Liberation and Micellarization of Carotenoids from Different Smoothies after Thermal and Ultrasound Treatments †
Abstract
1. Introduction
2. Reagents and Methods
2.1. Reagents
2.2. Sample Preparation
2.3. Microbial Analysis
2.4. Smoothie Color Measurement
2.5. In Vitro Digestion Model
2.6. UHPLC Analyses
2.7. Extraction and Quantification of Carotenoids
2.8. Treatments
2.9. Statistical Analysis
3. Results and Discussion
3.1. Undigested Samples, Immediately after Thermal and Ultrasound Treatments
3.2. Colour Analysis
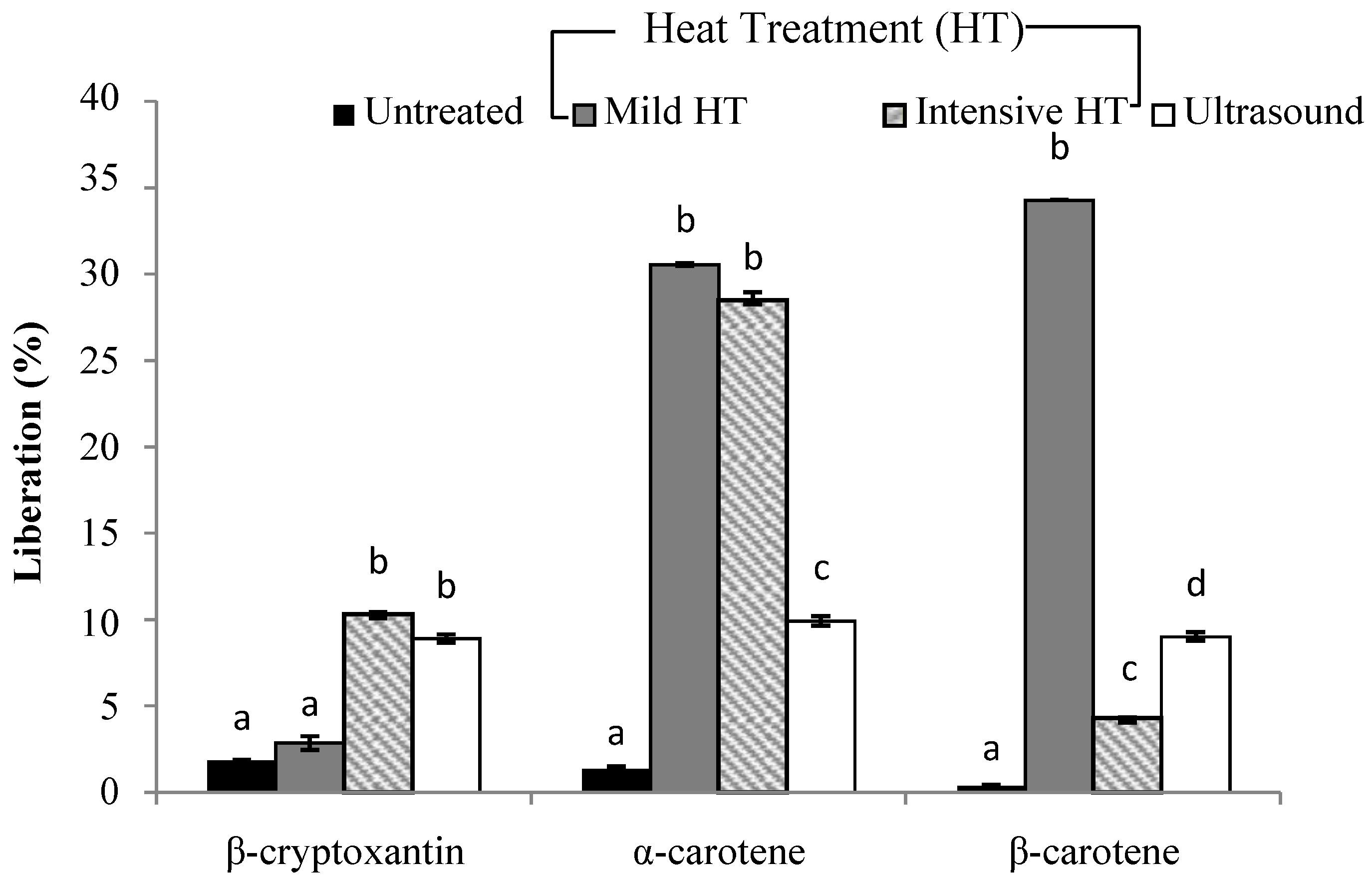
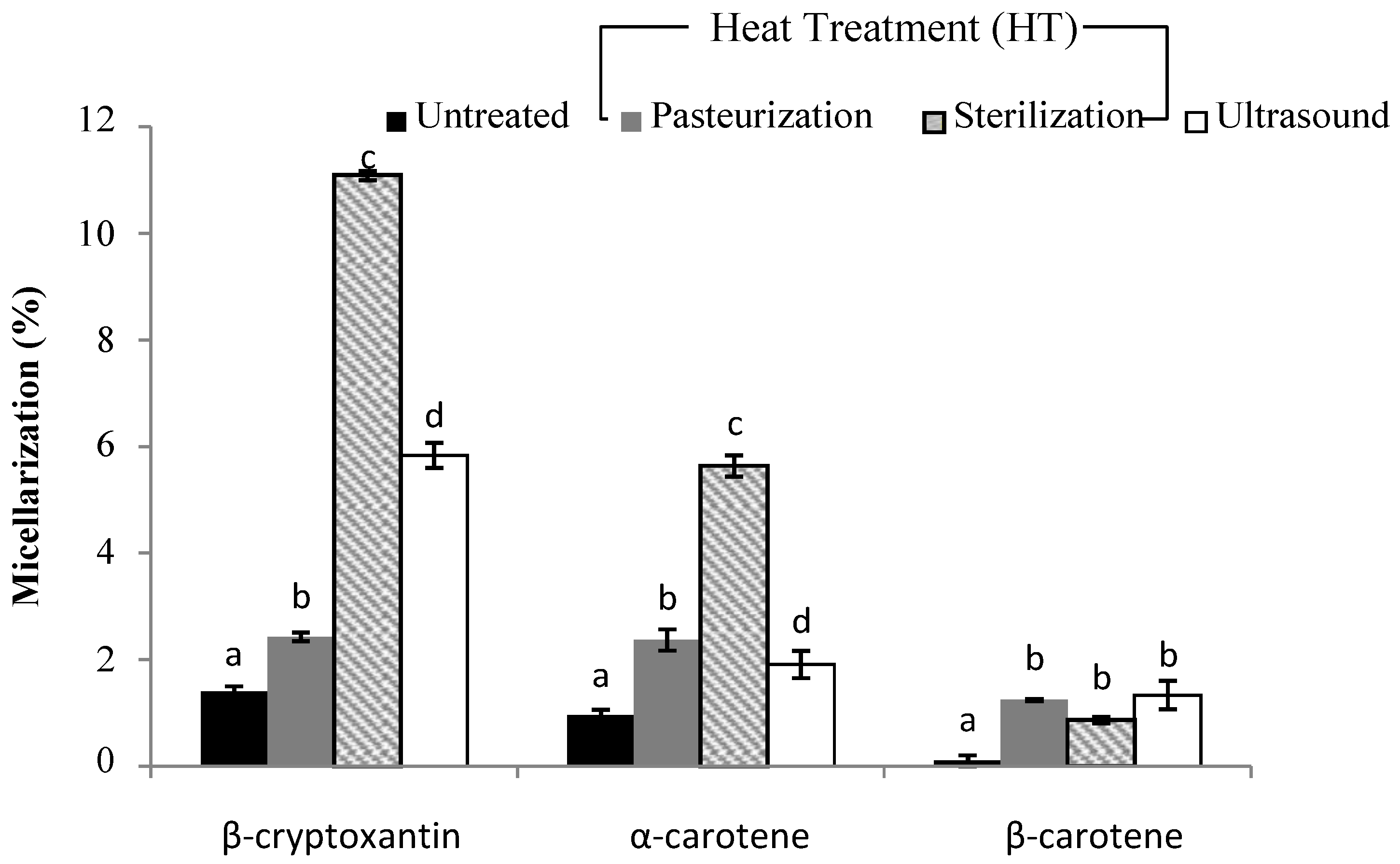
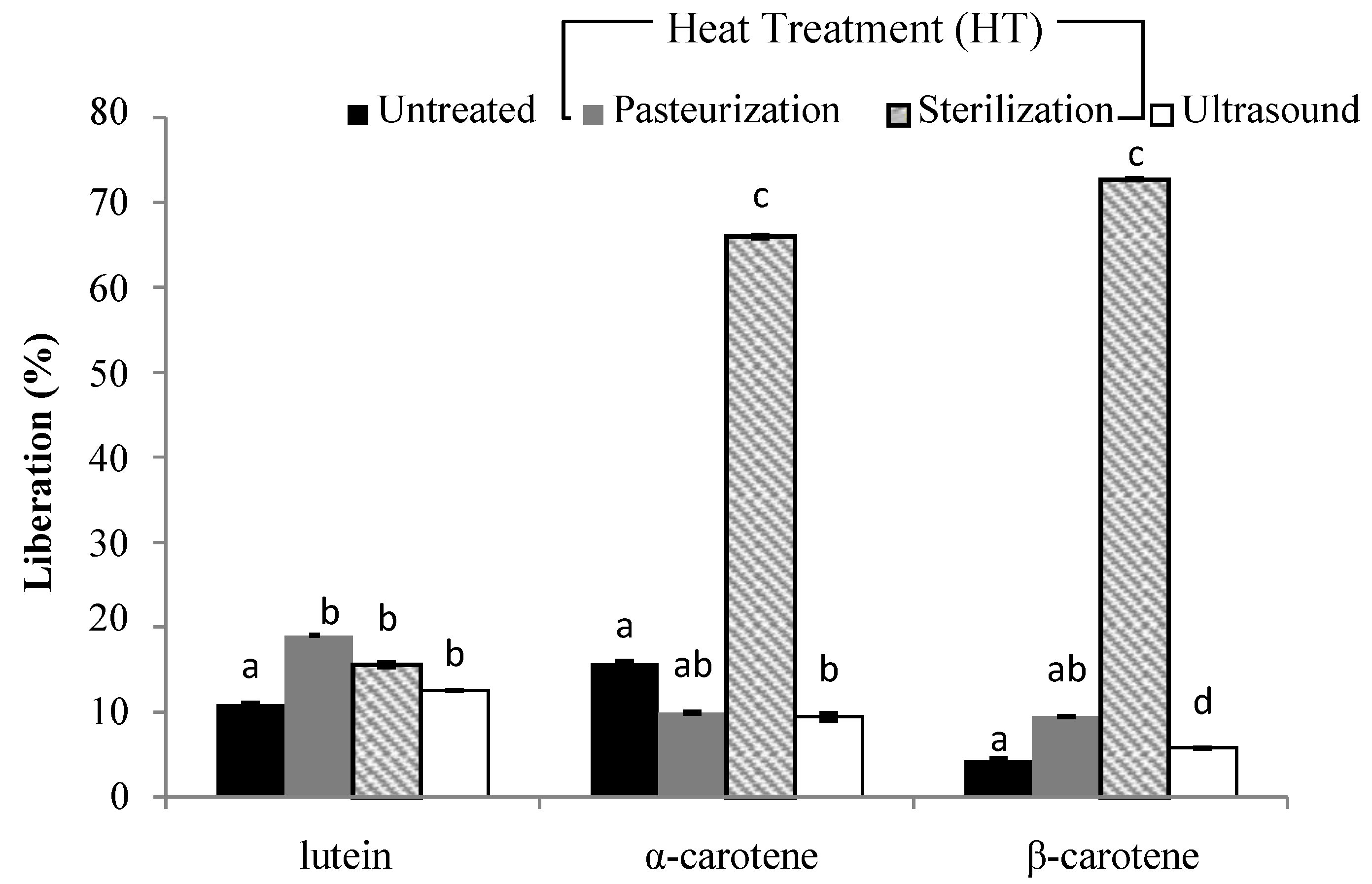
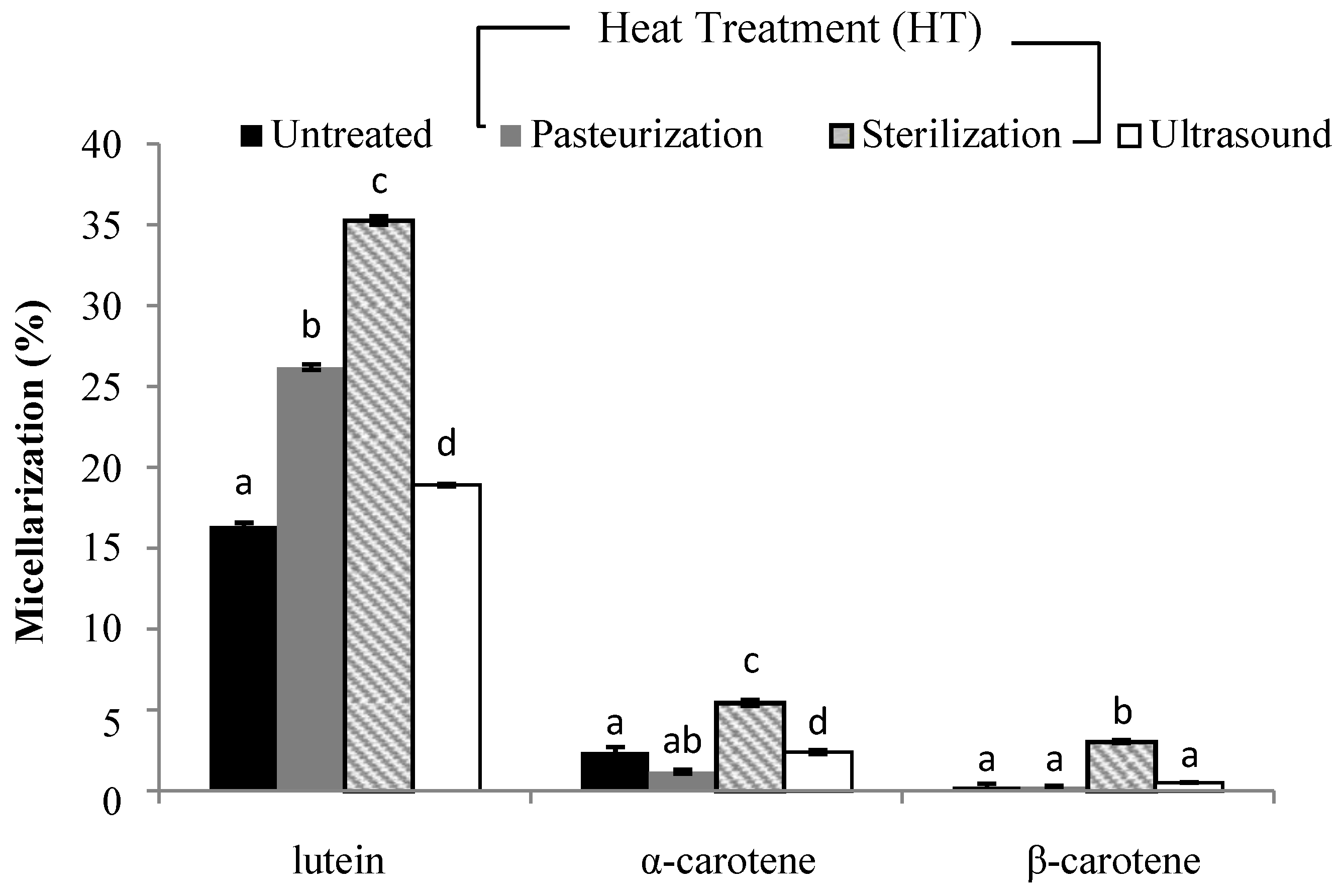
3.3. Liberation and Micellarization of Carotenoid Content from Different Smoothies Subjected to Thermal Processing and Ultrasound Treatment
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nagao, A. Absorption and function of dietary carotenoids. Food Factors Health Promot. 2009, 61, 55–63. [Google Scholar]
- Fiedor, J.; Burda, K. Potential Role of Carotenoids as Antioxidant in Human Health and Disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef]
- Sikora, E.; Cieslak, E.; Topolska, K. The sources of natural antioxidants. Acta Sci. Pol./Technol. Aliment. 2008, 7, 5–17. [Google Scholar]
- Gleise, O.; Dangles, J.; Landrier, C.C.; Veyrat, P.; Borel, A. Effects of physicochemical properties of carotenoids on their bioaccessibility, intestinal cell uptake, and blood and tissue concentrations. Mol. Nutr. Food Res. 2012, 56, 1385–1397. [Google Scholar]
- Fernández-García, E.; Carvajal-Lérida, I.; Jaren-Galán, M.; Garrido-Fernández, J.; Pérez-Galvez, A.; Hornero-Méndez, D. Carotenoids bioavailability from foods: From plant pigments to efficient biological activities. Food Res. Int. 2012, 46, 438–450. [Google Scholar] [CrossRef]
- Kopec, R.E.; Failla, M.L. Recent advances in the bioaccessibility and bioavailability of carotenoids and effects of other dietary lipophile. J. Food Compos. Anal. 2018, 68, 16–30. [Google Scholar] [CrossRef]
- Aschoff, J.K.; Kaufmann, S.; Kalkan, O.; Neidhart, S.; Carle, R.; Schweiggert, R.M. In vitro bioaccessibility of carotenoids, flavonoids, and vitamin C from differently processed oranges and orange juices [Citrus sinensis (L.) osbeck]. J. Agric. Food Chem. 2015, 63, 578–587. [Google Scholar] [CrossRef]
- Edwards, A.J.; Nguyen, C.H.; You, C.S.; Swanson, J.E.; Emenhiser, C.; Parker, R.S. α- and β-carotene from a commercial puree are more bioavailable to humans than from boiled-mashed carrots, as determined using an extrinsic stable isotope reference method. J. Nutr. 2002, 132, 159–167. [Google Scholar] [CrossRef]
- Hedrén, E.; Diaz, V.; Svamberg, U. Estimation of carotenoid accessibility from carrots determined by an in vitro digestion method. Eur. J. Clin. Nutr. 2002, 56, 425–430. [Google Scholar] [CrossRef]
- Tyssandier, V.; Lyan, B.; Borel, P. Main factors governing the transfer of carotenoids from emulsion lipid droplets to micelles. Biochim. Biophys. Acta 2001, 1533, 285–292. [Google Scholar] [CrossRef]
- Tiwari, B.K.; O’Donnell, C.P.; Cullen, P.J. Effect of sonication on retention of anthocyanins in blackberry juice. J. Food Eng. 2009, 93, 166–171. [Google Scholar] [CrossRef]
- Cortes, C.; Esteve, M.J.; Frigola, A. Color of orange juice treated by High Intensity Pulsed Electric Fields during refrigerated storage and comparison with pasteurized juice. Food Control 2008, 19, 151–158. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. Relation between particle size and carotenoid bioaccessibility in carrot- and tomato-derived suspensions. J. Agric. Food Chem. 2012, 60, 11995–12003. [Google Scholar]
- Kopf-Bolanz, K.A.; Schwander, F.; Gijs, M.; Vergères, G.; Portmann, R.; Lotti, E. Validation of an in vitro digestive system for studying macronutrient Decomposition in Humans. J. Nutr. 2012, 142, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Odriozola-Serrano, I.; Aguiló-Aguayo, I.; Saliva-Fortuny, R.; MartÍn-Belloso, O. Pulsed electric fields processing effects on quality and health-related constituents of plant-based foods. Trends Food Sci. Technol. 2013, 29, 98–107. [Google Scholar] [CrossRef]
- D’Evoli, L.; Lombardi-Boccia, G.; Lucarini, M. Influence of Heat treatments on carotenoid content of cherry tomatoes. Foods 2013, 2, 352–363. [Google Scholar] [CrossRef]
- Provesi, J.G.; Dias, C.O.; Amante, E.R. Changes in carotenoids during processing and storage of pumpkin puree. Food Chem. 2011, 128, 195–202. [Google Scholar] [CrossRef]
- Lee, H.S.; Coates, G.A. Effect of thermal mild heat treatment on Valencia orange juice color and pigments. LWT Food Sci. Technol. 2003, 36, 153–156. [Google Scholar] [CrossRef]
- Patras, A.; Brunton, N.P.; Da Pieve, S.; Butler, F. Impact of high pressure processing on total antioxidant activity, phenolic, ascorbic acid, anthocyanin content and colour of strawberry and blackberry purées. Innov. Food Sci. Emerg. Technol. 2009, 10, 308–313. [Google Scholar] [CrossRef]
- Barba, F.J.; Jäger, H.; Meneses, N.; Esteve, M.J.; Frígola, A.; Knorr, D. Evaluation of quality changes of blueberry juice during refrigerated storage after high-pressure and pulsed electric fields processing. Innov. Food Sci. Emerg. Technol. 2012, 14, 18–24. [Google Scholar] [CrossRef]
- Abid, M.; Jabbar, S.; Hu, B.; Hashim, M.M.; Wu, T.; Wu, Z.; Khan, M.A.; Zeng, X. Synergistic impact of sonication and high hydrostatic pressure on microbial and enzymatic inactivation of apple juice. Food Sci. Technol. 2014, 59, 70–76. [Google Scholar] [CrossRef]
- Rawson, A.; Tiwari, B.K.; Patras, A.; Brunton, N.; Brennan, C.; Cullen, P.J.; O’Donnell, C.P. Effect of thermosonication on bioactive compounds in watermelon juice. Food Res. Int. 2011, 44, 1168–1173. [Google Scholar] [CrossRef]
- Walkling-Ribeiro, M.; Noci, F.; Cronin, D.A.; Lyng, J.G.; Morgan, D.J. Shelf life and sensory attributes of a fruit smoothie-type beverage processed with moderate heat and pulsed electric fields. Food Sci. Technol. 2012, 43, 1067–1073. [Google Scholar] [CrossRef]
- Sengun, I.Y.; Karapinar, M. Effectiveness of Lemon Juice, Vinegar and Their Mixture in Elimination of Salmonella typhimurium on Carrots (Daucus carota L.). Int. J. Food Microbiol. 2004, 96, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Zenker, M.; Heinz, V.; Knorr, D. Application of ultrasound-assisted thermal processing for preservative and quality retention of liquid foods. J. Food Protect. 2003, 66, 1642–1649. [Google Scholar] [CrossRef]
- Pala, C.U.; Zorba, N.N.; Ozcan, G. Microbial inactivation and physicochemical properties of ultrasound processed pomegranate juice. J. Food Protect. 2015, 78, 531–539. [Google Scholar] [CrossRef]
- Lemmens, L.; Van Buggenhout, S.; Oey, I.; Van Loey, A.; Hendrickx, M.E. Towards a better understanding of the relationship between the β- carotene in vitro bio-accessibility and pectin structural changes: A case study on carrots. Food Res. Int. 2009, 42, 1323–1330. [Google Scholar] [CrossRef]
- Knockaert, G.; De Roeck, A.; Lemmens, L.; Van Buggenhout, S.; Hendrickx, M.; Van Loey, A. Effect of thermal and high pressure processes on structural and health-related properties of carrots (Daucus carota). Food Chem. 2011, 125, 903–912. [Google Scholar] [CrossRef]
- Stinco, C.M.; Fernández-Vázquez, R.; Escudero-Gilete, M.L.; Heredia, F.J.; Meléndez-Martínez, A.J.; Vicario, I.M. Effect of orange juices processing on the color, particle size, and bioaccessibility of carotenoids. J. Agric. Food Chem. 2012, 60, 1447–1455. [Google Scholar] [CrossRef]
- Bengtsson, A.; Brackmann, C.; Enejder, A.; Alminger, M.L.; Svanberg, U. Effects on thermal processing on the in vitro bioaccessibility and microstructure of β-carotene in orange-flashed sweet potato. J. Agric. Food Chem. 2010, 58, 11090–11096. [Google Scholar] [CrossRef]
- Bengtsson, A.; Alminger, M.; Svanberg, U. In vitro bioaccessibility of β-carotene from heat-processed orange-flashed sweet potato. J. Agric. Food Chem. 2009, 57, 9693–9696. [Google Scholar] [CrossRef]
- Schweiggert, R.M.; Mezger, D.; Schimpf, F.; Steingass, C.B.; Carle, R. Influence of chromoplast morphology on carotenoid bioaccessibility of carrot, mango, papaya, and tomato. Food Chem. 2012, 135, 2736–2742. [Google Scholar] [CrossRef]
- Gupta, R.; Kopec, R.E.; Schwartz, S.J.; Balasubramaniam, V.M. Combined pressure-temperature effects of carotenoid retention and bioaccessibility in tomato juice. J. Agric. Food Chem. 2011, 59, 7808–7817. [Google Scholar] [CrossRef]
- Granado-Lorencio, F.; Olmedilla-Alonso, B.; Herrero-Barbudo, C.; Blanco-Navarro, I.; Pérez-Sacristán, B.; Bláquez-García, S. In vitro bioaccessibility of carotenoids and tocopherols from fruits and vegetables. Food Chem. 2007, 102, 641–648. [Google Scholar] [CrossRef]
- Van het Hof, K.; West, C.; Weststrate, J.; Hautvast, J. Dietary factors that affect the bioavailability of carotenoids. J. Nutr. 2000, 130, 503–506. [Google Scholar] [CrossRef]
- Ryan, L.; O’Connell, O.; O’Sullivan, L.; Aherne, S.A.; O’Brien, N.M. Micellarisation of carotenoids from raw and cooked vegetables. Plant Foods Hum. Nutr. 2008, 63, 127–133. [Google Scholar] [CrossRef]
- Rich, G.T.; Bailey, A.L.; Faulks, R.M.; Parker, M.L.; Wickham, M.S.J.; Fillery-Travis, A. Solubilization of carotenoids from carrot juice and spinach in lipid phases: I. Modeling the gastric lumen. Lipids 2003, 38, 933–945. [Google Scholar] [CrossRef]
| Smoothie A: Carrot Juice-Papaya-Mango | ||||
| Untreated | Intensive Heat Treatment | Mild Heat Treatment | Ultrasound | |
| β-Cryptoxanthin | 0.21 ± 0.01 a | 0.10 ± 0.01 b | 0.15 ± 0.00 ab | 0.18 ± 0.01 a |
| α-Carotene | 1.98 ± 0.16 a | 1.66 ± 0.19 a | 1.88 ± 0.03 a | 1.92 ± 0.13 a |
| β-Carotene | 2.74 ± 0.24 a | 2.24 ± 0.25 a | 2.53 ± 0.05 a | 2.42 ± 0.10 a |
| Smoothie B: Carrot Juice-Pumpkin-Mango | ||||
| Untreated | Intensive Heat Treatment | Mild Heat Treatment | Ultrasound | |
| Lutein | 0.44 ± 0.01 a | 0.15 ± 0.01 b | 0.27 ± 0.01 ab | 0.38 ± 0.01 ab |
| α-Carotene | 1.83 ± 0.09 a | 1.53 ± 0.18 b | 1.80 ± 0.04 a | 1.86 ± 0.08 a |
| β-Carotene | 2.82 ± 0.16 a | 2.09 ± 0.21 a | 2.39 ± 0.05 a | 3.02 ± 0.09 b |
| Smoothie A: Carrot Juice-Papaya-Mango | ||||
| Untreated | Intensive Heat Treatment | Mild Heat Treatment | Ultrasound | |
| L* | 63.02 ± 0.03 a | 60.08 ± 0.13 a | 59.33 ± 0.07 ab | 59.66 ± 0.05 a |
| a* | 34.13 ± 0.11 a | 32.04 ± 0.05 ab | 34.15 ± 0.07 a | 33.60 ± 0.41 a |
| b* | 47.02 ± 0.02 a | 34.11 ± 0.15 b | 47.14 ± 0.05 a | 44.50 ± 0.28 ab |
| Smoothie B: Carrot Juice-Pumpkin-Mango | ||||
| Untreated | Intensive Heat Treatment | Mild Heat Treatment | Ultrasound | |
| L* | 63.02 ± 0.03 a | 59.08 ± 0.13 a | 59.33 ± 0.07 a | 64.66 ± 0.05 ab |
| a* | 32.14 ± 0.14 a | 31.81 ± 0.18 a | 32.17 ± 0.18 a | 31.22 ± 0.29 a |
| b* | 54.51 ± 0.44 a | 50.10 ± 0.09 b | 51.78 ± 0.01 ab | 55.20 ± 0.12 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buniowska, M.; Arrigoni, E.; Znamirowska, A.; Blesa, J.; Frígola, A.; Esteve, M.J. Liberation and Micellarization of Carotenoids from Different Smoothies after Thermal and Ultrasound Treatments. Foods 2019, 8, 492. https://doi.org/10.3390/foods8100492
Buniowska M, Arrigoni E, Znamirowska A, Blesa J, Frígola A, Esteve MJ. Liberation and Micellarization of Carotenoids from Different Smoothies after Thermal and Ultrasound Treatments. Foods. 2019; 8(10):492. https://doi.org/10.3390/foods8100492
Chicago/Turabian StyleBuniowska, Magdalena, Eva Arrigoni, Agata Znamirowska, Jesús Blesa, Ana Frígola, and María J. Esteve. 2019. "Liberation and Micellarization of Carotenoids from Different Smoothies after Thermal and Ultrasound Treatments" Foods 8, no. 10: 492. https://doi.org/10.3390/foods8100492
APA StyleBuniowska, M., Arrigoni, E., Znamirowska, A., Blesa, J., Frígola, A., & Esteve, M. J. (2019). Liberation and Micellarization of Carotenoids from Different Smoothies after Thermal and Ultrasound Treatments. Foods, 8(10), 492. https://doi.org/10.3390/foods8100492









