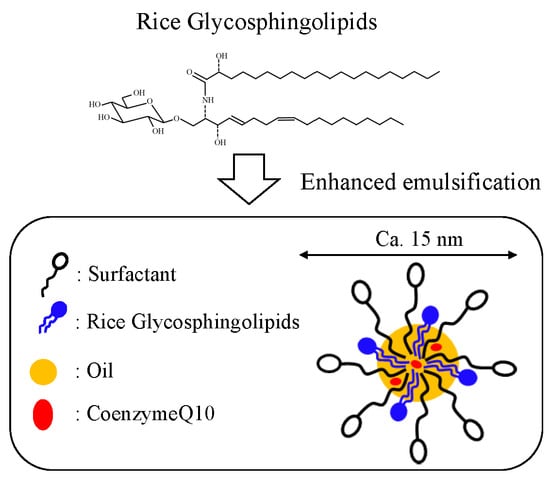
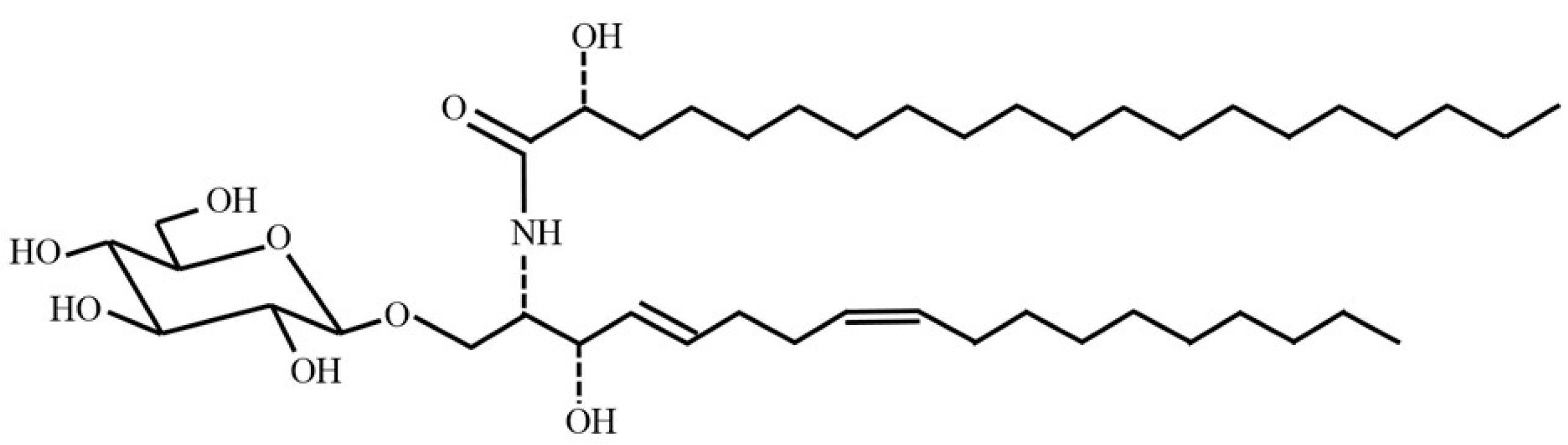
Formation of Food Grade Microemulsion with Rice Glycosphingolipids to Enhance the Oral Absorption of Coenzyme Q10
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Particle Size Measurement
2.3. Preparation of RGSLs, Polysorbate 80, or RGSLs/Polysorbate 80 particles
2.4. Preparation of Emulsion Without CoQ10
2.5. Preparation of CoQ10-Loaded Emulsion
2.6. Encapsulation Efficiency of CoQ10
2.7. Powder Preparation
2.8. Morphology of Freeze-Dried Particles
2.9. Re-Dispersibility of Freeze-Dried Particles
2.10. Solubility Test
2.11. Animal Study
2.12. Statistical Analysis
3. Results and Discussion
3.1. Evaluation of the Emulsion Without CoQ10
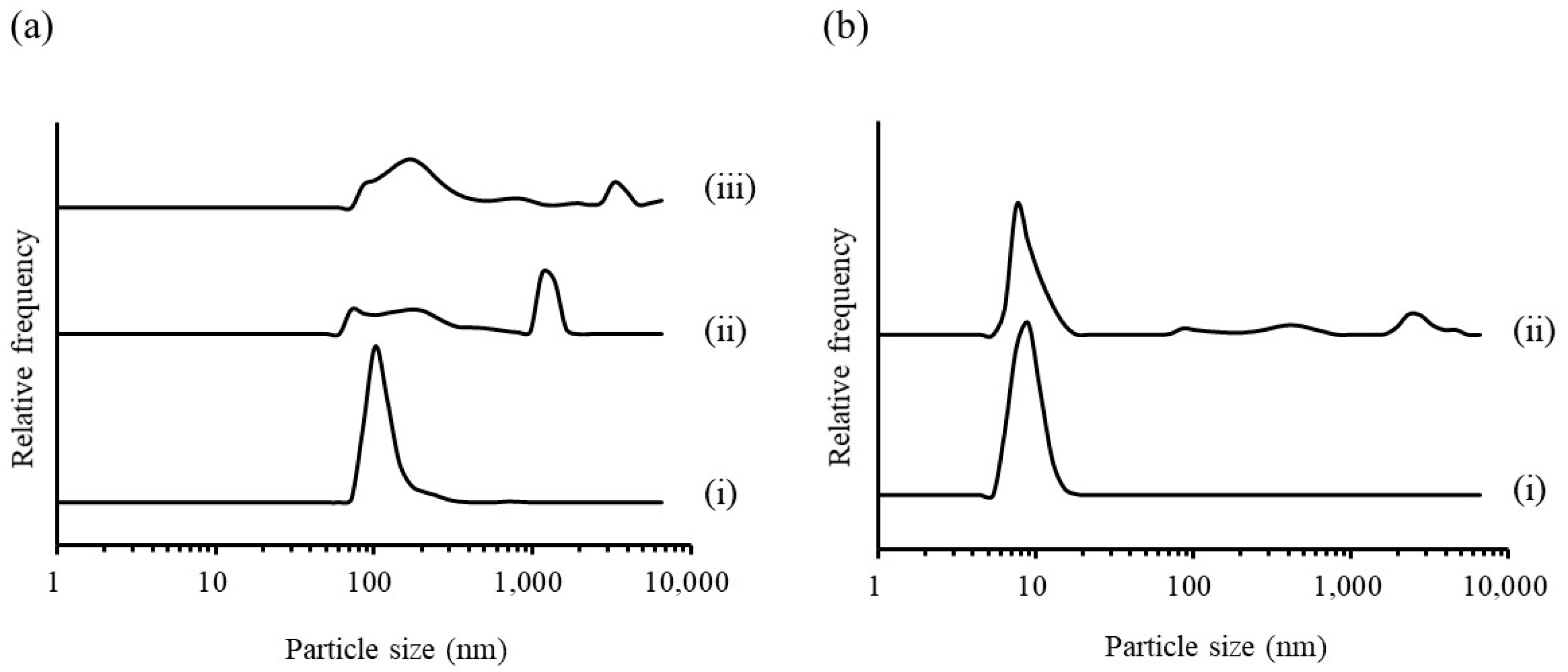
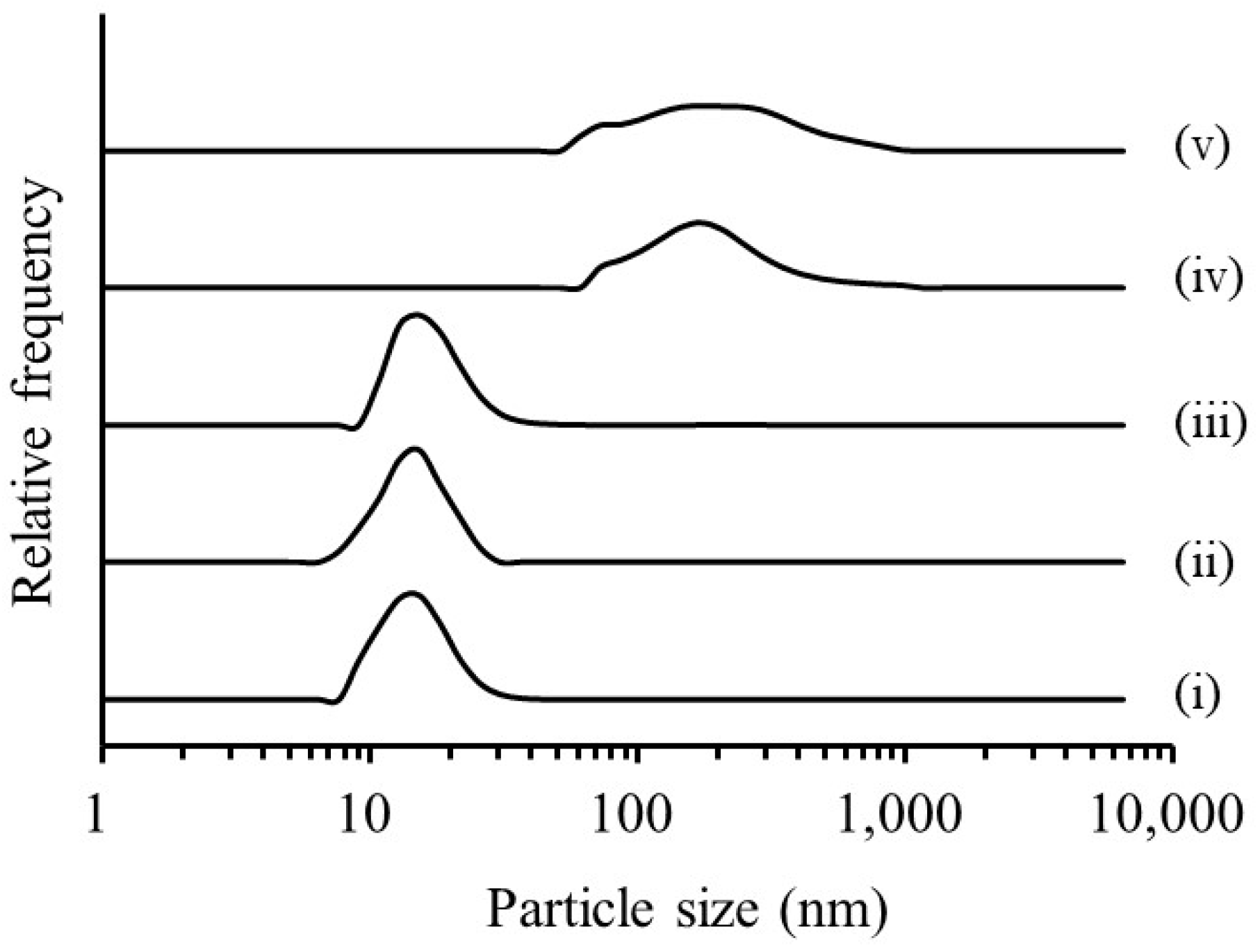
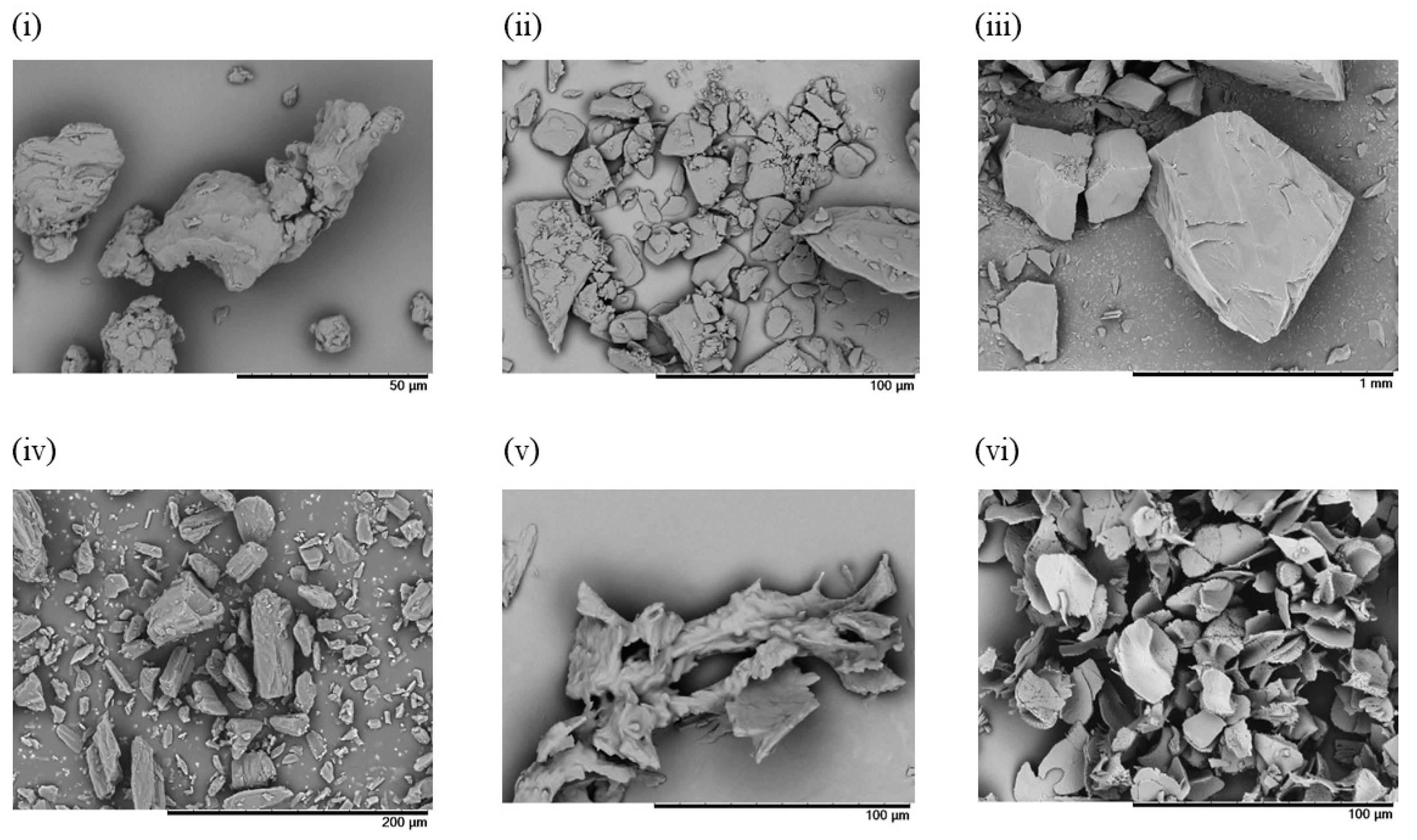
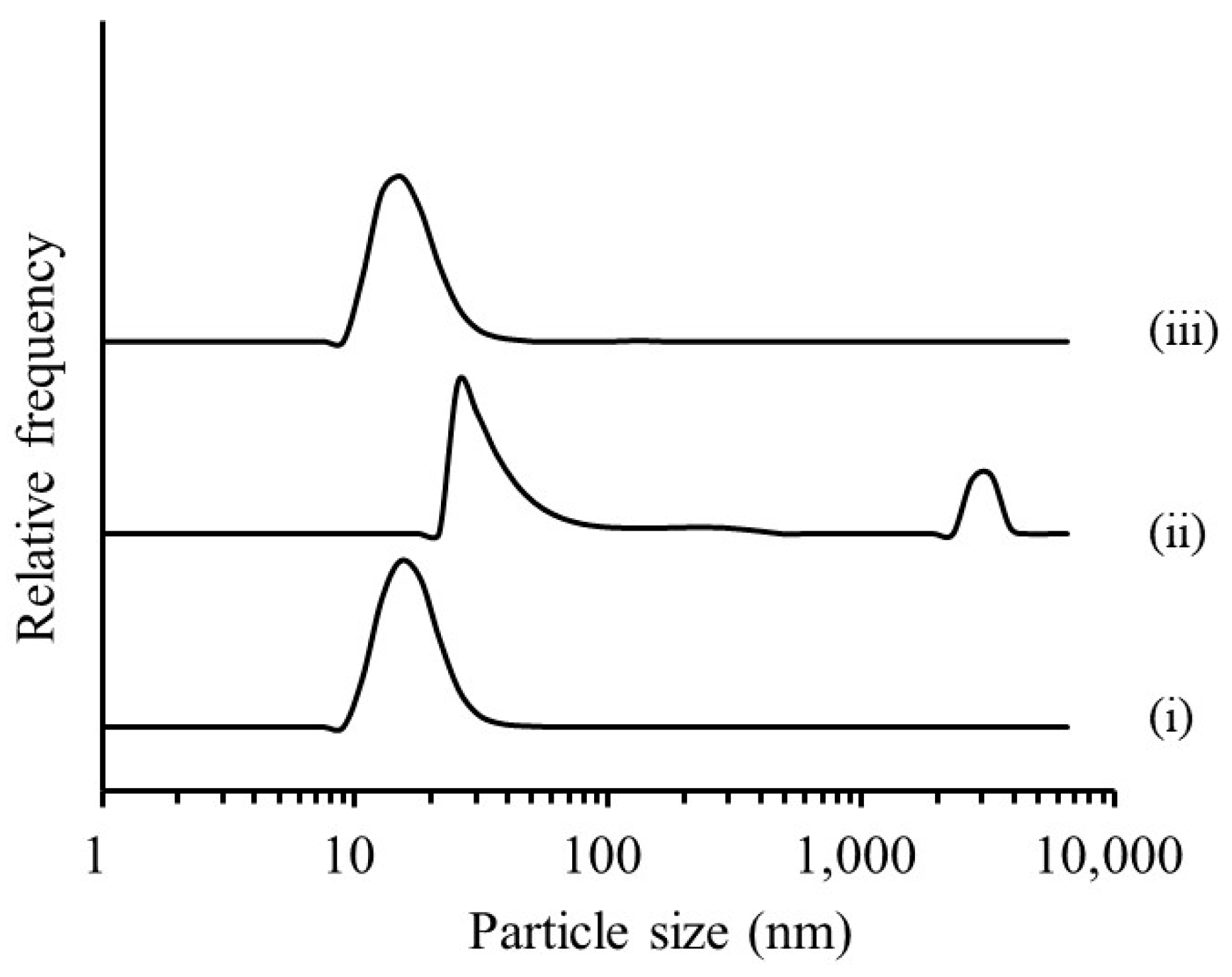
3.1.1. Evaluation of RGSLs, Polysorbate 80, and RGSLs/Polysorbate 80 Particles
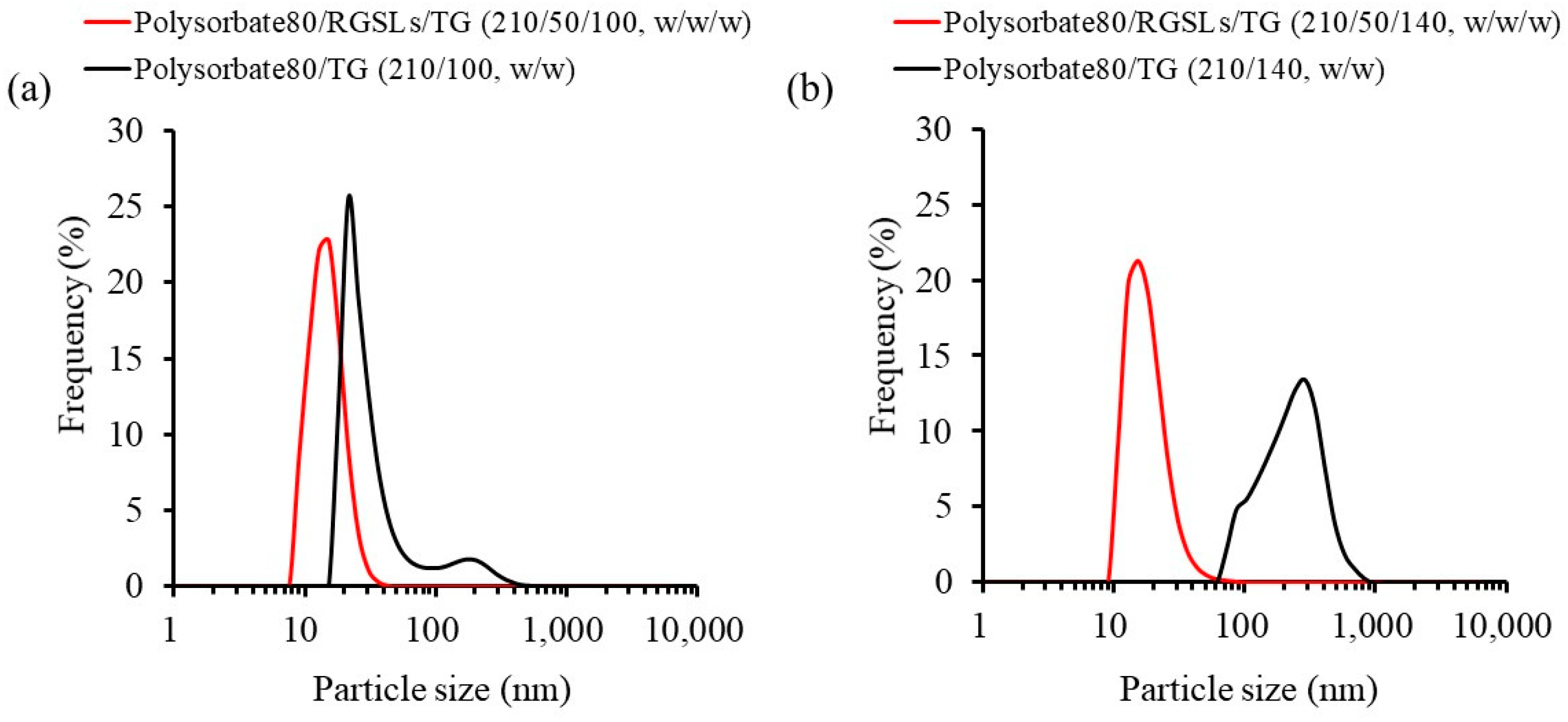
3.1.2. Evaluation of the TG/RGSLs/Polysorbate 80 Emulsion
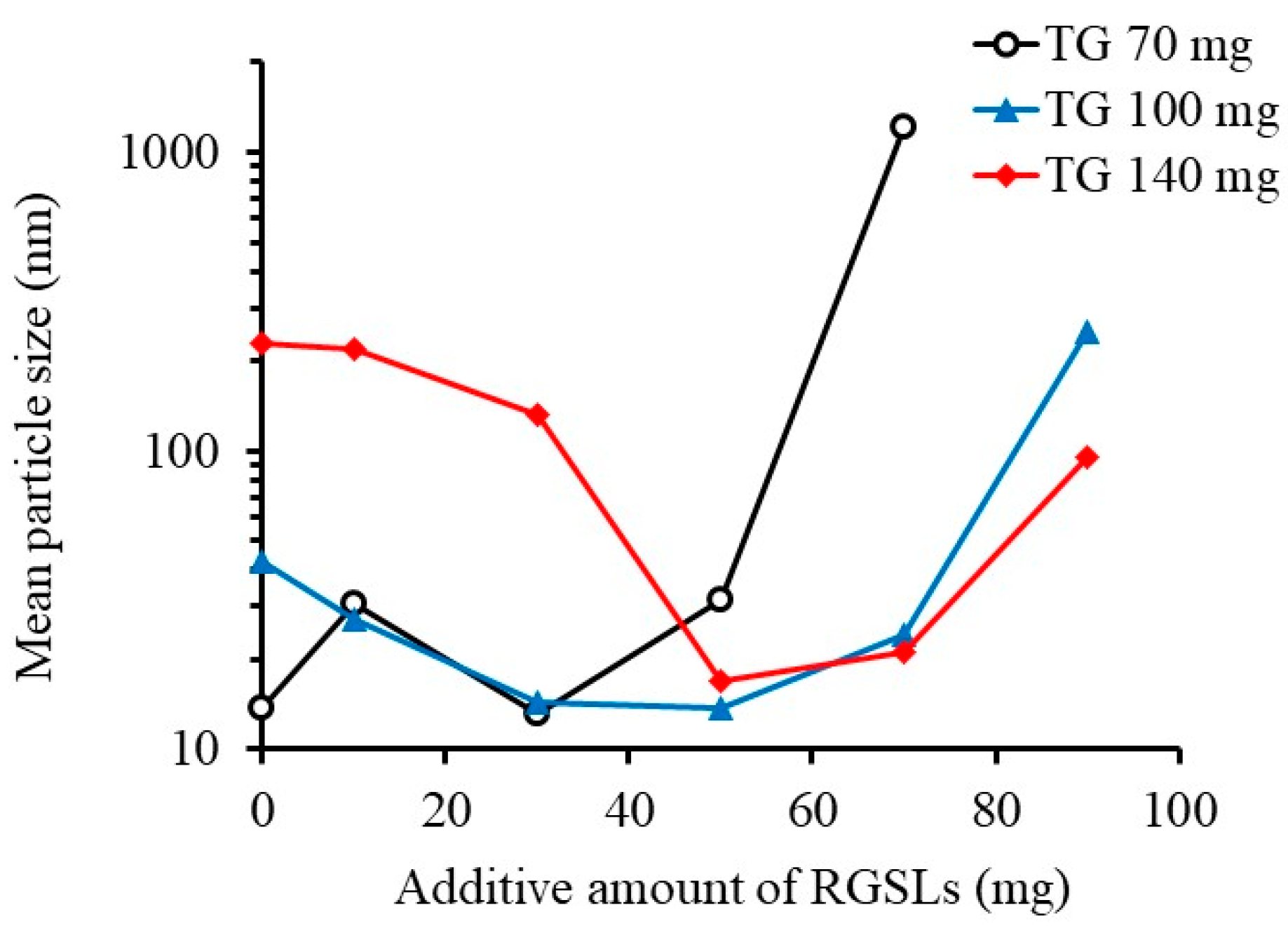
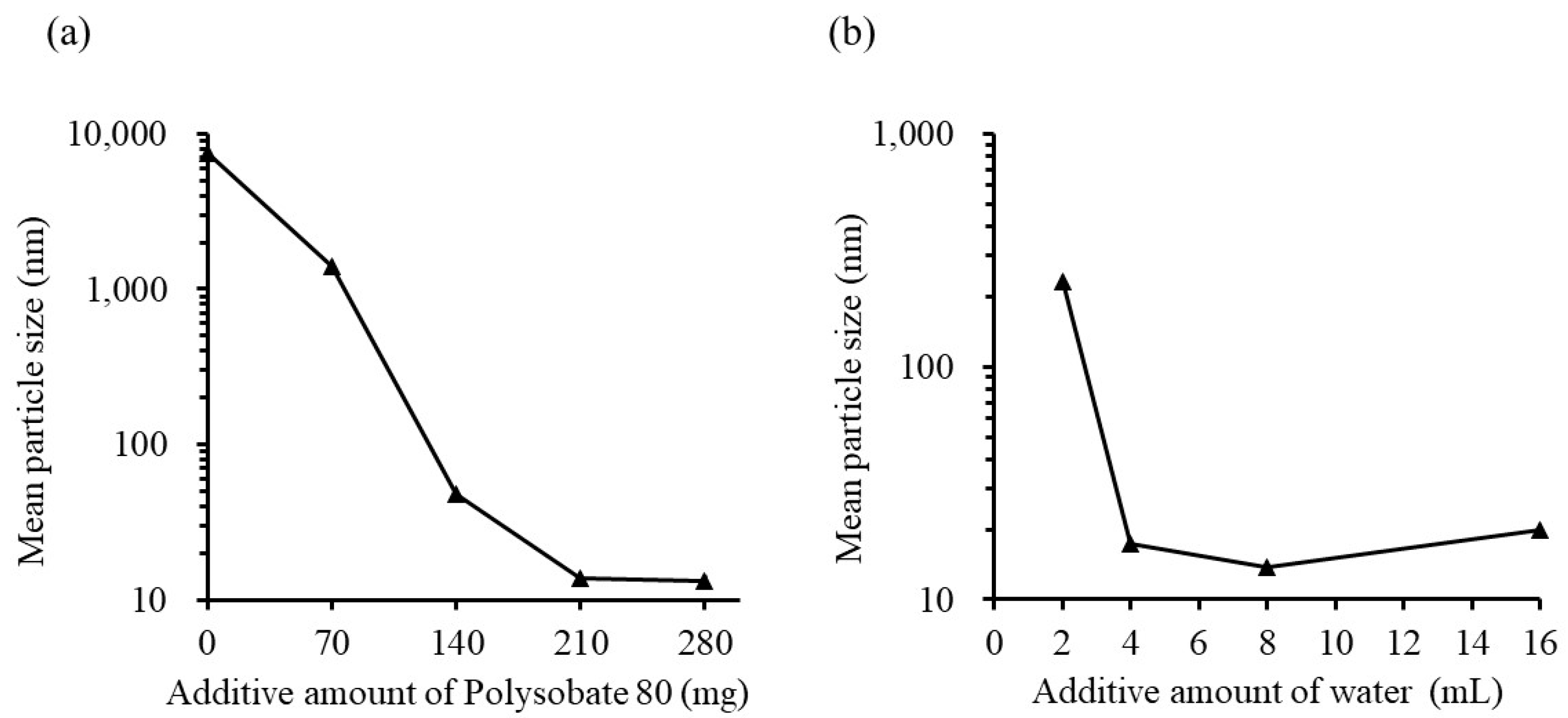
3.1.3. Effect of Surfactant Content and Water Volume on Emulsion Formation
3.2. Evaluation of CoQ10-Loaded Microemulsion
3.2.1. Preparation of CoQ10-Loaded Emulsion
3.2.2. Preparation of Freeze-Dried Powder Containing CoQ10-Loaded Microemulsion
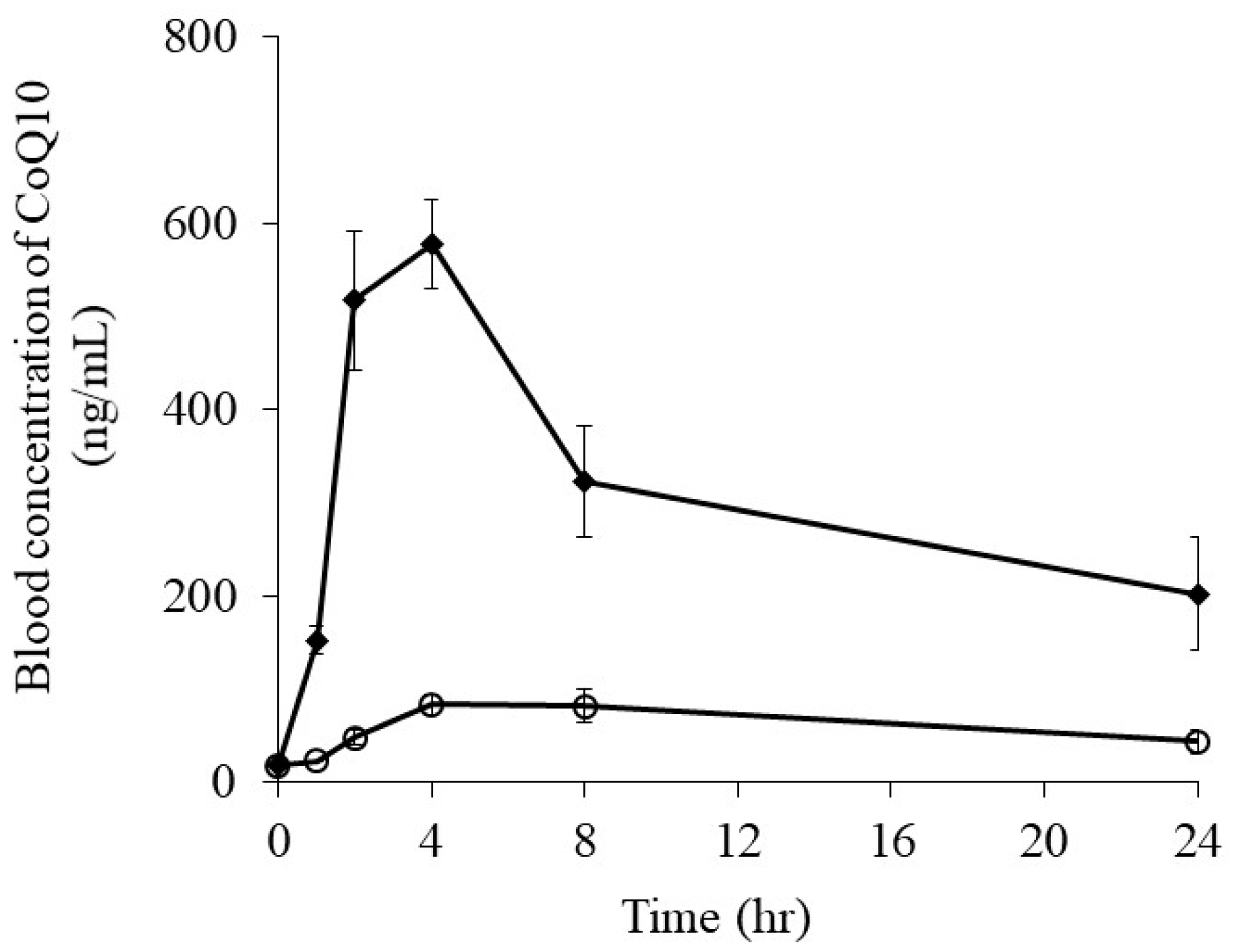
3.2.3. Oral Absorption of CoQ10 from CoQ10-Loaded Microemulsion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Christian, E.; Freye, K.; Strobel, H.-P. Coenzyme Q10 Supplements Which Increase ATP Synthesis within Mitochondria and Protect Against Toxic Sodium Azide. Acta Sci. Nutr. Health 2018, 2, 2–9. [Google Scholar]
- Cadenas, E.; Davies, K.J.A.; Witting, P.K.; Pettersson, K.; Letters, J.; Stocker, R. Anti-atherogenic effect of coenzyme Q10 in apolipoprotein E gene knockout mice. Free Radic. Biol. Med. 2000, 29, 295–305. [Google Scholar]
- Fuller, B.; Smith, D.; Howerton, A.; Kern, D. Anti-Inflammatory Effects of CoQ10 and Colorless Carotenoids. J. Cosmet. Dermatol. 2006, 5, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Crane, F.L. Biochemical Functions of Coenzyme Q10. J. Am. Coll. Nutr. 2001, 20, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Beal, M.F.; Shults, C.W. Effects of Coenzyme Q 10 in Huntington’s Disease and Early Parkinson’s Disease. BioFactors 2003, 18, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Terao, K.; Nakata, D.; Fukumi, H.; Schmid, G.; Arima, H.; Hirayama, F.; Uekama, K. Enhancement of Oral Bioavailability of Coenzyme Q10by Complexation with γ-Cyclodextrin in Healthy Adults. Nutr. Res. 2006, 26, 503–508. [Google Scholar] [CrossRef]
- Schulz, C.; Obermüller-Jevic, U.C.; Hasselwander, O.; Bernhardt, J.; Biesalski, H.K. Comparison of the Relative Bioavailability of Different Coenzyme Q10formulations with a Novel Solubilizate (SoluTM Q10). Int. J. Food Sci. Nutr. 2006, 57, 546–555. [Google Scholar] [CrossRef]
- Bala, V.; Rao, S.; Prestidge, C.A. Facilitating Gastrointestinal Solubilisation and Enhanced Oral Absorption of SN38 Using a Molecularly Complexed Silica-Lipid Hybrid Delivery System. Eur. J. Pharm. Biopharm. 2016, 105, 32–39. [Google Scholar] [CrossRef]
- Floury, J.; Desrumaux, A.; Lardières, J. Effect of High-Pressure Homogenization on Droplet Size Distributions and Rheological Properties of Model Oil-in-Water Emulsions. Innov. Food Sci. Emerg. Technol. 2000, 1, 127–134. [Google Scholar] [CrossRef]
- Ogawa, S.; Decker, E.A.; McClements, D.J. Production and Characterization of O/W Emulsions Containing Cationic Droplets Stabilized by Lecithin−Chitosan Membranes. J. Agric. Food Chem. 2003, 51, 2806–2812. [Google Scholar] [CrossRef]
- Joseph, S.; Bunjes, H. Preparation of Nanoemulsions and Solid Lipid Nanoparticles by Premix Membrane Emulsification. J. Pharm. Sci. 2012, 101, 2479–2489. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Decker, E.A.; McClements, D.J. Production and Characterization of O/W Emulsions Containing Droplets Stabilized by Lecithin-Chitosan-Pectin Multilayered Membranes. J. Agric. Food Chem. 2004, 52, 3595–3600. [Google Scholar] [CrossRef] [PubMed]
- Davidov-Pardo, G.; McClements, D.J. Nutraceutical Delivery Systems: Resveratrol Encapsulation in Grape Seed Oil Nanoemulsions Formed by Spontaneous Emulsification. Food Chem. 2015, 167, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Guttoff, M.; Saberi, A.H.; Mcclements, D.J. Formation of Vitamin D Nanoemulsion-Based Delivery Systems by Spontaneous Emulsification: Factors Affecting Particle Size and Stability. Food Chem. 2015, 171, 117–122. [Google Scholar] [CrossRef]
- Gulotta, A.; Saberi, A.H.; Nicoli, M.C.; McClements, D.J. Nanoemulsion-Based Delivery Systems for Polyunsaturated (ω-3) Oils: Formation Using a Spontaneous Emulsification Method. J. Agric. Food Chem. 2014, 62, 1720–1725. [Google Scholar] [CrossRef]
- Valicherla, G.R.; Dave, K.M.; Syed, A.A.; Riyazuddin, M.; Gupta, A.P.; Singh, A.; Wahajuddin; Mitra, K.; Datta, D.; Gayen, J.R. Formulation Optimization of Docetaxel Loaded Self-Emulsifying Drug Delivery System to Enhance Bioavailability and Anti-Tumor Activity. Sci. Rep. 2016, 6, 26895. [Google Scholar] [CrossRef]
- Kelmann, R.G.; Kuminek, G.; Teixeira, H.F.; Koester, L.S. Carbamazepine Parenteral Nanoemulsions Prepared by Spontaneous Emulsification Process. Int. J. Pharm. 2007, 342, 231–239. [Google Scholar] [CrossRef]
- Sjöström, B.; Westesen, K.; Bergenståhl, B. Preparation of submicron drug particles in lecithin-stabilized o/w emulsions. II. Characterization of cholesteryl acetate particles. Int. J. Pharm. 1992, 94, 89–101. [Google Scholar] [CrossRef]
- Zhou, H.; Yue, Y.; Liu, G.; Li, Y.; Zhang, J.; Gong, Q.; Yan, Z.; Duan, M. Preparation and Characterization of a Lecithin Nanoemulsion as a Topical Delivery System. Nanoscale Res. Lett. 2010, 5, 224–230. [Google Scholar] [CrossRef]
- Wennekes, T.; van den Berg, R.J.; Boot, R.G.; van der Marel, G.A.; Overkleeft, H.S.; Aerts, J.M. Glycosphingolipids—Nature, Function, and Pharmacological Modulation. Angew. Chem. Int. Ed. 2009, 48, 8848–8869. [Google Scholar] [CrossRef]
- Yamashita, T.; Wada, R.; Sasaki, T.; Deng, C.; Bierfreund, U.; Sandhoff, K.; Proia, R. A vital role for glycosphingolipid synthesis during development and differentiaion. Seikagaku 2000, 72, 1420–1425. [Google Scholar] [PubMed]
- Inoue, J.; Ideue, R.; Takahashi, D.; Kubota, M.; Kumazawa, Y. Liposomal Glycosphingolipids Activate Natural Killer T Cell-Mediated Immune Responses through the Endosomal Pathway. J. Control Release 2009, 133, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Mao-qiang, M.A.N.; Feingold, K.R.; Wang, F.; Thornfeldt, C.R.; Elias, P.M. A Natural Lipid Mixture Improves Barrier Function and Hydration in Human and Murine Skin. J. Soc. Cosmet. Chem. 1996, 47, 157–166. [Google Scholar]
- Galia, E.; Nicolaides, J.E.; Horter, D.; Lobenberg, R.; Reppas, C.; Dressman, J.B. Evaluation of Various Dissolution Media for Predicting in Vivo Performance of Class I and II Drugs. Pharm. Res. 1998, 15, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, K.; McDowell, A.; Ishii, Y.; Hook, S. Liposomal α-galactosylceramide is taken up by gut-associated lymphoid tissue and stimulates local and systemic immune responses. J. Pharm. Pharmacol. 2017, 69, 1724–1735. [Google Scholar] [CrossRef] [PubMed]
- Donsì, F.; Annunziata, M.; Vincensi, M.; Ferrari, G. Design of nanoemulsion-based delivery systems of natural antimicrobials: Effect of the emulsifier. J. Biotechnol. 2012, 159, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Seki, J.; Sonoke, S.; Saheki, A.; Fukui, H.; Sasaki, H.; Mayumi, T. A nanometer lipid emulsion, lipid nano-sphere (LNS®), as a parenteral drug carrier for passive drug targeting. Int. J. Pharm. 2004, 273, 75–83. [Google Scholar] [CrossRef]
- Trang, P.; Wiggins, J.F.; Daige, C.L.; Cho, C.; Omotola, M.; Brown, D.; Weidhaas, J.B.; Bader, A.G.; Slack, F.J. Systemic delivery of tumor suppressor microRNA mimics using a neutral lipid emulsion inhibits lung tumors in mice. Mol. Ther. 2011, 19, 1116–1122. [Google Scholar] [CrossRef]
- McClements, D.J. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter. 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Li, F.; Wang, T.; He, H.B.; Tang, X. The properties of bufadienolides-loaded nano-emulsion and submicro-emulsion during lyophilization. Int. J. Pharm. 2008, 349, 291–299. [Google Scholar] [CrossRef]
- Qin, B.; Liu, L.; Pan, Y.; Zhu, Y.; Wu, X.; Song, S.; Han, G. PEGylated Solanesol for Oral Delivery of Coenzyme Q 10. J. Agric. Food Chem. 2017, 65, 3360–3367. [Google Scholar] [CrossRef] [PubMed]
- Swarnakar, N.K.; Thanki, K.; Jain, S. Lyotropic liquid crystalline nanoparticles of CoQ10: Implication of lipase digestibility on oral bioavailability, in vivo antioxidant activity, and in vitro—In vivo relationships. Mol. Pharm. 2014, 11, 1435–1449. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, M.; McClements, D.J. Factors affecting lipase digestibility of emulsified lipids using an in vitro digestion model: Proposal for a standardised pH-stat method. Food Chem. 2011, 126, 498–505. [Google Scholar] [CrossRef]
| Storage Time (week) | Mean Particle Size (nm) | Encapsulation Efficiency (%) |
|---|---|---|
| 0 | 16.83 ± 0.32 | 100.34 ± 0.92 |
| 1 | 17.37 ± 0.90 | 98.71 ± 6.66 |
| 2 | 16.97 ± 1.40 | 102.3 ± 4.86 |
| 4 | 17.17 ± 1.81 | 105.80 ± 4.42 |
| Test Solution | Solubility of CoQ10 (μg/mL) | |
|---|---|---|
| CoQ10 Powder | CoQ10-Loaded Microemulsion | |
| Simulated gastric fluid | N.D. | 507.31 ± 54.00 |
| Simulated intestinal fluid | 0.44 ± 0.03 | 455.57 ± 51.91 |
| CoQ10 Powder | CoQ10 Loaded-Microemulsion | |
|---|---|---|
| Cmax (ng/mL) | 84.2 ± 17.8 | 578.1 ± 47.5 |
| Tmax (h) | 4.0 | 4.0 |
| AUC0-24 h (ng·h/mL) | 1050.9 ± 202.6 | 7503.6 ± 1079.3 ** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uchiyama, H.; Chae, J.; Kadota, K.; Tozuka, Y. Formation of Food Grade Microemulsion with Rice Glycosphingolipids to Enhance the Oral Absorption of Coenzyme Q10. Foods 2019, 8, 502. https://doi.org/10.3390/foods8100502
Uchiyama H, Chae J, Kadota K, Tozuka Y. Formation of Food Grade Microemulsion with Rice Glycosphingolipids to Enhance the Oral Absorption of Coenzyme Q10. Foods. 2019; 8(10):502. https://doi.org/10.3390/foods8100502
Chicago/Turabian StyleUchiyama, Hiromasa, Jisoon Chae, Kazunori Kadota, and Yuichi Tozuka. 2019. "Formation of Food Grade Microemulsion with Rice Glycosphingolipids to Enhance the Oral Absorption of Coenzyme Q10" Foods 8, no. 10: 502. https://doi.org/10.3390/foods8100502
APA StyleUchiyama, H., Chae, J., Kadota, K., & Tozuka, Y. (2019). Formation of Food Grade Microemulsion with Rice Glycosphingolipids to Enhance the Oral Absorption of Coenzyme Q10. Foods, 8(10), 502. https://doi.org/10.3390/foods8100502