Panel and Panelist Performance in the Sensory Evaluation of Black Ripe Olives from Spanish Manzanilla and Hojiblanca Cultivars
Abstract
:1. Introduction
2. Materials and Methods
2.1. Olives and Their Processing
2.2. Data Analysis
3. Results and Discussion
3.1. Overview of Results
3.2. Panel Performance
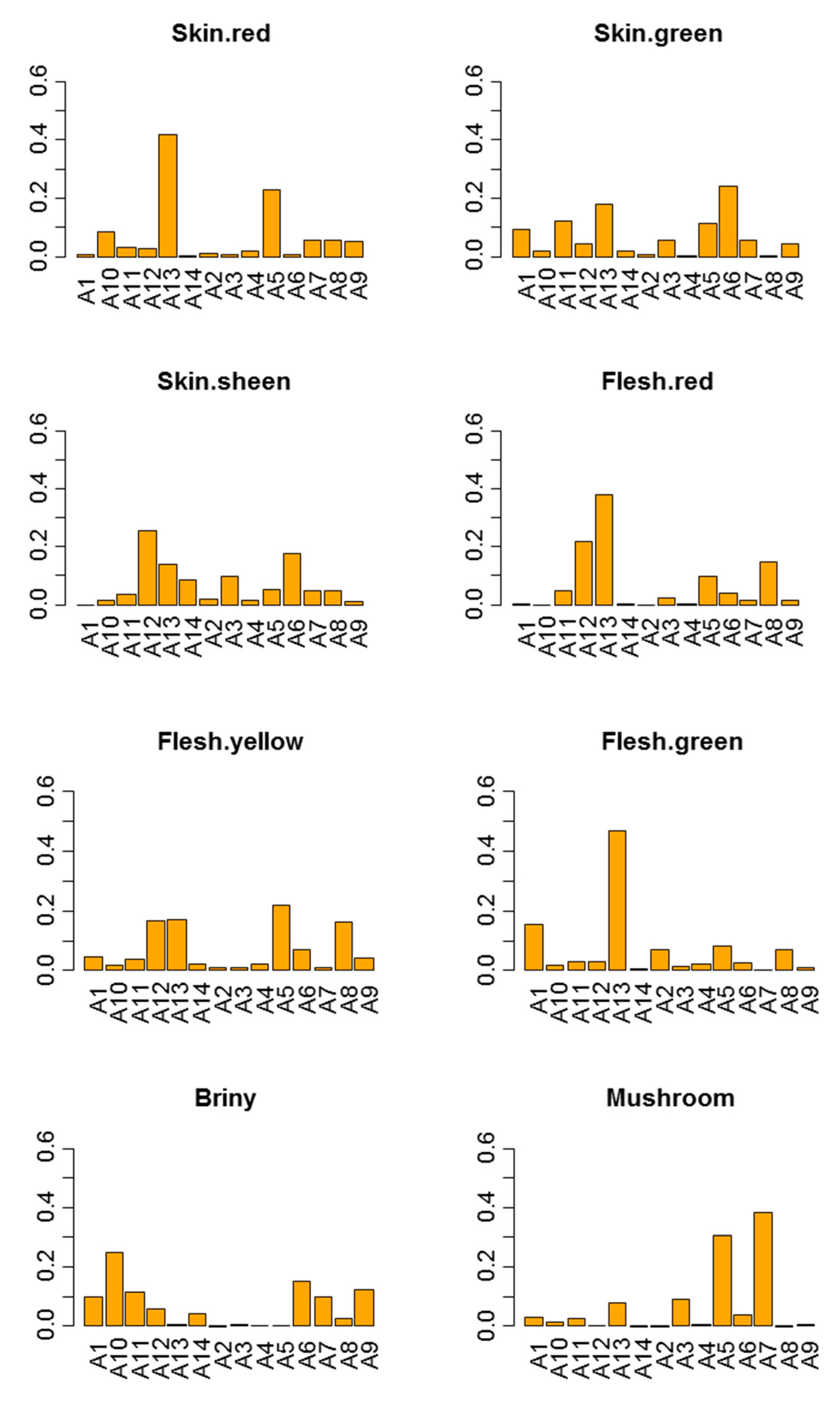
3.2.1. Effect of Sample (Power of Discrimination)
3.2.2. Effect of Panelist
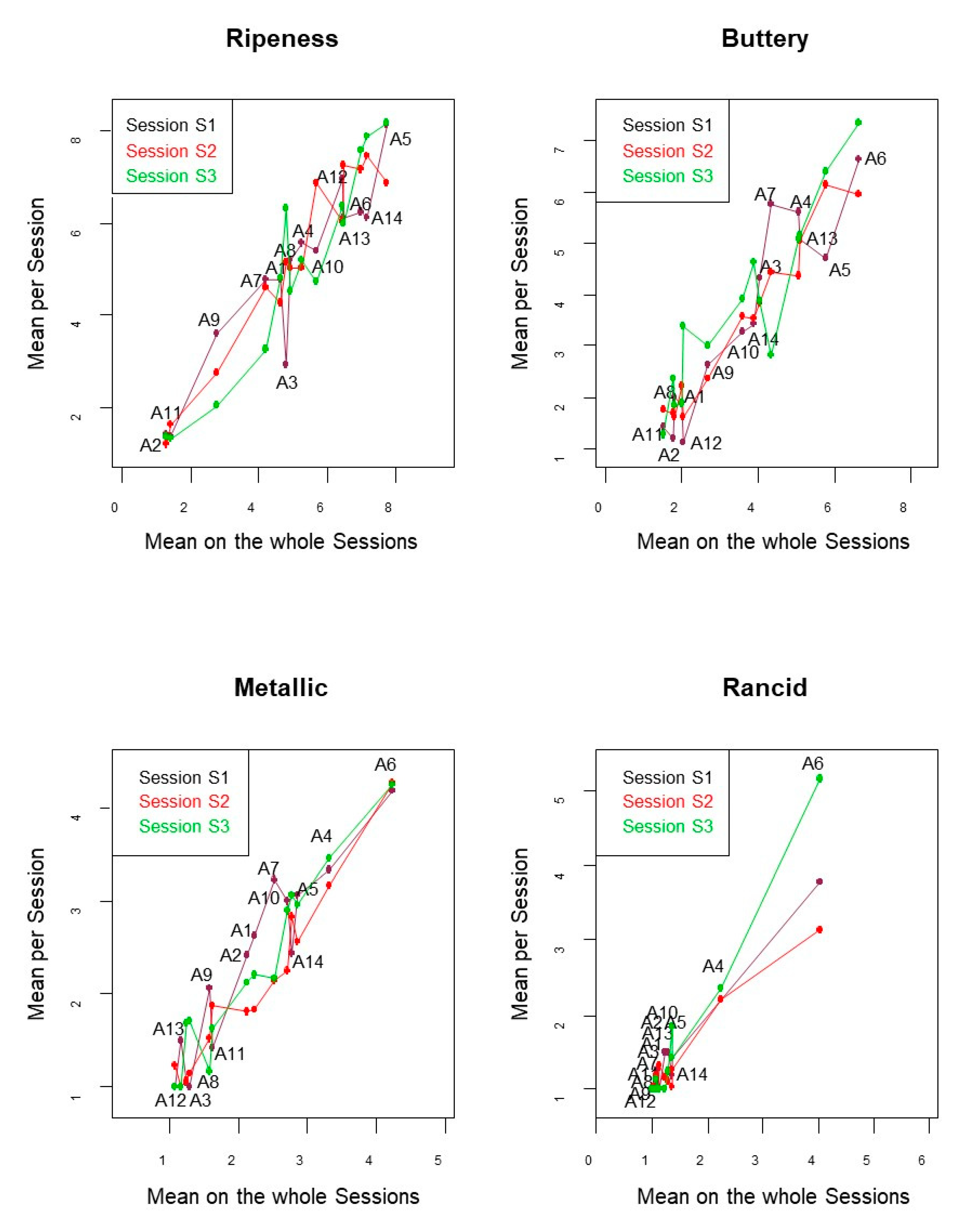
3.2.3. Effect of Session
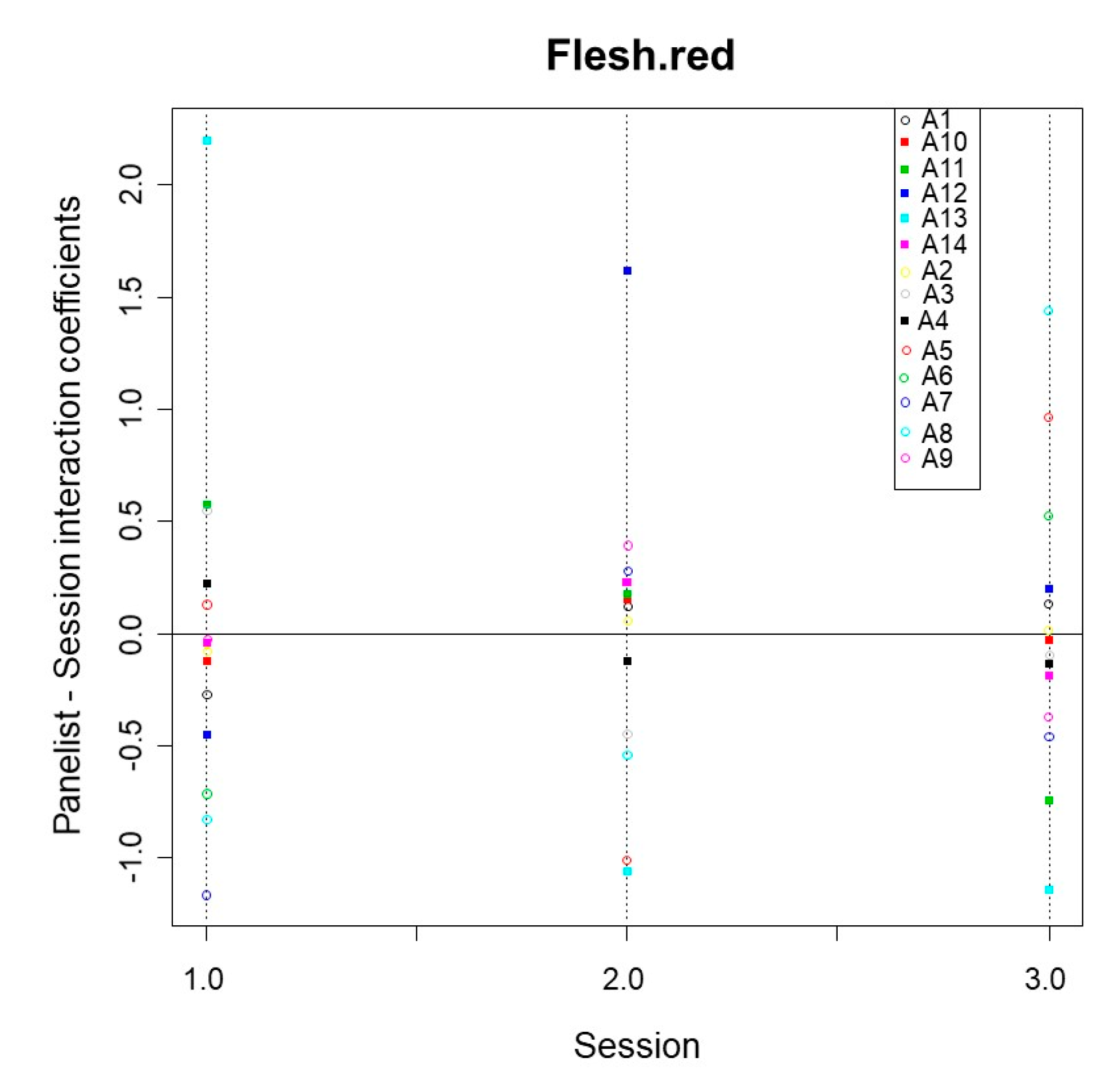
3.2.4. Sample·Panelist Interaction
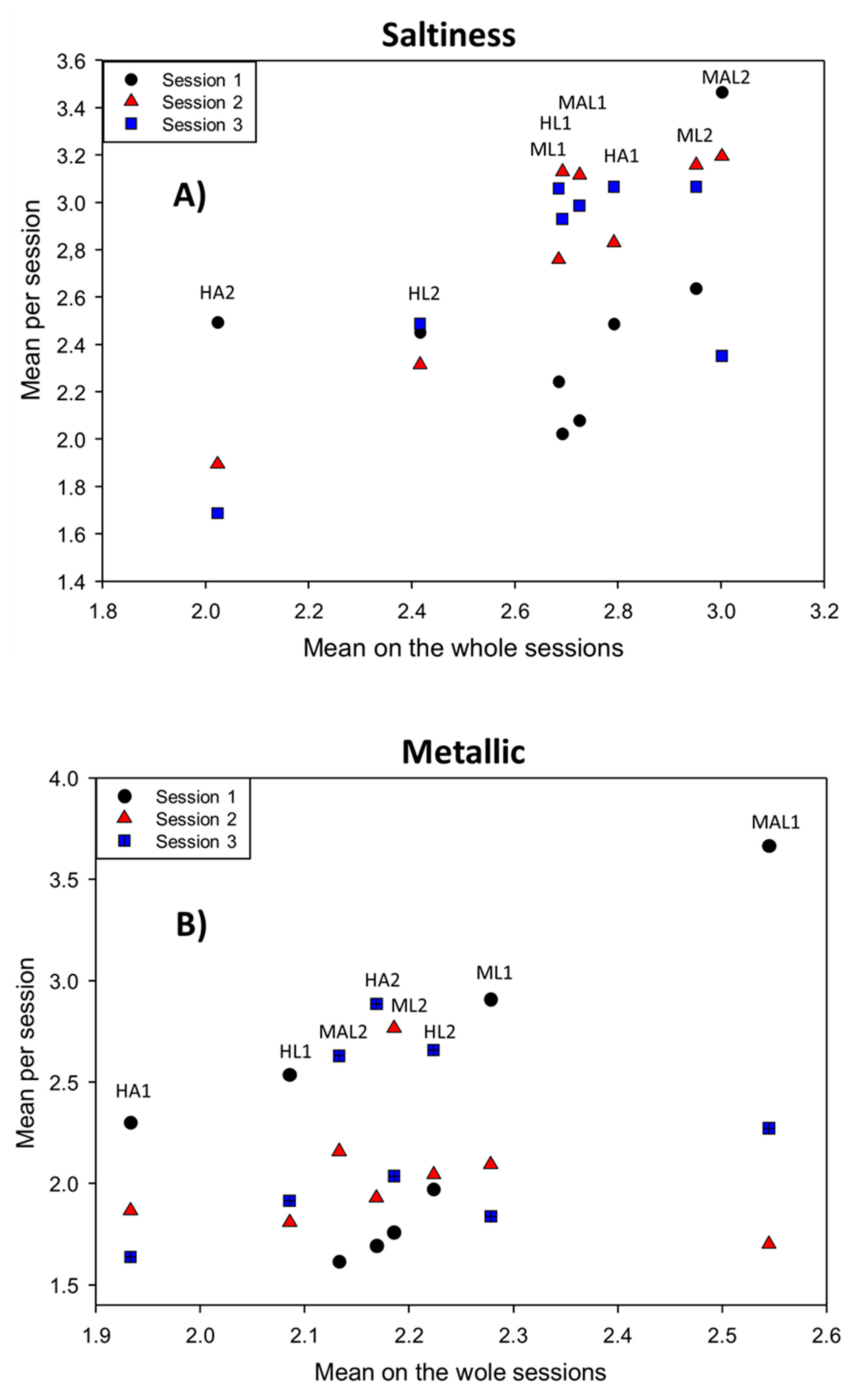
3.2.5. Sample·Session Interaction
3.2.6. Panelist·Session Interaction
3.3. Panelist Performance
3.3.1. Discrimination Power of Each Panelist
3.3.2. Panelist Repeatability
3.3.3. Panelist Reproducibility
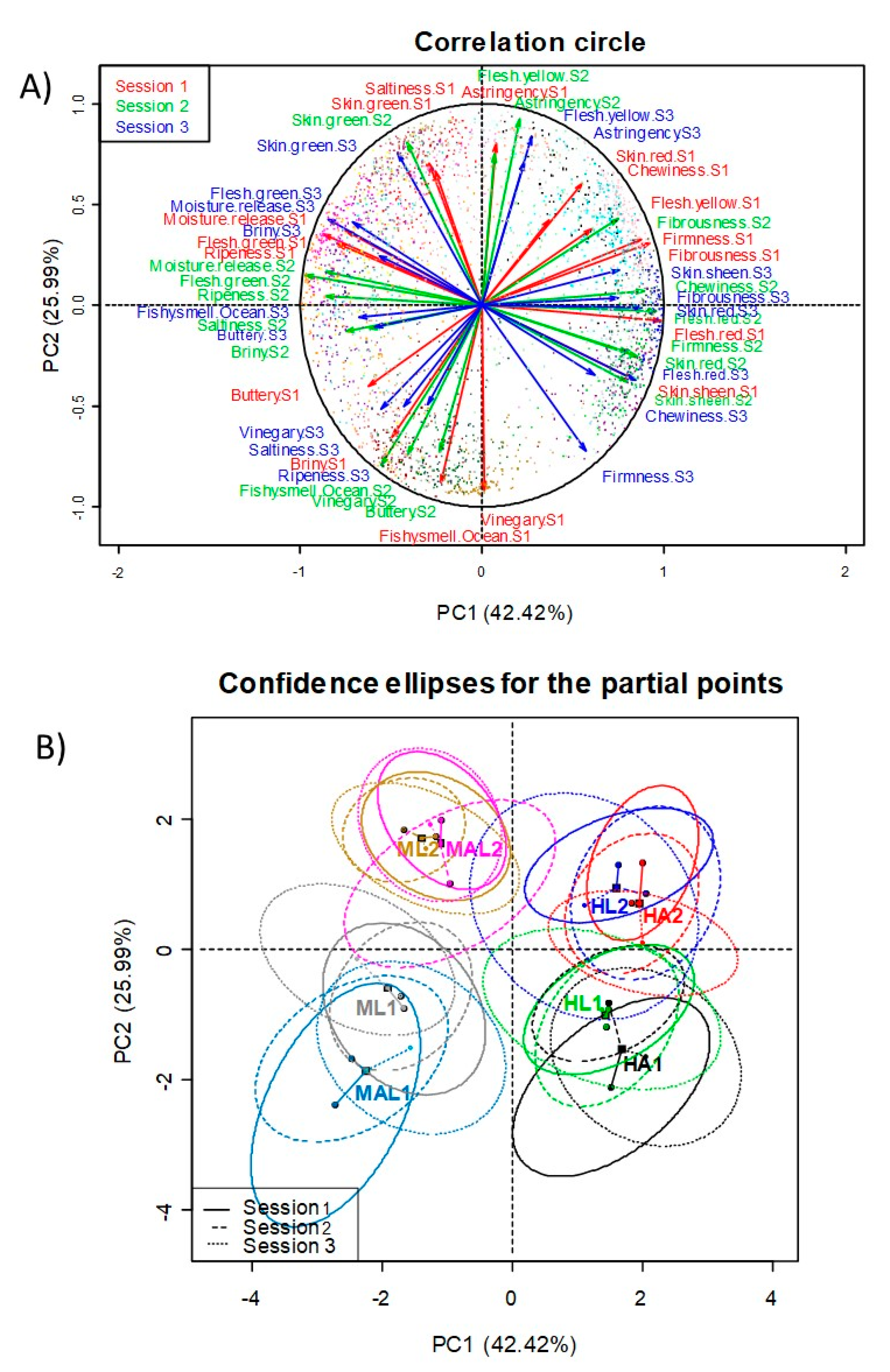
3.4. Multivariate Study of Panelists and Panel
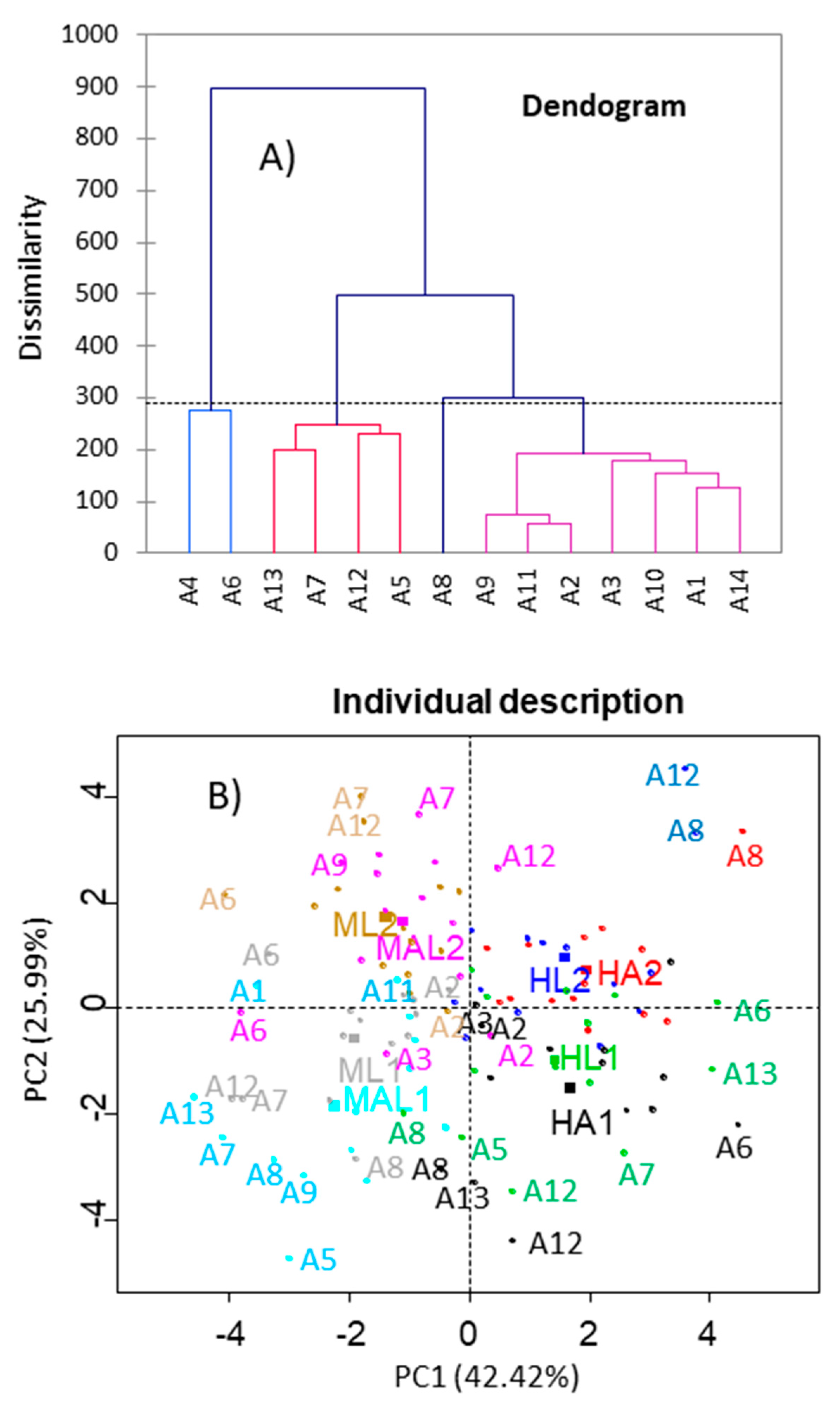
3.4.1. Clustering
3.4.2. Panelist Reproducibility
3.4.3. Panel Repeatability
Study by Variables Projection on the Correlation Circle According to Sessions
Study by Sample Projections According to Sessions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- International Olive Council (IOC). Online Reference Included in World Table Olives Figures: Production. 2018. Available online: http://www.internationaloliveoil.org/estaticos/view/132-world-table-olive-figures (accessed on 30 April 2019).
- Garrido-Fernández, A.; Fernández-Díez, M.J.; Adams, R.M. Table Olive Production and Processing; Chapman & Hall: London, UK, 1997. [Google Scholar]
- International Olive Council (IOC). Trade Standards Applying to Table Olives. IOC/OT/NC No. 1; International Olive Council: Madrid, Spain, 2004. [Google Scholar]
- International Olive Council (IOC). Method for the Sensory Analysis of Table Olives. COI/OT/MO No. 1/Rev.2 November 2011; International Olive Council: Madrid, Spain, 2011; Available online: http://www.internationaloliveoil.org/estaticos/view/70-metodos-de-evaluacion (accessed on 30 April 2018).
- Department of Agriculture. United States Standards for Grades of Ripe Olives; Agricultural Marketing Order, Department of Agriculture: Colombia, WA, USA, 1983. [Google Scholar]
- Alasalvar, C.; Pelvan, E.; Bahar, B.; Korel, F.; Ölmez, H. Flavour of natural and roasted Turkish hazelnut varieties (Corylus avellana L.) by descriptive sensory analysis, electronic nose and chemometrics. Int. J. Food Sci. Technol. 2012, 47, 122–131. [Google Scholar] [CrossRef]
- Dabbou, S.; Issaoui, M.; Brahmi, F.; Nakbi, A.; Chelab, H.; Mechri, R.; Hammani, M. Changes in volatile compounds during processing of Tunisian-style table olives. J. Am. Oil Chem. Soc. 2012, 89, 347–354. [Google Scholar] [CrossRef]
- Heyman, H.; Hopfer, H.; Bershaw, D. An exploration of the perception of minerality in white wines by projective mapping and descriptive analysis. J. Sens. Stud. 2014, 29, 1–13. [Google Scholar] [CrossRef]
- Lee, S.M.; Kitsawad, K.; Sigal, A.; Flynn, D.; Guinard, J.X. Sensory properties and consumer acceptance of imported and domestic sliced black ripe olives. J. Food Sci. 2012, 77, 439–448. [Google Scholar] [CrossRef] [PubMed]
- López-López, A.; Sánchez-Gómez, A.H.; Montaño, A.; Cortés-Delgado, A.; Garrido-Fernández, A. Sensory characterisation of black ripe table olives from Spanish Manzanilla and Hojiblanca cultivars. Food Res. Int. 2019, 116, 114–125. [Google Scholar] [CrossRef] [PubMed]
- López-López, A.; Sánchez-Gómez, A.H.; Montaño, A.; Cortés-Delgado, A.; Garrido-Fernández, A. Sensory profile of Green Spanish-style table olives according to cultivar and origin. Food Res. Int. 2018, 108, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Lanza, B.; Amoruso, F. Sensory analysis of natural table olives: Relationships between appearance of defect and gustatory-kinaesthetic sensation changes. LWT-Food Sci. Technol. 2016, 68, 365–372. [Google Scholar] [CrossRef]
- Yilmaz, E.; Aydeniz, B. Sensory evaluation and consumer perception of some commercial green table olives. Br. Food J. 2012, 114, 1085–1094. [Google Scholar] [CrossRef]
- Galán Soldevilla, H.; Ruiz Pérez-Cacho, P.; Hernández Campuzano, J.A. Determination of the sensory profiles of Aloreña table olives. Grasas Aceites 2013, 64, 442–452. [Google Scholar] [CrossRef]
- Marsilio, V.; Campestre, C.; Lanza, B.; De Angelis, M.; Russi, F. Sensory analysis of green table olives fermented in different saline solutions. Acta Hortic. 2002, 586, 617–620. [Google Scholar] [CrossRef]
- Lombardi, S.J.; Macciola, V.; Iorizzo, M.; De Leonardis, A. Effect of different storage conditions on the shelf life of natural green table olives. Ital. J. Food Sci. 2018, 30, 414–427. [Google Scholar]
- Lanza, B.; Amoruso, F. Panel performance, discrimination power of descriptors, and sensory characterization of table olive samples. J. Sens. Stud. 2019, e12542. [Google Scholar] [CrossRef]
- Rossi, F. Assessing sensory panelist performance using repeatability and reproducibility measures. Food Qual. Pref. 2001, 12, 467–479. [Google Scholar] [CrossRef]
- Kermit, M.; Lengard Almli, V. Assessing the performance of a sensory panel-panellist monitoring and tracking. J. Chemom. 2005, 19, 154–161. [Google Scholar] [CrossRef]
- Tomic, O.; Nilsen, A.; Martens, M.; Naes, T. Visualization of sensory profiling data for performance monitoring. LWT-Food Sci. Technol. 2007, 40, 262–269. [Google Scholar] [CrossRef]
- Tomic, O.; Forde, C.; Delahunty, C.; Naes, T. Performance indices in descriptive sensory analysis-A complimentary screening tool for assessor and panel performance. Food Qual. Pref. 2013, 28, 122–133. [Google Scholar] [CrossRef]
- Sipos, L.; Ladányi, M.; Gere, A.; Kókai, Z.; Kovács, S. Panel performance monitoring by Poincaré plot: A case study on flavoured bottled waters. Food Res. Int. 2017, 99, 198–205. [Google Scholar] [CrossRef] [PubMed]
- López-López, A.; Rodríguez-Gómez, F.; Cortés-Delgado, A.; Montaño, A.; Garrido-Fernández, A. Influence of ripe table olive processing on oils characteristics and composition as determined by chemometrics. J. Agric. Food Chem. 2009, 57, 8973–8981. [Google Scholar] [CrossRef] [PubMed]
- International Olive Council (IOC). Sensory Analysis of Olive Oil Standard Glass for Oil Tasting. COI/T20/Doc No. 5; International Olive Council: Madrid, Spain, 1987. [Google Scholar]
- Husson, F.; Lê, S. SensoMineR: Sensory Data Analysis with R. R Package Version 1.07. 2007. Available online: http://agrocampus-rennes.fr/math/SensoMinR (accessed on 08 November 2019).
- R Development Core Team. R: A Language and Environment for Statistical Computing; The R Foundation for Statistical Computing: Vienna, Austria, 2011. [Google Scholar]
- XLSTAT. Data Analysis and Statistical Solution for Microsoft Excel; Addinsoft: Paris, France, 2017. [Google Scholar]
- François, N.; Guyot-Declerck, C.; Hug, B.; Callemien, D.; Govaerst, B.; Collin, S. Beer astringency assessed by time-intensity and quantitative descriptive analysis: Influence of pH and accelerated aging. Food Qual. Pref. 2006, 17, 445–452. [Google Scholar] [CrossRef]
- Pense-Lheritier, A.-M.; Vallet, T.; Aubert, A.; Courne, M.-A.; Lavarde, M. Descriptive analysis of a complex product space: Drug-beverage mixtures. J. Sens. Stud. 2016, 31, 101–113. [Google Scholar] [CrossRef]
- Nyambaka, H.; Ryley, J. Multivariate analysis of the sensory change in the dehydrated cowpea leaves. Talanta 2004, 64, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Husson, F.; Lê, S.; Pagés, J. Confidence ellipse for the sensory profiles obtained by Principal Components Analysis. Food Qual. Pref. 2005, 16, 245–250. [Google Scholar] [CrossRef]
- Stone, H.; Sidel, J.; Oliver, S.; Woolsey, A.; Singleton, R.C. Sensory evaluation by quantitative descriptive analysis. Food Technol. 1974, 28, 24–34. [Google Scholar]
- Powers, J.J. Current practices and application of descriptive methods. In Sensory Analysis of Foods; Piggott, J.R., Ed.; Elsevier Applied Science: London, UK, 1988. [Google Scholar]
- Kim, M.K.; Lee, Y.-J.; Kwat, H.S.; Kang, M.W. Identification of sensory attributes that drive consumer liking of commercial orange juice products in Korea. J. Food Sci. 2013, 78, 1451–1458. [Google Scholar] [CrossRef] [PubMed]
- Rodrigue, N.; Guillet, M.; Fortin, J.; Martin, J.F. Comparing information obtaining for ranking and descriptive tests of four sweet corn products. Food Qual. Pref. 2000, 11, 47–54. [Google Scholar] [CrossRef]


| Sensory Attribute | Sample | Panelist | Session | Sample·Panelist | Sample·Session | Panelist·Session | Median |
|---|---|---|---|---|---|---|---|
| Skin green | 2.792 × 10−10 | 9.069 × 10−26 | 2.177 × 10−1 | 2.404 × 10−8 | 6.211 × 10−1 | 9.423 × 10−2 | 4.712 × 10−2 |
| Flesh green | 1.720 × 10−9 | 2.186 × 10−15 | 2.377 × 10−1 | 2.602 × 10−4 | 7.443 × 10−1 | 6.387 × 10−1 | 1.190 × 10−1 |
| Skin sheen | 1.900 × 10−6 | 1.742 × 10−28 | 2.497 × 10−1 | 1.011 × 10−4 | 6.362 × 10−1 | 2.689 × 10−1 | 1.249x 10−1 |
| Flesh red | 8.603 × 10−6 | 2.654 × 10−34 | 6.326 × 10−1 | 1.796 × 10−9 | 5.180 × 10−1 | 3.250 × 10−3 | 1.629 × 10−3 |
| Firmness | 1.033 × 10−4 | 4.141 × 10−35 | 3.320 × 10−1 | 1.881 × 10−3 | 9.960 × 10−2 | 3.305 × 10−2 | 1.747 × 10−2 |
| Fibrousness | 9.292 × 10−4 | 2.088 × 10−39 | 4.165 × 10−1 | 1.397 × 10−3 | 4.263 × 10−1 | 9.392 × 10−3 | 5.394 × 10−3 |
| Flesh yellow | 1.328 × 10−2 | 7.265 × 10−20 | 2.752 × 10−1 | 1.752 × 10−4 | 2.046 × 10−1 | 2.332 × 10−1 | 1.090 × 10−1 |
| Skin red | 1.760 × 10−2 | 3.535 × 10−51 | 6.585 × 10−1 | 1.511 × 10−10 | 2.695 × 10−1 | 1.342 × 10−1 | 7.590 × 10−2 |
| Vinegary | 1.833 × 10−2 | 3.683 × 10−30 | 1.613 × 10−1 | 1.794 × 10−3 | 6.612 × 10−2 | 2.908 × 10−2 | 2.370 × 10−2 |
| Moisture release | 2.978 × 10−2 | 4.515 × 10−33 | 9.058 × 10−1 | 9.558 × 10−6 | 5.027 × 10−1 | 6.828 × 10−2 | 4.903 × 10−2 |
| Fishy smell/Ocean | 3.060 × 10−2 | 1.312 × 10−12 | 3.342 × 10−1 | 3.912 × 10−1 | 7.507 × 10−1 | 7.249 × 10−1 | 3.627 × 10−1 |
| Saltiness | 3.117 × 10−2 | 1.680 × 10−48 | 3.191 × 10−1 | 2.575 × 10−3 | 3.940 × 10−3 | 1.068 × 10−3 | 3.258 × 10−3 |
| Astringency | 2.232 × 10−1 | 1.599 × 10−45 | 9.491 × 10−1 | 6.062 × 10−14 | 6.593 × 10−1 | 1.447 × 10−1 | 1.839 × 10−1 |
| Ripeness | 2.614 × 10−1 | 1.586 × 10−44 | 8.095 × 10−1 | 4.834 × 10−5 | 6.614 × 10−1 | 6.012 × 10−3 | 1.337 × 10−1 |
| Soapy smell/Medicinal | 2.636 × 10−1 | 2.478 × 10−51 | 4.467 × 10−1 | 4.560 × 10−1 | 6.792 × 10−1 | 2.451 × 10−2 | 3.552 × 10−1 |
| Bitterness | 2.710 × 10−1 | 4.434 × 10−38 | 2.556 × 10−1 | 5.075 × 10−1 | 1.940 × 10−1 | 2.364 × 10−3 | 2.248 × 10−1 |
| Chewiness | 3.334 × 10−1 | 1.538 × 10−39 | 7.989 × 10−1 | 4.862 × 10−11 | 2.964 × 10−1 | 2.947 × 10−3 | 1.497 × 10−1 |
| Briny | 3.567 × 10−1 | 1.708 × 10−37 | 7.944 × 10−1 | 4.671 × 10−6 | 8.067 × 10−1 | 2.152 × 10−2 | 1.891 × 10−1 |
| Natural fruity/Floral | 4.102 × 10−1 | 2.781 × 10−30 | 1.840 × 10−1 | 4.931 × 10−3 | 6.946 × 10−1 | 3.302 × 10−1 | 2.571 × 10−1 |
| Rancid | 4.867 × 10−1 | 1.815 × 10−41 | 3.093 × 10−1 | 2.110 × 10−1 | 6.873 × 10−1 | 2.669 × 10−2 | 2.601 × 10−1 |
| Nutty | 4.892 × 10−1 | 4.653 × 10−26 | 3.041 × 10−1 | 3.637 × 10−3 | 2.203 × 10−1 | 1.108 × 10−2 | 1.157 × 10−1 |
| Buttery | 5.223 × 10−1 | 7.225 × 10−42 | 3.749 × 10−1 | 1.292 × 10−9 | 4.572 × 10−1 | 6.488 × 10−3 | 1.907 × 10−1 |
| Oak barrel | 5.496 × 10−1 | 4.336 × 10−44 | 3.501 × 10−1 | 7.740 × 10−3 | 9.681 × 10−2 | 2.796 × 10−6 | 5.227 × 10−2 |
| Metallic | 5.778 × 10−1 | 1.374 × 10−24 | 1.115 × 10−1 | 1.010 × 10−1 | 5.508 × 10−6 | 8.859 × 10−1 | 1.062 × 10−1 |
| Alcohol | 6.690 × 10−1 | 9.806 × 10−60 | 8.765 × 10−2 | 2.337 × 10−1 | 7.464 × 10−1 | 1.730 × 10−1 | 2.033 × 10−1 |
| Mushroom | 6.795 × 10−1 | 5.867 × 10−26 | 4.712 × 10−1 | 3.910 × 10−6 | 1.256 × 10−1 | 6.242 × 10−7 | 6.280 × 10−2 |
| Mouth coating | 6.925 × 10−1 | 2.358 × 10−56 | 7.926 × 10−1 | 5.014 × 10−17 | 2.719 × 10−1 | 1.033 × 10−3 | 1.365 × 10−1 |
| Sourness | 7.219 × 10−1 | 1.227 × 10−24 | 6.145 × 10−1 | 1.206 × 10−2 | 8.290 × 10−1 | 8.804 × 10−4 | 3.133 × 10−1 |
| Earthy/Soil | 7.335 × 10−1 | 1.713 × 10−28 | 2.620 × 10−1 | 2.050 × 10−1 | 8.907 × 10−2 | 3.771 × 10−1 | 2.335 × 10−1 |
| Artificial fruity/Floral | 8.387 × 10−1 | 8.279 × 10−37 | 2.036 × 10−1 | 1.018 × 10−4 | 9.372 × 10−1 | 8.575 × 10−1 | 5.211 × 10−1 |
| Residual | 8.937 × 10−1 | 4.326 × 10−43 | 1.212 × 10−1 | 1.784 × 10−9 | 6.901 × 10−1 | 9.118 × 10−2 | 1.062 × 10−1 |
| Cheesy smell | 9.075 × 10−1 | 2.015 × 10−27 | 6.441 × 10−1 | 8.088 × 10−3 | 3.692 × 10−1 | 2.652 × 10−4 | 1.886 × 10−1 |
| Gassy smell | 9.389 × 10−1 | 3.652 × 10−33 | 5.786 × 10−1 | 1.249 × 10−1 | 9.484 × 10−1 | 7.670 × 10−1 | 6.728 × 10−1 |
| A14 | A4 | A2 | A3 | A6 | A5 | A8 | A1 | A12 | A13 | A7 | A11 | A10 | A9 | Median | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Skin green | 0.0007 | 0.0119 | 0.0011 | 0.0010 | 0.0016 | 0.0687 | 0.0002 | 0.1243 | 5.2 × 10−7 | 0.0402 | 0.0655 | 0.5177 | 0.6086 | 0.1772 | 0.0261 |
| Mouth coating | 0.0491 | 0.0188 | 0.2445 | 0.0023 | 0.0162 | 0.0063 | 4.8 × 10−7 | 0.0927 | 0.7896 | 0.0042 | 0.2880 | 0.4278 | 0.2185 | 0.8250 | 0.0709 |
| Flesh red | 0.0103 | 0.0294 | 0.448 | 0.0019 | 3.9 × 10−6 | 0.0073 | 0.6432 | 0.3536 | 0.0409 | 0.1384 | 2.4 × 10−5 | 0.1661 | 0.4706 | 0.1965 | 0.0897 |
| Briny | 0.0912 | 0.0490 | 0.0074 | 0.3023 | 0.0138 | 0.1940 | 0.0679 | 0.5585 | 0.0370 | 0.1132 | 0.4739 | 0.2358 | 0.1762 | 0.0419 | 0.1022 |
| Flesh green | 0.0564 | 0.0049 | 0.0885 | 0.0792 | 0.0001 | 0.2135 | 0.1192 | 0.1599 | 0.0240 | 0.1850 | 5.3 × 10−7 | 0.3497 | 0.1588 | 0.1216 | 0.1039 |
| Skin red | 6.7 × 10−6 | 0.1042 | 0.0225 | 1.0 × 10−6 | 0.0063 | 0.5103 | 0.1245 | 0.3750 | 0.0251 | 0.0046 | 0.2455 | 0.0883 | 0.4822 | 0.2204 | 0.0963 |
| Residual | 0.1602 | 0.0005 | 0.4853 | 0.1151 | 0.0480 | 0.0976 | 4.9 × 10−10 | 0.0514 | 0.5184 | 0.6976 | 0.3556 | 0.2728 | 0.7313 | 0.0727 | 0.1376 |
| Oak barrel | 0.1427 | 0.0006 | 0.9411 | 0.1950 | 0.2170 | 0.8693 | 0.4858 | 0.0194 | 0.1548 | 0.2937 | 0.4778 | 0.1655 | 0.1373 | 0.1540 | 0.1802 |
| Fibrousness | 0.1065 | 0.0220 | 6.4 × 10−5 | 0.3311 | 0.1991 | 0.3859 | 0.0055 | 0.1004 | 0.0410 | 0.5118 | 0.0525 | 0.6702 | 0.4188 | 0.6076 | 0.1528 |
| Ripeness | 0.1602 | 0.0005 | 0.4853 | 0.4853 | 0.0480 | 0.0976 | 4.9 × 10−10 | 0.0514 | 0.5184 | 0.6976 | 0.3556 | 0.2728 | 0.7313 | 0.0727 | 0.1376 |
| Firmness | 0.1345 | 0.0402 | 0.0444 | 0.0022 | 0.1130 | 0.7759 | 0.1552 | 0.4107 | 0.2639 | 0.1660 | 0.0009 | 0.3727 | 0.2481 | 0.8867 | 0.1606 |
| Skin sheen | 0.2700 | 0.2860 | 0.0323 | 0.0003 | 0.3672 | 0.0109 | 0.0269 | 7.7 × 10−5 | 0.1841 | 0.0009 | 0.6877 | 0.1887 | 0.1489 | 0.8764 | 0.1665 |
| Flesh yellow | 0.1231 | 0.0116 | 0.081 | 0.1643 | 0.7031 | 0.2925 | 0.0878 | 0.5186 | 0.0442 | 0.5177 | 6.6 × 10−5 | 0.3582 | 0.0754 | 0.2687 | 0.1437 |
| Bitterness | 0.0100 | 0.6474 | 0.0056 | 0.2079 | 0.5114 | 0.7813 | 0.2312 | 0.0501 | 0.2547 | 0.2341 | 0.9994 | 0.2390 | 0.1831 | 0.5097 | 0.2365 |
| Astringency | 0.5481 | 0.0240 | 0.0374 | 0.2538 | 0.6887 | 0.2064 | 1.5 × 10−8 | 0.0159 | 0.1436 | 0.7140 | 0.0508 | 0.9816 | 0.6175 | 0.4225 | 0.2301 |
| Moisture release | 0.1299 | 0.2333 | 0.2658 | 0.0004 | 0.0052 | 0.6328 | 0.0480 | 0.3207 | 0.0604 | 0.0330 | 0.102 | 0.5576 | 0.8638 | 0.2983 | 0.1816 |
| Nutty | 0.0392 | 0.0793 | 0.3598 | 0.2546 | 0.1677 | 0.0824 | 0.2438 | 0.2683 | 0.4706 | 0.8304 | 0.4658 | 0.1908 | 0.3928 | 0.1083 | 0.2492 |
| Buttery | 7.6 × 10−7 | 0.2047 | 0.2191 | 6.1 × 10−6 | 0.6094 | 0.1268 | 0.8115 | 0.0356 | 0.2662 | 0.1992 | 0.0634 | 0.5658 | 0.3017 | 0.9222 | 0.2119 |
| Mushroom | 0.8962 | 0.0003 | 0.6540 | 0.0006 | 0.6046 | 0.3799 | 0.7030 | 0.0476 | 0.4706 | 0.5158 | 0.0077 | 0.1854 | 0.5196 | 0.136 | 0.4253 |
| Chewiness | 0.0003 | 0.4448 | 0.7152 | 0.0156 | 0.2636 | 0.6584 | 6.5 × 10−9 | 0.0003 | 0.0422 | 0.2922 | 0.1441 | 0.6937 | 0.9195 | 0.6294 | 0.2779 |
| Sourness | 0.2963 | 0.6765 | 0.6530 | 0.4706 | 0.0155 | 0.1844 | 0.4783 | 0.2397 | 0.3977 | 0.2461 | 0.3597 | 0.8106 | 0.0778 | 0.7095 | 0.3787 |
| Saltiness | 0.5401 | 0.6428 | 0.4611 | 0.1292 | 0.0715 | 0.2307 | 0.0075 | 0.0147 | 0.1889 | 0.9875 | 0.7995 | 0.7577 | 0.4706 | 0.1714 | 0.3459 |
| Vinegary | 0.1315 | 0.6339 | 0.3024 | 0.3830 | 0.2282 | 0.1108 | 0.4706 | 0.3442 | 0.0716 | 0.5815 | 0.3609 | 0.4651 | 0.4706 | 0.5146 | 0.3719 |
| Nat. fruity/floral | 0.0598 | 0.7439 | 0.7778 | 0.0112 | 0.4959 | 0.4959 | 0.4706 | 0.3475 | 0.6836 | 0.2703 | 0.6252 | 0.3119 | 0.0554 | 0.7819 | 0.4091 |
| Earthy soil | 0.3278 | 0.0136 | 0.1162 | 0.6321 | 0.9603 | 0.2985 | 0.4706 | 0.561 | 0.4706 | 0.3367 | 0.5492 | 0.4013 | 0.5284 | 0.1496 | 0.4360 |
| Rancid | 0.09477 | 0.2697 | 0.2386 | 0.5123 | 0.3915 | 0.6038 | 0.4706 | 0.3878 | 0.4706 | 0.4822 | 0.5793 | 0.1436 | 0.5866 | 0.6142 | 0.4706 |
| Art. fruity/floral | 4.4 × 10−5 | 0.4568 | 0.3301 | 0.5810 | 0.0916 | 0.0457 | 0.0279 | 0.2441 | 0.4706 | 0.0880 | 0.4715 | 0.4706 | 0.4876 | 0.7257 | 0.3934 |
| Metallic | 0.2645 | 0.5882 | 0.0167 | 0.4980 | 0.5781 | 0.8309 | 0.5770 | 0.3776 | 0.4706 | 0.4706 | 0.3236 | 0.3508 | 0.4074 | 0.5429 | 0.4706 |
| Alcohol | 0.3289 | 0.4027 | 0.1303 | 0.5559 | 0.7270 | 0.2080 | 0.5836 | 0.4118 | 0.4706 | 0.3147 | 0.3841 | 0.4706 | 0.4706 | 0.7604 | 0.4412 |
| Cheesy smell | 0.5312 | 0.5468 | 0.1270 | 0.6647 | 0.3881 | 0.7116 | 0.4706 | 0.3594 | 0.4706 | 0.7706 | 0.1850 | 0.5121 | 0.0275 | 0.7174 | 0.4914 |
| Fishy smell | 0.7693 | 0.1763 | 0.1578 | 0.5570 | 0.2083 | 0.7899 | 0.4706 | 0.3009 | 0.4706 | 0.0289 | 0.4288 | 0.1892 | 0.7001 | 0.5644 | 0.4497 |
| Soapy smell/med | 0.6524 | 0.5964 | 0.1828 | 0.5373 | 0.7019 | 0.6411 | 0.6237 | 0.8154 | 0.4706 | 0.0145 | 0.4853 | 0.5051 | 0.7512 | 0.4998 | 0.5669 |
| Median | 0.1299 | 0.1763 | 0.1828 | 0.1950 | 0.2170 | 0.2307 | 0.2312 | 0.2583 | 0.2662 | 0.2703 | 0.3556 | 0.3582 | 0.4706 | 0.5097 | 0.2448 |
| A1 | A10 | A11 | A12 | A13 | A14 | A2 | A3 | A4 | A5 | A6 | A7 | A8 | A9 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Skin red | 1.63 | 2.27 | 0.88 | 1.85 | 1.91 | 1.03 | 0.60 | 1.06 | 0.94 | 2.74 | 1.69 | 2.06 | 1.41 | 1.03 |
| Skin green | 1.50 | 2.06 | 1.26 | 1.47 | 1.96 | 1.37 | 0.31 | 1.30 | 0.73 | 1.75 | 1.23 | 2.02 | 1.03 | 2.42 |
| Skin sheen | 0.98 | 1.57 | 1.27 | 3.20 | 1.61 | 1.15 | 0.51 | 1.43 | 1.03 | 1.39 | 1.82 | 1.81 | 0.77 | 1.63 |
| Flesh red | 1.74 | 0.06 | 1.41 | 2.89 | 3.10 | 1.23 | 0.84 | 1.64 | 0.94 | 1.58 | 1.11 | 1.61 | 1.86 | 1.27 |
| Flesh yellow | 1.22 | 1.90 | 1.38 | 2.77 | 1.16 | 1.16 | 0.67 | 0.21 | 0.67 | 1.55 | 2.41 | 0.78 | 1.75 | 0.91 |
| Flesh green | 1.76 | 2.05 | 1.46 | 2.76 | 2.64 | 1.65 | 0.54 | 1.06 | 0.88 | 2.88 | 1.40 | 1.11 | 1.85 | 2.08 |
| Briny | 1.37 | 1.58 | 1.09 | 2.32 | 1.34 | 0.91 | 0.46 | 1.00 | 0.39 | 1.41 | 1.45 | 1.74 | 1.27 | 1.75 |
| Mushroom | 0.58 | 0.91 | 1.06 | 0.09 | 1.35 | 1.09 | 0.75 | 1.13 | 0.53 | 1.08 | 2.19 | 1.65 | 1.41 | 0.88 |
| Earthy soil | 0.78 | 1.66 | 2.00 | 0.09 | 0.98 | 0.29 | 0.54 | 0.68 | 0.86 | 1.21 | 1.86 | 0.59 | <0.01 | 0.77 |
| Oak barrel | 0.41 | 1.93 | 0.65 | 1.33 | 1.03 | 0.36 | 0.89 | 0.53 | 0.59 | 1.63 | 1.88 | 1.06 | 1.93 | 0.79 |
| Nutty | 0.21 | 1.19 | 0.80 | 0.10 | 0.64 | 0.40 | 0.54 | 0.77 | 0.54 | 0.51 | 1.07 | 0.96 | 2.06 | 1.33 |
| Artificial fruity/floral | 0.18 | 0.93 | 0.02 | 0.12 | 0.72 | 0.36 | 0.59 | 0.15 | 0.78 | 1.14 | 1.19 | 1.19 | 0.79 | 0.96 |
| Natural fruity/floral | 1.13 | 1.74 | 0.07 | 1.03 | 1.06 | 0.87 | 0.87 | 0.79 | 0.80 | 1.61 | 1.95 | 1.50 | <0.01 | 1.05 |
| Vinegary | 0.24 | <0.01 | 0.28 | 1.55 | 1.20 | 0.38 | 0.53 | 0.39 | 0.53 | 2.31 | 1.98 | 0.59 | 0.41 | 0.61 |
| Alcohol | 0.20 | 0.13 | 0.07 | 0.14 | 0.61 | 0.67 | 0.55 | 0.26 | 0.60 | 2.03 | 1.73 | 1.09 | 1.04 | 1.00 |
| Fishy smell/ocean | 0.48 | 0.85 | 0.15 | 0.14 | 0.96 | 1.08 | 0.31 | 0.38 | 0.84 | 1.37 | 1.46 | 1.71 | 0.35 | 1.58 |
| Cheese smell | 0.44 | 1.00 | 0.09 | 0.20 | 0.86 | 0.09 | 0.29 | 0.09 | 0.47 | 0.76 | 1.39 | 0.55 | 0.02 | 0.15 |
| Sourness | 0.14 | 0.56 | 0.53 | 1.28 | 0.35 | 1.89 | 0.14 | 0.20 | 0.70 | 1.98 | 0.84 | 1.65 | 0.65 | 0.28 |
| Bitterness | 0.80 | 1.06 | 0.30 | 1.27 | 1.54 | 0.87 | 0.42 | 0.55 | 0.87 | 1.99 | 1.07 | 2.73 | 1.59 | 0.37 |
| Saltiness | 0.78 | 0.13 | 0.65 | 1.75 | 0.76 | 0.27 | 0.30 | 0.40 | 0.86 | 1.19 | 1.19 | 2.35 | 1.33 | 0.93 |
| Ripeness | 1.27 | 2.81 | 0.52 | 2.12 | 1.95 | 1.73 | 0.45 | 0.98 | 0.73 | 1.43 | 1.49 | 2.07 | 1.69 | 1.04 |
| Buttery | 0.71 | 2.26 | 0.68 | 1.41 | 1.71 | 0.91 | 0.53 | 1.18 | 0.77 | 1.09 | 1.87 | 1.57 | 1.26 | 2.12 |
| Metallic | 1.62 | 1.37 | 0.54 | 0.25 | 0.82 | 1.45 | 0.52 | 1.09 | 0.95 | 1.84 | 2.00 | 1.69 | 0.82 | 0.80 |
| Rancid | 0.36 | 0.73 | 0.04 | <0.01 | 0.45 | 0.56 | 0.37 | 0.33 | 0.74 | 0.70 | 2.01 | 0.26 | <0.01 | 0.05 |
| Soapy smell/medicinal | 0.30 | 1.37 | 0.46 | 0.42 | 1.21 | 0.05 | 0.30 | 0.85 | 0.81 | 0.91 | 2.50 | 1.14 | 0.66 | 0.71 |
| Gassy smell | 0.12 | 0.59 | 0.05 | 0.71 | <0.01 | <0.01 | 0.12 | 0.47 | 0.70 | 0.12 | 1.41 | 0.13 | 0.022 | 0.07 |
| Firmness | 1.29 | 1.36 | 0.64 | 1.12 | 2.36 | 1.68 | 0.38 | 1.14 | 0.82 | 1.85 | 1.83 | 1.10 | 1.03 | 1.01 |
| Fibrousness | 1.02 | 0.77 | 0.62 | 1.25 | 2.02 | 1.43 | 0.25 | 1.51 | 0.71 | 1.14 | 1.56 | 1.35 | 1.53 | 1.17 |
| Moisture release | 1.36 | 2.02 | 0.61 | 1.20 | 1.85 | 1.34 | 0.29 | 1.01 | 0.67 | 0.99 | 1.41 | 1.32 | 1.03 | 1.42 |
| Mouth coating | 0.85 | 1.73 | 0.60 | 0.65 | 1.30 | 1.32 | 0.52 | 1.20 | 0.64 | 0.72 | 1.15 | 1.00 | 0.84 | 0.92 |
| Chewiness | 0.75 | 1.46 | 0.64 | 1.10 | 1.94 | 1.11 | 0.36 | 1.10 | 1.26 | 1.36 | 1.73 | 1.04 | 0.84 | 0.92 |
| Astringency | 0.05 | 1.60 | 0.53 | 1.19 | 1.99 | 0.07 | 0.12 | 0.91 | 0.62 | 0.54 | 1.37 | 1.45 | 0.91 | 0.10 |
| Residual | 0.62 | 2.14 | 0.13 | 0.13 | 1.41 | 1.89 | 1.36 | 0.34 | 0.95 | 0.82 | 1.35 | 1.28 | 1.04 | 0.55 |
| A6 | A8 | A14 | A5 | A1 | A7 | A13 | A10 | A9 | A3 | A2 | A4 | A12 | A11 | Median | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Skin green | 0.674 | 0.828 | 0.792 | 0.810 | 0.660 | 0.603 | 0.932 | 0.547 | 0.814 | 0.808 | 0.853 | 0.874 | 0.791 | 0.860 | 0.800 |
| Skin sheen | 0.762 | 0.787 | 0.840 | 0.962 | 0.809 | 0.869 | 0.677 | 0.820 | 0.050 | 0.220 | 0.092 | 0.883 | 0.762 | 0.323 | 0.744 |
| Flesh red | 0.907 | 0.904 | 0.499 | 0.841 | 0.667 | 0.956 | 0.190 | −0.322 | 0.816 | 0.268 | 0.759 | 0.524 | 0.722 | 0.420 | 0.695 |
| Firmness | 0.335 | 0.678 | 0.600 | 0.296 | 0.754 | 0.758 | 0.789 | 0.696 | 0.500 | 0.216 | 0.720 | 0.875 | 0.335 | 0.803 | 0.687 |
| Flesh green | 0.960 | 0.601 | 0.152 | 0.198 | 0.752 | 0.550 | 0.727 | 0.615 | 0.505 | 0.751 | 0.344 | 0.764 | 0.097 | 0.572 | 0.637 |
| Fibrousness | 0.644 | 0.639 | 0.152 | 0.198 | 0.752 | 0.550 | 0.727 | 0.615 | 0.505 | 0.751 | 0.344 | 0.833 | 0.965 | −0.658 | 0.577 |
| Flesh yellow | 0.309 | 0.673 | 0.293 | 0.529 | −0.635 | 0.598 | 0.801 | 0.556 | 0.653 | 0.306 | 0.649 | 0.538 | −0.226 | 0.504 | 0.558 |
| Moist. release | 0.781 | 0.425 | 0.579 | 0.588 | 0.667 | 0.605 | 0.355 | −0.078 | 0.653 | 0.306 | 0.649 | 0.538 | −0.226 | 0.504 | 0.558 |
| Fishy smell | −0.018 | 0.777 | 0.885 | 0.434 | 0.336 | 0.366 | 0.723 | 0.216 | 0.650 | 0.886 | −0.532 | 0.886 | 0.774 | 0.245 | 0.542 |
| Nutty | −0.206 | 0.831 | 0.606 | 0.401 | −0.049 | −0.445 | 0.674 | −0.262 | 0.640 | 0.706 | 0.665 | 0.566 | −0.157 | −0.580 | 0.484 |
| Astringency | −0.387 | 0.921 | 0.488 | 0.769 | 0.470 | 0.850 | −0.412 | 0.576 | −0.254 | 0.497 | 0.730 | 0.373 | −0.249 | −0.471 | 0.479 |
| Briny | −0.250 | 0.478 | −0.043 | 0.470 | 0.620 | 0.560 | 0.386 | −0.477 | 0.530 | 0.513 | −0.133 | 0.183 | 0.609 | 0.735 | 0.474 |
| Ripeness | 0.420 | 0.093 | 0.674 | 0.454 | 0.760 | −0.309 | 0.690 | 0.559 | 0.618 | −0.106 | 0.832 | −0.057 | 0.444 | 0.373 | 0.473 |
| Buttery | 0.036 | 0.065 | 0.691 | −0.266 | 0.600 | 0.405 | 0.751 | 0.537 | 0.407 | −0.353 | 0.607 | 0.309 | 0.480 | 0.582 | 0.444 |
| Skin red | 0.862 | 0.356 | 0.551 | −0.063 | 0.676 | 0.257 | 0.444 | 0.154 | 0.585 | 0.387 | 0.517 | 0.228 | 0.776 | 0.223 | 0.416 |
| Chewiness | 0.135 | 0.269 | −0.045 | 0.485 | 0.235 | 0.568 | 0.646 | 0.476 | 0.311 | 0.414 | 0.046 | 0.677 | 0.406 | 0.811 | 0.410 |
| Vinegary | 0.989 | 0.255 | 0.798 | 0.853 | 0.392 | 0.079 | 0.422 | - | −0.277 | −0.723 | −0.511 | −0.696 | 0.982 | 0.594 | 0.392 |
| Oak barrel | 0.696 | 0.401 | 0.556 | 0.511 | 0.250 | 0.117 | −0.058 | 0.367 | 0.546 | 0.118 | 0.640 | 0.334 | −0.426 | −0.209 | 0.351 |
| Bitterness | −0.145 | 0.754 | 0.433 | −0.312 | 0.590 | 0.533 | 0.735 | 0.127 | 0.158 | 0.740 | 0.245 | 0.053 | −0.039 | 0.450 | 0.339 |
| Saltiness | 0.682 | 0.850 | 0.061 | 0.397 | 0.440 | 0.607 | −0.033 | 0.792 | 0.531 | 0.060 | −0.312 | 0.244 | 0.314 | 0.170 | 0.279 |
| Earthy soil | 0.703 | - | −0.843 | 0.013 | 0.462 | −0.133 | 0.478 | 0.544 | 0.328 | 0.239 | −0.283 | −0.067 | −0.392 | 0.540 | 0.329 |
| Mouth coating | 0.770 | 0.770 | −0.558 | 0.172 | −0.189 | 0.283 | −0.143 | −0.511 | 0.406 | 0.870 | 0.434 | 0.817 | −0.393 | −0.255 | 0.228 |
| Natural fruity/floral | −0.514 | - | 0.773 | 0.765 | 0.546 | 0.210 | −0.700 | 0.685 | −0.482 | 0.952 | 0.464 | −0.013 | 0.055 | −0.798 | 0.210 |
| Mushroom | −0.064 | 0.473 | 0.023 | 0.834 | 0.386 | 0.408 | 0.286 | 0.152 | 0.076 | 0.343 | −0.070 | 0.054 | 0.003 | 0.229 | 0.190 |
| Cheese smell | 0.237 | 0.050 | 0.258 | −0.058 | −0.037 | 0.379 | −0.241 | 0.830 | −0.403 | 0.846 | 0.478 | −0.652 | 0.090 | 0.358 | 0.164 |
| Gassy smell | 0.695 | 0.537 | - | −0.099 | 0.155 | −0.132 | - | 0.138 | −0.037 | 0.104 | −0.464 | 0.192 | 0.341 | 0.180 | 0.146 |
| Alcohol | −0.294 | −0.119 | 0.526 | 0.805 | −0.192 | 0.684 | 0.735 | −0.306 | 0.389 | 0.011 | −0.292 | −0.423 | 0.798 | 0.260 | 0.135 |
| Soapy smell/medicinal | 0.134 | −0.387 | −0.256 | 0.865 | 0.279 | 0.795 | 0.928 | −0.370 | 0.354 | 0.865 | −0.099 | −0.056 | 0.075 | 0.070 | 0.105 |
| Sourness | 0.605 | −0.037 | 0.532 | 0.540 | 0.320 | −0.213 | 0.123 | −0.149 | 0.061 | 0.241 | −0.056 | −0.414 | 0.667 | −0.287 | 0.092 |
| Rancid | 0.932 | - | −0.407 | 0.694 | 0.038 | 0.038 | −0.108 | 0.423 | −0.397 | 0.568 | 0.589 | −0.132 | - | 0.077 | 0.058 |
| Metallic | 0.466 | −0.117 | −0.169 | 0.307 | 0.700 | 0.915 | −0.601 | 0.543 | −0.311 | −0.061 | −0.153 | 0.776 | 0.087 | −0.527 | 0.028 |
| Artificial fruity/floral | 0.597 | 0.579 | 0.310 | 0.359 | 0.441 | −0.337 | −0.058 | 0.103 | −0.217 | −0.219 | −0.137 | 0.517 | −0.054 | −0.054 | 0.024 |
| Residual | 0.680 | 0.842 | −0.859 | 0.068 | −0.500 | −0.204 | −0.731 | 0.522 | −0.727 | 0.760 | −0.680 | 0.876 | −0.384 | −0.234 | −0.217 |
| Median | 0.597 | 0.558 | 0.493 | 0.470 | 0.441 | 0.408 | 0.404 | 0.395 | 0.385 | 0.387 | 0.344 | 0.334 | 0.324 | 0.245 | 0.400 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-López, A.; Sánchez-Gómez, A.H.; Montaño, A.; Cortés-Delgado, A.; Garrido-Fernández, A. Panel and Panelist Performance in the Sensory Evaluation of Black Ripe Olives from Spanish Manzanilla and Hojiblanca Cultivars. Foods 2019, 8, 562. https://doi.org/10.3390/foods8110562
López-López A, Sánchez-Gómez AH, Montaño A, Cortés-Delgado A, Garrido-Fernández A. Panel and Panelist Performance in the Sensory Evaluation of Black Ripe Olives from Spanish Manzanilla and Hojiblanca Cultivars. Foods. 2019; 8(11):562. https://doi.org/10.3390/foods8110562
Chicago/Turabian StyleLópez-López, Antonio, Antonio Higinio Sánchez-Gómez, Alfredo Montaño, Amparo Cortés-Delgado, and Antonio Garrido-Fernández. 2019. "Panel and Panelist Performance in the Sensory Evaluation of Black Ripe Olives from Spanish Manzanilla and Hojiblanca Cultivars" Foods 8, no. 11: 562. https://doi.org/10.3390/foods8110562
APA StyleLópez-López, A., Sánchez-Gómez, A. H., Montaño, A., Cortés-Delgado, A., & Garrido-Fernández, A. (2019). Panel and Panelist Performance in the Sensory Evaluation of Black Ripe Olives from Spanish Manzanilla and Hojiblanca Cultivars. Foods, 8(11), 562. https://doi.org/10.3390/foods8110562






