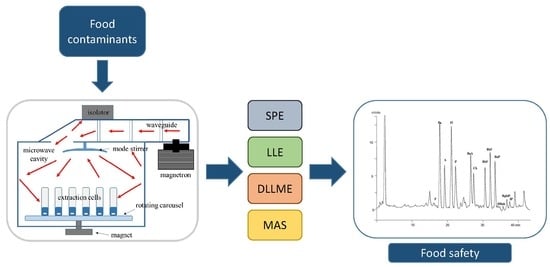
Microwave-Based Technique for Fast and Reliable Extraction of Organic Contaminants from Food, with a Special Focus on Hydrocarbon Contaminants
Abstract
1. Introduction
2. Principle of Microwave Heating
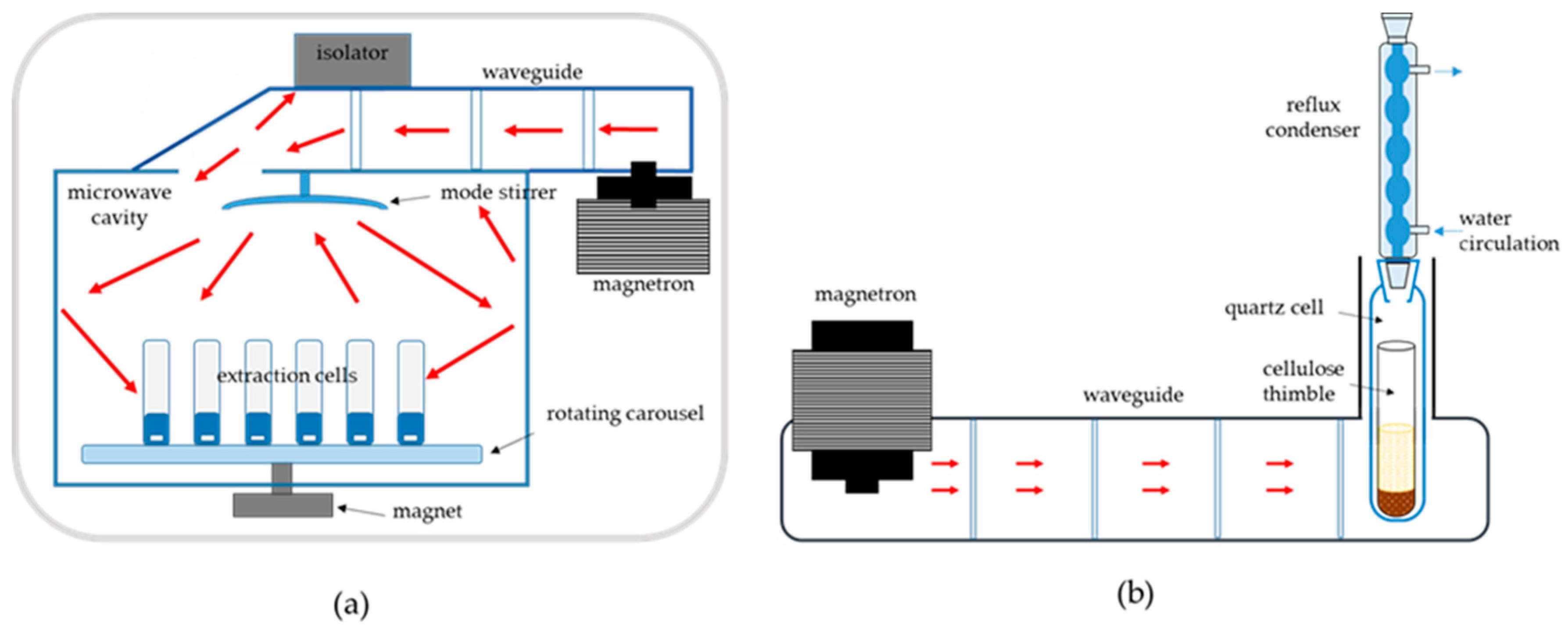
3. Instrumentation
4. Extraction Mode
5. Sample Processing
6. Parameters Affecting the Extraction
7. Combined Extraction/Purification Techniques Applied to Food Contaminants
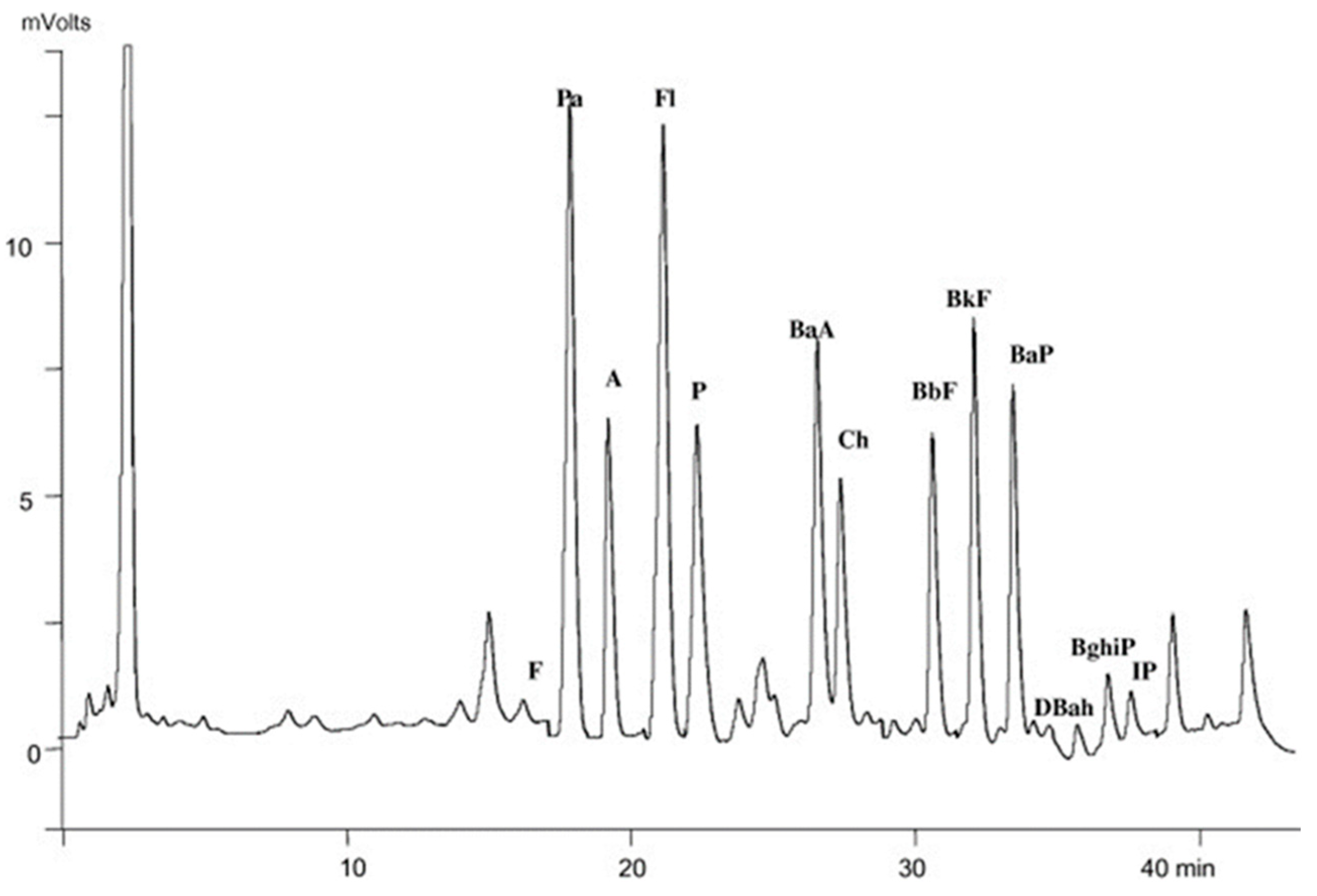
8. Applications Focusing on Hydrocarbon Contaminants
8.1. Focused Microwave-Assisted Extraction (FMAE)
8.2. Pressurized Microwave-Assisted Extraction (PMAE)
8.3. Microwave-Assisted Saponification (MAS)
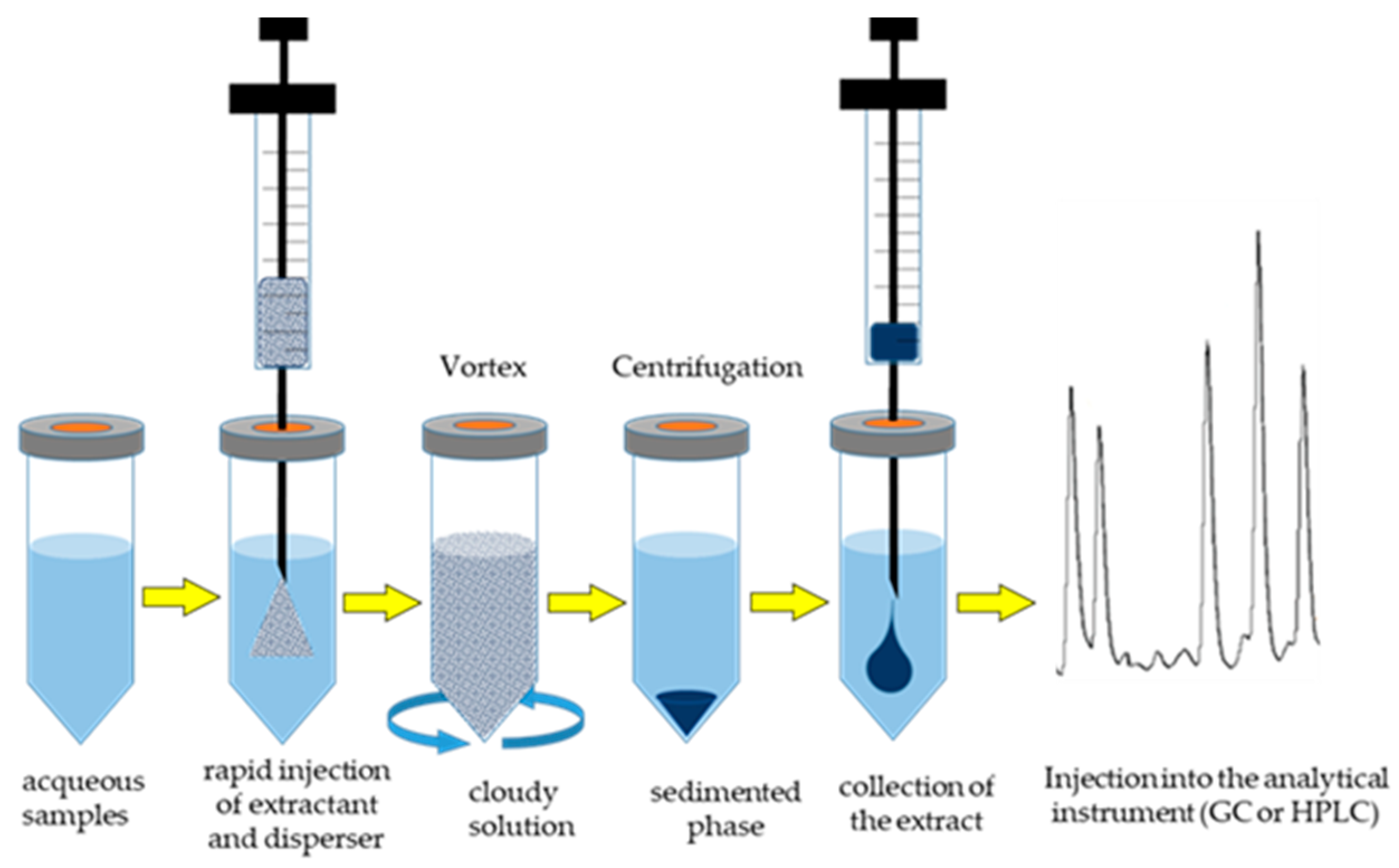
8.4. MAE Combined with DLLME
8.5. Ionic Liquids (ILs)
9. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| A | anthracene |
| ANNs | artificial neural networks |
| APMAE | atmospheric pressure microwave-assisted extraction |
| BaA | Benz (a) anthracene |
| BaP | Benzo (a) pyrene |
| BbF | Benzo (b) fluoranthene |
| BghiP | Benzo (g,h,i) perylene |
| BkF | Benzo (k) fluoranthene |
| Ch | chrysene |
| D-µ-SPE | dispersive solid phase micro-extraction |
| DAD | photodiode array detector |
| DBahA | Dibenz (a,h) anthracene |
| DLLME | dispersive liquid-liquid micro-extraction |
| ED | experimental design |
| EPA | Environmental Protection Agency |
| F | fluorene |
| FID | flame ionization detector |
| Fl | fluoranthene |
| FLD | spectrofluorometric detection |
| FMAE | focused microwave-assisted extraction |
| GC | gas chromatography |
| GC-MS | gas chromatography-mass spectrometry |
| GPC | gel permeation chromatography |
| HDBIm-Br | 1-hexadecyl-3-butylimidazolium bromide |
| HPLC | high performance liquid chromatography |
| IL | ionic liquid |
| IP | Indeno (1,2,3-cd) pyrene |
| ISO | International Standard Organization |
| LLE | liquid-liquid extraction |
| LOD | limit of detection |
| MAE | microwave-assisted extraction |
| MAME | microwave-assisted micellar extraction |
| MAS | microwave-assisted saponification |
| MASE | microwave-assisted solvent extraction |
| MOAH | mineral oil aromatic hydrocarbons |
| MOH | mineral oil hydrocarbons |
| MOSH | mineral oil saturated hydrocarbons |
| NP | normal phase |
| P | pyrene |
| Pa | phenanthrene |
| PAH | polycyclic aromatic hydrocarbons |
| PCBs | polychlorinated biphenyls |
| PEEK | polyetheretherketone |
| PMAE | pressurized microwave-assisted extraction |
| PTFE | polytetrafluoroethylene |
| RP | reversed phase |
| SIM | selected ion monitoring |
| SPE | solid phase extraction |
| UV | ultraviolet |
References
- Ganzler, K.; Salgó, A.; Valkó, K. Microwave extraction: A novel sample preparation method for chromatography. J. Chromatogr. A 1986, 371, 299–306. [Google Scholar] [CrossRef]
- Purcaro, G.; Barp, L.; Moret, S. Determination of hydrocarbon contamination in foods. A review. Anal. Methods 2016, 8, 5755–5772. [Google Scholar] [CrossRef]
- European Commission. Commission Recommendation of 4 February 2005 on the further investigation into the levels of polycyclic aromatic hydrocarbons in certain foods (notified under document number C (2005) 256). Off. J. Eur. Union 2005, 48, 43–45. [Google Scholar]
- European Commission. Commission Regulation (EU) No 836/2011 of 19 August 2011 amending Regulataion (EC) No 333/2007 laying down the methods of sampling and analysis for the official control of the levels lead, cadnium, mercury, inorganic tin, 3-MCPD and benzo (a) pyrene in foodstuffs. Off. J. Eur. Union 2011, 215, 9–16. [Google Scholar]
- EFSA (Panel on Contaminants in the Food Chain (CONTAM)). Scientific Opinion on Mineral Oil Hydrocarbons in Food. EFSA J. 2012, 10, 2704. [Google Scholar]
- Biedermann, M.; Fiselier, K.; Grob, K. Aromatic Hydrocarbons of Mineral Oil Origin in Foods: Method for Determining the Total Concentration and First Results. J. Agric. Food Chem. 2009, 57, 8711–8721. [Google Scholar] [CrossRef]
- Biedermann, M.; Munoz, C.; Grob, K. Update of on-line coupled liquid chromatography—Gas chromatography for the analysis of mineral oil hydrocarbons in foods and cosmetics. J. Chromatogr. A 2017, 1521, 140–149. [Google Scholar] [CrossRef]
- Eskilsson, C.S.; Björklund, E. Analytical-scale microwave-assisted extraction. J. Chromatogr. A 2000, 902, 227–250. [Google Scholar] [CrossRef]
- Dean, J.R. Microwave extraction. In Comprehensive Sampling and Sample Preparation; Elsevier: Amsterdam, The Netherlands, 2012; Volume 2, pp. 135–149. ISBN 9780123813749. [Google Scholar]
- Moret, S.; Purcaro, G.; Conte, L.S. Estrazione Assistita con Microonde (MAE); Springer-Verlag: Milan, Italy, 2014; pp. 81–104. [Google Scholar]
- Purcaro, G.; Moret, S.; Conte, L.; Conte, L. Optimisation of microwave assisted extraction (MAE) for polycyclic aromatic hydrocarbon (PAH) determination in smoked meat. Meat Sci. 2009, 81, 275–280. [Google Scholar] [CrossRef]
- Kovacs, A.; Ganzler, K.; Simon-Sarkadi, L. Microwave-assisted extraction of free amino acids from foods. Z. Lebensm. Forsch. A 1998, 207, 26–30. [Google Scholar] [CrossRef]
- Akpambang, V.; Purcaro, G.; Lajide, L.; Amoo, I.; Conte, L.; Moret, S.; Conte, L. Determination of polycyclic aromatic hydrocarbons (PAHs) in commonly consumed Nigerian smoked/grilled fish and meat. Food Addit. Contam. Part A 2009, 26, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Moret, S.; Scolaro, M.; Barp, L.; Purcaro, G.; Conte, L.S. Microwave assisted saponification (MAS) followed by on-line liquid chromatography (LC)–gas chromatography (GC) for high-throughput and high-sensitivity determination of mineral oil in different cereal-based foodstuffs. Food Chem. 2016, 196, 50–57. [Google Scholar] [CrossRef]
- Moreda-Piñeiro, J.; Moreda-Piñeiro, A. Combined assisted extraction techniques as green sample pre-treatments in food analysis. TrAC Trends Anal. Chem. 2019, 118, 1–18. [Google Scholar] [CrossRef]
- Li, N.; Wu, L.; Nian, L.; Song, Y.; Lei, L.; Yang, X.; Wang, K.; Wang, Z.; Zhang, L.; Zhang, H.; et al. Dynamic microwave assisted extraction coupled with dispersive micro-solid-phase extraction of herbicides in soybeans. Talanta 2015, 142, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gong, Z.; Zhang, T.; Feng, S.; Wang, J.; Zhang, Y. Simultaneous determination of 106 pesticides in nuts by LC-MS/MS using freeze-out combined with dispersive solid-phase extraction purification. J. Sep. Sci. 2017, 40, 2398–2405. [Google Scholar] [CrossRef]
- Wang, Y.; Dai, X.; He, X.; Chen, L.; Hou, X. MIL-101(Cr)@GO for dispersive micro-solid-phase extraction of pharmaceutical residue in chicken breast used in microwave-assisted coupling with HPLC–MS/MS detection. J. Pharm. Biomed. Anal. 2017, 145, 440–446. [Google Scholar] [CrossRef]
- Du, L.J.; Chu, C.; Warner, E.; Wang, Q.Y.; Hu, Y.H.; Chai, K.J.; Cao, J.; Peng, L.Q.; Chen, Y.B.; Yang, J.; et al. Rapid microwave-assisted dispersive micro-solid phase extraction of mycotoxins in food using zirconia nanoparticles. J. Chromatogr. A 2018, 1561, 1–12. [Google Scholar] [CrossRef]
- Huang, P.; Zhao, P.; Dai, X.; Hou, X.; Zhao, L.; Liang, N. Trace determination of antibacterial pharmaceuticals in fishes by microwave-assisted extraction and solid-phase purification combined with dispersive liquid–liquid microextraction followed by ultra-high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2016, 1011, 136–144. [Google Scholar]
- Ramezani, H.; Hosseini, H.; Kamankesh, M.; Ghasemzadeh-Mohammadi, V.; Mohammadi, A. Rapid determination of nitrosamines in sausage and salami using microwave-assisted extraction and dispersive liquid–liquid microextraction followed by gas chromatography–mass spectrometry. Eur. Food Res. Technol. 2014, 240, 441–450. [Google Scholar] [CrossRef]
- Chisvert, A.; Cárdenas, S.; Lucena, R. Dispersive micro-solid phase extraction. TrAC Trends Anal. Chem. 2019, 112, 226–233. [Google Scholar] [CrossRef]
- Reyes-Gallardo, E.M.; Lucena, R.; Cardenas, S.; Valcarcel, M. Magnetic nanoparticles-nylon 6 composite for the dispersive micro solid phase extraction of selected polycyclic aromatic hydrocarbons from water samples. J. Chromatogr. A 2014, 1345, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Rezaee, M.; Assadi, Y.; Hosseini, M.R.M.; Aghaee, E.; Ahmadi, F.; Berijani, S. Determination of organic compounds in water using dispersive liquid–liquid microextraction. J. Chromatogr. A 2006, 1116, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Soclo, H.H.; Budzinski, H.; Garrigues, P.; Matsuzawa, S. Biota accumulation of polycyclic aromatic hydrocarbons in benin coastal waters. Polycycl. Aromat. Compd. 2008, 28, 112–127. [Google Scholar] [CrossRef]
- Li, X.Y.; Li, N.; Luo, H.D.; Lin, L.R.; Zou, Z.X.; Jia, Y.Z.; Li, Y.Q. A Novel Synchronous Fluorescence Spectroscopic Approach for the Rapid Determination of Three Polycyclic Aromatic Hydrocarbons in Tea with Simple Microwave-Assisted Pretreatment of Sample. J. Agric. Food Chem. 2011, 59, 5899–5905. [Google Scholar] [CrossRef]
- Alarcón, F.; Báez, M.E.; Bravo, M.; Richter, P.; Fuentes, E. Screening of edible oils for polycyclic aromatic hydrocarbons using microwave-assisted liquid–liquid and solid phase extraction coupled to one- to three-way fluorescence spectroscopy analysis. Talanta 2012, 100, 439–446. [Google Scholar] [CrossRef]
- Alarcón, F.; Báez, M.E.; Bravo, M.; Richter, P.; Escandar, G.M.; Olivieri, A.C.; Fuentes, E. Feasibility of the determination of polycyclic aromatic hydrocarbons in edible oils via unfolded partial least-squares/residual bilinearization and parallel factor analysis of fluorescence excitation emission matrices. Talanta 2013, 103, 361–370. [Google Scholar] [CrossRef]
- Germán-Hernández, M.; Pino, V.; Anderson, J.L.; Afonso, A.M. Use of ionic liquid aggregates of 1-hexadecyl-3-butyl imidazolium bromide in a focused-microwave assisted extraction method followed by high-performance liquid chromatography with ultraviolet and fluorescence detection to determine the 15 +1 EU priority PAHs in toasted cereals (“gofios”). Talanta 2011, 85, 1199–1206. [Google Scholar]
- Hernández-Póveda, G.F.; Morales-Rubio, A.; Pastor-García, A.; De La Guardia, M. Extraction of polycyclic aromatic hydrocarbons from cookies: A comparative study of ultrasound and microwave-assisted procedures. Food Addit. Contam. Part A 2008, 25, 356–363. [Google Scholar] [CrossRef]
- Moret, S.; Conte, L.S. A rapid method for polycyclic aromatic hydrocarbon determination in vegetable oils. J. Sep. Sci. 2002, 25, 96–100. [Google Scholar] [CrossRef]
- Zhang, H.; Xue, M.; Dai, Z. Determination of polycyclic aromatic hydrocarbons in aquatic products by HPLC-fluorescence. J. Food Compos. Anal. 2010, 23, 469–474. [Google Scholar] [CrossRef]
- Ramalhosa, M.J.; Paíga, P.; Morais, S.; Sousa, A.M.; Gonçalves, M.P.; Delerue-Matos, C.; Oliveira, M.B.P.P. Analysis of polycyclic aromatic hydrocarbons in fish: Optimisation and validation of microwave-assisted extraction. Food Chem. 2012, 135, 234–242. [Google Scholar] [CrossRef]
- Gharbi, I.; Moret, S.; Chaari, O.; Issaoui, M.; Conte, L.S.; Lucci, P.; Hammami, M. Evaluation of hydrocarbon contaminants in olives and virgin olive oils from Tunisia. Food Control 2017, 75, 160–166. [Google Scholar] [CrossRef]
- Xiong, G.; He, X.; Zhang, Z. Microwave-assisted extraction or saponification combined with microwave-assisted decomposition applied in pretreatment of soil or mussel samples for the determination of polychlorinated biphenyls. Anal. Chim. Acta 2000, 413, 49–56. [Google Scholar] [CrossRef]
- Carro, N.; Garcìa, I.; Ignacio, M.C.; Llompart, M.; Yebra, M.C.; Mouteira, A. Microwave-assisted extraction and mild saponification for determination of organochlorine pesticides in oyster samples. Anal. Bioanal. Chem. 2002, 374, 547–553. [Google Scholar]
- Fajar, N.; Carro, A.; Lorenzo, R.; Fernandez, F.; Cela, R.; Fajar, N. Optimization of microwave-assisted extraction with saponification (MAES) for the determination of polybrominated flame retardants in aquaculture samples. Food Addit. Contam. Part A 2008, 25, 1015–1023. [Google Scholar] [CrossRef]
- García-Falcón, M.S.; Simal-Gándara, J.; Carril-González-Barros, S.T. Analysis of benzo [a] pyrene in spiked fatty foods by second derivative synchronous spectrofluorimetry after microwave-assisted treatment of samples. Food Addit. Contam. 2000, 17, 957–964. [Google Scholar] [CrossRef]
- Gfrerer, M.; Lankmayr, E. Microwave-assisted saponification for the determination of 16 polycyclic aromatic hydrocarbons from pumpkin seed oils. J. Sep. Sci. 2003, 26, 1230–1236. [Google Scholar] [CrossRef]
- Hernández-Borges, J.; Rodriguez-Delgado, M.A.; García-Montelongo, F.J. Optimization of the Microwave-Assisted Saponification and Extraction of Organic Pollutants from Marine Biota Using Experimental Design and Artificial Neural Networks. Chromatographia 2006, 63, 155–160. [Google Scholar] [CrossRef]
- Pena, T.; Pensado, L.; Casais, C.; Mejuto, C.; Phan-Tan-Luu, R.; Cela, R.; Casais, M.C. Optimization of a microwave-assisted extraction method for the analysis of polycyclic aromatic hydrocarbons from fish samples. J. Chromatogr. A 2006, 1121, 163–169. [Google Scholar] [CrossRef]
- Navarro, P.; Cortazar, E.; Bartolomé, L.; Deusto, M.; Raposo, J.; Zuloaga, O.; Arana, G.; Etxebarria, N. Comparison of solid phase extraction, saponification and gel permeation chromatography for the clean-up of microwave-assisted biological extracts in the analysis of polycyclic aromatic hydrocarbons. J. Chromatogr. A 2006, 1128, 10–16. [Google Scholar] [CrossRef]
- Moret, S.; Purcaro, G.; Conte, L.S. Polycyclic aromatic hydrocarbons (PAHs) levels in propolis and propolis-based dietary supplements from the Italian market. Food Chem. 2010, 122, 333–338. [Google Scholar] [CrossRef]
- Akpambang, V.O.E.; Purcaro, G.; Labunmi, L.; Amoo, I.A.; Conte, L.S.; Moret, S. Polycyclic Aromatic Hydrocarbons in Some Nigerian Roasted Plant Foods. Front. Food Nutr. Res. 2015, 1, 1–5. [Google Scholar]
- Biedermann-Brem, S.; Grob, K. Removal of mineral oil migrated from paperboard packing during cooking of foods in boiling water. Eur. Food Res. Technol. 2011, 232, 1035–1041. [Google Scholar] [CrossRef]
- Ghasemzadeh-Mohammadi, V.; Mohammadi, A.; Hashemi, M.; Khaksar, R.; Haratian, P. Microwave-assisted extraction and dispersive liquid–liquid microextraction followed by gas chromatography–mass spectrometry for isolation and determination of polycyclic aromatic hydrocarbons in smoked fish. J. Chromatogr. A 2012, 1237, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Ghasemzadeh-Mohammadi, V.; Haratian, P.; Khaksar, R.; Chaichi, M. Determination of polycyclic aromatic hydrocarbons in smoked fish samples by a new microextraction technique and method optimisation using response surface methodology. Food Chem. 2013, 141, 2459–2465. [Google Scholar] [CrossRef] [PubMed]
- Kamankesh, M.; Mohammadi, A.; Hosseini, H.; Tehrani, Z.M. Rapid determination of polycyclic aromatic hydrocarbons in grilled meat using microwave-assisted extraction and dispersive liquid–liquid microextraction coupled to gas chromatography–mass spectrometry. Meat Sci. 2015, 103, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudpour, M.; Mohtadinia, J.; Mousavi, M.M.; Ansarin, M.; Nemati, M. Application of the Microwave-Assisted Extraction and Dispersive Liquid–Liquid Microextraction for the Analysis of PAHs in Smoked Rice. Food Anal. Methods 2017, 10, 277–286. [Google Scholar] [CrossRef]
- Rostampour, R.; Kamalabadi, M.; Kamankesh, M.; Hadian, Z.; Jazaeri, S.; Mohammadi, A.; Zolgharnein, J. An efficient, sensitive and fast microextraction method followed by gas chromatography-mass spectrometry for the determination of polycyclic aromatic hydrocarbons in bread samples. Anal. Methods 2017, 9, 6246–6253. [Google Scholar] [CrossRef]
- Mahmoudpour, M.; Pilevar, Z.; Javanmardi, F.; Taram, F.; Mousavi, M.M. PAHs in toasted bread: Determination using microwave-assisted extraction and dispersive liquid–liquid microextraction followed by high-performance liquid chromatography. Anal. Methods 2018, 10, 2398–2404. [Google Scholar] [CrossRef]
- Kamalabadi, M.; Mohammadi, A.; Alizadeh, N. Simultaneous Determination of Seven Polycyclic Aromatic Hydrocarbons in Coffee Samples Using Effective Microwave-Assisted Extraction and Microextraction Method Followed by Gas Chromatography-Mass Spectrometry and Method Optimization Using Central Composit. Food Anal. Methods 2018, 11, 781–789. [Google Scholar] [CrossRef]
- Tan, Y.H.; Chai, M.K.; Wong, L.S. The Effects of Parameters on the Efficiency of DLLME in Extracting of PAHs from Vegetable Samples. Int. J. Eng. Technol. 2018, 7, 15–21. [Google Scholar] [CrossRef]
- Mohammadi, A.; Jahani, S.M.M.; Kamankesh, M.; Jazaeri, S.; Eivani, M.; Esmaeili, S.; Abdi, S. Determination of Polycyclic Aromatic Hydrocarbons in Edible Oil Using Fast and Sensitive Microwave-assisted Extraction and Dispersive Liquid–Liquid Microextraction Followed by Gas Chromatography-Mass Spectrometry. Polycycl. Aromat. Compd. 2018, 20, 1–9. [Google Scholar] [CrossRef]
- Ekezie, F.G.C.; Sun, D.W.; Cheng, J.H. Acceleration of microwave-assisted extraction processes of food components by integrating technologies and applying emerging solvents: A review of latest developments. Trends Food Sci. Technol. 2017, 67, 160–172. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Kong, J.; Nie, C.; Lin, X. Application of ionic liquids in the microwave-assisted extraction of quercetin from Chinese herbal medicine. Anal. Methods 2012, 4, 1012–1018. [Google Scholar] [CrossRef]
- Wu, L.; Hu, M.; Li, Z.; Song, Y.; Yu, C.; Zhang, Y.; Zhang, H.; Yu, A.; Ma, Q.; Wang, Z. Determination of triazine herbicides in fresh vegetables by dynamic microwave-assisted extraction coupled with homogeneous ionic liquid microextraction high performance liquid chromatography. Anal. Bioanal. Chem. 2015, 407, 1753–1762. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Li, D.; Wu, L.; Han, J.; Lian, W.; Wang, K.; Yang, H. Determination of triazine herbicides in juice samples by microwave-assisted ionic liquid/ionic liquid dispersive liquid-liquid microextraction coupled with high-performance liquid chromatography. J. Sep. Sci. 2017, 40, 2950–2958. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, H.; Es-Haghi, A.; Basiripour, F.; Es-Haghi, A. Microwave-assisted extraction and high-throughput monolithic-polymer-based micro-solid-phase extraction of organophosphorus, triazole, and organochlorine residues in apple. J. Sep. Sci. 2016, 39, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Su, P.; Yang, L.; Yang, Y. Microwave-assisted preparation of poly (ionic liquids)-modified magnetic nanoparticles for pesticide extraction. J. Sep. Sci. 2014, 37, 1503–1510. [Google Scholar] [CrossRef]
- Germán-Hernández, M.; Pino, V.; Anderson, J.L.; Afonso, A.M. A novel in situ preconcentration method with ionic liquid-based surfactants resulting in enhanced sensitivity for the extraction of polycyclic aromatic hydrocarbons from toasted cereals. J. Chromatogr. A 2012, 1227, 29–37. [Google Scholar] [CrossRef]
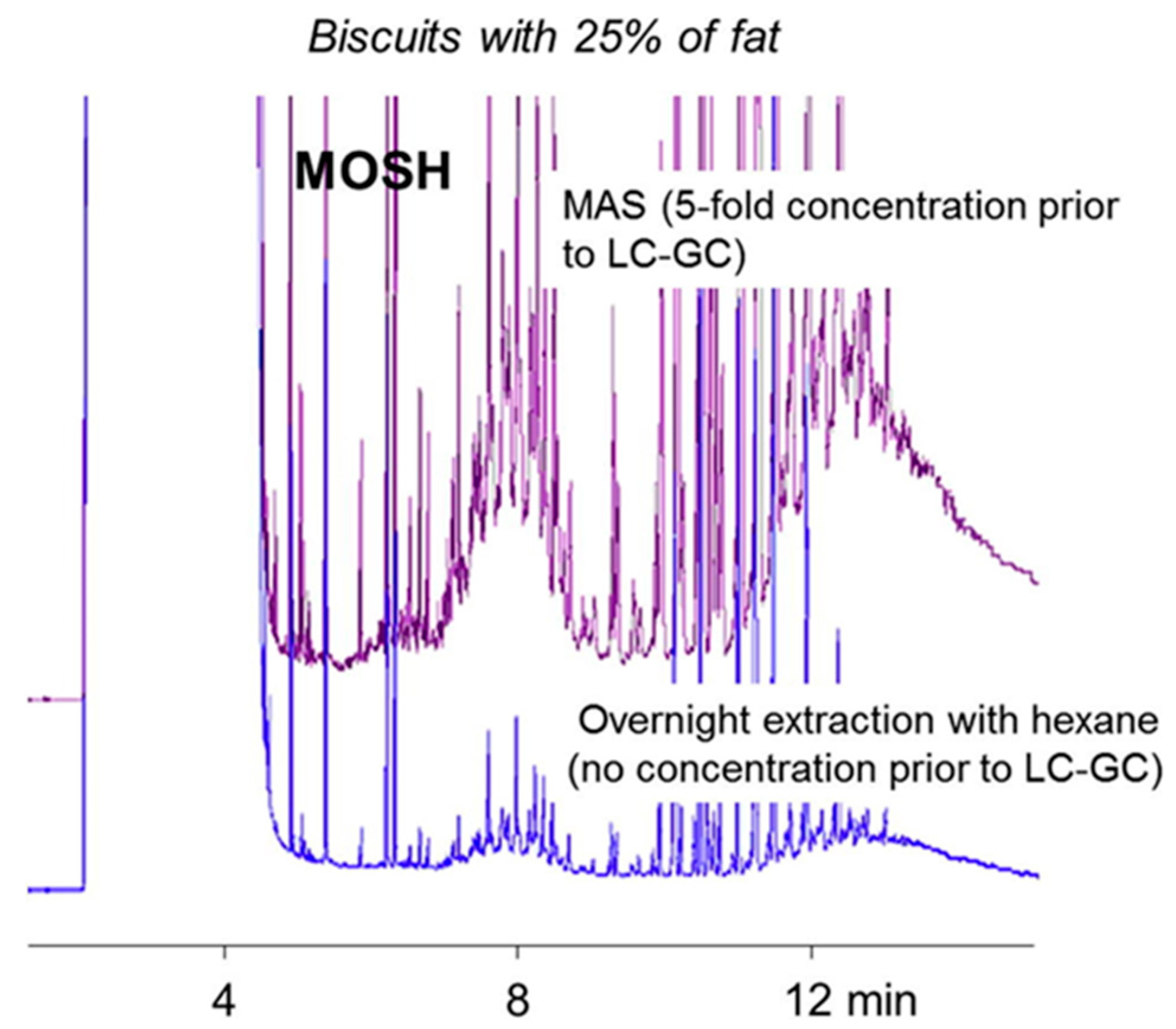
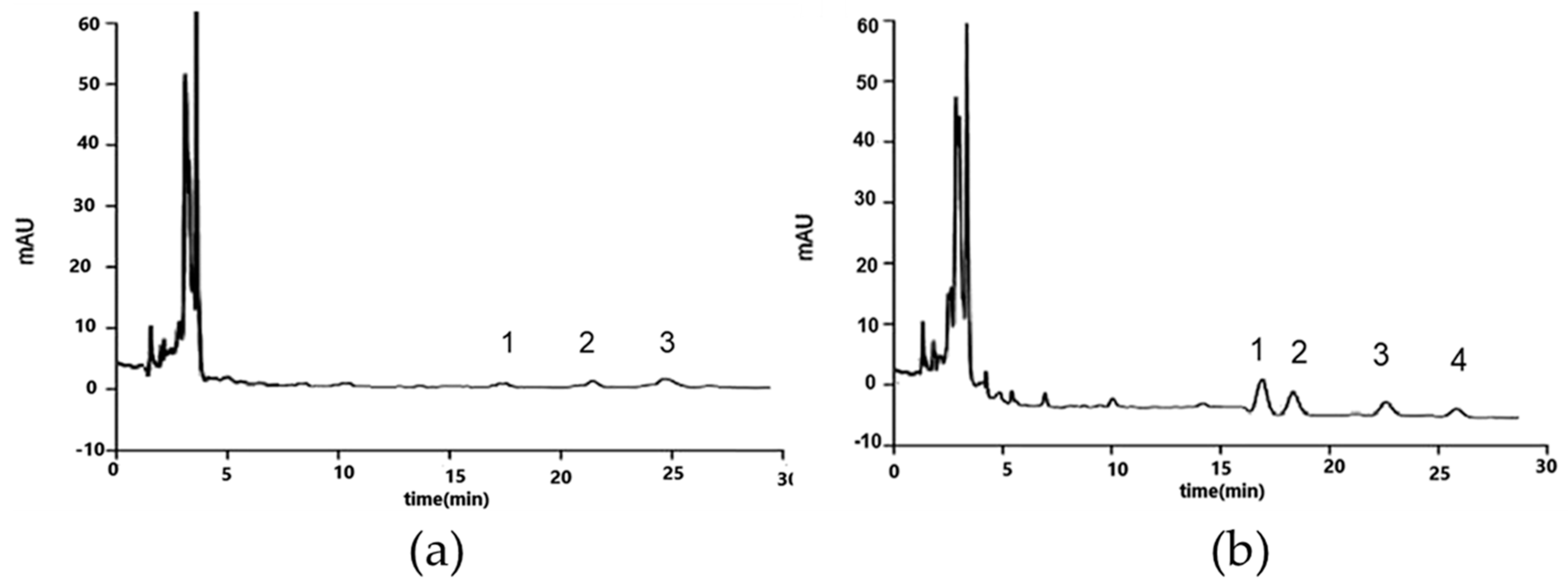
| Compounds and Food | MAS Condition | Sample Post Treatment | Analysis | Recovery (%) | LODng/g (or mL) | Reference |
|---|---|---|---|---|---|---|
| EPA PAH Pumpkin seed oil | 1 g sample + 35 mL of 1.5 M KOH in methanol; microwaved at 130 °C for 10 min | LLE, SPE (on 0.5 g of silica gel) | GC-MS | >83 | [39] | |
| Aliphatic hydrocarb PAH Marine biota | 20 g sample + 50 mL ethanolic KOH (12%); microwaved at 600 W for 45 min (T max 85 °C) | MAE (3 × 40 mL n-hexane at 100 W for 12 min), LLE. SPE on 5 g Florisil | GC-FID | >90, except for lighter PAH | [40] | |
| 6 heavy PAH Fish and mussels | 1 g of fresh fish (or 0.2 g lyophilized + 0.8 mL of water) + 4 mL of saturated KOH in methanol + 10 mL of n-hexane; microwaved at 129 °C for 17 min. | SPE clean-up on silica gel (0.5 g) | HPLC-DAD/FLD | 85-100 except for IP, DBahA, BghiP* | 0.1–0.5 | [41] |
| 10 PAH Fish and mussels | 2 g of lyophilized sample + 12 mL saturated methanolic KOH + 12 mL n-hexane; microwaved for 15 min at 80% of the power | No sample post-treatment | GC-MS | 90–115, | [42] | |
| EPA-PAH Smoked fish and meat | 0.4 g of lyophilized sample + 1.6 mL of water + 8 mL of saturated KOH in methanol + 20 mL of n-hexane; microwaved at 120 °C for 20 min | SPE clean-up on silica gel (0.5 g) | HPLC-FLD | 75–109 | <0.1 | [13] |
| EPA-PAH Propolis and propolis- extracts | 0.2 g of propolis (or 0.5 g of propolis extract) + 8 mL of a saturated solution of KOH in methanol + 20 mL of n-hexane; microw. at 120 °C for 20 min | No sample post-treatment | HPLC-FLD | 91–102 | <0.1 | [43] |
| EPA-PAH Roasted plants | 0.4 g of lyophilized sample + 1.6 mL of water + 8 mL of saturated KOH in methanol + 20 mL of n-hexane; microwaved at 120 °C for 20 min | SPE clean-up on silica gel (0.5 g) | HPLC-FLD | <74 (for PAH8) | <0.1 | [44] |
| Mineral oils Cereal based products | 5 g sample + I.S.+ 10–20 mL saturated KOH in methanol (depending on the fat content) + 10 mL n-hexane; microwaved at 120 °C for 20 min | Rapid wash with water/methanol; follows sample concentration | on-line HPLC-GC | 89–104 (MOSH) 85–108 (MOAH) | 30 | [14] |
| Compounds and Food | MAE Conditions | Pre-Treatment before DLLME | Extractant/Disperser | Analysis | Enrichment Factors | Recovery (%) | LODng/g (or mL) | Reference |
|---|---|---|---|---|---|---|---|---|
| EPA PAH Smoked fish | 1 g sample + 12 mL KOH (2 M)/ethanol (50:50) | pH 6.5 | Ethylene tetrachloride (100 μL)/Acetone (500 uL) | GC-MS | 244–373 | 82–106 | 0.11–0.43 | [46] |
| EPA PAH Smoked fish | 1 g sample + 10 mL KOH (2 M)/ethanol (50:50); microw. at 500 MHz for 2 min. | pH 5 + Carrez | Ethylene tetrachloride (150 μL)/Acetone (500 uL) | GC-MS | [47] | |||
| EPA PAH Grilled meat | 1 g sample + 10 mL of KOH (2 M)/ethanol (50:50); microwaved at 500 MHz for 1.5 min | pH 6 + Carrez | Ethylene tetrachloride (80 μL)/Acetone (300 μL) | GC-MS | 110–265 | 85–104 | 0.15–0.3 | [48] |
| PAH 4Smoked rice | 1 g of sample + 10 mL of KOH (2 M)/ethanol 1:1); microwaved at 500 MHz for 1.5 min | pH 5 + Carrez | Chloroform (250 μL)/Acetonitrile (1.2 mL) | HPLC-UV | 258–307 | 87–98 | 0.05–0.12 | [49] |
| EPA PAH Bread | 1 g of sample + 10 mL of KOH (1 M)/ethanol (60:40); microwaved at 500 MHz for 1.5 min | pH 6.5 + Carrez | Ethylene tetrachloride (80 μL)/Acetone (300 μL) | GC-MS | 200–300 | 85–104 | 0.1–0.3 | [50] |
| PAH4 Toasted bread | 2 g of sample + 10 mL of KOH (1 M)/ethanol (50:50); microwaved at 500 MHz for 1.5 min | pH 6 + Carrez | Chloroform (180 μL)/Acetonitrile (0.9 mL) | HPLC | 255–312 | 87–104 | 0.03–0.19 | [51] |
| Heavy PAH Coffee | 0.5 g sample + 0.5 mLwater + 10 mL KOH (85% v/v) in ethanol; microw. at 520 MHz for 8 min | pH 6 + Carrez | Ethylene tetrachloride (80 μL)/Acetone (300 μL) | GC-MS | 155–248 | 88–101 | 0.1–0.3 | [52] |
| PAH vegetables | 10 mL homogenate sample + 4 mL acetone; microw. at 400 W for 1.5 min | 1-bromo-3-methylbutane (30 µL)/Acetone (800 µL) | GC-FID | [53] | ||||
| PAH Edible oils | Sample + acetonitrile/acetone (1:1) and methanolic KOH (in two steps). | Ethylene tetrachloride/Ethanol | GC-MS | 81–124 | 0.2–2.7 | [54] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moret, S.; Conchione, C.; Srbinovska, A.; Lucci, P. Microwave-Based Technique for Fast and Reliable Extraction of Organic Contaminants from Food, with a Special Focus on Hydrocarbon Contaminants. Foods 2019, 8, 503. https://doi.org/10.3390/foods8100503
Moret S, Conchione C, Srbinovska A, Lucci P. Microwave-Based Technique for Fast and Reliable Extraction of Organic Contaminants from Food, with a Special Focus on Hydrocarbon Contaminants. Foods. 2019; 8(10):503. https://doi.org/10.3390/foods8100503
Chicago/Turabian StyleMoret, Sabrina, Chiara Conchione, Ana Srbinovska, and Paolo Lucci. 2019. "Microwave-Based Technique for Fast and Reliable Extraction of Organic Contaminants from Food, with a Special Focus on Hydrocarbon Contaminants" Foods 8, no. 10: 503. https://doi.org/10.3390/foods8100503
APA StyleMoret, S., Conchione, C., Srbinovska, A., & Lucci, P. (2019). Microwave-Based Technique for Fast and Reliable Extraction of Organic Contaminants from Food, with a Special Focus on Hydrocarbon Contaminants. Foods, 8(10), 503. https://doi.org/10.3390/foods8100503