The Optimization of Plasma-Activated Water Treatments to Inactivate Salmonella Enteritidis (ATCC 13076) on Shell Eggs
Abstract
:1. Introduction
2. Material and Methods
2.1. Bacterial Strain and Cell Suspension Preparation
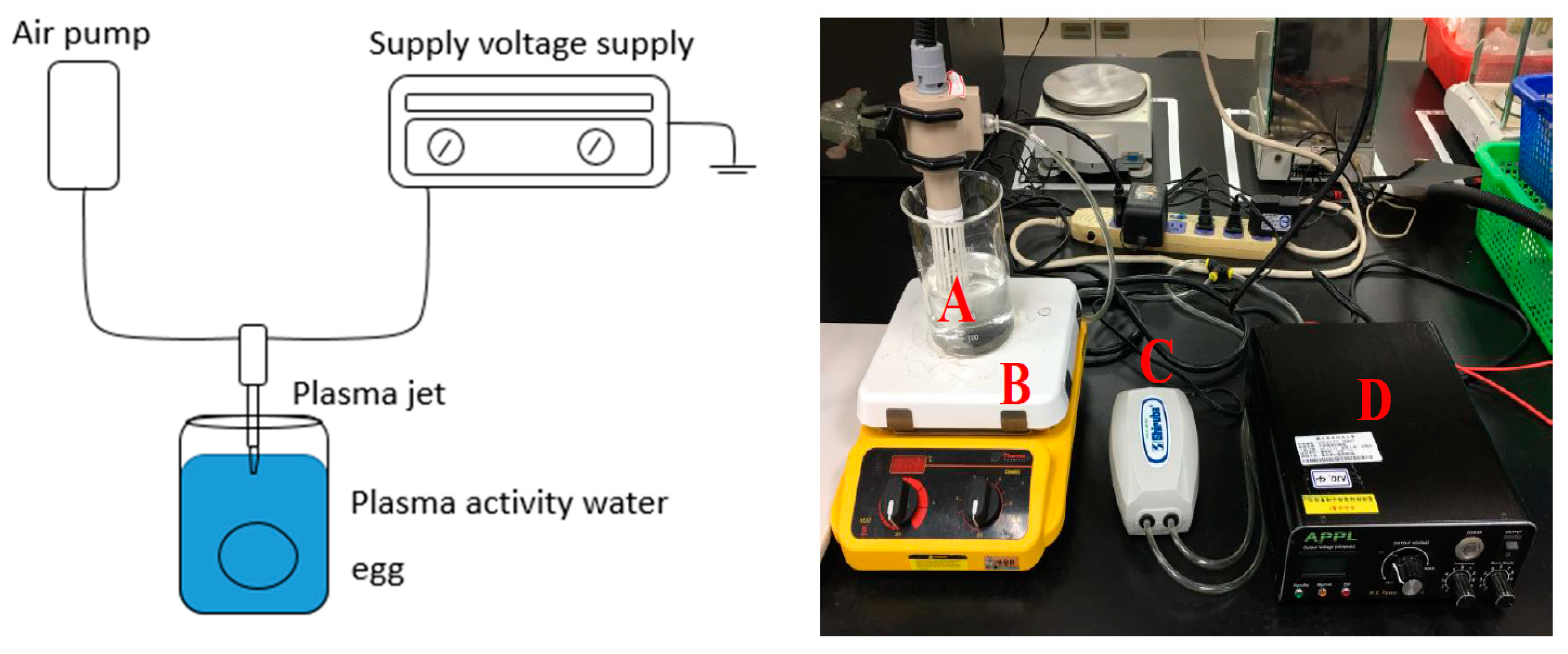
2.2. The Preparation of Plasma-Activated Water (PAW)
2.3. Inactivation of Salmonella spp. on Eggs by PAW
2.4. Determination of Egg Freshness
2.4.1. Preparation of Solutions with Different Specific Gravities
2.4.2. Measurements of Yolk and Albumen Indexes
2.4.3. Measurement of Weight-Loss Rate
2.5. Observations via Scanning Electron Microscope (SEM)
2.6. Statistical Analyses
3. Results
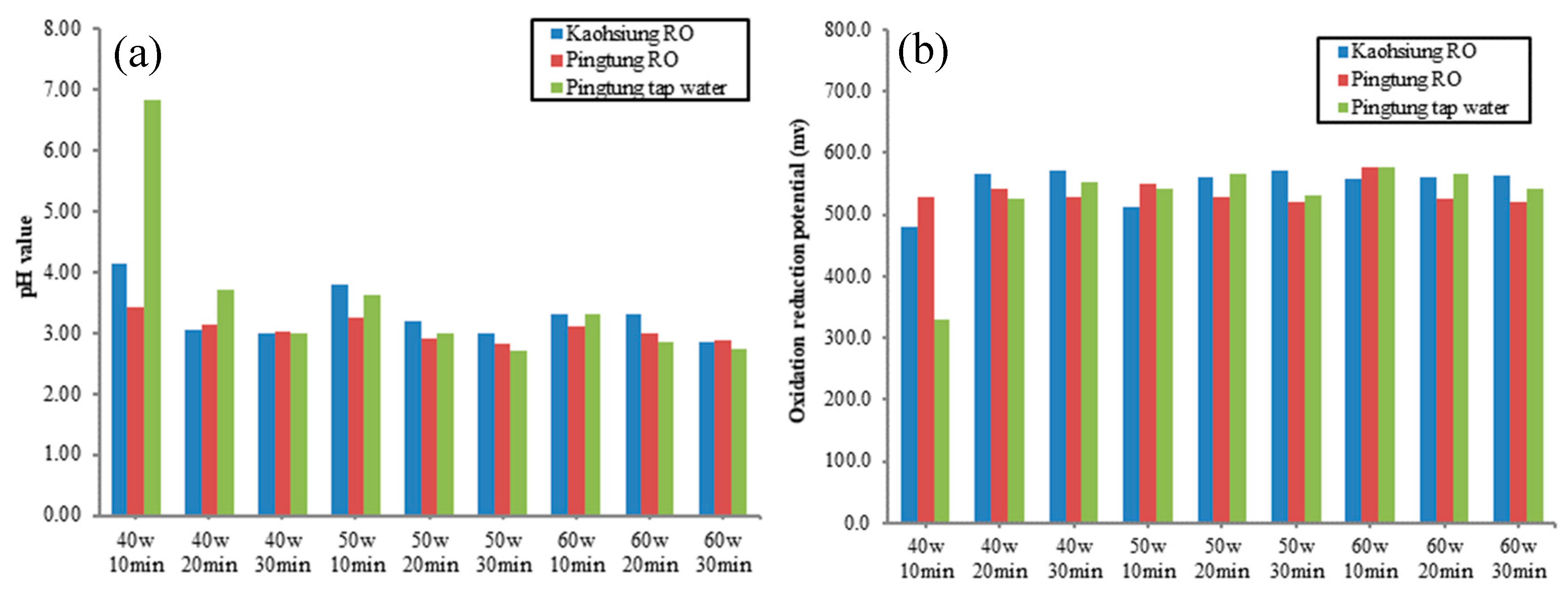
3.1. The Effects of Water Source, Power (watt), and Treatment Time on the Oxidation/Reduction Potential (ORP) and pH Values
3.2. Bacterial Populations in the Suspension and on the Egg Surface after PAW Treatment
3.3. Freshness Index of the Eggs
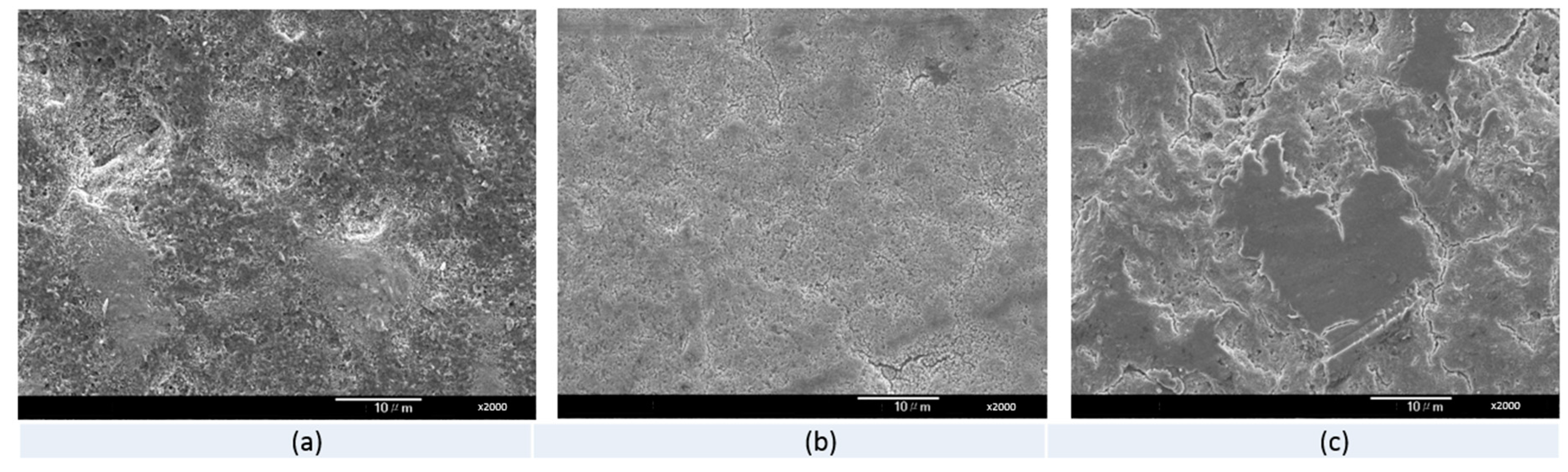
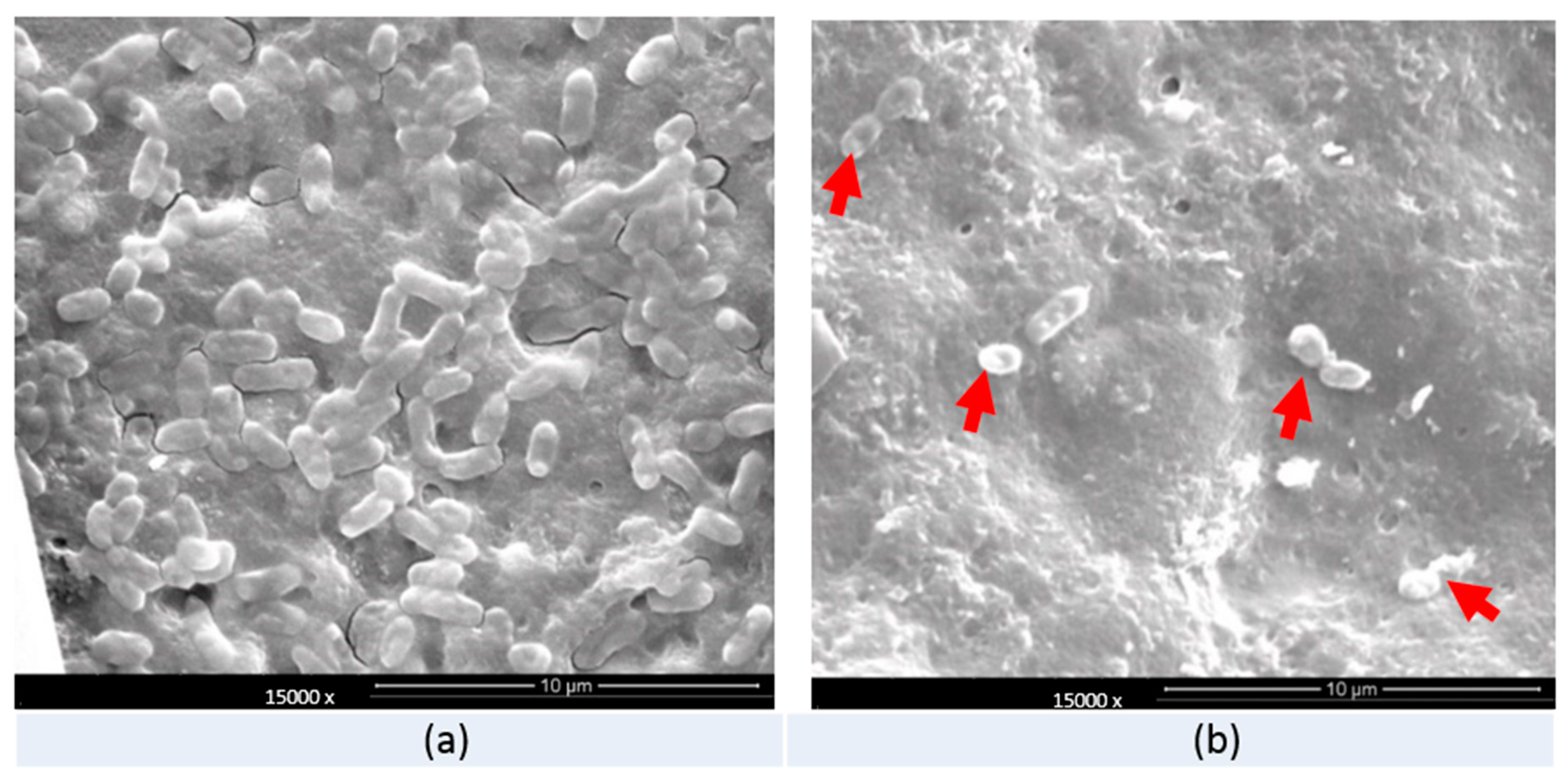
3.4. Scanning Electronic Microscope (SEM) Observation
4. Discussions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Surai, P.F.; Fisinin, V.I. 8. Natural multi-nutrient enriched eggs: Production and role in health. In Handbook of Eggs in Human Function; Wageningen Academic Publishers: Wageningen, The Netherlands, 2015; Volume 9, pp. 133–152. [Google Scholar]
- Sass, C.A.B.; Kuriya, S.; da Silva, G.V.; Silva, H.L.A.; da Cruz, A.G.; Esmerino, E.A.; Freitas, M.Q. Completion task to uncover consumer's perception: A case study using distinct types of hen’s eggs. Poult. Sci. 2018, 97, 2591–2599. [Google Scholar] [CrossRef] [PubMed]
- Jakočiūnė, D.; Herrero-Fresno, A.; Jelsbak, L.; Olsen, J.E. Highly expressed amino acid biosynthesis genes revealed by global gene expression analysis of salmonella enterica serovar enteritidis during growth in whole egg are not essential for this growth. Int. J. Food Microbiol. 2016, 224, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Deng, X.; Brown, E.W.; Hammack, T.S.; Ma, L.M.; Zhang, G. Evaluation of roka atlas salmonella method for the detection of salmonella in egg products in comparison with culture method, real-time pcr and isothermal amplification assays. Food Control 2018, 94, 123–131. [Google Scholar] [CrossRef]
- Han, J.; Gokulan, K.; Barnette, D.; Khare, S.; Rooney, A.W.; Deck, J.; Nayak, R.; Stefanova, R.; Hart, M.E.; Foley, S.L. Evaluation of virulence and antimicrobial resistance in salmonella enterica serovar enteritidis isolates from humans and chicken- and egg-associated sources. Foodborne Pathog. Dis. 2013, 10, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Foley, S.L.; Nayak, R.; Hanning, I.B.; Johnson, T.J.; Han, J.; Ricke, S.C. Population dynamics of salmonella enterica serotypes in commercial egg and poultry production. Appl. Environ. Microbiol. 2011, 77, 4273–4279. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-C.; Yeh, H.-D. Trihalomethane species forecast using optimization methods: Genetic algorithms and simulated annealing. J. Comput. Civ. Eng. 2005, 19, 248–257. [Google Scholar] [CrossRef]
- Kolb, J.F.; Mohamed, A.A.H.; Price, R.O.; Swanson, R.J.; Bowman, A.; Chiavarini, R.L.; Stacey, M.; Schoenbach, K.H. Cold atmospheric pressure air plasma jet for medical applications. Appl. Phys. Lett. 2008, 92, 241501. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.; Sun, K.; Liang, Y.; Sun, P.; Yang, X.; Wang, J.; Zhang, J.; Zhu, W.; Fang, J.; Becker, K.H. Cold plasma therapy of a tooth root canal infected with enterococcus faecalis biofilms in vitro. J. Endod. 2013, 39, 105–110. [Google Scholar] [CrossRef]
- Xu, Y.; Tian, Y.; Ma, R.; Liu, Q.; Zhang, J. Effect of plasma activated water on the postharvest quality of button mushrooms, agaricus bisporus. Food Chem. 2016, 197, 436–444. [Google Scholar] [CrossRef]
- Joshi, S.G.; Cooper, M.; Yost, A.; Paff, M.; Ercan, U.K.; Fridman, G.; Friedman, G.; Fridman, A.; Brooks, A.D. Nonthermal dielectric-barrier discharge plasma-induced inactivation involves oxidative DNA damage and membrane lipid peroxidation in Escherichia coli. Antimicrob. Agents Chemother. 2011, 55, 1053. [Google Scholar] [CrossRef]
- Thirumdas, R.; Kothakota, A.; Annapure, U.; Siliveru, K.; Blundell, R.; Gatt, R.; Valdramidis, V.P. Plasma activated water (paw): Chemistry, physico-chemical properties, applications in food and agriculture. Trends Food Sci. Technol. 2018, 77, 21–31. [Google Scholar] [CrossRef]
- Ma, S.; Kim, K.; Huh, J.; Hong, Y. Characteristics of microdischarge plasma jet in water and its application to water purification by bacterial inactivation. Sep. Purif. Technol. 2017, 188, 147–154. [Google Scholar] [CrossRef]
- Scholtz, V.; Pazlarova, J.; Souskova, H.; Khun, J.; Julak, J. Nonthermal plasma—A tool for decontamination and disinfection. Biotechnol. Adv. 2015, 33, 1108–1119. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Wang, G.; Tian, Y.; Wang, K.; Zhang, J.; Fang, J. Non-thermal plasma-activated water inactivation of food-borne pathogen on fresh produce. J. Hazard. Mater. 2015, 300, 643–651. [Google Scholar] [CrossRef]
- Qian, J.; Zhuang, H.; Nasiru, M.M.; Muhammad, U.; Zhang, J.; Yan, W. Action of plasma-activated lactic acid on the inactivation of inoculated salmonella enteritidis and quality of beef. Innov. Food Sci. Emerg. Technol. 2019, 57, 102196. [Google Scholar] [CrossRef]
- Liao, X.; Su, Y.; Liu, D.; Chen, S.; Hu, Y.; Ye, X.; Wang, J.; Ding, T. Application of atmospheric cold plasma-activated water (paw) ice for preservation of shrimps (metapenaeus ensis). Food Control 2018, 94, 307–314. [Google Scholar] [CrossRef]
- Bagheri, S.; Janmohammadi, H.; Maleki, R.; Ostadrahimi, A.; Kianfar, R. Laying hen performance, egg quality improved and yolk 5-methyltetrahydrofolate content increased by dietary supplementation of folic acid. Anim. Nutr. 2019, 5, 130–133. [Google Scholar] [CrossRef]
- Xiang, Q.; Kang, C.; Zhao, D.; Niu, L.; Liu, X.; Bai, Y. Influence of organic matters on the inactivation efficacy of plasma-activated water against E. coli o157:H7 and S. aureus. Food Control 2019, 99, 28–33. [Google Scholar] [CrossRef]
- Choi, E.J.; Park, H.W.; Kim, S.B.; Ryu, S.; Lim, J.; Hong, E.J.; Byeon, Y.S.; Chun, H.H. Sequential application of plasma-activated water and mild heating improves microbiological quality of ready-to-use shredded salted kimchi cabbage (Brassica pekinensis L.). Food Control 2019, 98, 501–509. [Google Scholar] [CrossRef]
- Georgescu, N.; Apostol, L.; Gherendi, F. Inactivation of salmonella enterica serovar typhimurium on egg surface, by direct and indirect treatments with cold atmospheric plasma. Food Control 2017, 76, 52–61. [Google Scholar] [CrossRef]
- Dasan, B.G.; Yildirim, T.; Boyaci, I.H. Surface decontamination of eggshells by using non-thermal atmospheric plasma. Int. J. Food Microbiol. 2018, 266, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Vukusic, T.; Shi, M.; Herceg, Z.; Rogers, S.; Estifaee, P.; Thagard, S.M. Liquid-phase electrical discharge plasmas with a silver electrode for inactivation of a pure culture of escherichia coli in water. Innov. Food Sci. Emerg. Technol. 2016, 38, 407–413. [Google Scholar] [CrossRef]
- Royintarat, T.; Seesuriyachan, P.; Boonyawan, D.; Choi, E.H.; Wattanutchariya, W. Mechanism and optimization of non-thermal plasma-activated water for bacterial inactivation by underwater plasma jet and delivery of reactive species underwater by cylindrical dbd plasma. Curr. Appl. Phys. 2019, 19, 1006–1014. [Google Scholar] [CrossRef]
| Storage Days (Day 0) | Storage Days (Day 1) | |||||
|---|---|---|---|---|---|---|
| Treatment | Bacterial Population | ORP | pH | Bacterial Population | ORP | pH |
| Sterile water | 5.98 ± 0.35 dA | 348.7 ± 9.1 aA | 6.50 ± 0.08 aA | 5.77 ± 0.63 dA | 396.3 ± 8.93 aA | 6.49 ± 0.15 aA |
| 40 w 30 min | 5.27 ± 0.23 cA | 517.1 ± 12.3 bA | 3.05 ± 0.16 bA | 5.41 ± 0.32 cA | 449.6 ± 10.4 bB | 2.80 ± 0.23 bA |
| 50 w 20 min | 4.90 ± 0.21 bA | 556.7 ± 11.3 bA | 3.19 ± 0.11 bA | 4.82 ± 0.25 bA | 504.4 ± 12.5 bB | 2.92 ± 0.16 bA |
| 60 w 10 min | 3.88 ± 0.13 bA | 569.6 ± 8.3 bA | 3.35 ± 0.20 bA | 4.62 ± 0.14 bB | 516.8 ± 12.4 bB | 3.07 ± 0.18 bA |
| 60 w 20 min | 2.18 ± 0.10 aA | 567.5 ± 10.3 bA | 3.13 ± 0.14 bA | 3.63 ± 0.33 aB | 532.5 ± 11.5 bB | 2.89 ± 0.15 bA |
| Treatments | Bacterial Populations | Reduction |
|---|---|---|
| Unwashed | 7.68 ± 0.13 e | |
| Sterile water 60 s | 6.91 ± 0.18 d | 0.77 |
| PAW 30 s | 5.30 ± 0.16 c | 2.38 |
| PAW 60 s | 3.28 ± 0.31 b | 4.40 |
| PAW 90 s | 3.69 ± 0.12 b | 3.99 |
| PAW 120 s | 2.17 ± 0.17 a | 5.51 |
| Treatments | Storage Day | Weight Loss (%) | Specific Gravity A | Yolk Index | Albumen Index |
|---|---|---|---|---|---|
| PAW—60 s | 0 | 0 | 3 | 0.40 ± 0.02 a | 0.10 ± 0.02 a |
| 7 | 0.28 ± 0.00 a | 3 | 0.33 ± 0.01 b | 0.06 ± 0.01 b | |
| 14 | 0.69 ± 0.04 b | 3 | 0.22 ± 0.02 c | 0.06 ± 0.02 b | |
| PAW—120 s | 0 | 0 | 3 | 0.41 ± 0.03 a | 0.10 ± 0.02 a |
| 7 | 0.29 ± 0.02 a | 3 | 0.32 ± 0.01 b | 0.07 ± 0.02 b | |
| 14 | 0.66 ± 0.06 b | 3 | 0.21 ± 0.03 c | 0.06 ± 0.01 b | |
| Commercially washed | 0 | 0 | 3 | 0.38 ± 0.02 a | 0.08 ± 0.03 a |
| 7 | 0.68 ± 0.06 b | 2 | 0.21 ± 0.01 c | 0.06 ± 0.00 b | |
| 14 | 1.06 ± 0.08 c | 2 | 0.18 ± 0.03 d | 0.04 ± 0.00 c | |
| unwashed | 0 | 0 | 3 | 0.39 ± 0.09 a | 0.09 ± 0.04 a |
| 7 | 0.31 ± 0.03 a | 3 | 0.32 ± 0.01 b | 0.06 ± 0.00 b | |
| 14 | 0.72 ± 0.04 b | 3 | 0.25 ± 0.00 c | 0.05 ± 0.04 b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-M.; Chu, Y.-C.; Hsiao, C.-P.; Wu, J.-S.; Hsieh, C.-W.; Hou, C.-Y. The Optimization of Plasma-Activated Water Treatments to Inactivate Salmonella Enteritidis (ATCC 13076) on Shell Eggs. Foods 2019, 8, 520. https://doi.org/10.3390/foods8100520
Lin C-M, Chu Y-C, Hsiao C-P, Wu J-S, Hsieh C-W, Hou C-Y. The Optimization of Plasma-Activated Water Treatments to Inactivate Salmonella Enteritidis (ATCC 13076) on Shell Eggs. Foods. 2019; 8(10):520. https://doi.org/10.3390/foods8100520
Chicago/Turabian StyleLin, Chia-Min, Yu-Chi Chu, Chun-Ping Hsiao, Jong-Shinn Wu, Chang-Wei Hsieh, and Chih-Yao Hou. 2019. "The Optimization of Plasma-Activated Water Treatments to Inactivate Salmonella Enteritidis (ATCC 13076) on Shell Eggs" Foods 8, no. 10: 520. https://doi.org/10.3390/foods8100520
APA StyleLin, C.-M., Chu, Y.-C., Hsiao, C.-P., Wu, J.-S., Hsieh, C.-W., & Hou, C.-Y. (2019). The Optimization of Plasma-Activated Water Treatments to Inactivate Salmonella Enteritidis (ATCC 13076) on Shell Eggs. Foods, 8(10), 520. https://doi.org/10.3390/foods8100520








