3.1. Physicochemical Parameter Values for Acacia Honeys
Acacia honey in general has a low water content [
14,
32,
33,
34,
35], which can be observed also in this study. The values obtained are within the limits pertaining to the honeys found in the temperate climate, especially in Europe.
In order to carry out a primary interpretation of the data obtained for this parameter and to have a clear image of its variability in accordance with the geographical origin, a box plot graph of these values was constructed for each zone. The box plot diagram summarizes the charts for the five values specific to their spread in each zone (the minimum, the first quartile, the median, the third quartile and the maximum) and the extreme values. In this chart, one can observe a central line for each box plot, representing the median of the values measured for each zone. If the median is closer to the lower margin, the value spread leans towards the left, and if it is closer to the higher margin, the spread leans towards the right. For Zone 1, the maximum of the median was found to be 16.704, for Zone 2, 17.416, and for zone 3, the median value was 17.088. The box plot shows 50% of the values, and its size shows result variability. The horizontal lines above and below the box are traced from between the minimum and the maximum for each zone, so that the minimum values are 15.4, 15.8 and 17.1 for zones 1, 2, 3, respectively. The extreme values are placed outside the box plot and are marked with “*”, accompanied by the sample number. In the case of water content determination, two extreme values have been found, samples S15 (belonging to Zone 1) and S25 (Zone 2) (
Supplementary Figure S2).
The values obtained for water activity ranged between 0.540 (S51, Zone 1) and 0.690 (S25, Zone 2) (
Table 1).
A water activity value of 0.562 was recorded for Slovakian acacia honey [
36], 0.490 for Czech honey [
33] and 0.81 for Romanian honey [
37].
The L* value shows the brightness level, positive values of the parameter a* show red zone, negative values for a* show the green component, positive values for b* show the yellow zone and negative values for b* indicate the blue component. The values for L* (
Table 2) were between 50.19 and 62.70, and the average obtained after measuring all the samples was 58.96. These values are in accordance with those measured by Bertoncelj et al. [
38] for Slovenian honey (64.4), some Spanish honeys (51.62–56.11) [
38] but are much higher than those found for Slovakian honey (11.29) [
36] and other Romanian acacia honeys (48.96) [
37].
The value for parameter a* (
Table 2) lies between –1.28 and 5.80. The average obtained by the measuring of all the samples was 0.08 ± 1.12. A wide distribution can be observed when determining this parameter, as samples were situated in the green zone (negative a*), as well as in the red zone (positive a*). Only positive values were found for this parameter by Kasperová et al. (4.07) [
35] in the case of Slovakian honey, Romanian honey [
37], whereas Bertoncelj et al. [
38] found only negative values for Slovenian acacia honey (−2.82), as well as Karabagias et al. [
8] for Portuguese honey. As it can be observed in
Table 2, the positive and negative values obtained for this parameter cannot be linked to geographical origin or with the sample year of harvest. Each zone has shown both negative and positive values. The same characteristic presented Spanish honey [
39].
As for b* parameter values (
Table 2), the recorded minimum was 6.23, and the maximum 30.41. The measurement average for all zones was 15.70. Despite the fact that the zone between the minimum and maximum values of the b* parameter is relatively vast, the average obtained for acacia honey in correlated to the value found for Slovenian acacia honey [
38] (17.95) and to that of Slovakian honey (14.66) [
36], but much higher than other Romanian acacia honeys [
37].
The highest median value for the L* parameter was recorded in the case of the samples from Zone 1 (59.315), a value close to the one from Zone 3 (59.062), while the value for Zone 2 has shown the lowest value (58.177). Variance values are higher than one and show a greater degree of variation. The results of the ANOVA
p = 0.5662 and
F = 0.575 test show that there are no significant differences between samples when measuring this parameter. Thus, the samples are homogenous (
Table 3).
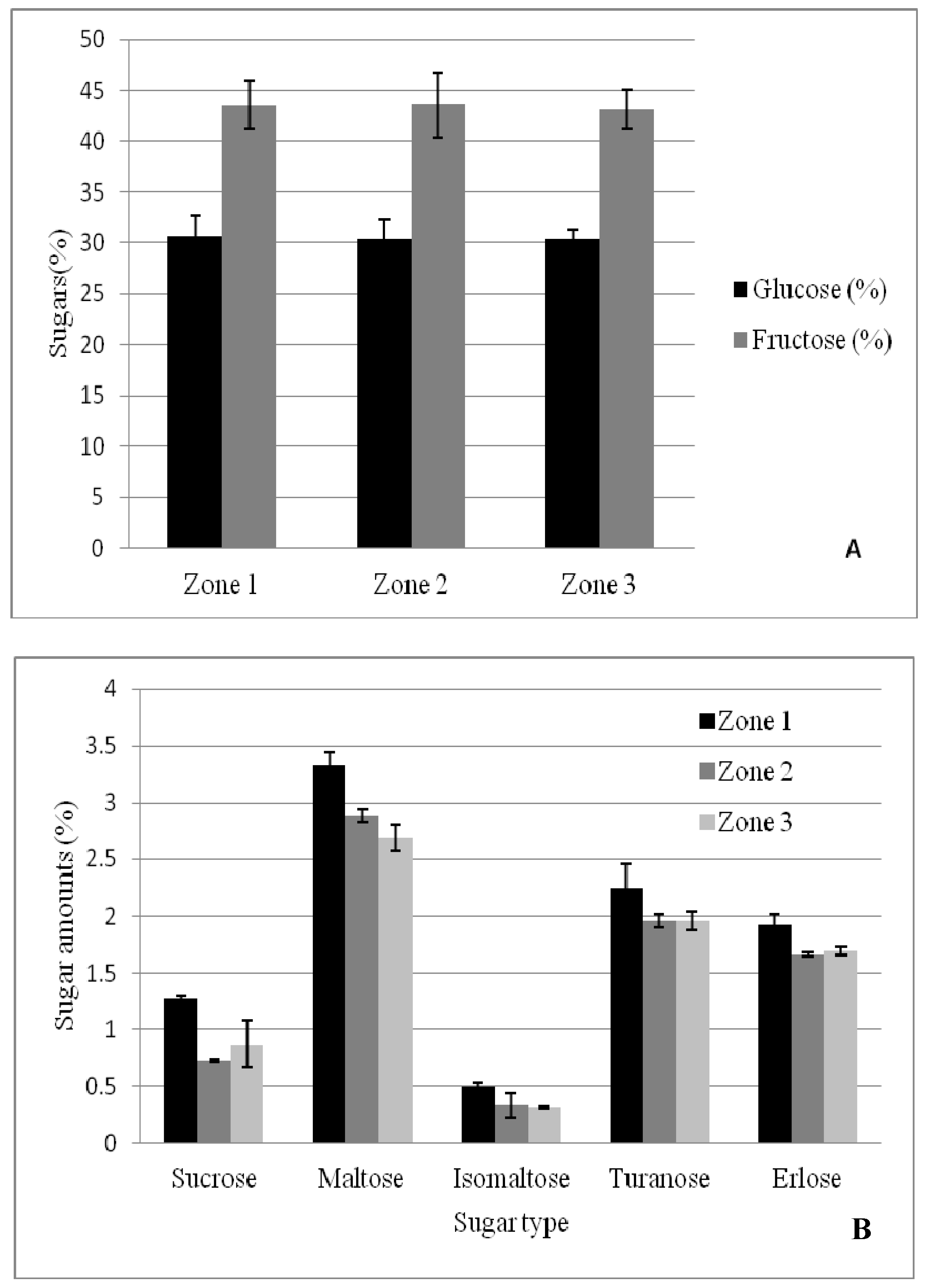
The results obtained for each sugar are presented in
Figure 2A, B. Each identified and quantified sugar was individually discussed, as the values obtained are extremely important to determine and authenticate the botanical origin of the honey samples.
As far as the glucose content is concerned, the values obtained for the 50 analyzed samples ranged between 22.32% and 43.71%. The mean value obtained for every zone was: 30.57 (Zone 1), 30.40 (Zone 2) and 30.37% (Zone 3).
Generally, acacia honey has an average glucose content of 26.3% [
40]. Romanian acacia honey has values ranging from 30.87% [
41] and 32.58% [
14]. The results obtained by analyzing these samples fell within these limits and proved to be very similar to other acacia honeys in Europe [
42,
43].
The fructose content is a very important parameter in analyzing acacia honey. The fluid state of this type of honey is due to a high fructose content and implicitly to a higher than 1.2 fructose/glucose ratio. The results obtained following the HPLC analysis of fructose in the honey samples (
Figure 2A) ranged between 33.80% and 48.16%. The mean value of the fructose content for each zone was: 43.55 (Zone 1), 43.58 (Zone 2) and 43.15% (Zone 3). While determining this parameter, we could observe the fact that only 5 samples out of 50 had a lower than 40% fructose content. Similar values were obtained in the case of other acacia honey samples from Romania [
14,
41] or other acacia honey samples in Europe [
42,
43]. As it has already been mentioned, the fructose/glucose ratio is very important in acacia honey analysis. Sabatini et al. [
40] found about a 1.66 ± 0.14 ratio in the case of acacia honey. The results obtained following the HPLC analysis of individual carbohydrates in the analyzed samples highlight (with three exceptions) the supraunitary values of this ratio, while 50% of the samples showed a > 1.5 ratio.
Sucrose is a very important indicator of honey authenticity, as standards require a maximum of 5% in authentic honey. The samples analyzed have significantly lower values, under no suspicion of falsification. The values registered ranged between 0.0 and 5.51%, with an average value of 1.0% (mean of all zones) (
Figure 2B). Only one sample had a concentration higher than 5% (sample S58, Zone 1). Some of the analyzed samples had a sucrose content that proved to be under the detection limit of the method and device (0.02%). The results obtained in this study confirmed earlier studies [
41,
44,
45,
46,
47].
The ANOVA analysis (the ratio between the variance produced by differences inside the groups and the variance produced by differences outside the group) shows that there are no statistic differences (p = 0.3010 > 0.05 and F = 1.23174 < Fcrit) between the samples harvested from the three production zones.
Maltose is an important disaccharide found in honey with determined values ranging between 1.3% [
40], 1.9% [
48] and 2.5% [
49] or a medium value of 3.53% obtained by Mărghitaş et al. [
14] for acacia honey in Romania (Transylvania). It is generally found in nectar honey and particularly in acacia honey.
The values obtained after analyzing the samples in this study varied between 0.68 and 4.16%. The ANOVA variance analysis shows that the value of p is lower than 0.05 (p = 0.012177), but F is bigger than Fcrit. (F = 4.848788 and Fcrit = 3.195056). Consequently, the hypothesis fails because there are significant differences between the samples.
Isomaltose is a disaccharide found in a relatively small quantity in honey, generally less than 1%. Depending on the carbohydrate determination method chosen (HPLC, GC or FTIR), but also on the botanical origin of honey, the quantity may differ, but remains low. The values obtained in the case of acacia honey varied between 0.14 and 0.79% and the mean value of the 50 samples was 0.39%. Similar values were obtained for the acacia honey in Italy [
40], other acacia honeys in Romania [
14] or the honey in Poland [
49]. However, it was easy to observe that this disaccharide presented values two times higher for the samples collected in geographical Zone 1 (
Figure 2B). The two other zones presented similar values, although geographically they are very different (Zone 2 being located on the south-west of the country, while Zone 3 represents the eastern part of the country).
Turanose is a sugar found in small quantities in honey. The use of high performance liquid chromatography, gas chromatography or nuclear magnetic resonance, made it possible for this disaccharide to be found by different researchers in small quantities in Portuguese heather honey [
50], in Polish nectar honey [
49], in 5 types of Italian honey [
51], in Hungarian acacia honey [
52] and in Turkish nectar and honeydew honey [
53].
Turanose was quantified between 0.38 and 2.77%, with an average of 2.08% in the acacia honey samples used in this study. These values are generally higher than the ones found by the aforementioned authors who have analyzed acacia honey, but similar to other honey types analyzed.
Erlose is a trisaccharide found in honey in different quantities, in accordance with its botanical origin. The studies conducted at the APHIS Laboratory (USAMV Cluj-Napoca) have shown a rather high concentration of erlose in acacia honey, compared to other honey types [
41].
Although Goldschmidt and Burckert [
54] highlighted erlose as a honey component since 1955, many researchers did not report this trisaccharide when using high performance liquid chromatography as a determination method [
49,
52,
55]. The values obtained for the analyzed honey samples ranged between 0.09 and 3.19%.
The glucidic spectrum analyzed by refractive index high performance liquid chromatography has shown the presence of seven saccharides specific to acacia honey. The samples fall between the limits established for this honey type, due to the high fructose content, >1.2 fructose/glucose (F/G) ratio, small sucrose content and higher than one maltose quantities [
40,
42,
43].
In conclusion, the glucidic spectrum for acacia honey was established, and carbohydrate concentrations, especially fructose and F/G ratio, which show high values for this honey type. In order to illustrate the correlations between carbohydrates and to determine which samples are outliers, a graphical representation was made for all carbohydrates, called a matrix plot (
Figure 3).
Due to the vast database, high sample and analysis number, it is fit to use a Pearson correlation matrix in order to identify the correlation indices. These indices have a value ranging between (–1 to 1).
Table 4,
Table 5 and
Table 6 show positive values, which means that there is a positive association of parameters, while negative values show an inverse dependence of the two parameters. The values colored in blue are significant for the 0.01 level, while the ones in yellow are significant of the 0.05 level.
For Zone 1, the best correlation was established between ash content and color. Also, positive correlations were found between different sugars, 0.72 for turanose–maltose and 0.79 for saccharose–erlose. The most significant negative correlation found for Zone 1 was between water activity–color (−0.841).
For Zone 2, significant positive correlations were found between sugars, 0.93 for turanose–maltose and 0.90 for sucrose–erlose. The most significant negative correlation was identified between the color parameters L*–a* (−0.94).
For Zone 3, the best correlation was established between water content and water activity, 0.92. The negative correlation found for Zone 3 was between color parameters (−0.80).
3.2. Volatile Compounds
The GC-MS analysis method is a combination of two analytical techniques: capillary column GC, which separates the components of a mixture and mass spectrometry (MS), which supplies the information necessary in the structural determination of each constituent. The results obtained following the SPME/GC-MS analysis is shown as chromatograms (
Supplementary Figure S3). Their interpretation is achieved by identifying mass spectrum and the retention time. These two parameters significantly contribute to the identification of each volatile compound found in honey. Also, retention time has a determining role in many cases where the constituents show identical mass spectra. Following the SPME/GC-MS analysis, 79 constituents were identified the honey samples: 72 compounds samples from Transylvania (Zone 1), 57 compounds in samples from Southern Romania (Zone 2) and 66 compounds in samples from Eastern Romania (Zone 3). From all identified compounds, only 54 are common to all 50 samples. The chemotype changes from one region to another, and the difference is constituted by the 6 compounds found only in one region and 9 compounds in two of the regions.
The interpretation of the chromatograms is achieved using the “Data Analysis” program. The noise level is set to 50 for the x-coordinate and the program goes over the chromatogram signal by signal according to this ratio. Identification of the compounds is achieved by comparing standard mass spectra from the “Pal 600” spectra library. The surface is calculated following the “apexing mass” model, which means measuring the surfaces in its entire mass.
The next stage in the qualitative analysis is the calculation of the Kovats indexes. We have used this retention index in order to eliminate the relativity of the retention parameters by inserting some reference points in order to compare the retention times of chromatographic separation for each volatile compound found in honey. In this respect, a mixture of homologues n-alkanes (C
7–C
30) was used under the same chromatographic conditions (Sigma, Darmstadt, Germany), whose retention indices showed values between 600 and 2200. The retention indices specific to each compound is calculated as follows:
where: RI: compound retention index, tr
i: compound retention time, tr
n: retention time of the adjacent and anterior alkane, tr
n+1: retention time of the adjacent and subsequent alkane.
The expression of the retention index uses two reference points: the alkane with the same number of carbon atoms in its molecule (
n) as the volatile compound identified as i, which will elute before the compound, and the second reference will be the alkane with
n+1 carbon atoms in its molecule, which will elute after the i compound.
Table 7 comparatively shows the retention indices calculated RI
b and the retention indexes found in literature. RI
a corresponds to each volatile compound found and the type of column used (polar column). The main bibliographical source used was the PHEROBASE database, which is one of the most comprehensive and up-to-date.
The main volatile compounds of acacia honey identified undoubtedly are: 3-methyl-3-buten-1-ol for Zone 1, ethanol, acetic acid, 5-ethenyldihydro-5-furanone for Zone 2, acetone, 3-methyl-3-buten-1-ol, trans-linalool oxide, benzemethanol for Zone 3. Among the secondary compounds identified in a percentage of 80–90% of the samples one can find:
Dimethyl sulfide, acetone, 3-hydroxy-2-butanone, linalool oxide, acetic acid, 2-furancarboxaldehyde, benzaldehyde, linalool L, ho-trienol, 4-ketoisophorone, epoxylinalool, benzenemethanol for Zone 1;
Dimethyl sulfide, acetone, 3-hydroxy-2-butanone, 2-methyl-2-buten-1-ol, nonanal, linalool, 2-furancarboxaldehyde, 5-methyl-2-furancarboxaldehyde, butanoic acid, epoxy-linalool, 3-hydroxy-2-butanone, methylpentanoic acid, benzenethanol for Zone 2;
Dimethyl sulfide, ethanol, isoamyl alcohol, 2-methyl-2-buten-1-ol, cis-3-hexen-1-ol, linalool oxide, acetic acid, 2-furancarboxaldehyde, benzaldehyde, propanoic acid, hotrienol, butyrolactone, benzeneacetaldehyde, phenol for Zone 3.
Thus, once the qualitative analysis was completed, the volatile profile of the Romanian acacia honey was outlined.
The results revealed that there is a remarkable influence of the production zone on the volatile profile of the honey samples. Of all the identified compounds, only 56 are common to all the 50 samples. The chemotype changes from one region to another, and the difference resides in the 23 compounds found only in certain regions. The compounds found only in Zone 1 are: 2-butanol, 5-methyl-2-hexanone, 2-heptanone, octanal, 2,2-dymethyl propanoic acid, naphthalene, nonanoic and octanoic acids. The origin of these compounds can be either vegetal (nectar and poliniferous plants) or animal, products secreted by the bee. For example, octanol is found in
Houttuynia cordata and in
Lavandula angustifolia [
56]. The 2-heptanone compound was identified in the secretion of bees by Shearer [
57], but it is also found in
Artemisia dracunculus and
Larrea tridentate [
58,
59]. In regard to Zone 2, borneol and HMF compounds have been identified. Borneol is a monoterpenic compound found in medicinal plants [
60]. Borneol is used as an additive in cosmetic products due to its analgesic and antibacterial effects [
61]. The presence of the HMF compound is a hint towards honey freshness [
62]. In Zone 3, pentanoic acid was found. Also, it was found that Zone 1 shows a volatile profile similar to Zone 3, a relevant example being the existing common compounds,
beta-damascenone, hotrienol, benzyl nitrile, 2,5-furancarboxaldehyde. Out of these compounds,
beta-damascenone was found in
Ipomoea pes caprae with an antispastic effect [
63]. Additionally, hotrienol was found to be a component of many honey varieties, and of essential oils of many plants. This compound was found to be specific to citrus, lavender, and mint honey [
64,
65,
66].
By using the peak area, it is possible to determine the percentage of each compound, compared to the total surface of the areas.
The calibration and concentration determination methods for volatile compounds are:
Gauging of the calibration curve method (area normalization);
Internal standard method [
67].
For a better interpretation of the results, the first method was used, the concentration of each compound being calculated according to the following expression:
where: A
x: compound surface, ∑A
i: the sum of all identified compound surfaces.
Table 8 shows the semi-quantification results for each product, as well as product chemical classification. The volatile compounds identified in the acacia honey samples stemming from the three geographical areas are classified into 10 chemical subclasses: Sulphur compounds, ketones, esters, aldehydes, alcohols, nitrate compounds, aliphatic hydrocarbons, carboxylic acids, aromatic acids and ethers. From the collected data, we can see that the honey samples harvested from Zone 1 (Transylvania) are the richest in volatile compounds, as all of these compound classes are present in the samples. Keeping in mind that the number of compounds was so high, only the majorities were discussed.
Regarding the presence of acetic acid, this compound was found in samples from all three zones, but in different quantities. Acetic and butyric acids could be produced by bee metabolism [
68]. This is the main compound of the honey samples, and its percentages vary between 12.26% for Zone 3, 16.64% for Zone 1 and 24.73% for Zone 2. The previous studies show that the percentage of this compound can vary between 17.32% and 36.15% for different Chilean honey types [
19], while having a percentage of 0.01% in Spanish honey [
64]. We must mention that the two studies have used different extraction methods, ultrasound extraction and thermal desorption. Kadar et al. [
21] have found that Romanian acacia honey has linalool oxide as the main compound, without having identified any acetic acid.
Ethanol is another important constituent of honey, all honey samples have shown a high ethanol content, especially those from Zone 2 and Zone 3, as their content reached 17.91% and 13.07%, respectively. In the case of Transylvanian honey (Zone 1), there was a relatively low ethanol content found, of 4.11%. Bastos and Alves [
68] issue the hypothesis that the high ethanol content is due to the presence of ferments. Ethanol was found to be a marker for lavender honey [
65]. Furthermore, ethanol was found in honeys of different geographical and botanical origins [
8,
9,
22,
64].
The volatile profile found depends on the extraction method. Therefore, the fiber used shows a great affinity for compounds with low molecular mass, like ethanol or acetic acid [
69], which justifies the significant presence of all these compounds in the honey samples, from a quantitative point of view.
Another major compound was 2-propanone, present in a high concentration in Zone 3 (20.60%), as opposed to its presence in honey stemming from Zone 1 and Zone 2, where its values were 2.83% and 5.11%, respectively. Transylvanian honeys (Zone 1) have shown the lowest 2-propanone or acetone content, these differences being the result of the discrepancy between geographical, climatic and especially floral conditions. In a recent study, acetone concentration in Chilean honey was determined, according to geographical area. In the eastern part, the zone percentage was between 1.57–17.15%, in the central area, 7.8–9.63%, and in the western zone, 5.00–13.13% [
19]. Acetone is considered a marker for pine honey (
Albies spp.) [
68]. Additionally, acetone was found to be a major compound in acacia and rosemary monofloral honey [
65]. Linalool oxide and 2–furancarboxaldehyde are usually found in the honey samples collected from Zone 2 (8.61% and 9.73). Linalool oxide is a compound of floral origin (Pherobase) and is considered to be a secondary product of linalool. Jerkovic et al. [
70] determined a 2.23% concentration of this product in acacia honey, whereas in chestnut honey, the concentration was of 0.40%.
Furfural is a compound derived from furan, which is considered to be an indicator of the thermal and storage processes [
64]. On the other hand, furfural was identified as being a relevant compound in unifloral lime tree, lavender and acacia honey [
65]. Furfural is present in all regions with an area percentage of 9.73% for Zone 2, 7.26% for Zone 1 and 5.24% for Zone 3. Radovic et al. [
71] established furfural as being a marker for rape honey. These volatile compounds, furfural and 5-methyl-furfural were identified in fresh citrus honey. Moreover, the evolution of the concentration of these compounds was observed and it intensified during storage and temperature increase from 10 to 40 °C [
64]. The mild heating of the samples during the SPME analysis recommended for the improvement of the extraction result and the reduction of the balance time, may be responsible for some of these compounds [
72].
We can also observe a higher benzaldehyde content, especially in Zone 1 honey (7.23%), as compared to the one in Zone 2 (3.89%) and Zone 3 (4.68%). Benzaldehyde was identified as being a relevant compound in lavender, acacia and rosemary honey [
65,
72]. Yang et al. [
73] identified benzaldehyde as being a volatile marker in Corsican chestnut honey with a 10.8% concentration. Furthermore, we can also note the presence of benzeneethanol in significant amounts in the case of the honey samples from Zone 2 (3.58%), Zone 1 (2.78%) and Zone 3 (2.37%). Benzenethanol and benzoic acid were identified as rape honey markers [
74].
Alcohols constitute an important part of Romanian honey samples reaching values of 28.35% in Zone 3, 23.87% in Zone 2, 19.13% in Zone 1. The alcohols present in all the zones were ethanol, 2-propanol, 2-methyl-3-butene-2-ol, isoamyl alcohol, 3-methyl-3-butene-1-ol, 2-methyl-2-butene-1-ol,
cis-3-hexen-1-ol, 2-ethyl-1-hexanol, linalool, hotrienol,
alfa-terpienol, benzeneethanol, benzenemethanol, phenol. Among the identified alcohols, ethanol, benzeneethanol, 3-methyl-3-butene-1-ol proved to have the highest area percentage of all the analyzed samples (
Table 8).
Alcohols represent important markers of honey; alcohols, such as 3-methyl-3-butene-1-ol and 2-methyl-2-butene-1-ol were described as adding a fresh aroma, their presence in honey being associated with different floral origins [
64]. Ethanol, 2-methyl-propanol and 3-methyl-3-butene-1-ol were described as being very important compounds in monofloral lavender honey [
65]. The identification of 3-methyl-3-butene-1-ol was also associated with monofloral rosemary honey [
75].
As far as aldehydes are concerned, it can be observed that the honey samples from Zone 1 present the highest concentration, 27.63%, followed by the ones from Zone 2 with a 22.34% concentration. The samples in Zone 3 have a relative aldehyde concentration of 13.43%. The aldehydes identified in the samples from all the three production areas are: 3-methyl-butanal, heptanal, 3-methyl-2-butanal, nonanal, furfural, decanal, benzaldehyde, lilac aldehyde, 5-methyl-2-furancarboxaldehyde, acetaldehyde and 3-phenylfenil-propenal. Plutowska et al. [
76] have established hexanal as a specific marker for the acacia honey produced in Poland, while methyl butanol and lilac aldehyde proved to be a specific marker for the Polish honeydew honey. The following substances from the aldehyde group were also identified in the composition of these honeys: hexanal, nonanal, decanal, acetaldehyde and lilac aldehydes.
Ketones, sulfur compounds, esters and aliphatic hydrocarbons were also identified in small amounts, but none of them could be considered markers for acacia honey.
As other studies presented the idea that minor compounds also influence honey flavor, the table was organized in order to summarize the specific aromas of all the identified volatile compounds. Thus, it can be observed that the honey aroma is very complex and depends on the specific flora of each production zone, but also on the compounds secreted by the bees or on the transformations that occur during storage.