High Oxygen Modified Atmosphere Packaging Negatively Influences Consumer Acceptability Traits of Pork
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Design and Pork Loins
2.2. Sample Cutting and Packaging
2.3. Display and Storage
2.4. Colour Measurement
2.5. Objective Measurements of Texture and Water Loss
- Hardness: the force work required for the initial penetration,
- Cohesiveness (or called ease of break down): the reduced force work required for second penetration from work needed for the initial penetration,
- Chewiness: the force required to achieve hardness and cohesiveness.
2.6. TBARS Analysis
2.7. Sensory Assessment
2.8. Statistical Analysis
3. Results
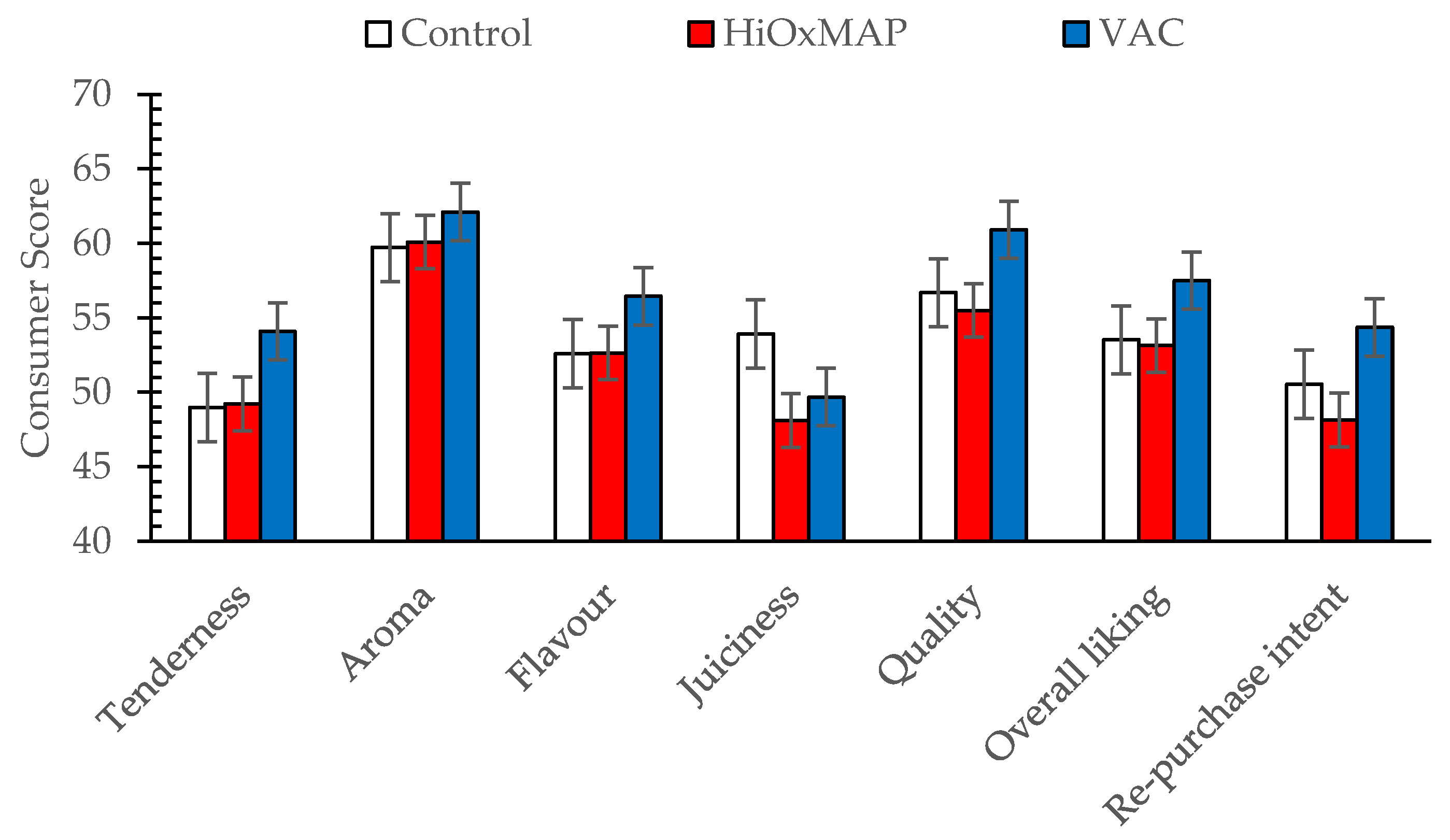
3.1. Sensory Assessment
3.2. WBSF and Compression
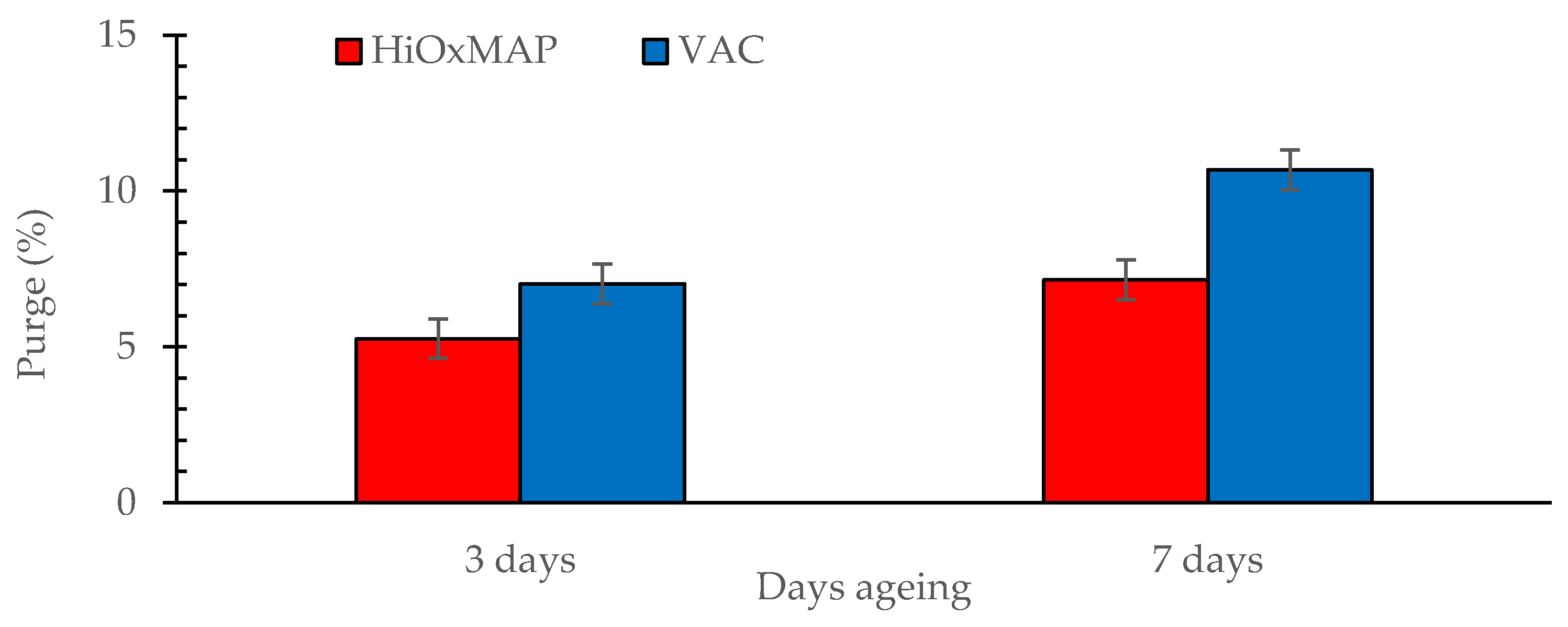
3.3. Purge Loss
3.4. Cooking Loss
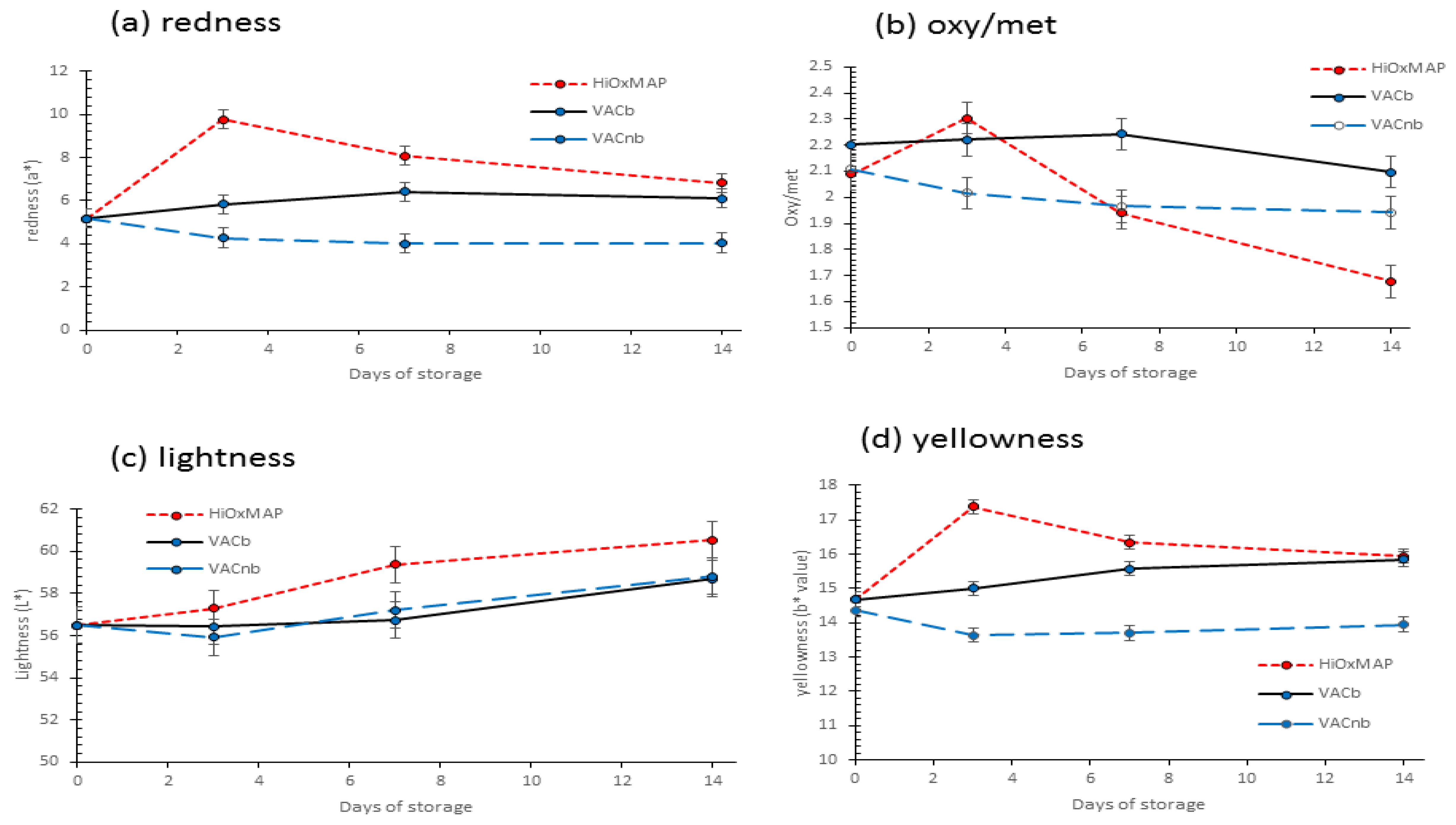
3.5. Colour
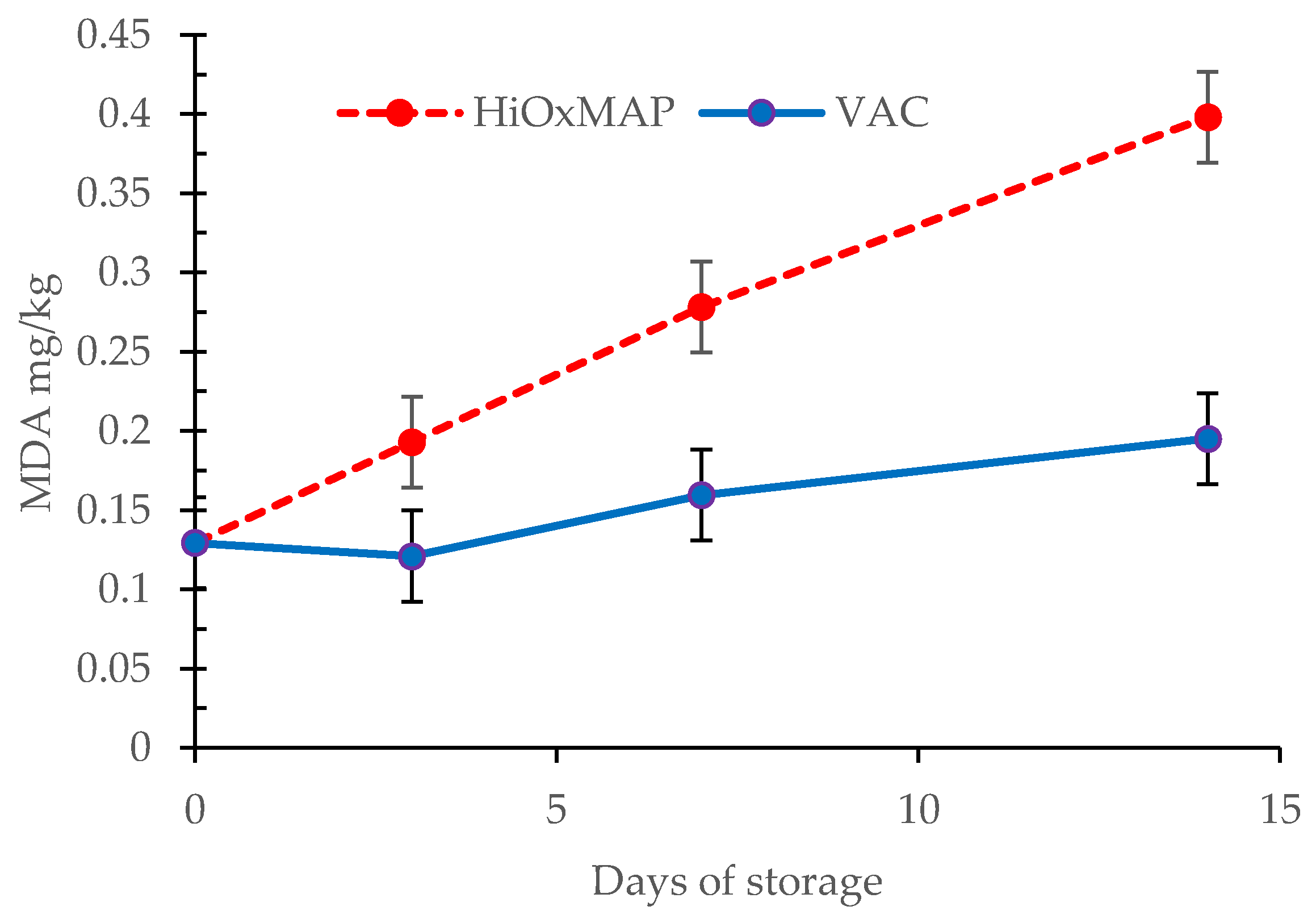
3.6. TBARS
4. Summary and Recommendations
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Simultaneous Improvement of Meat Quality and Growth and Carcass Traits in Pigs. Available online: http://wcgalp.org/system/files/proceedings/2002/simultaneous-improvement-meat-quality-and-growth-and-carcass-traits-pigs.pdf (accessed on 16 July 2001).
- Warner, R.D.; Greenwood, P.L.; Pethick, D.W.; Ferguson, D.M. Genetic and environmental effects on meat quality. Meat Sci. 2010, 86, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Producing Consistent Quality Meat from the Modern Pig. Available online: https://www.taylorfrancis.com/books/e/9781351114493/chapters/10.1201/9781351114493-9 (accessed on 15 June 2018).
- Channon, H.A.; Hamilton, A.J.; D’Souza, D.N.; Dunshea, F.R. Estimating the impact of various pathway parameters on tenderness, flavour and juiciness of pork using Monte Carlo simulation methods. Meat Sci. 2016, 116, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Channon, H.A.; D’Souza, D.N.; Dunshea, F.R. Developing a cuts-based system to improve consumer acceptability of pork: Impact of gender, ageing period, endpoint temperature and cooking method. Meat Sci. 2016, 121, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Delivering Consistent Quality Australian Pork to Consumers—A Systems Approach. Available online: https://www.researchgate.net/publication/239732001_Delivering_consistent_quality_australian_pork_to_consumers_-_a_systems_approach (accessed on 1 November 2011).
- Jonsäll, A.; Johansson, L.; Lundström, K. Effects of red clover silage and RN genotype on sensory quality of prolonged frozen stored pork (M. Longissimus dorsi). Food Qual. Prefer. 2000, 11, 371–376. [Google Scholar] [CrossRef]
- Nam, Y.J.; Choi, Y.M.; Lee, S.H.; Choe, J.H.; Jeong, D.W.; Kim, Y.Y.; Kim, B.C. Sensory evaluations of porcine longissimus dorsi muscle: Relationships with postmortem meat quality traits and muscle fiber characteristics. Meat Sci. 2009, 83, 731–736. [Google Scholar] [CrossRef]
- Cayuela, J.M.; Gil, M.D.; Bañón, S.; Garrido, M.D. Effect of vacuum and modified atmosphere packaging on the quality of pork loin. Eur. Food Res. Technol. 2004, 219, 316–320. [Google Scholar] [CrossRef]
- Lund, M.N.; Lametsch, R.; Hviid, M.S.; Jensen, O.N.; Skibsted, L.H. High-oxygen packaging atmosphere influences protein oxidation and tenderness of porcine longissimus dorsi during chill storage. Meat Sci. 2007, 77, 295–303. [Google Scholar] [CrossRef]
- Lavieri, N.; Williams, S.K. Effects of packaging systems and fat concentrations on microbiology, sensory and physical properties of ground beef stored at 4 ± 1 °C for 25 days. Meat Sci. 2014, 97, 534–541. [Google Scholar] [CrossRef]
- Dransfield, E.; Jones, R.C.D. Tenderising in m longissimus dorsi of beef veal rabbit lamb and pork. Meat Sci. 1980, 5, 139–147. [Google Scholar] [CrossRef]
- Lagerstedt, Å.; Ahnström, M.L.; Lundström, K. Vacuum skin pack of beef—A consumer friendly alternative. Meat Sci. 2011, 88, 391–396. [Google Scholar] [CrossRef] [PubMed]
- McMillin, K.W. Where is MAP Going? A review and future potential of modified atmosphere packaging for meat. Meat Sci. 2008, 80, 43–65. [Google Scholar] [CrossRef] [PubMed]
- Geesink, G.; Robertson, J.; Ball, A. The effect of retail packaging method on objective and consumer assessment of beef quality traits. Meat Sci. 2015, 104, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Mancini, R.A.; Hunt, M.C. Current research in meat color. Meat Sci. 2005, 71, 100–121. [Google Scholar] [CrossRef] [PubMed]
- Hunt, M.C.; King, A.J. AMSA Meat Colour Measurement Guidelines; Barbut, S., Claus, J., Cornforth, D.P., Hanson, D., Lindahl, G., Mancini, R.A., Milkowski, A., Mohan, A., Pohlman, F., Raines, C.R., et al., Eds.; American Meat Science Association: Champaignb, IL, USA, 2012; pp. 1–135. [Google Scholar]
- Warner, R.D.; Kearney, G.; Hopkins, D.L.; Jacob, R. Retail colour stability of lamb meat is influenced by packaging, breed type, muscle and iron concentration. Meat Sci. 2017, 129, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Hood, D.E.; Riordan, E.B. Discolouration in pre-packaged beef: Measurement by reflectance spectrophotometry and shopper discrimination. J. Food Sci. Technol. 1973, 8, 333–343. [Google Scholar] [CrossRef]
- Jakobsen, M.; Bertelsen, G. Colour stability and lipid oxidation of fresh beef. Development of a response surface model for predicting the effects of temperature, storage time, and modified atmosphere composition. Meat Sci. 2000, 54, 49–57. [Google Scholar] [CrossRef]
- Nerin, C.; Tovar, L.; Djenane, D.; Camo, J.; Salafranca, J.; Beltran, J.A.; Roncales, P. Stabilization of beef meat by a new active packaging containing natural antioxidants. J. Agric. Food Chem. 2006, 54, 7840–7846. [Google Scholar] [CrossRef]
- Trevisan, M.; Browne, R.; Ram, M.; Muti, P.; Freudenheim, J.; Carosella, A.M.; Armstrong, D. Correlates of Markers of Oxidative Status in the General Population. Am. J. Epidemiol. 2001, 154, 348–356. [Google Scholar] [CrossRef]
- Warner, R. Measurement Of Meat Quality|Measurements of Water-holding Capacity and Color: Objective and Subjective in Encyclopedia of Meat Sciences, 2nd ed.; Devine, C., Dikeman, M., Eds.; Academic Press: Oxford, UK, 2014; pp. 164–171. [Google Scholar] [CrossRef]
- Warner, R.D.; Ponnampalam, E.N.; Kearney, G.A.; Hopkins, D.L.; Jacob, R.H. Genotype and age at slaughter influence the retail shelf-life of the loin and knuckle from sheep carcasses. Aust. J. Exp. Agric. 2007, 47, 1190–1200. [Google Scholar] [CrossRef]
- Mortimer, S.I.; Jacob, R.H.; Kearney, G.; Hopkins, D.L.; Warner, R.D. Genetic variation in colour stability traits of lamb cuts under two packaging systems. Meat Sci. 2019, 157, 107870. [Google Scholar] [CrossRef]
- Jacob, R.H.; D’Antuono, M.F.; Gilmour, A.R.; Warner, R.D. Phenotypic characterization of colour stability of lamb meat. Meat Sci. 2014, 96, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- Warner, R.D.; Ferguson, D.M.; McDonagh, M.B.; Channon, H.A.; Cottrell, J.J.; Dunshea, F.R. Acute exercise stress and electrical stimulation influence the consumer perception of sheep meat eating quality and objective quality traits. Aust. J. Exp. Agric. 2005, 45, 553–560. [Google Scholar] [CrossRef]
- Channon, H.A.; Taverner, M.R.; D’Souza, D.N.; Warner, R.D. Aitchbone hanging and ageing period are additive factors influencing pork eating quality. Meat Sci. 2014, 96, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Bouton, P.E.; Harris, P.V.; Shorthose, W.R. Effect of ultimate pH upon the water-holding capacity and tenderness of mutton. J. Food Sci. 1971, 37, 358–360. [Google Scholar] [CrossRef]
- Bouton, P.E.; Harris, P.V. A comparison of some objective methods used to assess meat tenderness. J. Food Sci. 1972, 37, 218–221. [Google Scholar] [CrossRef]
- Witte, V.C.; Krause, G.F.; Bailey, M.E. A new extraction method for determining 2-thiobarbituric acid values of pork and beef during storage. J. Food Sci. 1970, 35, 582–585. [Google Scholar] [CrossRef]
- Warner, R.D.; Ferguson, D.M.; Cottrell, J.J.; Knee, B.W. Acute stress induced by the preslaughter use of electric prodders causes tougher beef meat. Aust. J. Exp. Agric. 2007, 47, 782–788. [Google Scholar] [CrossRef]
- Ferguson, D.M.; Warner, R.D.; Walker, P.J.; Knee, B. Effect of cattle marketing method on beef quality and palatability. Aust. J. Exp. Agric. 2007, 47, 774–781. [Google Scholar] [CrossRef]
- Watson, R.; Gee, A.; Polkinghorne, R.; Porter, M. Consumer assessment of eating quality—Development of protocols for Meat Standards Australia (MSA) testing. Aust. J. Exp. Agric. 2008, 48, 1360–1367. [Google Scholar] [CrossRef]
- Colditz, I.G.; Ferguson, D.M.; Greenwood, P.L.; Doogan, V.J.; Petherick, J.C.; Kilgour, R.J. Regrouping unfamiliar animals in the weeks prior to slaughter has few effects on physiology and meat quality in Bos taurus feedlot steers. Aust. J. Exp. Agric. 2007, 47, 763–769. [Google Scholar] [CrossRef]
- Ha, M.; McGilchrist, P.; Polkinghorne, R.; Huynh, L.; Galletly, J.; Kobayashi, K.; Nishimura, T.; Bonney, S.; Kelman, K.R.; Warner, R.D. Effects of different ageing methods on colour, yield, oxidation and sensory qualities of Australian beef loins consumed in Australia and Japan. Food Res. Int. 2019, 125, 108528. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.M.; Polkinghorne, R.J.; Gee, A.M.; Motiang, D.; Strydom, P.E.; Mashau, M.; Ng’ambi, J.; DeKock, R.; Burrow, H.M. Beef Palatability in the Republic of South Africa: Implications for Niche-Marketing Strategies; ACIAR Technical Reports No. 72; Australian Centre for International Agricultural Research: Canberra, Australia, 2010.
- Thompson, J.M.; Polkinghorne, R.; Hwang, I.H.; Gee, A.M.; Cho, S.H.; Park, B.Y.; Lee, J.M. Beef quality grades as determined by Korean and Australian consumers. Aust. J. Exp. Agric. 2008, 48, 1380–1386. [Google Scholar] [CrossRef]
- Huffman, K.L.; Miller, M.F.; Hoover, L.C.; Wu, C.K.; Brittin, H.C.; Ramsey, C.B. Effect of beef tenderness on consumer satisfaction with steaks consumed in the home and restaurant. J. Anim. Sci. 1996, 74, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Channon, H.A.; Kerr, M.G.; Walker, P.J. Effect of Duroc content, sex and ageing period on meat and eating quality attributes of pork loin. Meat Sci. 2004, 66, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Tornberg, E. Effects of heat on meat proteins—Implications on structure and quality of meat products. Meat Sci. 2005, 70, 493–508. [Google Scholar] [CrossRef]
- Frank, D.C.; Geesink, G.; Alvarenga, T.I.R.C.; Polkinghorne, R.; Stark, J.; Lee, M.; Warner, R. Impact of high oxygen and vacuum retail ready packaging formats on lamb loin and topside eating quality. Meat Sci. 2017, 123, 126–133. [Google Scholar] [CrossRef]
- Sorheim, O.; Wahlgren, N.M.; Nilsen, B.N.; Lea, P. Effects of high oxygen packaging on tenderness and quality characteristics of beef longissimus muscles. In Proceedings of the 51st International Congress of Meat Science and Technology, Helsinki, Finland, 8–13 August 2004. [Google Scholar]
- Aaslyng, M.D.; Bejerholm, C.; Ertbjerg, P.; Bertram, H.C.; Andersen, H.J. Cooking loss and juiciness of pork in relation to raw meat quality and cooking procedure. Food Qual. Prefer. 2003, 14, 277–288. [Google Scholar] [CrossRef]
- Brewer, M.S.; McKeith, F.K. Consumer-rated Quality Characteristics as Related to Purchase Intent of Fresh Pork. J. Food Sci. 1999, 64, 171–174. [Google Scholar] [CrossRef]
- O’Sullivan, M.G.; Byrne, D.V.; Martens, H.; Gidskehaug, L.H.; Andersen, H.J.; Martens, M. Evaluation of pork colour: Prediction of visual sensory quality of meat from instrumental and computer vision methods of colour analysis. Meat Sci. 2003, 65, 909–918. [Google Scholar] [CrossRef]
- Van Oeckel, M.J.; Warnants, N.; Boucqué, C.V. Measurement and prediction of pork colour. Meat Sci. 1999, 52, 347–354. [Google Scholar] [CrossRef]
- Chizzolini, R.; Novelli, E.; Badiani, A.; Rosa, P.; Delbono, G. Objective measurements of pork quality: Evaluation of various techniques. Meat Sci. 1993, 34, 49–77. [Google Scholar] [CrossRef]
- Warner, R.D.; Jacob, R.H.; Hocking Edwards, J.E.H.; McDonagh, M.; Pearce, K.; Geesink, G.; Kearney, G.; Allingham, P.; Hopkins, D.L.; Pethick, D.W. Quality of lamb meat from the Information Nucleus Flock. Anim. Prod. Sci. 2010, 50, 1123–1134. [Google Scholar] [CrossRef]
- Karamucki, T.; Gardzielewska, J.; Rybarczyk, A.; Jakubowska, M.; Natalczyk-Szymkowska, W. Usefulness of Selected Methods of Colour Change Measurement for Pork Quality Assessment. Czech J. Food Sci. 2011, 29, 212–218. [Google Scholar] [CrossRef]
- Chmiel, M.; Słowiński, M.; Dasiewicz, K. Lightness of the color measured by computer image analysis as a factor for assessing the quality of pork meat. Meat Sci. 2011, 88, 566–570. [Google Scholar] [CrossRef]
- Cannon, J.E.; Morgan, J.B.; Schmidt, G.R.; Tatum, J.D.; Sofos, J.N.; Smith, G.C.; Delmore, R.J.; Williams, S.N. Growth and fresh meat quality characteristics of pigs supplemented with vitamin E. J. Anim. Sci. 1996, 74, 98–105. [Google Scholar] [CrossRef]
- Nam, K.C.; Du, M.; Jo, C.; Ahn, D.U. Cholesterol oxidation products in irradiated raw meat with different packaging and storage time Journal Paper No. J-18976 of the Iowa Agriculture and Home Economics Experiment Station, Ames, IA 50011. Project No. 3322, supported by S-292 Regional Project and Iowa Egg Council. Meat Sci. 2001, 58, 431–435. [Google Scholar] [CrossRef]
- Faustman, C.; Sun, Q.; Mancini, R.; Suman, S.P. Myoglobin and lipid oxidation interactions: Mechanistic bases and control. Meat Sci. 2010, 86, 86–94. [Google Scholar] [CrossRef]
- Lin, T.-Z.; Hultin, H.O. Oxidation of myoglobin in vitro mediated by lipid oxidation in microsomal fractions of muscle. J. Food Sci. 1977, 42, 136–140. [Google Scholar] [CrossRef]
- Campo, M.M.; Nute, G.R.; Hughes, S.I.; Enser, M.; Wood, J.D.; Richardson, R.I. Flavour perception of oxidation in beef. Meat Sci. 2006, 72, 303–311. [Google Scholar] [CrossRef]
- Casaburi, A.; Piombino, P.; Nychas, G.-J.; Villani, F.; Ercolini, D. Bacterial populations and the volatilome associated to meat spoilage. Food Microbiol. 2015, 45, 83–102. [Google Scholar] [CrossRef]
- Greene, B.E.; Cumuze, T.H. Relationships between TBA Numbers and Inexperienced panelists assessments of oxidized flavor in cooked beef. J. Food Sci. 1981, 47, 52–58. [Google Scholar] [CrossRef]
- Polkinghorne, R.J.; Philpott, J.; Perovic, J.; Lau, J.; Davies, L.; Mudannayake, W.; Watson, R.; Tarr, G.; Thompson, J.M. The effect of packaging on consumer eating quality of beef. Meat Sci. 2018, 142, 59–64. [Google Scholar] [CrossRef] [PubMed]
| Position in Both Loins | 0 Days | 3 Days VAC 1 & HiOxMAP 1 | 7 Days VAC & HiOxMAP | 14 Days VAC & HiOxMAP |
|---|---|---|---|---|
| Anterior section 5 × 4 cm | Warner-Bratzler, Compression, cooking loss | Warner-Bratzler, Compression, cooking loss | Warner-Bratzler, Compression, cooking loss | |
| Middle section 3 × 12.5 cm | Consumer sensory | Consumer sensory | ||
| Posterior 7 × 2.5 cm | Colour, TBARS | Colour, TBARS | Colour, TBARS | Colour, TBARS |
| Treatment | Control | HiOxMAP | Vacuum | SED 1 | F-values | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Days Ageing | 0 Days | 3 Days | 7 Days | 3 Days | 7 Days | Control | Pack | Age | Pack.Age | |
| Cook loss (%) | 19.28 | 19.54 | 18.97 | 16.73 | 18.45 | 0.590 | <0.001 | <0.001 | 0.337 | 0.017 |
| Chewiness | 10.00 | 10.12 | 10.29 | 9.74 | 9.15 | 0.452 | 0.111 | 0.021 | 0.505 | 0.238 |
| Cohesiveness | 0.435 | 0.412 | 0.436 | 0.427 | 0.421 | 0.0076 | 0.14 | 0.515 | 0.362 | 0.043 |
| Hardness | 34.18 | 34.53 | 34.82 | 31.59 | 30.65 | 0.874 | 0.001 | <0.001 | 0.599 | 0.329 |
| WBSF (N) | 29.23 | 21.56 | 22.39 | 20.23 | 17.94 | 0.973 | <0.001 | <0.001 | 0.292 | 0.027 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Y.; Adhiputra, K.; Padayachee, A.; Channon, H.; Ha, M.; Warner, R.D. High Oxygen Modified Atmosphere Packaging Negatively Influences Consumer Acceptability Traits of Pork. Foods 2019, 8, 567. https://doi.org/10.3390/foods8110567
Peng Y, Adhiputra K, Padayachee A, Channon H, Ha M, Warner RD. High Oxygen Modified Atmosphere Packaging Negatively Influences Consumer Acceptability Traits of Pork. Foods. 2019; 8(11):567. https://doi.org/10.3390/foods8110567
Chicago/Turabian StylePeng, Yunling, Karunia Adhiputra, Anneline Padayachee, Heather Channon, Minh Ha, and Robyn Dorothy Warner. 2019. "High Oxygen Modified Atmosphere Packaging Negatively Influences Consumer Acceptability Traits of Pork" Foods 8, no. 11: 567. https://doi.org/10.3390/foods8110567
APA StylePeng, Y., Adhiputra, K., Padayachee, A., Channon, H., Ha, M., & Warner, R. D. (2019). High Oxygen Modified Atmosphere Packaging Negatively Influences Consumer Acceptability Traits of Pork. Foods, 8(11), 567. https://doi.org/10.3390/foods8110567






