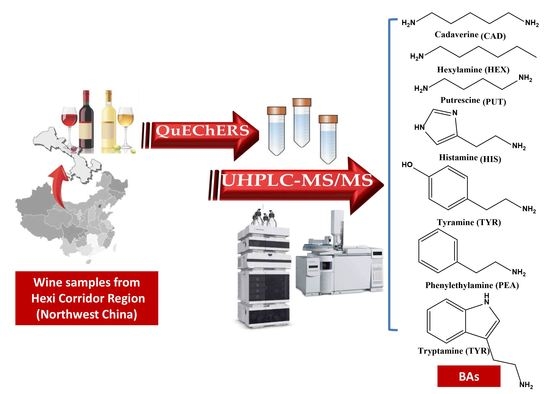
Development and Application of a New QuEChERS Method in UHPLC-QqQ-MS/MS to Detect Seven Biogenic Amines in Chinese Wines
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Standards
2.2. Optimization of the Extraction and Instrument Method
2.2.1. Optimization of the Extraction Method
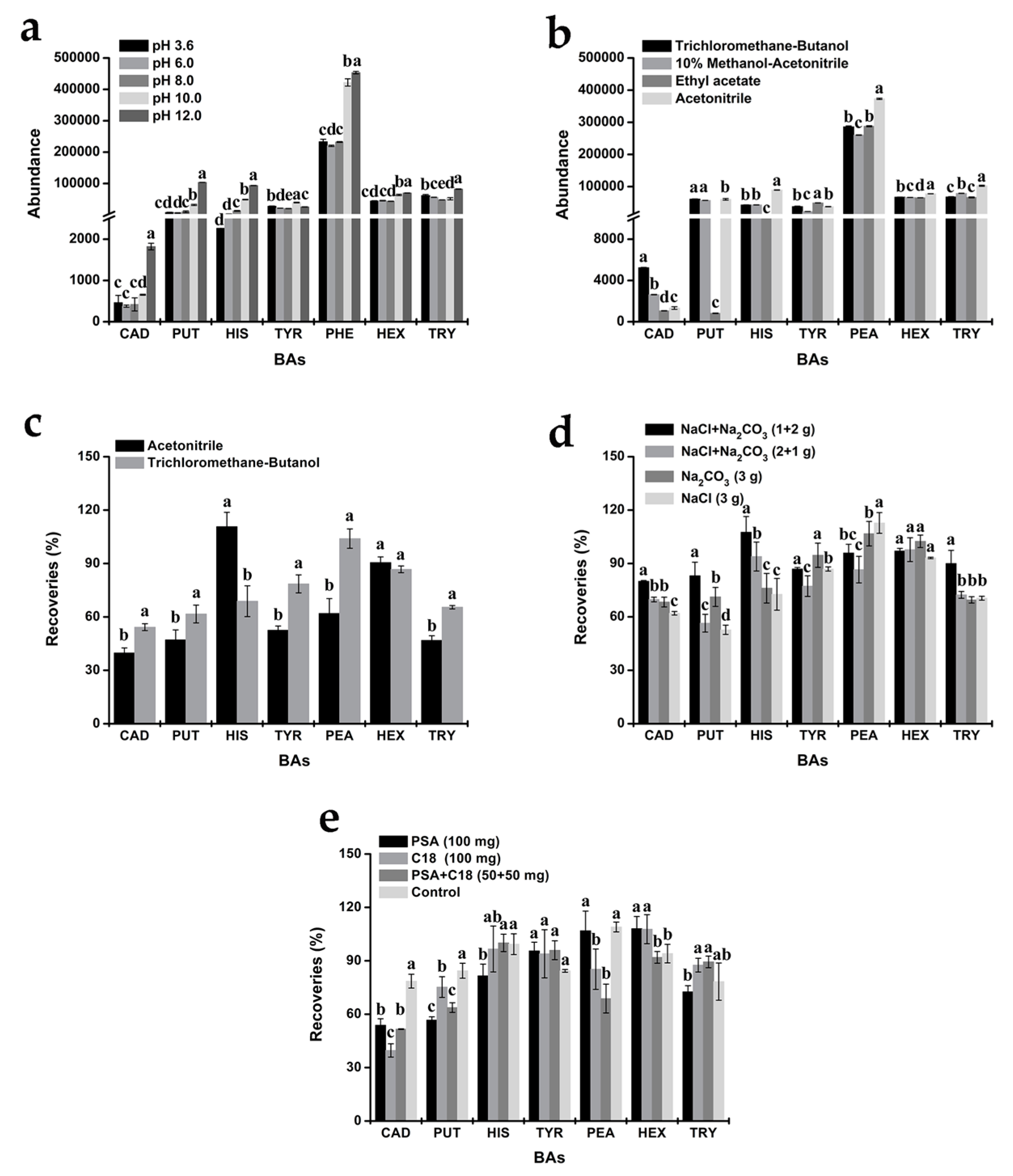
The pH Optimization
The Extraction Solvent Optimization
The Amount and Type of Salt Optimization
The Clean-Up Optimization
Optimized QuEChERS Procedure
2.2.2. Optimization of the Instrument Method
2.3. Method Validation
2.4. Sample Preparation
2.5. Data Analysis
3. Results
3.1. Optimization of the UHPLC-QqQ-MS/MS Conditions
3.2. Optimization of the QuEChERS Procedure
3.3. Method Validation
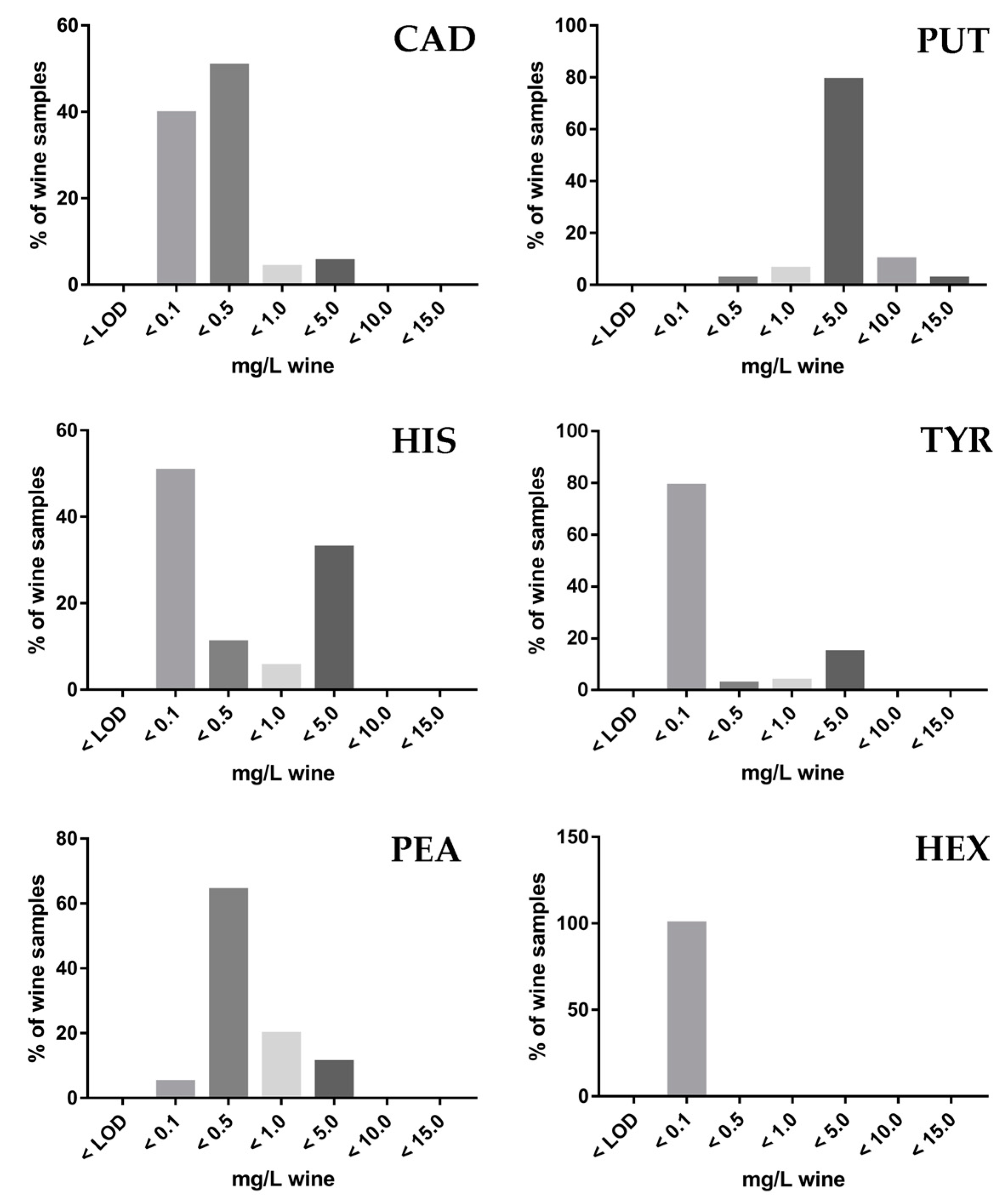
3.4. Application to Wine Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Omanovic-Miklicanin, E.; Valzacchi, S. Development of new chemiluminescence biosensors for determination of biogenic amines in meat. Food Chem. 2017, 235, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, L.; Xu, J.; Preininger, C.; Tse Sum Bui, B.; Haupt, K. Competitive fluorescent pseudo-immunoassay exploiting molecularly imprinted polymers for the detection of biogenic amines in fish matrix. Talanta 2018, 181, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Arribas, M.V.; Polo, M.C. Wine Chemistry and Biochemistry; Springer: New York, NY, USA, 2009. [Google Scholar]
- Linares, D.M.; Martín, M.; Ladero, V.; Alvarez, M.A.; Fernández, M. Biogenic amines in dairy products. Crit. Rev. Food Sci. Nutr. 2011, 51, 691–703. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Carou, M.C.; Veciana-Nogués, M.T.; Latorre-Moratala, M.L.; Bover-Cid, S. Biogenic Amines: Risks and Control. In Handbook of Fermented Meat and Poultry; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2008. [Google Scholar]
- Kim, S.H.; Eun, J.B.; Chen, T.Y.; Wei, C.I.; Clemens, R.A.; An, H. Evaluation of histamine and other biogenic amines and bacterial isolation in canned anchovies recalled by the USFDA. J. Food Sci. 2010, 69, M157–M162. [Google Scholar] [CrossRef]
- Costa, M.P.; Rodrigues, B.L.; Frasao, B.S.; Conte-Junior, C.A. Chapter 2-Biogenic Amines as Food Quality Index and Chemical Risk for Human Consumption. In Food Quality: Balancing Health and Disease; Holban, A.M., Grumezescu, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 75–108. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, F.; Skandamis, P.; Valdramidis, V. Quantitative Methods for Food Safety and Quality in the Vegetable Industry; Springer: New York, NY, USA, 2018. [Google Scholar]
- Ladero, V.; Calles-Enriquez, M.; Fernandez, M.; Alvarez, M.A. Toxicological effects of dietary biogenic amines. Curr. Nutr. Food Sci. 2010, 6, 145–156. [Google Scholar] [CrossRef]
- Hong, J.Y.; Na, H.P.; Oh, M.S.; Lee, H.S.; Pyo, H.; Hong, J. Profiling analysis of biogenic amines and their acidic metabolites in mouse brain tissue using gas chromatography–tandem mass spectrometry. J. Chromatogr. B 2013, 940, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Ščavničar, A.; Rogelj, I.; Kočar, D.; Kӧse, S.; Pompe, M. Determination of biogenic amines in cheese by ion chromatography with tandem mass spectrometry detection. J. AOAC Int. 2018, 101, 1542–1547. [Google Scholar] [CrossRef]
- Marcobal, A.; Polo, M.C.; Martín-Álvarez, P.J.; Moreno-Arribas, M.V. Biogenic amine content of red Spanish wines: Comparison of a direct ELISA and an HPLC method for the determination of histamine in wines. Food Res. Int. 2005, 38, 387–394. [Google Scholar] [CrossRef]
- Xu, J.J.; Ma, C.L.; Guo, C.F. A comparative analysis of derivatization strategies for the determination of biogenic amines in sausage and cheese by HPLC. Food Chem. 2018, 266, 275–283. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Ye, D.Q.; Zhu, B.Q.; Wu, G.F.; Duan, C.Q. Rapid HPLC analysis of amino acids and biogenic amines in wines during fermentation and evaluation of matrix effect. Food Chem. 2014, 163, 6–15. [Google Scholar] [CrossRef]
- Manetta, A.C.; Giuseppe, L.D.; Tofalo, R.; Martuscelli, M.; Schirone, M.; Giammarco, M.; Suzzi, G. Evaluation of biogenic amines in wine: Determination by an improved HPLC-PDA method. Food Control 2016, 62, 351–356. [Google Scholar] [CrossRef]
- Jain, A.; Gupta, M.; Verma, K.K. Salting-out assisted liquid–liquid extraction for the determination of biogenic amines in fruit juices and alcoholic beverages after derivatization with 1-naphthylisothiocyanate and high performance liquid chromatography. J. Chromatogr. A 2015, 1422, 60–72. [Google Scholar] [CrossRef]
- Huang, K.J.; Wei, C.Y.; Liu, W.L.; Xie, W.Z.; Zhang, J.F.; Wang, W. Ultrasound-assisted dispersive liquid–liquid microextraction combined with high-performance liquid chromatography-fluorescence detection for sensitive determination of biogenic amines in rice wine samples. J. Chromatogr. A 2009, 1216, 6636–6641. [Google Scholar] [CrossRef]
- Fernández-Franzón, M.; Berardinis, F.D.; Sagratini, G.; Vittori, S.; Font, G.; Mañes, J. Simultaneous determination of eight biogenic amines in fish by liquid chromatography with fluorescence detector and tandem mass spectrometry. Toxicol. Lett. 2010, 196, 342. [Google Scholar] [CrossRef]
- Papageorgiou, M.; Lambropoulou, D.; Morrison, C.; Kłodzińska, E.; Namieśnik, J.; Płotka-Wasylka, J. Literature update of analytical methods for biogenic amines determination in food and beverages. Trends Anal. Chem. 2018, 98, 128–142. [Google Scholar] [CrossRef]
- Dong, H.; Xiao, K. Modified QuEChERS combined with ultra high performance liquid chromatography tandem mass spectrometry to determine seven biogenic amines in Chinese traditional condiment soy sauce. Food Chem. 2017, 229, 502–508. [Google Scholar] [CrossRef]
- Lozanov, V.; Benkova, B.; Mateva, L.; Petrov, S.; Popov, E.; Slavov, C.; Mitev, V. Liquid chromatography method for simultaneous analysis of amino acids and biogenic amines in biological fluids with simultaneous gradient of pH and acetonitrile. J. Chromatogr. B 2007, 860, 92–97. [Google Scholar] [CrossRef]
- Cai, Y.; Sun, Z.; Chen, G.; Liu, X.; You, J.; Zhang, C. Rapid analysis of biogenic amines from rice wine with isotope-coded derivatization followed by high performance liquid chromatography-tandem mass spectrometry. Food Chem. 2016, 192, 388–394. [Google Scholar] [CrossRef]
- Cong, L.; Huang, B.; Chen, Q.; Lu, B.; Zhang, J.; Ren, Y. Determination of trace amount of microcystins in water samples using liquid chromatography coupled with triple quadrupole mass spectrometry. Anal. Chim. Acta 2006, 569, 157–168. [Google Scholar] [CrossRef]
- Ramos, R.M.; Valente, I.M.; Rodrigues, J.A. Analysis of biogenic amines in wines by salting-out assisted liquid–liquid extraction and high-performance liquid chromatography with fluorimetric detection. Talanta 2014, 124, 146–151. [Google Scholar] [CrossRef]
- Marques, A.P.; Leitão, M.C.; San Romão, M.V. Biogenic amines in wines: Influence of oenological factors. Food Chem. 2008, 107, 853–860. [Google Scholar] [CrossRef]
- García-Marino, M.; Trigueros, Á.; Escribano-Bailón, T. Influence of oenological practices on the formation of biogenic amines in quality red wines. J. Food Compos. Anal. 2010, 23, 455–462. [Google Scholar] [CrossRef]
- Peña-Gallego, A.; Hernández-Orte, P.; Cacho, J.; Ferreira, V. Biogenic amine determination in wines using solid-phase extraction: A comparative study. J. Chromatogr. A 2009, 1216, 3398–3401. [Google Scholar] [CrossRef] [PubMed]
- Lucas, P.M.; Olivier, C.; Aline, L.F. High frequency of histamine-producing bacteria in the enological environment and instability of the histidine decarboxylase production phenotype. Appl. Environ. Microbiol. 2008, 74, 811. [Google Scholar] [CrossRef]
- Jovanov, P.; Guzsvány, V.; Franko, M.; Lazić, S.; Sakač, M.; Milovanović, I.; Nedeljković, N. Development of multiresidue DLLME and QuEChERS based LC–MS/MS method for determination of selected neonicotinoid insecticides in honey liqueur. Food Res. Int. 2014, 55, 11–19. [Google Scholar] [CrossRef]
- Prete, V.D.; Costantini, A.; Cecchini, F.; Morassut, M.; Garcia-Moruno, E. Occurrence of biogenic amines in wine: The role of grapes. Food Chem. 2009, 112, 474–481. [Google Scholar] [CrossRef]
- Cecchini, F.; Morassut, M. Effect of grape storage time on biogenic amines content in must. Food Chem. 2010, 123, 263–268. [Google Scholar] [CrossRef]
- Ortega-Heras, M.; Pérez-Magariño, S.; Del-Villar-Garrachón, V.; González-Huerta, C.; Moro Gonzalez, L.C.; Guadarrama, R.A.; Villanueva, S.S.; Gallo, G.R.; Martín, D.L.H.S. Study of the effect of vintage, maturity degree, and irrigation on the amino acid and biogenic amine content of a white wine from Verdejo variety. J. Sci. Food Agric. 2014, 94, 2073–2082. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Y.; Wu, G. Effect of cluster thinning on concentration of biogenic amines in Cabernet sauvignon grape berries and wines. J. Chin. Inst. Food Sci. Technol. 2015, 15, 199–205. [Google Scholar]
- Sacchi, K.L.; Bisson, L.F.; Adams, D.O. A review of the effect of winemaking techniques on phenolic extraction in red wines. Am. J. Enol. Vitic. 2005, 56, 197–206. [Google Scholar]
- Soleas, G.J.; Carey, M.; Goldberg, D.M. Method development and cultivar-related differences of nine biogenic amines in Ontario wines. Food Chem. 1999, 64, 49–58. [Google Scholar] [CrossRef]
- Lorenzo, C.; Bordiga, M.; Pérez-Álvarez, E.P.; Travaglia, F.; Arlorio, M.; Salinas, M.R.; Coïsson, J.D.; Garde-Cerdán, T. The impacts of temperature, alcoholic degree and amino acids content on biogenic amines and their precursor amino acids content in red wine. Food Res. Int. 2017, 99, 328–335. [Google Scholar] [CrossRef]
- Ordóñez, J.L.; Callejón, R.M.; Troncoso, A.M.; García–Parrilla, M.C. Evaluation of biogenic amines profile in opened wine bottles: Effect of storage conditions. J. Food Compos. Anal. 2017, 63, 139–147. [Google Scholar] [CrossRef]
- Bach, B.; Le Quere, S.; Vuchot, P.; Grinbaum, M.; Barnavon, L. Validation of a method for the analysis of biogenic amines: Histamine instability during wine sample storage. Anal. Chim. Acta 2012, 732, 114–119. [Google Scholar] [CrossRef]
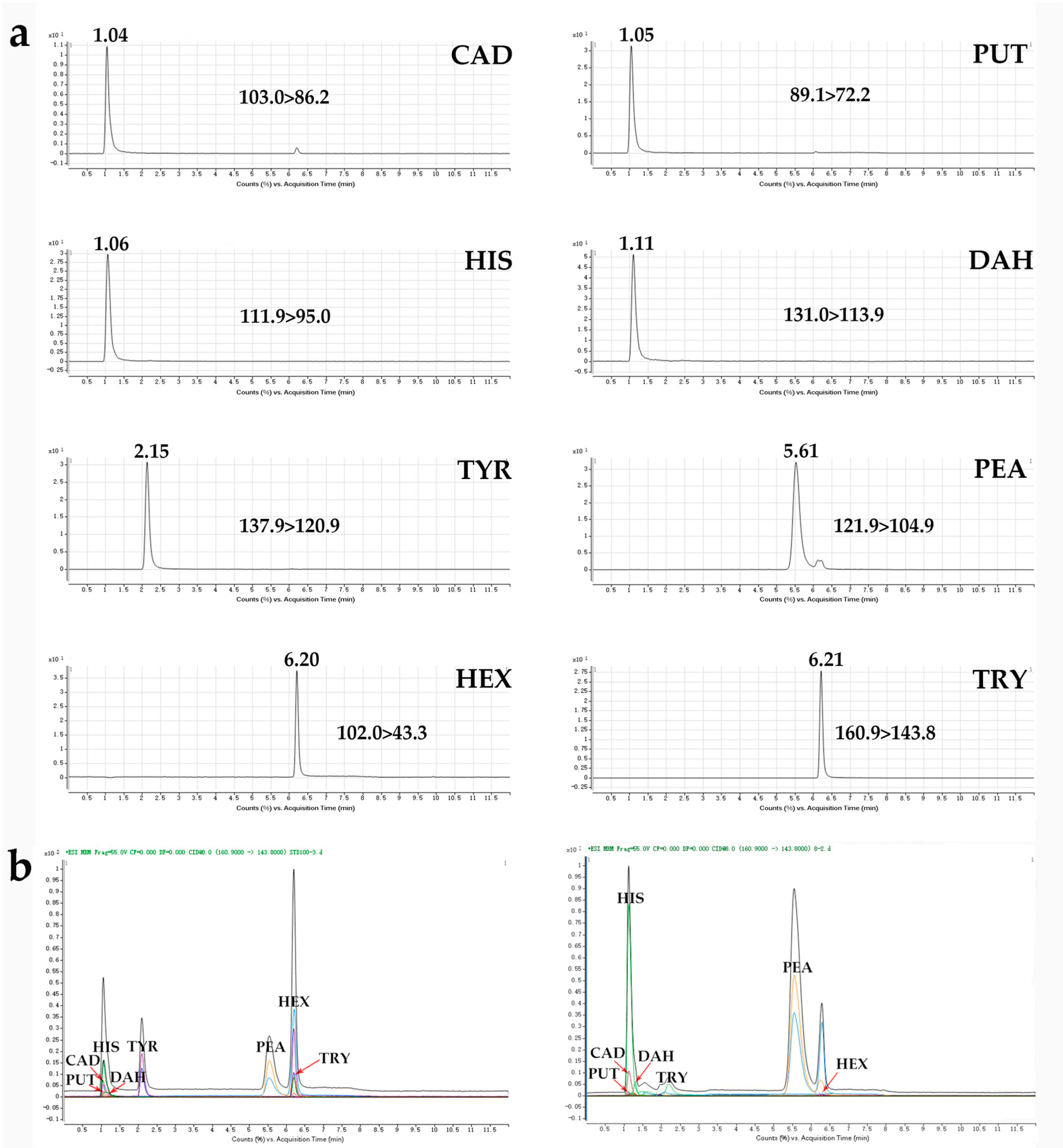
| Compounds | Abbr | Precursor Ion (m/z) | Product Ion b (m/z) | Fragmentor (V) | Collision Energy (V) |
|---|---|---|---|---|---|
| Cadaverine | CAD | 103.0 | 86.2/41.2 | 50 | 5/22 |
| Putrescine | PUT | 89.1 | 72.2/30.2 | 45 | 5/20 |
| Histamine | HIS | 111.9 | 95.0/68.2 | 50 | 24/12 |
| 1,7-diaminoheptane | DAH | 131.0 | 113.9/55.2 | 70 | 10/20 |
| Tyramine | TYR | 137.9 | 120.9/77.1 | 45 | 5/30 |
| Phenylethylamine | PEA | 121.9 | 104.9/77.1 | 50 | 10/30 |
| Hexylamine | HEX | 102.0 | 43.3/41.2 | 50 | 15/28 |
| Tryptamine | TRY | 160.9 | 143.8/114.9 | 55 | 40/8 |
| Compounds b | LOD b (µg/L) | LOQ (µg/L) | Linearity | Precision (RSD %) c | Recovery (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Calibration Equations | R2 | Range (μg/L) | Intra-day (n = 6) | Inter-day (n = 6) | 50 (μg/L) | 100 (μg/L) | 500 (μg/L) | |||
| CAD | 0.67 | 2.21 | Y = 0.0072X + 0.1485 | 0.9976 | 0.5–800 | 2.72–6.24 | 1.13–9.74 | 83.03 ± 9.49 | 79.41 ± 6.87 | 83.23 ± 7.81 |
| PUT | 0.67 | 2.21 | Y = 0.0176X + 0.6442 | 0.9903 | 0.5–800 | 2.41–4.45 | 3.34–4.81 | 80.34 ± 2.50 | 93.71 ± 4.88 | 91.61 ± 6.30 |
| HIS | 1.00 | 3.30 | Y = 0.0421X + 0.0967 | 0.9988 | 0.5–800 | 3.03–3.81 | 2.31–4.53 | 96.38 ± 0.70 | 77.18 ± 7.61 | 90.58 ± 9.73 |
| TYR | 0.75 | 2.50 | Y = 0.0682X − 0.1523 | 0.9991 | 0.5–800 | 3.30–4.64 | 3.26–5.27 | 91.69 ± 11.42 | 88.96 ± 8.07 | 92.23 ± 13.65 |
| PEA | 0.50 | 1.65 | Y = 0.1307X + 0.1676 | 0.9991 | 0.5–800 | 2.82–3.36 | 1.95–2.62 | 97.67 ± 7.32 | 86.87 ± 10.06 | 99.80 ± 15.40 |
| HEX | 1.00 | 3.30 | Y = 0.0636X − 0.2141 | 0.9996 | 0.5–800 | 3.69–8.88 | 4.99–6.29 | 101.69 ± 12.03 | 95.96 ± 16.55 | 101.17 ± 10.13 |
| TRY | 0.75 | 2.50 | Y = 0.0491X + 0.0345 | 0.9990 | 0.5–800 | 0.00–7.04 | 0.00–3.49 | 93.23 ± 10.38 | 90.21 ± 7.76 | 93.52 ± 13.86 |
| Type of Wine | CAD b | PUT | HIS | TYR | PEA | HEX | TRY a | |
|---|---|---|---|---|---|---|---|---|
| Dry Red Wine | Number of positive samples/analyzed | 57/62 | 62/62 | 60/62 | 62/62 | 62/62 | 35/62 | - |
| Mean (range) µg/L | 238.36 (15.03–1337.65) | 3525.21 (405.91–10787.44) | 931.12 (4.24–4704.05) | 335.76 (14.65–2807.30) | 448.02 (9.45–2378.34) | 26.77 (17.68–59.47) | - | |
| White Ice Wine | Number of positive samples/analyzed | 5/6 | 6/6 | 3/6 | 6/6 | 6/6 | 3/6 | - |
| Mean (range) µg/L | 275.97 (21.34–448.73) | 2319.46 (967.79–4780.97) | 733.86 (2.82–2173.29) | 470.49 (14.29–2725.91) | 633.86 (214.96–1179.09) | 26.60 (20.88–37.46) | - | |
| Red Ice Wine | Number of positive samples/analyzed | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 | 3/6 | - |
| Mean (range) µg/L | 380.84 (21.97–1632.95) | 3032.69 (295.04–6083.07) | 353.50 (5.33–1942.15) | 249.25 (12.86–1369.54) | 487.74 (126.36–905.96) | 35.68 (27.55–50.25) | - | |
| Dry White Wine | Number of positive samples/analyzed | 2/3 | 3/3 | 2/3 | 3/3 | 3/3 | 1/3 | - |
| Mean (range) µg/L | 48.54 (25.52–71.55) | 2227.71 (1627.65–2530.45) | 22.66 (10.61–34.71) | 15.68 (12.76–19.62) | 202.42 (127.49–343.69) | 30.39 | - | |
| Sweet Red Wine | Number of positive samples/analyzed | 2/3 | 3/3 | 1/3 | 3/3 | 3/3 | 2/3 | - |
| Mean (range) µg/L | 86.95 (70.86–103.04) | 2647.07 (999.86–5074.44) | 1046.44 | 45.73 (12.82–104.44) | 498.58 (12.71–937.60) | 19.32 (18.24–20.43) | - | |
| Semi-Sweet Red Wine | Number of positive samples/analyzed | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 | - |
| Mean (range) µg/L | 67.21 | 1832.62 | 36.68 | 19.52 | 135.75 | 23.83 | - | |
| Type of Wine a | Grape Variety | Storage Time | pH | Alcoholic Degree | Residual Sugar | PUT b | HEX | CAD | HIS | PEA | TYR | Total BAs | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type of Wine | 1 | ||||||||||||
| Grape Variety | −0.052 | 1 | |||||||||||
| Storage Time | 0.219 * | 0.022 | 1 | ||||||||||
| pH | −0.397 ** | −0.179 | −0.347 ** | 1 | |||||||||
| Alcoholic Degree | −0.058 | 0.341 ** | −0.068 | 0.299 ** | 1 | ||||||||
| Residual Sugar | −0.035 | 0.053 | 0.075 | 0.190 | 0.172 | 1 | |||||||
| PUT | −0.030 | 0.148 | −0.167 | −0.058 | 0.116 | −0.199 | 1 | ||||||
| HEX | −0.010 | 0.022 | 0.080 | 0.120 | 0.019 | 0.133 | 0.009 | 1 | |||||
| CAD | −0.158 | 0.098 | −0.046 | 0.046 | −0.125 | 0.053 | 0.041 | 0.246 * | 1 | ||||
| HIS | 0.084 | 0.236 * | −0.133 | −0.026 | 0.417 ** | −0.148 | 0.176 | 0.006 | 0.024 | 1 | |||
| PEA | 0.282 * | 0.026 | 0.258 * | −0.287 ** | −0.080 | 0.131 | 0.043 | 0.350 ** | 0.310 ** | 0.042 | 1 | ||
| TYR | −0.052 | −0.004 | 0.038 | −0.188 | −0.143 | −0.006 | −0.066 | 0.023 | 0.228 * | 0.476 ** | 0.205 | 1 | |
| Total BAs | 0.026 | 0.214 | −0.131 | −0.134 | 0.191 | −0.179 | 0.797 ** | 0.097 | 0.242 * | 0.640 ** | 0.280 * | 0.423 ** | 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, S.-Y.; Hao, L.-L.; Shi, X.; Niu, J.-M.; Zhang, B. Development and Application of a New QuEChERS Method in UHPLC-QqQ-MS/MS to Detect Seven Biogenic Amines in Chinese Wines. Foods 2019, 8, 552. https://doi.org/10.3390/foods8110552
Han S-Y, Hao L-L, Shi X, Niu J-M, Zhang B. Development and Application of a New QuEChERS Method in UHPLC-QqQ-MS/MS to Detect Seven Biogenic Amines in Chinese Wines. Foods. 2019; 8(11):552. https://doi.org/10.3390/foods8110552
Chicago/Turabian StyleHan, Shun-Yu, Lan-Lan Hao, Xiao Shi, Jian-Ming Niu, and Bo Zhang. 2019. "Development and Application of a New QuEChERS Method in UHPLC-QqQ-MS/MS to Detect Seven Biogenic Amines in Chinese Wines" Foods 8, no. 11: 552. https://doi.org/10.3390/foods8110552
APA StyleHan, S.-Y., Hao, L.-L., Shi, X., Niu, J.-M., & Zhang, B. (2019). Development and Application of a New QuEChERS Method in UHPLC-QqQ-MS/MS to Detect Seven Biogenic Amines in Chinese Wines. Foods, 8(11), 552. https://doi.org/10.3390/foods8110552