Bioactive Candy: Effects of Licorice on the Cardiovascular System
Abstract
1. The Sweet “Father of Herbal Medicine”
2. Pharmacological Effects of Licorice
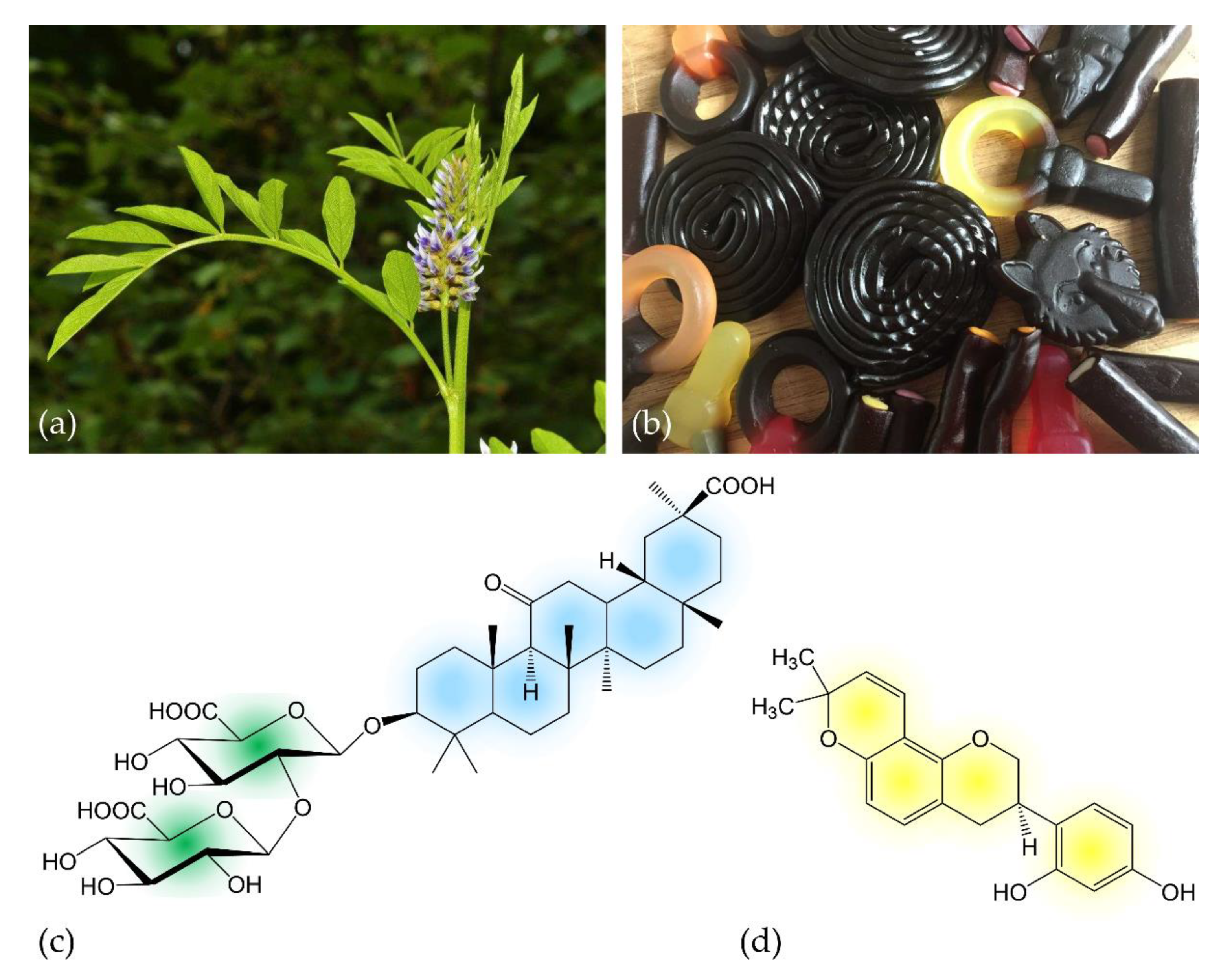
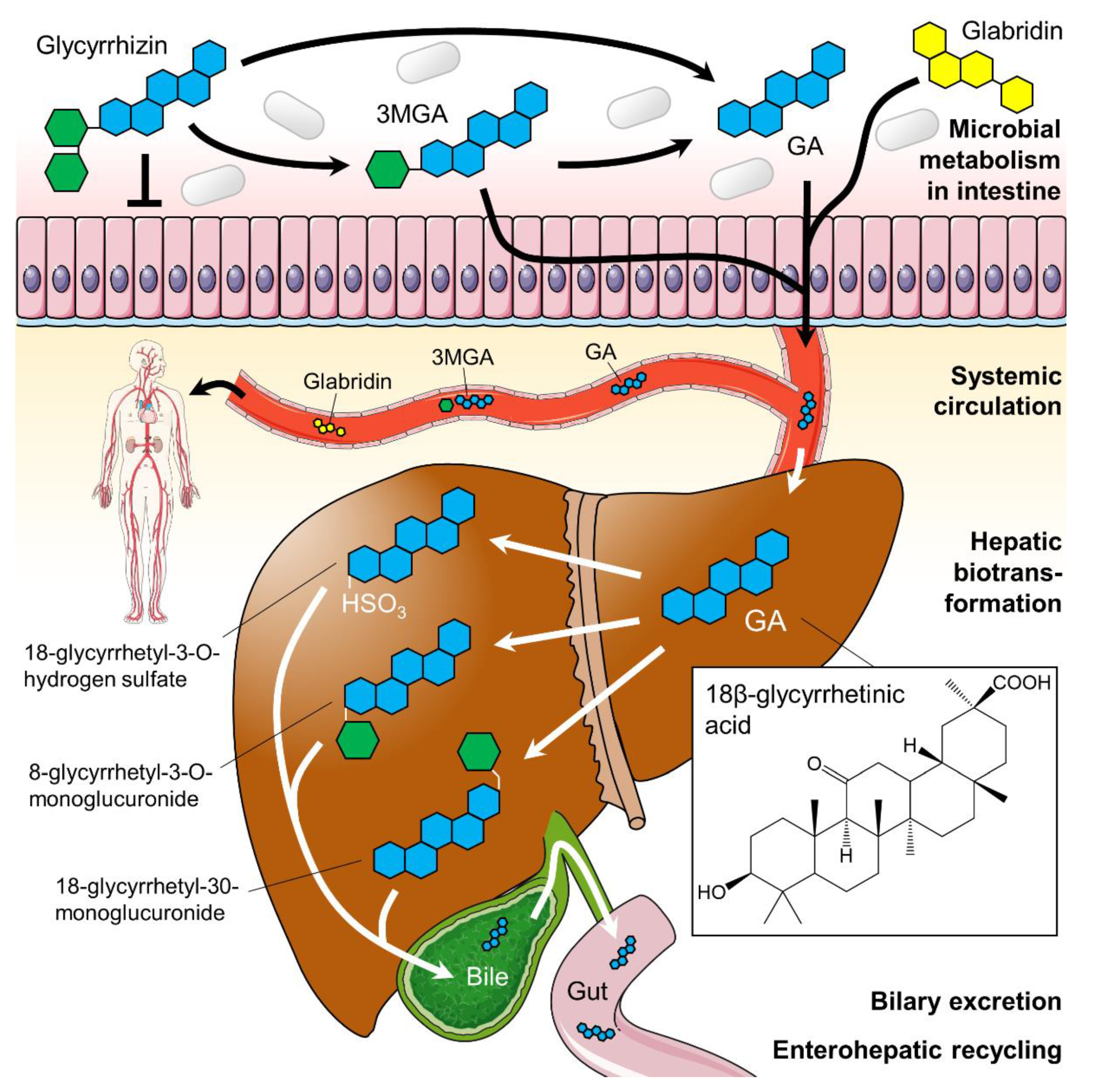
2.1. Licorice Digestion and Chemistry of Metabolites
2.2. Pharmacodynamics of Licorice Constituents and Metabolites
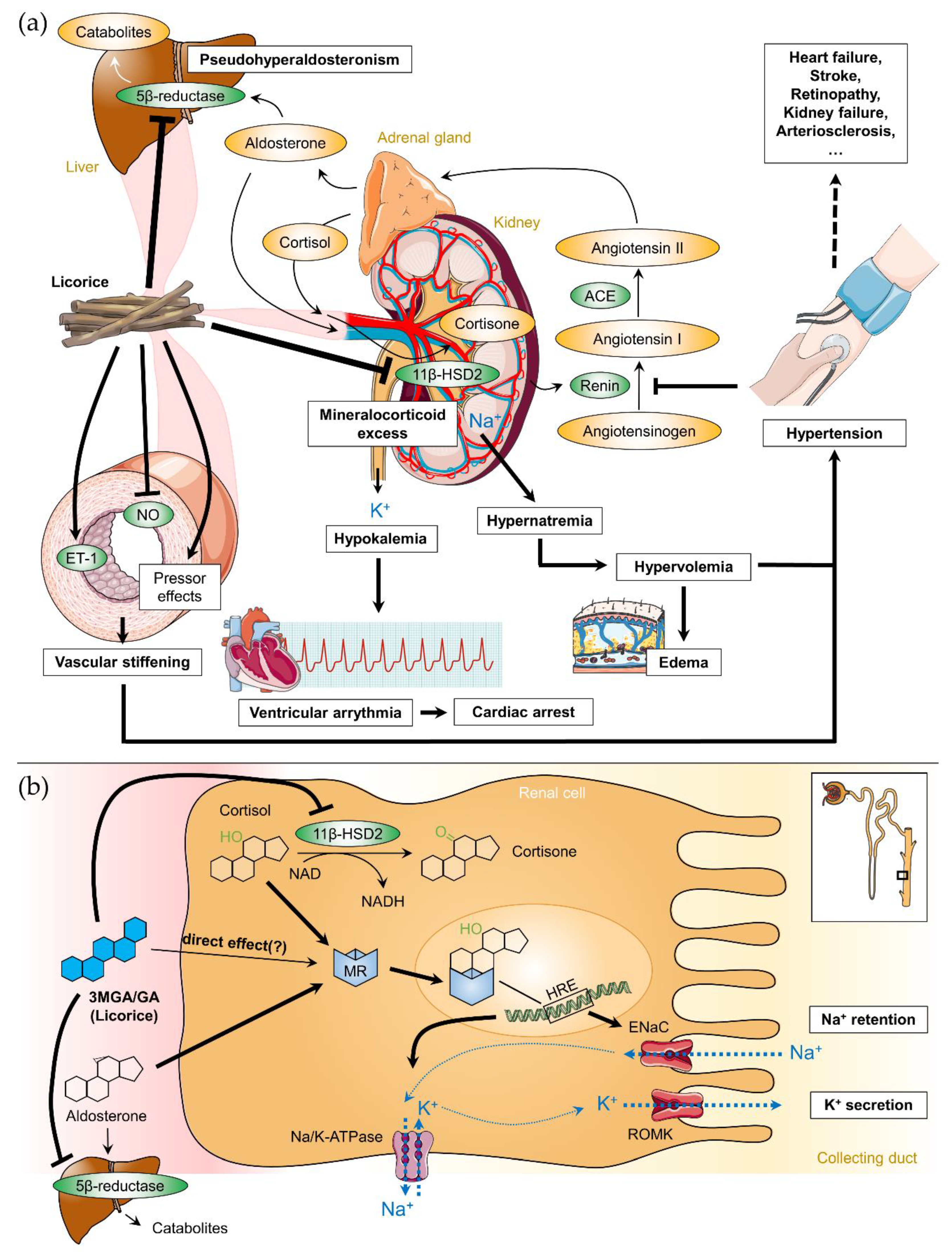
2.3. Licorice-Induced Hypertension
2.3.1. Meta-analyses of Human Trials
2.3.2. Treatment
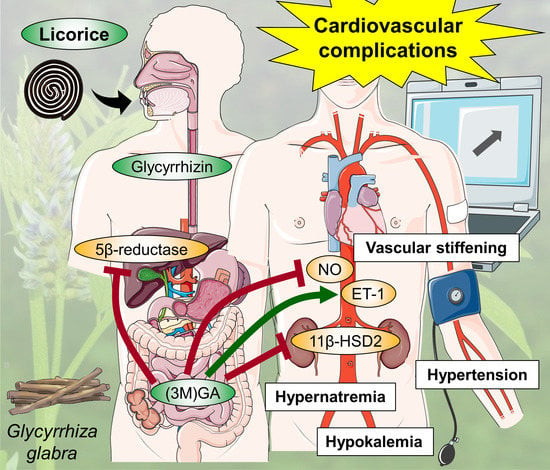
2.4. Cardiovascular Effects of Licorice
2.5. Interaction of Licorice with Prescribed Drugs
2.6. Contraindications and Effects of Licorice Overconsumption
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 11β-HSD2 | 11-β-hydrogenase type II enzyme |
| 3MGA | 3β-monoglucuronyl-18β-glycyrrhetinic acid |
| ACE | Angiotensin converting enzyme |
| ANP | Atrial natriuretic peptide |
| ATP | Adenosine triphosphate |
| BP | Blood pressure |
| CI | Confidence interval |
| CVD | Cardiovascular disease |
| CYP3A4 | Cytochrome P450 3A4 |
| DBP | Diastolic blood pressure |
| ENaC | Epithelial sodium channel |
| ESC | European Society of Cardiology |
| ET-1 | Endothelin 1 |
| GA | 18β-glycyrrhetinic acid |
| HRE | Hormone response element |
| MR | Mineralocorticoid receptor |
| NAD(H) | Nicotinamide adenine dinucleotide |
| NO | Nitric oxide |
| ROMK | Renal outer medullary potassium channel |
| SBP | Systolic blood pressure |
| VSMC | Vascular smooth muscle cell |
References
- Foster, C.A.; Church, K.S.; Poddar, M.; Van Uum, S.H.; Spaic, T. Licorice-induced hypertension: A case of pseudohyperaldosteronism due to jelly bean ingestion. Postgrad. Med. 2017, 129, 3293–3331. [Google Scholar] [CrossRef] [PubMed]
- Fenwick, G.R.; Lutomski, J.; Nieman, C. Liquorice, Glycyrrhiza glabra L.—Composition, uses and analysis. Food Chem. 1990, 38, 1191–1243. [Google Scholar] [CrossRef]
- Kao, T.C.; Wu, C.H.; Yen, G.C. Bioactivity and potential health benefits of licorice. J. Agric. Food Chem. 2014, 62, 542–553. [Google Scholar] [CrossRef] [PubMed]
- Fiore, C.; Eisenhut, M.; Ragazzi, E.; Zanchin, G.; Armanini, D. A history of the therapeutic use of liquorice in europe. J. Ethnopharmacol. 2005, 99, 317–324. [Google Scholar] [CrossRef]
- Allcock, E.; Cowdery, J. Hypertension induced by liquorice tea. BMJ Case Rep. 2015. [Google Scholar] [CrossRef]
- Isbrucker, R.A.; Burdock, G.A. Risk and safety assessment on the consumption of licorice root (Glycyrrhiza sp.), its extract and powder as a food ingredient, with emphasis on the pharmacology and toxicology of glycyrrhizin. Regul. Toxicol. Pharmacol. 2006, 46, 167–192. [Google Scholar] [CrossRef]
- NCCIH. Licorice Root. Available online: http://nccih.nih.gov/health/licoriceroot (accessed on 24 September 2019).
- Sabbadin, C.; Bordin, L.; Donà, G.; Manso, J.; Avruscio, G.; Armanini, D. Licorice: From pseudohyperaldosteronism to therapeutic uses. Front. Endocrinol. 2019. [Google Scholar] [CrossRef]
- Volqvartz, T.; Vestergaard, A.L.; Aagaard, S.K.; Andreasen, M.F.; Lesnikova, I.; Uldbjerg, N.; Larsen, A.; Bor, P. Use of alternative medicine, ginger and licorice among Danish pregnant women—A prospective cohort study. BMC Complement. Altern. Med. 2019, 19, 5. [Google Scholar] [CrossRef]
- Yang, R.; Wang, L.Q.; Yuan, B.C.; Liu, Y. The pharmacological activities of licorice. Planta Med. 2015, 81, 1654–1669. [Google Scholar] [CrossRef]
- Aly, A.M.; Al-Alousi, L.; Salem, H.A. Licorice: A possible anti-inflammatory and anti-ulcer drug. AAPS PharmSciTech 2005, 6, E74–E82. [Google Scholar] [CrossRef]
- Jalilzadeh-Amin, G.; Najarnezhad, V.; Anassori, E.; Mostafavi, M.; Keshipour, H. Antiulcer properties of Glycyrrhiza glabra L. Extract on experimental models of gastric ulcer in mice. Iranian J. Pharm. Res. 2015, 14, 1163–1170. [Google Scholar]
- Yang, R.; Yuan, B.C.; Ma, Y.S.; Zhou, S.; Liu, Y. The anti-inflammatory activity of licorice, a widely used chinese herb. Pharm. Biol. 2017, 55, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, R.; Yuan, B.; Liu, Y.; Liu, C. The antiviral and antimicrobial activities of licorice, a widely-used Chinese herb. Acta Pharm. Sin. B 2015, 5, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Fukuchi, K.; Okudaira, N.; Adachi, K.; Odai-Ide, R.; Watanabe, S.; Ohno, H.; Yamamoto, M.; Kanamoto, T.; Terakubo, S.; Nakashima, H.; et al. Antiviral and antitumor activity of licorice root extracts. In Vivo 2016, 30, 777–785. [Google Scholar] [CrossRef]
- Huo, H.Z.; Wang, B.; Liang, Y.K.; Bao, Y.Y.; Gu, Y. Hepatoprotective and antioxidant effects of licorice extract against CCl4-induced oxidative damage in rats. Int. J. Mol. Sci. 2011, 12, 6529–6543. [Google Scholar] [CrossRef]
- Jung, J.-C.; Lee, Y.-H.; Kim, S.H.; Kim, K.-J.; Kim, K.-M.; Oh, S.; Jung, Y.-S. Hepatoprotective effect of licorice, the root of Glycyrrhiza uralensis Fischer, in alcohol-induced fatty liver disease. BMC Complement. Altern. Med. 2016, 16, 19. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Nixon, D.W. Licorice and cancer. Nutr. Cancer 2001, 39, 1–11. [Google Scholar] [CrossRef]
- Rahnama, M.; Mehrabani, D.; Japoni, S.; Edjtehadi, M.; Saberi Firoozi, M. The healing effect of licorice (Glycyrrhiza glabra) on Helicobacter pylori infected peptic ulcers. J. Res. Med. Sci. 2013, 18, 532–533. [Google Scholar]
- Momeni, A.; Rahimian, G.; Kiasi, A.; Amiri, M.; Kheiri, S. Effect of licorice versus bismuth on eradication of Helicobacter pylori in patients with peptic ulcer disease. Pharmacogn. Res. 2014, 6, 341–344. [Google Scholar] [CrossRef]
- Wu, F.; Jin, Z.; Jin, J. Hypoglycemic effects of glabridin, a polyphenolic flavonoid from licorice, in an animal model of diabetes mellitus. Mol. Med. Rep. 2013, 7, 1278–1282. [Google Scholar] [CrossRef]
- Simmler, C.; Pauli, G.F.; Chen, S.N. Phytochemistry and biological properties of glabridin. Fitoterapia 2013, 90, 160–184. [Google Scholar] [CrossRef] [PubMed]
- Stepien, M.; Kujawska-Luczak, M.; Szulinska, M.; Kregielska-Narozna, M.; Skrypnik, D.; Suliburska, J.; Skrypnik, K.; Regula, J.; Bogdanski, P. Beneficial dose-independent influence of Camellia sinensis supplementation on lipid profile, glycemia, and insulin resistance in an NaCl-induced hypertensive rat model. J. Physiol. Pharmacol. 2018. [Google Scholar] [CrossRef]
- Sontia, B.; Mooney, J.; Gaudet, L.; Touyz, R.M. Pseudohyperaldosteronism, liquorice, and hypertension. J. Clin. Hypertens. 2008, 10, 153–157. [Google Scholar] [CrossRef]
- Varma, R.; Ross, C.N. Liquorice: A root cause of secondary hypertension. JRSM Open 2017, 8, 2054270416685208. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.J. Liquorice: New insights into mineralocorticoid and glucocorticoid hypertension. R. I. Med. 1993, 76, 251–254. [Google Scholar]
- Sigurjónsdóttir, H.Á.; Franzson, L.; Manhem, K.; Ragnarsson, J.; Sigurdsson, G.; Wallerstedt, S. Liquorice-induced rise in blood pressure: A linear dose-response relationship. J. Hum. Hypertens. 2001, 15, 549–552. [Google Scholar] [CrossRef]
- Leskinen, M.H.; Hautaniemi, E.J.; Tahvanainen, A.M.; Koskela, J.K.; Päällysaho, M.; Tikkakoski, A.J.; Kähönen, M.; Kööbi, T.; Niemelä, O.; Mustonen, J.; et al. Daily liquorice consumption for two weeks increases augmentation index and central systolic and diastolic blood pressure. PLoS ONE 2014, 9, e105607. [Google Scholar] [CrossRef]
- Falet, J.P.; Elkrief, A.; Green, L. Hypertensive emergency induced by licorice tea. CMAJ 2019, 191, E581–E583. [Google Scholar] [CrossRef]
- Omar, H.R. The cardiovascular complications of licorice. Cardiovasc. Endocrinol. 2013, 2, 46–49. [Google Scholar] [CrossRef]
- Forouzanfar, M.H.; Liu, P.; Roth, G.A.; Ng, M.; Biryukov, S.; Marczak, L.; Alexander, L.; Estep, K.; Hassen Abate, K.; Akinyemiju, T.F.; et al. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm hg, 1990–2015. JAMA 2017, 317, 165–182. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 esc/esh guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- Charles, L.; Triscott, J.; Dobbs, B. Secondary hypertension: Discovering the underlying cause. Am. Fam. Phys. 2017, 96, 453–461. [Google Scholar]
- Wang, Q.; Qian, Y.; Wang, Q.; Yang, Y.F.; Ji, S.; Song, W.; Qiao, X.; Guo, D.A.; Liang, H.; Ye, M. Metabolites identification of bioactive licorice compounds in rats. J. Pharm. Biomed. Anal. 2015, 115, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Nieman, C. Licorice. Adv. Food Res. 1957, 7, 339–381. [Google Scholar]
- Kim, D.-H.; Lee, S.-W.; Han, M.J. Biotransformation of glycyrrhizin to 18β-glycyrrhetinic acid-3-o-β-d-glucuronide by streptococcus lj-22, a human intestinal bacterium. Biol. Pharm. Bull. 1999, 22, 320–322. [Google Scholar] [CrossRef]
- Hattori, M.; Sakamoto, T.; Yamagishi, T.; Sakamoto, K.; Konishi, K.; Kobashi, K.; Namba, T. Metabolism of glycyrrhizin by human intestinal flora. Ii. Isolation and characterization of human intestinal bacteria capable of metabolizing glycyrrhizin and related compounds. Chem. Pharm Bull. 1985, 33, 210–217. [Google Scholar] [CrossRef]
- Armanini, D.; Nacamulli, D.; Francini-Pesenti, F.; Battagin, G.; Ragazzi, E.; Fiore, C. Glycyrrhetinic acid, the active principle of licorice, can reduce the thickness of subcutaneous thigh fat through topical application. Steroids 2005, 70, 538–542. [Google Scholar] [CrossRef]
- Feng, X.; Ding, L.; Qiu, F. Potential drug interactions associated with glycyrrhizin and glycyrrhetinic acid. Drug Metab. Rev. 2015, 47, 229–238. [Google Scholar] [CrossRef]
- Omar, H.R.; Komarova, I.; El-Ghonemi, M.; Fathy, A.; Rashad, R.; Abdelmalak, H.D.; Yerramadha, M.R.; Ali, Y.; Helal, E.; Camporesi, E.M. Licorice abuse: Time to send a warning message. Ther. Adv. Endocrinol. 2012, 3, 125–138. [Google Scholar] [CrossRef]
- Ploeger, B.; Mensinga, T.; Sips, A.; Meulenbelt, J.; DeJongh, J. A human physiologically-based model for glycyrrhzic acid, a compound subject to presystemic metabolism and enterohepatic cycling. Pharm. Res. 2000, 17, 1516–1525. [Google Scholar] [CrossRef]
- Cao, J.; Chen, X.; Liang, J.; Yu, X.Q.; Xu, A.L.; Chan, E.; Wei, D.; Huang, M.; Wen, J.Y.; Yu, X.Y.; et al. Role of p-glycoprotein in the intestinal absorption of glabridin, an active flavonoid from the root of glycyrrhiza glabra. Drug Metab. Dispos. 2007, 35, 539–553. [Google Scholar] [CrossRef]
- Ito, C.; Oi, N.; Hashimoto, T.; Nakabayashi, H.; Aoki, F.; Tominaga, Y.; Yokota, S.; Hosoe, K.; Kanazawa, K. Absorption of dietary licorice isoflavan glabridin to blood circulation in rats. J. Nutr. Sci. Vitaminol. 2007, 53, 358–365. [Google Scholar] [CrossRef][Green Version]
- Aoki, F.; Nakagawa, K.; Tanaka, A.; Matsuzaki, K.; Arai, N.; Mae, T. Determination of glabridin in human plasma by solid-phase extraction and lc-ms/ms. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2005, 828, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Raggi, M.A.; Maffei, F.; Bugamelli, F.; Cantelli Forti, G. Bioavailability of glycyrrhizin and licorice extract in rat and human plasma as detected by a hplc method. Pharmazie 1994, 49, 269–272. [Google Scholar]
- Cantelli-Forti, G.; Maffei, F.; Hrelia, P.; Bugamelli, F.; Bernardi, M.; D’Intino, P.; Maranesi, M.; Raggi, M.A. Interaction of licorice on glycyrrhizin pharmacokinetics. Environ. Health Perspect. 1994, 102 (Suppl. 9), 65–68. [Google Scholar] [CrossRef]
- Ishiuchi, K.; Morinaga, O.; Ohkita, T.; Tian, C.; Hirasawa, A.; Mitamura, M.; Maki, Y.; Kondo, T.; Yasujima, T.; Yuasa, H.; et al. 18beta-glycyrrhetyl-3-o-sulfate would be a causative agent of licorice-induced pseudoaldosteronism. Sci. Rep. 2019, 9, 1587. [Google Scholar] [CrossRef]
- Morinaga, O.; Ishiuchi, K.; Ohkita, T.; Tian, C.; Hirasawa, A.; Mitamura, M.; Maki, Y.; Yasujima, T.; Yuasa, H.; Makino, T. Isolation of a novel glycyrrhizin metabolite as a causal candidate compound for pseudoaldosteronism. Sci. Rep. 2018, 8, 15568. [Google Scholar] [CrossRef]
- Armanini, D.; Karbowiak, I.; Funder, J.W. Affinity of liquorice derivatives for mineralocorticoid and glucocorticoid receptors. Clin. Endocrinol. 1983, 19, 609–612. [Google Scholar] [CrossRef] [PubMed]
- Calo, L.A.; Zaghetto, F.; Pagnin, E.; Davis, P.A.; De Mozzi, P.; Sartorato, P.; Martire, G.; Fiore, C.; Armanini, D. Effect of aldosterone and glycyrrhetinic acid on the protein expression of pai-1 and p22(phox) in human mononuclear leukocytes. J. Clin. Endocrinol. Metab. 2004, 89, 1973–1976. [Google Scholar] [CrossRef]
- Størmer, F.C.; Reistad, R.; Alexander, J. Glycyrrhizic acid in liquorice—Evaluation of health hazard. Food Chem. Toxicol. 1993, 31, 303–312. [Google Scholar] [CrossRef]
- Stewart, P.M.; Wallace, A.M.; Valentino, R.; Burt, D.; Shackleton, C.H.; Edwards, C.R. Mineralocorticoid activity of liquorice: 11-beta-hydroxysteroid dehydrogenase deficiency comes of age. Lancet 1987, 2, 821–824. [Google Scholar] [CrossRef]
- Ferrari, P. The role of 11beta-hydroxysteroid dehydrogenase type 2 in human hypertension. Biochim. Biophys. Acta 2010, 1802, 1178–1187. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Ignatova, I.D.; Nashev, L.G.; Dick, B.; Ferrari, P.; Frey, F.J.; Odermatt, A. Impaired protein stability of 11beta-hydroxysteroid dehydrogenase type 2: A novel mechanism of apparent mineralocorticoid excess. J. Am. Soc. Nephrol. 2007, 18, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Christy, C.; Hadoke, P.W.; Paterson, J.M.; Mullins, J.J.; Seckl, J.R.; Walker, B.R. 11beta-hydroxysteroid dehydrogenase type 2 in mouse aorta: Localization and influence on response to glucocorticoids. Hypertension 2003, 42, 580–587. [Google Scholar] [CrossRef]
- Lombes, M.; Alfaidy, N.; Eugene, E.; Lessana, A.; Farman, N.; Bonvalet, J.P. Prerequisite for cardiac aldosterone action. Mineralocorticoid receptor and 11 beta-hydroxysteroid dehydrogenase in the human heart. Circulation 1995, 92, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Hadoke, P.W.; Christy, C.; Kotelevtsev, Y.V.; Williams, B.C.; Kenyon, C.J.; Seckl, J.R.; Mullins, J.J.; Walker, B.R. Endothelial cell dysfunction in mice after transgenic knockout of type 2, but not type 1, 11beta-hydroxysteroid dehydrogenase. Circulation 2001, 104, 2832–2837. [Google Scholar] [CrossRef]
- Van Uum, S.H. Liquorice and hypertension. Neth. J. Med. 2005, 63, 119–120. [Google Scholar]
- Monder, C.; Stewart, P.M.; Lakshmi, V.; Valentino, R.; Burt, D.; Edwards, C.R. Licorice inhibits corticosteroid 11 beta-dehydrogenase of rat kidney and liver: In vivo and in vitro studies. Endocrinology 1989, 125, 1046–1053. [Google Scholar] [CrossRef]
- Tanahashi, T.; Mune, T.; Morita, H.; Tanahashi, H.; Isomura, Y.; Suwa, T.; Daido, H.; Gomez-Sanchez, C.E.; Yasuda, K. Glycyrrhizic acid suppresses type 2 11 beta-hydroxysteroid dehydrogenase expression in vivo. J. Steroid Biochem. Mol. Biol. 2002, 80, 441–447. [Google Scholar] [CrossRef]
- Kato, H.; Kanaoka, M.; Yano, S.; Kobayashi, M. 3-monoglucuronyl-glycyrrhetinic acid is a major metabolite that causes licorice-induced pseudoaldosteronism. J. Clin. Endocrinol. Metab. 1995, 80, 1929–1933. [Google Scholar] [CrossRef]
- Hammer, F.; Stewart, P.M. Cortisol metabolism in hypertension. Best Pract. Res. Clin. Endocrinol. Metab. 2006, 20, 337–353. [Google Scholar] [CrossRef] [PubMed]
- Souness, G.W.; Brem, A.S.; Morris, D.J. 11 beta-hydroxysteroid dehydrogenase antisense affects vascular contractile response and glucocorticoid metabolism. Steroids 2002, 67, 195–201. [Google Scholar] [CrossRef]
- Latif, S.A.; Conca, T.J.; Morris, D.J. The effects of the licorice derivative, glycyrrhetinic acid, on hepatic 3α- and 3β-hydroxysteroid dehydrogenases and 5α- and 5β-reductase pathways of metabolism of aldosterone in male rats. Steroids 1990, 55, 52–58. [Google Scholar] [CrossRef]
- Chanda, D.; Prieto-Lloret, J.; Singh, A.; Iqbal, H.; Yadav, P.; Snetkov, V.; Aaronson, P.I. Glabridin-induced vasorelaxation: Evidence for a role of bkca channels and cyclic gmp. Life Sci. 2016, 165, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Farese, R.V., Jr.; Biglieri, E.G.; Shackleton, C.H.; Irony, I.; Gomez-Fontes, R. Licorice-induced hypermineralocorticoidism. N. Engl. J. Med. 1991, 325, 1223–1227. [Google Scholar] [CrossRef]
- Tarjus, A.; Amador, C.; Michea, L.; Jaisser, F. Vascular mineralocorticoid receptor and blood pressure regulation. Curr. Opin. Pharmacol. 2015, 21, 138–144. [Google Scholar] [CrossRef]
- Ohtake, M.; Hattori, T.; Murase, T.; Takahashi, K.; Takatsu, M.; Ohtake, M.; Miyachi, M.; Watanabe, S.; Cheng, X.W.; Murohara, T.; et al. Glucocorticoids activate cardiac mineralocorticoid receptors in adrenalectomized dahl salt-sensitive rats. Nagoya J. Med. Sci. 2014, 76, 59–72. [Google Scholar]
- Quaschning, T.; Ruschitzka, F.; Shaw, S.; Luscher, T.F. Aldosterone receptor antagonism normalizes vascular function in liquorice-induced hypertension. Hypertension 2001, 37, 801–805. [Google Scholar] [CrossRef]
- Schiffrin, E.L.; Deng, L.Y.; Sventek, P.; Day, R. Enhanced expression of endothelin-1 gene in resistance arteries in severe human essential hypertension. J. Hypertens. 1997, 15, 57–63. [Google Scholar] [CrossRef]
- Ergul, S.; Parish, D.C.; Puett, D.; Ergul, A. Racial differences in plasma endothelin-1 concentrations in individuals with essential hypertension. Hypertension 1996, 28, 652–655. [Google Scholar] [CrossRef]
- Gomez-Sanchez, E.P.; Gomez-Sanchez, C.E. Central hypertensinogenic effects of glycyrrhizic acid and carbenoxolone. Am. J. Physiol. 1992, 263, E1125–E1130. [Google Scholar] [CrossRef] [PubMed]
- Hautaniemi, E.J.; Tahvanainen, A.M.; Koskela, J.K.; Tikkakoski, A.J.; Kähönen, M.; Uitto, M.; Sipilä, K.; Niemelä, O.; Mustonen, J.; Pörsti, I.H. Voluntary liquorice ingestion increases blood pressure via increased volume load, elevated peripheral arterial resistance, and decreased aortic compliance. Sci. Rep. 2017, 7, 10947. [Google Scholar] [CrossRef] [PubMed]
- Penninkilampi, R.; Eslick, E.M.; Eslick, G.D. The association between consistent licorice ingestion, hypertension and hypokalaemia: A systematic review and meta-analysis. J. Hum. Hypertens. 2017, 31, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Van Gelderen, C.E.; Bijlsma, J.A.; van Dokkum, W.; Savelkoul, T.J. Glycyrrhizic acid: The assessment of a no effect level. Hum. Exp. Toxicol. 2000, 19, 434–439. [Google Scholar] [CrossRef]
- Basso, A.; Dalla Paola, L.; Erle, G.; Boscaro, M.; Armanini, D. Licorice ameliorates postural hypotension caused by diabetic autonomic neuropathy. Diabetes Care 1994, 17, 1356. [Google Scholar] [CrossRef]
- Van der Zwan, A. Hypertension encephalopathy after liquorice ingestion. Clin. Neurol. Neurosurg. 1993, 95, 35–37. [Google Scholar] [CrossRef]
- Russo, S.; Mastropasqua, M.; Mosetti, M.A.; Persegani, C.; Paggi, A. Low doses of liquorice can induce hypertension encephalopathy. Am. J. Nephrol. 2000, 20, 145–148. [Google Scholar] [CrossRef]
- Bramont, C.; Lestradet, C.; Godart, L.; Faivre, R.; Narboni, G. cerebral vascular accident caused by alcohol-free licorice. Presse Med. 1985, 14, 746. [Google Scholar]
- Chamberlain, J.J.; Abolnik, I.Z. Pulmonary edema following a licorice binge. West J. Med. 1997, 167, 184–185. [Google Scholar]
- Chamberlain, T.J. Licorice poisoning, pseudoaldosteronism, and heart failure. JAMA 1970, 213, 1343. [Google Scholar] [CrossRef]
- Hasegawa, J.; Suyama, Y.; Kinugawa, T.; Morisawa, T.; Kishimoto, Y. Echocardiographic findings of the heart resembling dilated cardiomyopathy during hypokalemic myopathy due to licorice-induced pseudoaldosteronism. Cardiovasc. Drugs Ther. 1998, 12, 599–600. [Google Scholar] [CrossRef] [PubMed]
- Sailler, L.; Juchet, H.; Ollier, S.; Nicodeme, R.; Arlet, P. generalized edema caused by licorice: A new syndrome. Apropos of 3 cases. Rev. Med. Interne 1993, 14, 984. [Google Scholar] [CrossRef]
- Johns, C. Glycyrrhizic acid toxicity caused by consumption of licorice candy cigars. CJEM 2009, 11, 94–96. [Google Scholar] [CrossRef] [PubMed]
- Francini-Pesenti, F.; Puato, M.; Piccoli, A.; Brocadello, F. Liquorice-induced hypokalaemia and water retention in the absence of hypertension. Phytother. Res. 2008, 22, 563–565. [Google Scholar] [CrossRef] [PubMed]
- Negro, A.; Rossi, E.; Regolisti, G.; Perazzoli, F. Liquorice-induced sodium retention. Merely an acquired condition of apparent mineralocorticoid excess? A case report. Ann. Ital. Med. Int. 2000, 15, 296–300. [Google Scholar]
- Luis, A.; Domingues, F.; Pereira, L. Metabolic changes after licorice consumption: A systematic review with meta-analysis and trial sequential analysis of clinical trials. Phytomedicine 2018, 39, 17–24. [Google Scholar] [CrossRef]
- Forslund, T.; Fyhrquist, F.; Froseth, B.; Tikkanen, I. Effects of licorice on plasma atrial natriuretic peptide in healthy volunteers. J. Intern. Med. 1989, 225, 95–99. [Google Scholar] [CrossRef]
- Mattarello, M.J.; Benedini, S.; Fiore, C.; Camozzi, V.; Sartorato, P.; Luisetto, G.; Armanini, D. Effect of licorice on PTH levels in healthy women. Steroids 2006, 71, 403–408. [Google Scholar] [CrossRef]
- Sigurjonsdottir, H.A.; Axelson, M.; Johannsson, G.; Manhem, K.; Nyström, E.; Wallerstedt, S. The liquorice effect on the RAAS differs between the genders. Blood Press 2006, 15, 169–172. [Google Scholar] [CrossRef]
- Ruszymah, B.H.; Nabishah, B.M.; Aminuddin, S.; Khalid, B.A. Effects of glycyrrhizic acid on right atrial pressure and pulmonary vasculature in rats. Clin. Exp. Hypertens. 1995, 17, 575–591. [Google Scholar] [CrossRef]
- Yang, P.-S.; Kim, D.-H.; Lee, Y.J.; Lee, S.-E.; Kang, W.J.; Chang, H.-J.; Shin, J.-S. Glycyrrhizin, inhibitor of high mobility group box-1, attenuates monocrotaline-induced pulmonary hypertension and vascular remodeling in rats. Respir. Res. 2014, 15, 148. [Google Scholar] [CrossRef] [PubMed]
- Ottenbacher, R.; Blehm, J. An unusual case of licorice-induced hypertensive crisis. S. D. Med. 2015, 68, 346–347. [Google Scholar] [PubMed]
- Schulze zur Wiesch, C.; Sauer, N.; Aberle, J. hypertension and hypokalemia—A reninoma as the cause of suspected liquorice-induced arterial hypertension. Dtsch. Med. Wochenschr. 2011, 136, 882–884. [Google Scholar] [CrossRef] [PubMed]
- Epstein, M.T.; Espiner, E.A.; Donald, R.A.; Hughes, H. Liquorice toxicity and the renin-angiotensin-aldosterone axis in man. BMJ 1977, 1, 209–210. [Google Scholar] [CrossRef] [PubMed]
- Sigurjonsdottir, H.A.; Manhem, K.; Axelson, M.; Wallerstedt, S. Subjects with essential hypertension are more sensitive to the inhibition of 11 beta-hsd by liquorice. J. Hum. Hypertens. 2003, 17, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Epstein, M.T.; Espiner, E.A.; Donald, R.A.; Hughes, H. Effect of eating liquorice on the renin-angiotensin aldosterone axis in normal subjects. Br. Med. J. 1977, 1, 488–490. [Google Scholar] [CrossRef][Green Version]
- MacKenzie, M.A.; Hoefnagels, W.H.; Jansen, R.W.; Benraad, T.J.; Kloppenborg, P.W. The influence of glycyrrhetinic acid on plasma cortisol and cortisone in healthy young volunteers. J. Clin. Endocrinol. Metab. 1990, 70, 1637–1643. [Google Scholar] [CrossRef]
- Kageyama, Y.; Suzuki, H.; Saruta, T. Glycyrrhizin induces mineralocorticoid activity through alterations in cortisol metabolism in the human kidney. J. Endocrinol. 1992, 135, 147–152. [Google Scholar] [CrossRef]
- Bernardi, M.; D’Intino, P.E.; Trevisani, F.; Cantelli-Forti, G.; Raggi, M.A.; Turchetto, E.; Gasbarrini, G. Effects of prolonged ingestion of graded doses of licorice by healthy volunteers. Life Sci. 1994, 55, 863–872. [Google Scholar] [CrossRef]
- Armanini, D.; Lewicka, S.; Pratesi, C.; Scali, M.; Zennaro, M.C.; Zovato, S.; Gottardo, C.; Simoncini, M.; Spigariol, A.; Zampollo, V. Further studies on the mechanism of the mineralocorticoid action of licorice in humans. J. Endocrinol. Invest. 1996, 19, 624–629. [Google Scholar] [CrossRef]
- Sobieszczyk, P.; Borlaug, B.A.; Gornik, H.L.; Knauft, W.D.; Beckman, J.A. Glycyrrhetinic acid attenuates vascular smooth muscle vasodilatory function in healthy humans. Clin. Sci. 2010, 119, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.H.; He, Y.J.; Chen, Y.; Fan, L.; Zhang, W.; Tan, Z.R.; Huang, Y.F.; Guo, D.; Hu, D.L.; Wang, D.; et al. Effect of glycyrrhizin on the activity of cyp3a enzyme in humans. Eur. J. Clin. Pharmacol. 2010, 66, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Fang, P.-F.; Li, H.-D.; Xu, P.; Liu, Y.-P.; Wang, F.; Cai, H.-L.; Tan, Q.-Y. Lack of effect of continuous glycyrrhizin administration on the pharmacokinetics of the p-glycoprotein substrate talinolol in healthy volunteers. Eur. J. Clin. Pharmacol. 2013, 69, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Bocker, D.; Breithardt, G. induction of arrhythmia by licorice abuse. Z. Kardiol. 1991, 80, 389–391. [Google Scholar] [PubMed]
- Eriksson, J.W.; Carlberg, B.; Hillorn, V. Life-threatening ventricular tachycardia due to liquorice-induced hypokalaemia. J. Intern. Med. 1999, 245, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Bannister, B.; Ginsburg, R.; Shneerson, J. Cardiac arrest due to liquorice induced hypokalaemia. BMJ 1977, 2, 738–739. [Google Scholar] [CrossRef]
- Crean, A.M.; Abdel-Rahman, S.E.; Greenwood, J.P. A sweet tooth as the root cause of cardiac arrest. Can. J. Cardiol. 2009, 25, e357–e358. [Google Scholar] [CrossRef][Green Version]
- Campana, A.; Manzo, M.; Brigante, M.; Marrazzo, N.; Melchiorre, G. an unusual cause of cardiac arrest. Ital. Heart J. Suppl. 2003, 4, 510–513. [Google Scholar]
- Konik, E.; Kurtz, E.G.; Sam, F.; Sawyer, D. Coronary artery spasm, hypertension, hypokalemia and licorice. J. Clin. Case Rep. 2012, 2, 143. [Google Scholar] [CrossRef]
- Tąpolska, M.; Spałek, M.; Szybowicz, U.; Domin, R.; Owsik, K.; Sochacka, K.; Skrypnik, D.; Bogdański, P.; Owecki, M. Arterial Stiffness Parameters Correlate with Estimated Cardiovascular Risk in Humans: A Clinical Study. Int. J. Environ. Res. Public Health 2019, 16, 2547. [Google Scholar] [CrossRef]
- Banerjee, A.; Giri, R. Chapter 9—Nutraceuticals in gastrointestinal disorders. In Nutraceuticals; Gupta, R.C., Ed.; Academic Press: Boston, MA, USA, 2016; pp. 109–122. [Google Scholar]
- Ojha, S.K.; Sharma, C.; Golechha, M.J.; Bhatia, J.; Kumari, S.; Arya, D.S. Licorice treatment prevents oxidative stress, restores cardiac function, and salvages myocardium in rat model of myocardial injury. Toxicol. Ind. Health 2015, 31, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Ojha, S.; Golechha, M.; Kumari, S.; Bhatia, J.; Arya, D.S. Glycyrrhiza glabra protects from myocardial ischemia–reperfusion injury by improving hemodynamic, biochemical, histopathological and ventricular function. Exp. Toxicol. Pathol. 2013, 65, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Haleagrahara, N.; Varkkey, J.; Chakravarthi, S. Cardioprotective effects of glycyrrhizic acid against isoproterenol-induced myocardial ischemia in rats. Int. J. Mol. Sci. 2011, 12, 7100–7113. [Google Scholar] [CrossRef] [PubMed]
- Fuhrman, B.; Buch, S.; Vaya, J.; Belinky, P.A.; Coleman, R.; Hayek, T.; Aviram, M. Licorice extract and its major polyphenol glabridin protect low-density lipoprotein against lipid peroxidation: In vitro and ex vivo studies in humans and in atherosclerotic apolipoprotein e-deficient mice. Am. J. Clin. Nutr. 1997, 66, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Rosenblat, M.; Belinky, P.; Vaya, J.; Levy, R.; Hayek, T.; Coleman, R.; Merchav, S.; Aviram, M. Macrophage enrichment with the isoflavan glabridin inhibits nadph oxidase-induced cell-mediated oxidation of low density lipoprotein. A possible role for protein kinase C. J. Biol. Chem. 1999, 274, 13790–13799. [Google Scholar] [CrossRef]
- Somjen, D.; Knoll, E.; Vaya, J.; Stern, N.; Tamir, S. Estrogen-like activity of licorice root constituents: Glabridin and glabrene, in vascular tissues in vitro and in vivo. J. Steroid Biochem. Mol. Biol. 2004, 91, 147–155. [Google Scholar] [CrossRef]
- Somjen, D.; Kohen, F.; Jaffe, A.; Amir-Zaltsman, Y.; Knoll, E.; Stern, N. Effects of gonadal steroids and their antagonists on DNA synthesis in human vascular cells. Hypertension 1998, 32, 39–45. [Google Scholar] [CrossRef]
- Huang, K.; Liu, Y.; Tang, H.; Qiu, M.; Li, C.; Duan, C.; Wang, C.; Yang, J.; Zhou, X. Glabridin prevents doxorubicin-induced cardiotoxicity through gut microbiota modulation and colonic macrophage polarization in mice. Front. Pharmacol. 2019. [Google Scholar] [CrossRef]
- Meyer, R. Pseudohyperaldosteronismus: Lakritzverzehr mit Folgen. Dtsch. Arztebl. Int. 2000, 97, A-596. [Google Scholar]
- Wang, X.; Zhang, H.; Chen, L.; Shan, L.; Fan, G.; Gao, X. Liquorice, a unique “guide drug” of traditional chinese medicine: A review of its role in drug interactions. J. Ethnopharmacol. 2013, 150, 781–790. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Aburatani, M.; Yoshida, T.; Yamashita, Y.; El-Beih, A.A.; Ohta, T. Cyp3a4 inhibitors isolated from licorice. Biol. Pharm Bull. 2005, 28, 2000–2002. [Google Scholar] [CrossRef] [PubMed]
- Kent, U.M.; Aviram, M.; Rosenblat, M.; Hollenberg, P.F. The licorice root derived isoflavan glabridin inhibits the activities of human cytochrome p450s 3a4, 2b6, and 2c9. Drug Metab. Dispos. 2002, 30, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.L.; Wang, G.H.; Chen, S.H.; Hu, L.; Zhang, X.; Ying, G.; Qin, C.Z.; Zhou, H.H. In vitro and in vivo inhibitory effects of glycyrrhetinic acid in mice and human cytochrome p450 3a4. Int. J. Environ. Res. Public Health 2015, 13, 84. [Google Scholar] [CrossRef] [PubMed]
- Paolini, M.; Barillari, J.; Broccoli, M.; Pozzetti, L.; Perocco, P.; Cantelli-Forti, G. Effect of liquorice and glycyrrhizin on rat liver carcinogen metabolizing enzymes. Cancer Lett. 1999, 145, 35–42. [Google Scholar] [CrossRef]
- Paolini, M.; Pozzetti, L.; Sapone, A.; Cantelli-Forti, G. Effect of licorice and glycyrrhizin on murine liver cyp-dependent monooxygenases. Life Sci. 1998, 62, 571–582. [Google Scholar] [CrossRef]
- Hou, Y.C.; Lin, S.P.; Chao, P.D. Liquorice reduced cyclosporine bioavailability by activating p-glycoprotein and cyp 3a. Food Chem. 2012, 135, 2307–2312. [Google Scholar] [CrossRef]
- Heck, A.M.; DeWitt, B.A.; Lukes, A.L. Potential interactions between alternative therapies and warfarin. Am. J. Health Syst. Pharm. 2000, 57, 1221–1227, quiz 1228–1230. [Google Scholar] [CrossRef]
- Matsumoto, T.; Kaifuchi, N.; Mizuhara, Y.; Warabi, E.; Watanabe, J. Use of a caco-2 permeability assay to evaluate the effects of several kampo medicines on the drug transporter p-glycoprotein. J. Nat. Med. 2018, 72, 897–904. [Google Scholar] [CrossRef]
- Kerstens, M.N.; Dullaart, R.P. 11 beta-hydroxysteroid-dehydrogenase: Characteristics and the clinical significance of a key enzyme in cortisol metabolism. Ned. Tijdschr. Geneeskd. 1999, 143, 509–514. [Google Scholar]
- Buhl, L.F.; Pedersen, F.N.; Andersen, M.S.; Glintborg, D. Licorice-induced apparent mineralocorticoid excess compounded by excessive use of terbutaline and high water intake. BMJ Case Rep. 2018. [Google Scholar] [CrossRef]
- Harada, T.; Ohtaki, E.; Misu, K.; Sumiyoshi, T.; Hosoda, S. Congestive heart failure caused by digitalis toxicity in an elderly man taking a licorice-containing chinese herbal laxative. Cardiology 2002, 98, 218. [Google Scholar] [CrossRef] [PubMed]
- Scientific Committee on Food. Opinion of the Scientific Committee on Food on Glycyrrhizinic acid and Its Ammonium Salt; Scientific Committee on Food: Brussels, Belgium, 2003. [Google Scholar]
- Shimada, S.; Arai, T.; Tamaoka, A.; Homma, M. Liquorice-induced hypokalaemia in patients treated with yokukansan preparations: Identification of the risk factors in a retrospective cohort study. BMJ Open 2017, 7, e014218. [Google Scholar] [CrossRef] [PubMed]
- Nazari, S.; Rameshrad, M.; Hosseinzadeh, H. Toxicological effects of Glycyrrhiza glabra (licorice): A review. Phytother. Res. 2017, 31, 1635–1650. [Google Scholar] [CrossRef]
- Steinberg, D.; Sgan-Cohen, H.D.; Stabholz, A.; Pizanty, S.; Segal, R.; Sela, M.N. The anticariogenic activity of glycyrrhizin: Preliminary clinical trials. Isr. J. Dent. Sci. 1989, 2, 153–157. [Google Scholar] [PubMed]
- Segal, R.; Pisanty, S.; Wormser, R.; Azaz, E.; Sela, M.N. Anticariogenic activity of licorice and glycyrrhizine i: Inhibition of in vitro plaque formation by streptococcus mutans. J. Pharm. Sci. 1985, 74, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Li, Y.; Lei, Z.; Hao, Y.; Wu, Y.; Zhao, Q.; Wang, H.; Ma, L.; Liu, J.; Zhao, C.; et al. Relaxative effect of core licorice aqueous extract on mouse isolated uterine horns. Pharm. Biol. 2013, 51, 744–748. [Google Scholar] [CrossRef]
- Yang, L.; Chai, C.Z.; Yan, Y.; Duan, Y.D.; Henz, A.; Zhang, B.L.; Backlund, A.; Yu, B.Y. Spasmolytic mechanism of aqueous licorice extract on oxytocin-induced uterine contraction through inhibiting the phosphorylation of heat shock protein 27. Molecules 2017, 22, 1392. [Google Scholar] [CrossRef]
- Peskar, B.M. Effect of carbenoxolone on prostaglandin synthesizing and metabolizing enzymes and correlation with gastric mucosal carbenoxolone concentrations. Scand. J. Gastroenterol. Suppl. 1980, 65, 109–114. [Google Scholar]
- Wang, L.J.; Geng, C.A.; Ma, Y.B.; Huang, X.Y.; Luo, J.; Chen, H.; Zhang, X.M.; Chen, J.J. Synthesis, biological evaluation and structure-activity relationships of glycyrrhetinic acid derivatives as novel anti-hepatitis b virus agents. Bioorg. Med. Chem. Lett. 2012, 22, 3473–3479. [Google Scholar] [CrossRef]
- Hibasami, H.; Iwase, H.; Yoshioka, K.; Takahashi, H. Glycyrrhizin induces apoptosis in human stomach cancer kato iii and human promyelotic leukemia hl-60 cells. Int. J. Mol. Med. 2005, 16, 233–236. [Google Scholar] [CrossRef]
- Asl, M.N.; Hosseinzadeh, H. Review of pharmacological effects of Glycyrrhiza sp. And its bioactive compounds. Phytother. Res. 2008, 22, 709–724. [Google Scholar] [CrossRef] [PubMed]
| Author (Year), Country | Study Design | n | Drug | Daily Dose | Duration | Relevant Results |
|---|---|---|---|---|---|---|
| Epstein et al. (1977) [97], New Zealand | Pre-post intervention | 14 | Licorice | 100–200 g | 1–4 weeks | Serious metabolic effects due to modest licorice intake. |
| Forslund et al. (1989) [88], Finland | Pre-post intervention | 15 | Licorice | 100 g | 8 weeks | Increase in plasma ANP; Decrease in antidiuretic hormone, aldosterone, and plasma renin activity. |
| MacKenzie et al. (1990) [98], The Netherlands | Pre-post intervention | 10 | GA | 500 mg | 8 days | Inhibition of 11β-HSD2. |
| Kageyama et al. (1992) [99], Japan | Pre-post intervention | 58 | Glycyrrhizin | 225 mg | 7 days | Changes in cortisol metabolism. |
| Bernadini (1994) [100], Italy | Pre-post intervention | Licorice root extract | 108-814 mg glycyrrhizin | 14 days | Depression of plasma renin activity favored by subclinical disease. | |
| Armanini et al. (1996) [101], Italy | Pre-post intervention | 6 | Licorice concentrate | 7 g (500 mg GA) | 7 days | Decreased activity of 11β-HSD2. |
| van Gelderen et al. (2000) [75], USA | Double-blind randomized controlled | 39 | GA | 0–4 mg per kg | 8 weeks | No-effect level: 2 mg/kg GA per day. |
| Sigurjónsdóttir et al. (2001) [27], Iceland/Sweden | Pre-post intervention | 24 | Licorice | 50–200 g | 2–4 weeks | Increase in SBP. |
| Sigurjónsdóttir et al. (2003) [96], Sweden | Pre-post intervention | 25 | Licorice | 100 g | 4 weeks | Increase in SBP and DBP. Subjects with essential hypertension are more sensitive to licorice-induced rise in BP. |
| Sigurjónsdóttir et al. (2006) [90], Sweden | Pre-post intervention | 25 | Licorice | 100 g | 4 weeks | The effect on aldosterone secretion differs between the genders. |
| Sobieszcyk et al. (2010) [102], USA | Randomized double-blind placebo-controlled crossover | 15 | GA | 130 mg | 14 days | Attenuated vasodilatory function on VSMCs. |
| Tu et al. (2010) [103], China | Two-phase randomized crossover | 16 | Glycyrrhizin | 2 × 150 mg | 14 days | Induction of CYP3A. |
| Yan et al. (2013) [104], China | Two-phase randomized crossover | 14 | Glycyrrhizin (salt tablet) | 3 × 75 mg | 6 days | No induction of P-glycoprotein. |
| Leksinen et al. (2014) [28], Finland ClinicalTrials: NCT01742702 | Non-randomized, controlled open label | 20 | Licorice | 290–370 mg glycyrrhizin | 14 days | Increase in SBP, DBP, extracellular volume and amplified pressure wave reflection from the periphery. |
| Hautaniemi et al. (2017) [73], Finland | Non-randomized, controlled open label | 22 | Licorice | 290–370 mg glycyrrhizin | 14 days | Increase in SBP, DBP, central pulse pressure, extracellular fluid volume and aortic to popliteal pulse wave velocity. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deutch, M.R.; Grimm, D.; Wehland, M.; Infanger, M.; Krüger, M. Bioactive Candy: Effects of Licorice on the Cardiovascular System. Foods 2019, 8, 495. https://doi.org/10.3390/foods8100495
Deutch MR, Grimm D, Wehland M, Infanger M, Krüger M. Bioactive Candy: Effects of Licorice on the Cardiovascular System. Foods. 2019; 8(10):495. https://doi.org/10.3390/foods8100495
Chicago/Turabian StyleDeutch, Mikkel R., Daniela Grimm, Markus Wehland, Manfred Infanger, and Marcus Krüger. 2019. "Bioactive Candy: Effects of Licorice on the Cardiovascular System" Foods 8, no. 10: 495. https://doi.org/10.3390/foods8100495
APA StyleDeutch, M. R., Grimm, D., Wehland, M., Infanger, M., & Krüger, M. (2019). Bioactive Candy: Effects of Licorice on the Cardiovascular System. Foods, 8(10), 495. https://doi.org/10.3390/foods8100495