry | 1.67 | 1.67 | 0.83 | 0.35 | 1.67 | 1.67 |
| Meadowsweet | 3.33 | 2.78 | 6.67 | 1.39 | 1.67 | 3.33 |
| Woodland strawberry | 3.33 | 3.33 | 1.67 | 1.24 | 3.33 | 3.33 |
| Yellow bedstraw | n.d. | n.d. | 1.67 | 8.08 | 6.66 | 6.66 |
| Herb Robert | 5.55 | 5.55 | 3.33 | 2.77 | 6.66 | 6.66 |
| St. Johns wort | 6.66 | 6.66 | 0.83 | 1.00 | 3.33 | 3.33 |
| Walnut | 5.24 | 5.24 | 3.33 | 1.70 | 3.33 | 3.33 |
| Bay laurel | 3.33 | 3.33 | 1.67 | 1.39 | 1.67 | 3.33 |
| Sweet basil | n.d. | n.d. | 1.67 | 5.56 | 6.67 | 6.67 |
| Olive | n.d. | n.d. | 3.33 | 2.78 | 6.67 | 6.67 |
| Knotweed | n.d. | n.d. | 1.67 | 2.78 | 6.66 | 6.66 |
| Winter savory | n.d. | n.d. | 6.66 | 2.44 | 6.66 | 6.66 |
| Mountain Germander | n.d. | n.d. | 3.33 | 3.07 | 6.66 | 6.66 |
| Nettle | n.d. | n.d. | 0.83 | 2.78 | 1.67 | 3.33 |
| Common speedwell | n.d. | n.d. | 0.83 | 2.78 | 3.33 | 6.67 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Generalić Mekinić, I.; Skroza, D.; Ljubenkov, I.; Katalinić, V.; Šimat, V. Antioxidant and Antimicrobial Potential of Phenolic Metabolites from Traditionally Used Mediterranean Herbs and Spices. Foods 2019, 8, 579. https://doi.org/10.3390/foods8110579
Generalić Mekinić I, Skroza D, Ljubenkov I, Katalinić V, Šimat V. Antioxidant and Antimicrobial Potential of Phenolic Metabolites from Traditionally Used Mediterranean Herbs and Spices. Foods. 2019; 8(11):579. https://doi.org/10.3390/foods8110579
Chicago/Turabian StyleGeneralić Mekinić, Ivana, Danijela Skroza, Ivica Ljubenkov, Višnja Katalinić, and Vida Šimat. 2019. "Antioxidant and Antimicrobial Potential of Phenolic Metabolites from Traditionally Used Mediterranean Herbs and Spices" Foods 8, no. 11: 579. https://doi.org/10.3390/foods8110579
APA StyleGeneralić Mekinić, I., Skroza, D., Ljubenkov, I., Katalinić, V., & Šimat, V. (2019). Antioxidant and Antimicrobial Potential of Phenolic Metabolites from Traditionally Used Mediterranean Herbs and Spices. Foods, 8(11), 579. https://doi.org/10.3390/foods8110579