Narrow-Banded UVB Affects the Stability of Secondary Plant Metabolites in Kale (Brassica oleracea var. sabellica) and Pea (Pisum sativum) Leaves Being Added to Lentil Flour Fortified Bread: A Novel Approach for Producing Functional Foods
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Plant Material
2.3. Breadmaking Experiments
2.4. Antioxidant Activity
2.4.1. Trolox Equivalent Antioxidant Activity (TEAC) Assay
2.4.2. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Assay
2.5. Analysis of Carotenoids and Chlorophylls by Ultrahigh Perfomance Liquid Chromatography-Diode Array Detection-Time of Flight-Mass Spectrometry (UHPLC-DAD-ToF-MS)
2.6. Analysis of Flavonoid Glycosides and Hydroxycinnamic Acid Derivatives by High Perfomance Liquid Chromatography-Diode Array Detection-Electrospray Ionisation-Mass Spectrometry (HPLC-DAD-ESI-MSN)
2.7. Analysis of Protein Content by the Kjeldahl Method
2.8. Data Handling and Statistical Analysis
3. Results and Discussion
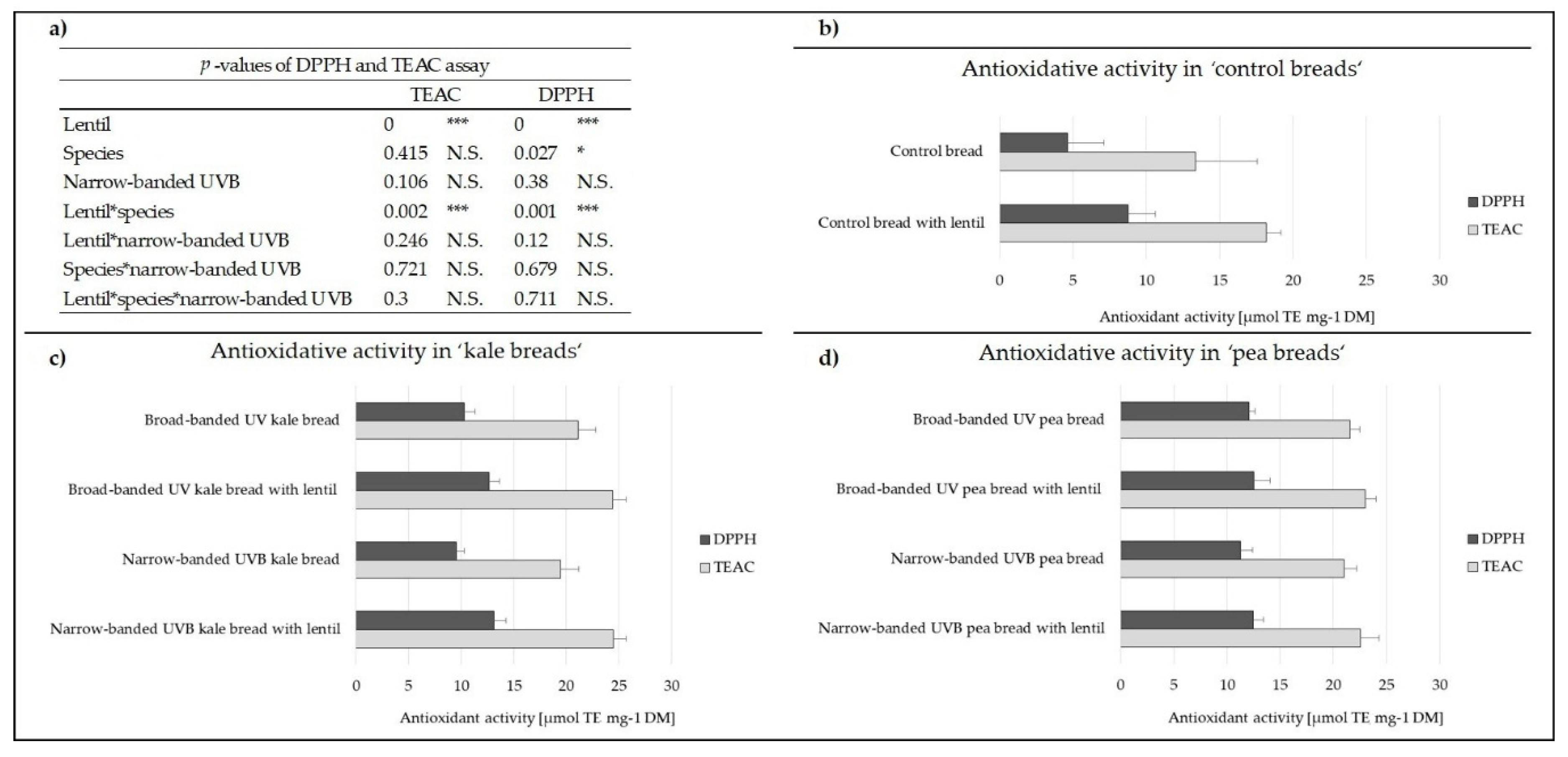
3.1. Antioxidant Activity of the Breads
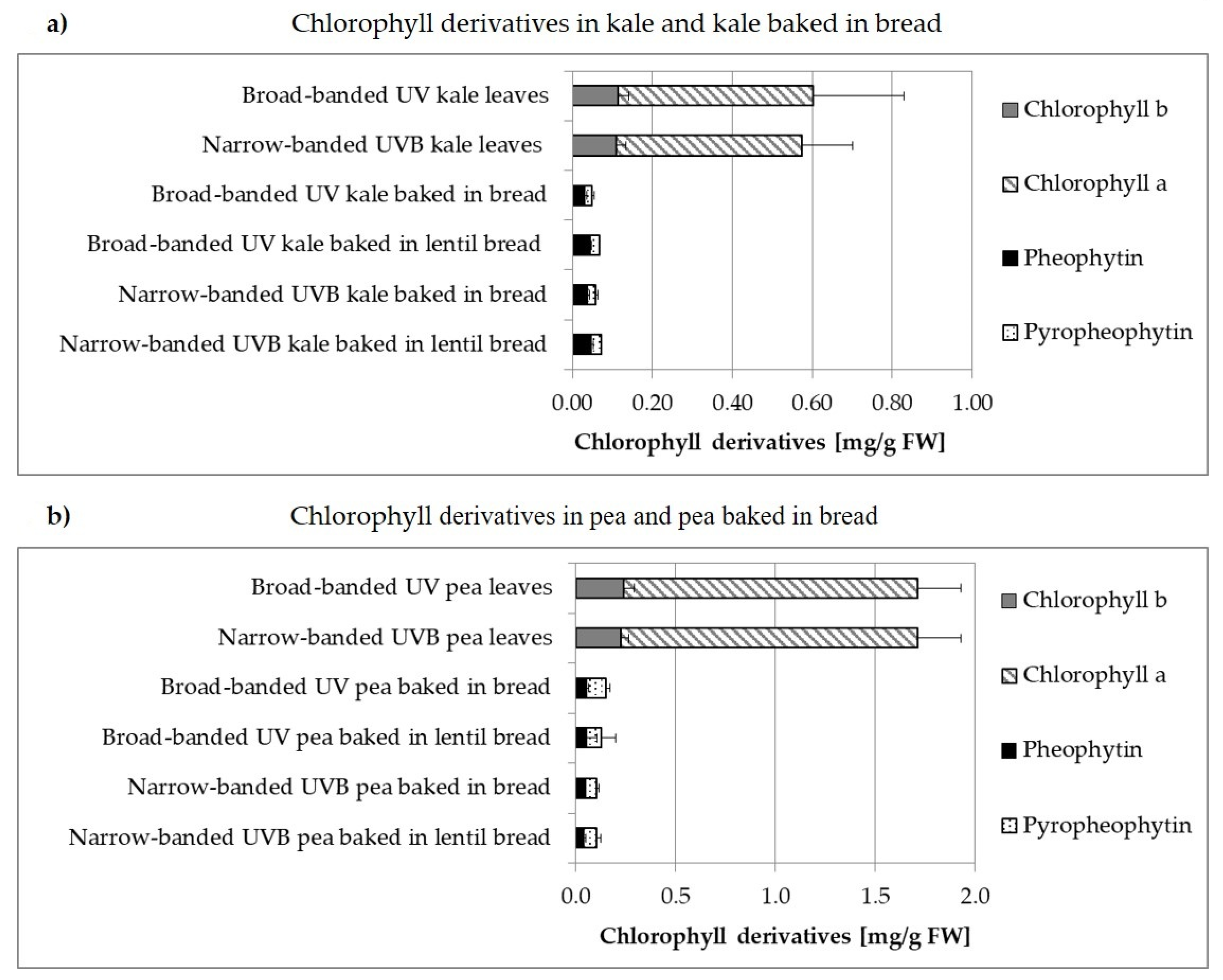
3.2. Carotenoids and Chlorophylls
3.2.1. Broad-Banded UV Light versus Narrow-Banded UVB Light
3.2.2. Non-Processed versus Processed
3.3. Flavonoid Glycosides and Hydroxycinnamic Acid Derivatives
3.3.1. Broad-Banded UV-Light versus Narrow-Banded UVB-Light
3.3.2. Non-Processed versus Processed
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Martirosyan, D.M.; Singh, J. A new definition of functional food by FFC: What makes a new definition unique? Funct. Food Health Dis. 2015, 5, 209–223. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Chao, P.-Y.; Hu, S.-P.; Yang, C.-M. The antioxidant and free radical scavenging activities of chlorophylls and pheophytins. Food Nutr. Sci. 2013, 4, 1. [Google Scholar] [CrossRef]
- Kaulmann, A.; Bohn, T. Carotenoids, inflammation, and oxidative stress—implications of cellular signaling pathways and relation to chronic disease prevention. Nutr. Res. 2014, 34, 907–929. [Google Scholar] [CrossRef] [PubMed]
- Agati, G.; Brunetti, C.; Di Ferdinando, M.; Ferrini, F.; Pollastri, S.; Tattini, M. Functional roles of flavonoids in photoprotection: New evidence, lessons from the past. Plant Physiol Biochem. 2013, 72, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects–A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Levine, M.E.; Suarez, J.A.; Brandhorst, S.; Balasubramanian, P.; Cheng, C.-W.; Madia, F.; Fontana, L.; Mirisola, M.G.; Guevara-Aguirre, J.; Wan, J. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 2014, 19, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Fung, T.T.; Hu, F.B.; Willett, W.C.; Longo, V.D.; Chan, A.T.; Giovannucci, E.L. Association of animal and plant protein intake with all-cause and cause-specific mortality. JAMA Intern. Med. 2016, 176, 1453–1463. [Google Scholar] [CrossRef]
- Zhang, B.; Deng, Z.; Ramdath, D.D.; Tang, Y.; Chen, P.X.; Liu, R.; Liu, Q.; Tsao, R. Phenolic profiles of 20 Canadian lentil cultivars and their contribution to antioxidant activity and inhibitory effects on α-glucosidase and pancreatic lipase. Food Chem. 2015, 172, 862–872. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Polyphenol-rich lentils and their health promoting effects. Int. J. Mol. Sci. 2017, 18, 2390. [Google Scholar] [CrossRef]
- Ribas-Agusti, A.; Martin-Belloso, O.; Soliva-Fortuny, R.; Elez-Martinez, P. Food processing strategies to enhance phenolic compounds bioaccessibility and bioavailability in plant-based foods. Crit. Rev. Food Sci. Nutr. 2017, 58, 1–18. [Google Scholar] [CrossRef]
- Rohn, S. Possibilities and limitations in the analysis of covalent interactions between phenolic compounds and proteins. Food Res. Int. 2014, 65, 13–19. [Google Scholar] [CrossRef]
- Heinze, M.; Hanschen, F.S.; Wiesner-Reinhold, M.; Baldermann, S.; Gräfe, J.; Schreiner, M.; Neugart, S. Effects of Developmental Stages and Reduced UVB and Low UV Conditions on Plant Secondary Metabolite Profiles in Pak Choi (Brassica rapa subsp. chinensis). J. Agric. Food Chem. 2018, 66, 1678–1692. [Google Scholar] [CrossRef] [PubMed]
- Moreira-Rodríguez, M.; Nair, V.; Benavides, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. UVA, UVB Light, and Methyl Jasmonate, Alone or Combined, Redirect the Biosynthesis of Glucosinolates, Phenolics, Carotenoids, and Chlorophylls in Broccoli Sprouts. Int. J. Mol. Sci. 2017, 18, 2330. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, M.; Mewis, I.; Huyskens-Keil, S.; Jansen, M.; Zrenner, R.; Winkler, J.; O’Brien, N.; Krumbein, A. UV-B-induced secondary plant metabolites-potential benefits for plant and human health. Crit. Rev. Plant Sci. 2012, 31, 229–240. [Google Scholar] [CrossRef]
- Ilić, Z.S.; Fallik, E. Light quality manipulation improves vegetable quality at harvest and postharvest: A review. Environ. Exp. Bot. 2017, 139, 79–90. [Google Scholar] [CrossRef]
- Caldwell, M.M. Solar UV irradiation and the growth and development of higher plants. In Photophysiology; Academic Press: New York, NY, USA; London, UK, 1971; Volume 6, pp. 131–177. [Google Scholar]
- Neugart, S.; Schreiner, M. UVB and UVA as eustressors in horticultural and agricultural crops. Sci. Hortic. 2018, 234, 370–381. [Google Scholar] [CrossRef]
- Singh, D.; Basu, C.; Meinhardt-Wollweber, M.; Roth, B. LEDs for energy efficient greenhouse lighting. Renew. Sust. Energy Rev. 2015, 49, 139–147. [Google Scholar] [CrossRef]
- Ahmed, M.; Eun, J.-B. Flavonoids in fruits and vegetables after thermal and nonthermal processing: A review. Crit. Rev. Food Sci. Nutr. 2017, 58, 1–30. [Google Scholar] [CrossRef]
- Turkmen, N.; Poyrazoglu, E.S.; Sari, F.; Sedat Velioglu, Y. Effects of cooking methods on chlorophylls, pheophytins and colour of selected green vegetables. Int. J. Food Sci. Technol. 2006, 41, 281–288. [Google Scholar] [CrossRef]
- Klopsch, R.; Baldermann, S.; Hanschen, F.S.; Voss, A.; Rohn, S.; Schreiner, M.; Neugart, S. Brassica-enriched wheat bread: Unraveling the impact of ontogeny and breadmaking on bioactive secondary plant metabolites of pak choi and kale. Food Chem. 2019, 295, 412–422. [Google Scholar] [CrossRef]
- Klopsch, R.; Baldermann, S.; Voss, A.; Rohn, S.; Schreiner, M.; Neugart, S. Bread enriched with legume microgreens and leaves–ontogenetic and baking-driven changes in the profile of secondary plant metabolites. Front. Chem. 2018, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Buscemi, S.; Corleo, D.; Di Pace, F.; Petroni, M.; Satriano, A.; Marchesini, G. The Effect of Lutein on Eye and Extra-Eye Health. Nutrients 2018, 10, 1321. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Zietz, M.; Schreiner, M.; Rohn, S.; Kroh, L.W.; Krumbein, A. Identification of complex, naturally occurring flavonoid glycosides in kale (Brassica oleracea var. sabellica) by high-performance liquid chromatography diode-array detection/electrospray ionization multi-stage mass spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 2009–2022. [Google Scholar] [CrossRef] [PubMed]
- Neugart, S.; Rohn, S.; Schreiner, M. Identification of complex, naturally occurring flavonoid glycosides in Vicia faba and Pisum sativum leaves by HPLC-DAD-ESI-MS n and the genotypic effect on their flavonoid profile. Food Res. Int. 2015, 76, 114–121. [Google Scholar] [CrossRef]
- Csepregi, K.; Neugart, S.; Schreiner, M.; Hideg, É. Comparative evaluation of total antioxidant capacities of plant polyphenols. Molecules 2016, 21, 208. [Google Scholar] [CrossRef] [PubMed]
- Fiol, M.; Weckmüller, A.; Neugart, S.; Schreiner, M.; Rohn, S.; Krumbein, A.; Kroh, L.W. Thermal-induced changes of kale’s antioxidant activity analyzed by HPLC–UV/Vis-online-TEAC detection. Food Chem. 2013, 138, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 15th ed.; Helrich, K., Ed.; Association of Analytical Chemists: Washington, DC, USA, 1990; Volume 15, p. 771. [Google Scholar]
- Llorente, B.; Martinez-Garcia, J.F.; Stange, C.; Rodriguez-Concepcion, M. Illuminating colors: Regulation of carotenoid biosynthesis and accumulation by light. Curr. Opin. Plant Biol. 2017, 37, 49–55. [Google Scholar] [CrossRef]
- Reif, C.; Arrigoni, E.; Berger, F.; Baumgartner, D.; Nyström, L. Lutein and β-carotene content of green leafy Brassica species grown under different conditions. LWT Food Sci. Technol. 2013, 53, 378–381. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Kopsell, D.E. Accumulation and bioavailability of dietary carotenoids in vegetable crops. Trends Plant Sci. 2006, 11, 499–507. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W. Carotenoid composition in sun and shade leaves of plants with different life forms. Plant Cell Environ. 1992, 15, 411–419. [Google Scholar] [CrossRef]
- Mewis, I.; Schreiner, M.; Nguyen, C.N.; Krumbein, A.; Ulrichs, C.; Lohse, M.; Zrenner, R. UV-B irradiation changes specifically the secondary metabolite profile in broccoli sprouts: Induced signaling overlaps with defense response to biotic stressors. Plant Cell Physiol. 2012, 53, 1546–1560. [Google Scholar] [CrossRef] [PubMed]
- Becatti, E.; Petroni, K.; Giuntini, D.; Castagna, A.; Calvenzani, V.; Serra, G.; Mensuali-Sodi, A.; Tonelli, C.; Ranieri, A. Solar UV-B radiation influences carotenoid accumulation of tomato fruit through both ethylene-dependent and-independent mechanisms. J. Agric. Food Chem. 2009, 57, 10979–10989. [Google Scholar] [CrossRef] [PubMed]
- Lazzeri, V.; Calvenzani, V.; Petroni, K.; Tonelli, C.; Castagna, A.; Ranieri, A. Carotenoid profiling and biosynthetic gene expression in flesh and peel of wild-type and hp-1 tomato fruit under UV-B depletion. J. Agric. Food Chem. 2012, 60, 4960–4969. [Google Scholar] [CrossRef] [PubMed]
- Canjura, F.L.; Schwartz, S.J.; Nunes, R.V. Degradation kinetics of chlorophylls and chlorophyllides. J. Food Sci. 1991, 56, 1639–1643. [Google Scholar] [CrossRef]
- Teng, S.; Chen, B. Formation of pyrochlorophylls and their derivatives in spinach leaves during heating. Food Chem. 1999, 65, 367–373. [Google Scholar] [CrossRef]
- Schwartz, S.; Von Elbe, J. Kinetics of chlorophyll degradation to pyropheophytin in vegetables. J. Food Sci. 1983, 48, 1303–1306. [Google Scholar] [CrossRef]
- Onkokesung, N.; Reichelt, M.; van Doorn, A.; Schuurink, R.C.; van Loon, J.J.; Dicke, M. Modulation of flavonoid metabolites in Arabidopsis thaliana through overexpression of the MYB75 transcription factor: Role of kaempferol-3, 7-dirhamnoside in resistance to the specialist insect herbivore Pieris brassicae. J. Exp. Bot. 2014, 65, 2203–2217. [Google Scholar] [CrossRef]
- Le Roy, J.; Huss, B.; Creach, A.; Hawkins, S.; Neutelings, G. Glycosylation is a major regulator of phenylpropanoid availability and biological activity in plants. Front. Plant. Sci. 2016, 7, 735. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; El-Said, A.M.; Khalifa, S.A.; Göransson, U.; Bohlin, L.; Borg-Karlson, A.-K.; Verpoorte, R. Biosynthesis, natural sources, dietary intake, pharmacokinetic properties, and biological activities of hydroxycinnamic acids. J. Agric. Food Chem. 2012, 60, 10877–10895. [Google Scholar] [CrossRef]
- Majer, P.; Neugart, S.; Krumbein, A.; Schreiner, M.; Hideg, É. Singlet oxygen scavenging by leaf flavonoids contributes to sunlight acclimation in Tilia platyphyllos. Environ. Exp. Bot. 2014, 100, 1–9. [Google Scholar] [CrossRef]
- Neugart, S.; Fiol, M.; Schreiner, M.; Rohn, S.; Zrenner, R.; Kroh, L.W.; Krumbein, A. Interaction of moderate UV-B exposure and temperature on the formation of structurally different flavonol glycosides and hydroxycinnamic acid derivatives in kale (Brassica oleracea var. sabellica). J. Agric. Food Chem. 2014, 62, 4054–4062. [Google Scholar] [CrossRef] [PubMed]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Rechner, O.; Neugart, S.; Schreiner, M.; Wu, S.; Poehling, H.-M. Different narrow-band light ranges alter plant secondary metabolism and plant defense response to aphids. J. Chem. Ecol. 2016, 42, 989–1003. [Google Scholar] [CrossRef] [PubMed]
- Guidi, L.; Brunetti, C.; Fini, A.; Agati, G.; Ferrini, F.; Gori, A.; Tattini, M. UV radiation promotes flavonoid biosynthesis, while negatively affecting the biosynthesis and the de-epoxidation of xanthophylls: Consequence for photoprotection? Environ. Exp. Bot. 2016, 127, 14–25. [Google Scholar] [CrossRef]
- Chaaban, H.; Ioannou, I.; Chebil, L.; Slimane, M.; Gérardin, C.; Paris, C.; Charbonnel, C.; Chekir, L.; Ghoul, M. Effect of heat processing on thermal stability and antioxidant activity of six flavonoids. J. Food Process. Preserv. 2017, 41, e13203. [Google Scholar] [CrossRef]
- Rohn, S.; Buchner, N.; Driemel, G.; Rauser, M.; Kroh, L.W. Thermal degradation of onion quercetin glucosides under roasting conditions. J. Agric. Food Chem. 2007, 55, 1568–1573. [Google Scholar] [CrossRef] [PubMed]
| (a) | Kale Leaves | Kale Bread | Kale Bread with Lentil | |||
|---|---|---|---|---|---|---|
| mg/g FW in Plant Tissue | Recovery in Kale Bread (%) | |||||
| Lutein | broad-banded UV | 0.034 A | ± | 0.009 | 1.8 a | 2 a |
| narrow-banded UVB | 0.028 A | ± | 0.008 | 1.9 a | 2.1 a | |
| α-Carotene | broad-banded UV | 0.001 A | ± | 0.001 | N.D. | N.D. |
| narrow-banded UVB | 0.002 B | ± | 0.001 | |||
| ß-Carotene | broad-banded UV | 0.011 A | ± | 0.004 | N.D. | N.D. |
| narrow-banded UVB | 0.009 A | ± | 0.003 | |||
| Neoxanthin derivative 1 | broad-banded UV | 0.01 A | ± | 0.003 | N.D. | N.D. |
| narrow-banded UVB | 0.009 A | ± | 0.003 | |||
| Neoxanthin derivative 2 | broad-banded UV | 0.004 A | ± | 0.001 | N.D. | N.D. |
| narrow-banded UVB | 0.005 B | ± | 0.001 | |||
| Violaxanthin | broad-banded UV | 0.001 A | ± | 0.001 | N.D. | N.D. |
| narrow-banded UVB | 0.001 A | ± | 0.001 | |||
| Total carotenoids | broad-banded UV | 0.06 A | ± | 0.019 | 1.2 a | 1.36 a |
| narrow-banded UVB | 0.055 A | ± | 0.017 | 1 a | 1.1 a | |
| (b) | Pea Leaves | Pea Bread | Pea Bread with Lentil | |||
| mg/g FW in Plant Tissue | Recovery in Pea Bread (%) | |||||
| Lutein | broad-banded UV | 0.102 A | ± | 0.023 | 15.1 a | 11 a |
| narrow-banded UVB | 0.106 A | ± | 0.020 | 9 b | 11.3 b | |
| ß-Carotene | broad-banded UV | 0.052 A | ± | 0.008 | N.D. | N.D. |
| narrow-banded UVB | 0.052 A | ± | 0.009 | |||
| Neoxanthin derivative 1 | broad-banded UV | 0.023 A | ± | 0.006 | N.D. | N.D. |
| narrow-banded UVB | 0.021 A | ± | 0.005 | |||
| Neoxanthin derivative 2 | broad-banded UV | 0.003 A | ± | 0.001 | N.D. | N.D. |
| narrow-banded UVB | 0.003 A | ± | 0.001 | |||
| Violaxanthin derivative 1 | broad-banded UV | 0.037 A | ± | 0.015 | N.D. | N.D. |
| narrow-banded UVB | 0.038 A | ± | 0.012 | |||
| Violaxanthin derivative 2 | broad-banded UV | 0.005 A | ± | 0.001 | N.D. | N.D. |
| narrow-banded UVB | 0.006 A | ± | 0.001 | |||
| Total carotenoids | broad-banded UV | 0.223 A | ± | 0.053 | 7 a | 6.9 a |
| narrow-banded UVB | 0.226 A | ± | 0.047 | 4.2 a | 5.3 b | |
| (a) Hydroxycinnamic Acid Derivatives | Kale | Kale Bread | Kale Bread with Lentil | |||
|---|---|---|---|---|---|---|
| mg/g FW in Plant Tissue | Recovery in Kale Bread (%) | |||||
| 3-Caffeoylquinic acid | broad-banded UV | 0.309 A | ± | 0.065 | 14 a | 14 a |
| narrow-banded UVB | 0.259 A | ± | 0.044 | 15.7 b | 14.5 b | |
| Caffeoylglucoside | broad-banded UV | 0.078 A | ± | 0.020 | N.D. | N.D. |
| narrow-banded UVB | 0.072 A | ± | 0.009 | |||
| Sinapic acid-glucoside | broad-banded UV | 0.185 A | ± | 0.037 | 21 a | 21.2 a |
| narrow-banded UVB | 0.239 B | ± | 0.026 | 15.7 b | 16.8 b | |
| Sinapoyl-feruloylgentiobiose | broad-banded UV | 0.257 A | ± | 0.049 | 15.1 a | 15.7 a |
| narrow-banded UVB | 0.204 B | ± | 0.034 | 18.3 b | 19 b | |
| Disinapoyl-feruloylgentiobiose | broad-banded UV | 0.095 A | ± | 0.018 | 33.5 a | 33.8 a |
| narrow-banded UVB | 0.092 A | ± | 0.012 | 34.6 b | 34.7 a | |
| Sinapoyl-hydroxyferuloylgentiobiose | broad-banded UV | 0.089 A | ± | 0.018 | 38.5 a | 38.5 a |
| narrow-banded UVB | 0.082 A | ± | 0.015 | 39.8 b | 40.5 b | |
| Disinapoyl-gentiobiose | broad-banded UV | 0.268 A | ± | 0.051 | 17.2 a | 17.8 a |
| narrow-banded UVB | 0.263 A | ± | 0.038 | 17.7 a | 19.4 a | |
| Trisinapoyl-gentiobiose | broad-banded UV | 0.184 A | ± | 0.045 | 26 a | 25.6 a |
| narrow-banded UVB | 0.203 A | ± | 0.032 | 23.6 b | 26.2 a | |
| Hydroxycinnamic acid derivatives | broad-banded UV | 1.465 A | ± | 0.302 | 19.2 a | 19.4 a |
| narrow-banded UVB | 1.413 A | ± | 0.211 | 19.4 a | 20.2 a | |
| (b) Kaempferol Glycosides | Kale | Kale Bread | Kale Bread with Lentil | |||
| mg/g FW in Plant Tissue | Recovery in Kale Bread (%) | |||||
| K-3-O-dirha-7-O-rha | broad-banded UV | 0.153 A | ± | 0.024 | 39.9 a | 37.6 a |
| narrow-banded UVB | 0.128 B | ± | 0.026 | 48.8 b | 47.5 b | |
| K-3-O-soph-7-O-ᴅ-glc | broad-banded UV | 0.163 A | ± | 0.052 | 10.1 a | 10.7 a |
| narrow-banded UVB | 0.124 A | ± | 0.040 | 6.1 b | 7 b | |
| K-3-O-cou-soph-7-O-ᴅ-glc | broad-banded UV | 0.08 A | ± | 0.023 | 19.1 a | 20.2 a |
| narrow-banded UVB | 0.035 B | ± | 0.011 | 33.6 b | 31.8 b | |
| K-3-O-caf-soph-7-O-ᴅ-glc | broad-banded UV | 0.529 A | ± | 0.141 | 17.1 a | 16.8 a |
| narrow-banded UVB | 0.388 B | ± | 0.129 | 17.3 a | 17.1 a | |
| K-3-O-fer-soph-7-O-glc | broad-banded UV | 0.17 A | ± | 0.045 | 16.4 a | 17.9 a |
| narrow-banded UVB | 0.1 B | ± | 0.031 | 20.4 b | 21.7 b | |
| K-3-O-hfer-soph-7-O-glc | broad-banded UV | 1.204 A | ± | 0.302 | 11.4 a | 11.8 a |
| narrow-banded UVB | 0.936 B | ± | 0.192 | 9.2 b | 9.5 b | |
| K-3-O-sin-soph-7-O-glc | broad-banded UV | 0.26 A | ± | 0.091 | 19.9 a | 21.3 a |
| narrow-banded UVB | 0.235 A | ± | 0.076 | 13.6 b | 15.4 b | |
| Kaempferol glycosides | broad-banded UV | 2.56 A | ± | 0.678 | 15.6 a | 16 a |
| narrow-banded UVB | 1.945 B | ± | 0.504 | 14.8 a | 15.1 a | |
| (c) Quercetin glycosides | Kale | Kale Bread | Kale Bread with Lentil | |||
| mg/g FW in Plant Tissue | Recovery in Kale Bread (%) | |||||
| Q-3-O-triglc | broad-banded UV | 0.495 A | ± | 0.69 | 10.3 a | 10.2 a |
| narrow-banded UVB | 0.624 B | ± | 0.109 | 7.2 b | 8.5 b | |
| Q-3,7,4´-O-ᴅ-triglc | broad-banded UV | 0.145 A | ± | 0.034 | 23.6 a | 25.5 a |
| narrow-banded UVB | 0.132 A | ± | 0.040 | 23.1 a | 22.6 b | |
| Q-3-O-caf-soph-7-O-glc | broad-banded UV | 0.15 A | ± | 0.047 | 40.2 a | 35.1 a |
| narrow-banded UVB | 0.195 B | ± | 0.047 | 15.5 b | 16.1 b | |
| Q-3-O-fer-soph-7-O-ᴅ-glc | broad-banded UV | 0.094 A | ± | 0.024 | N.D. | N.D. |
| narrow-banded UVB | 0.109 A | ± | 0.036 | |||
| Q-3-O-hfer-sophtr-7-O-glc | broad-banded UV | 0.188 A | ± | 0.040 | 75.6 a | 77.2 a |
| narrow-banded UVB | 0.176 B | ± | 0.041 | 63.1 b | 72 a | |
| Q-3-O-sin-soph-7-O-ᴅ-glc | broad-banded UV | 0.131 A | ± | 0.044 | 38.2 a | 38.1 a |
| narrow-banded UVB | 0.175 B | ± | 0.091 | 18.7 b | 21.4 b | |
| Quercetin glycosides | broad-banded UV | 1.203 A | ± | 0.305 | 28.1 a | 27.9 a |
| narrow-banded UVB | 1.41 A | ± | 0.363 | 17.7 a | 19.7 b | |
| (a) Hydroxycinnamic Acid Glycosides | Pea | Pea Bread | Pea Bread with Lentil | ||||||
|---|---|---|---|---|---|---|---|---|---|
| mg/g FW in Plant Tissue | Recovery in Pea Bread (%) | ||||||||
| Coumaroyl-glucoside | broad-banded UV | 0.022 A | ± | 0.008 | 63.5 a | 84.5 a | |||
| narrow-banded UVB | 0.016 B | ± | 0.004 | 47.45 b | 79.1 a | ||||
| Caffeoyl-glucoside 1 | broad-banded UV | 0.006 A | ± | 0.001 | N.D. | N.D. | |||
| narrow-banded UVB | 0.006 A | ± | 0.001 | ||||||
| Caffeoyl-glucoside 2 | broad-banded UV | 0.007 A | ± | 0.001 | N.D. | N.D. | |||
| narrow-banded UVB | 0.007 A | ± | 0.002 | ||||||
| Caffeoyl-glucoside 3 | broad-banded UV | 0.006 A | ± | 0.001 | N.D. | N.D. | |||
| narrow-banded UVB | 0.005 A | ± | 0.001 | ||||||
| Hydroxycinnamic acid derivatives | broad-banded UV | 0.041 A | ± | 0.012 | 34.3 a | 39.7 a | |||
| narrow-banded UVB | 0.034 A | ± | 0.005 | 25.7 a | 37.2 b | ||||
| (b) Kaempferol Glycosides | Pea | Pea Bread | Pea Bread with Lentil | ||||||
| mg/g FW in Plant Tissue | Recovery in Pea Bread (%) | ||||||||
| K-3-O-sophtr 1 | broad-banded UV | 0.086 A | ± | 0.013 | 111 a | 94.4 a | |||
| narrow-banded UVB | 0.098 B | ± | 0.01 | 109.7 a | 107.6 b | ||||
| K-3-O-sophtr 2 | broad-banded UV | 0.024 A | ± | 0.014 | N.D. | N.D. | |||
| narrow-banded UVB | 0.011 B | ± | 0.005 | ||||||
| K-3-O-cou-sophtr 1 | broad-banded UV | 0.681 A | ± | 0.108 | 92.4 a | 77.5 a | |||
| narrow-banded UVB | 0.742 A | ± | 0.114 | 82.1 a | 85.5 b | ||||
| K-3-O-cou-sophtr 2 | broad-banded UV | 0.043 A | ± | 0.008 | 61.5 a | 66.5 a | |||
| narrow-banded UVB | 0.039 A | ± | 0.004 | 52.3 a | 71.2 a | ||||
| K-3-O-sin-sophtr | broad-banded UV | 0.089 A | ± | 0.019 | 99.9 a | 89.4 a | |||
| narrow-banded UVB | 0.093 A | ± | 0.013 | 86.8 b | 89.2 a | ||||
| Kaempferol glycosides | broad-banded UV | 0.922 A | ± | 0.128 | 91.1 a | 79 a | |||
| narrow-banded UVB | 0.984 A | ± | 0.13 | 81.6 b | 86.5 a | ||||
| (c) Quercetin Glycosides | Pea | Pea Bread | Pea Bread with Lentil | ||||||
| mg/g FW in Plant Tissue | Recovery in Pea Bread (%) | ||||||||
| Q-3-O-sophtr 1 | broad-banded UV | 0.354 A | ± | 0.061 | 111.1 a | 93.8 a | |||
| narrow-banded UVB | 0.409 B | ± | 0.02 | 106.6 a | 112 a | ||||
| Q-3-O-sophtr 2 | broad-banded UV | 0.065 A | ± | 0.028 | 178.9 a | 163.1 a | |||
| narrow-banded UVB | 0.07 A | ± | 0.018 | 168.5 a | 187.6 b | ||||
| Q-3-O-cou-sophtr 1 | broad-banded UV | 3.007 A | ± | 0.28 | 100.3 a | 92.4 a | |||
| narrow-banded UVB | 2.896 A | ± | 0.208 | 75.9 b | 91.9 a | ||||
| Q-3-O-cou-sophtr 2 | broad-banded UV | 0.114 A | ± | 0.016 | 109.6 a | 91.6 a | |||
| narrow-banded UVB | 0.122 A | ± | 0.041 | 99.1 a | 103.6 a | ||||
| Q-3-O-cou-sophtr 3 | broad-banded UV | 0.169 A | ± | 0.045 | 68.9 a | 78.9 a | |||
| narrow-banded UVB | 0.137 A | ± | 0.019 | 50.1 b | 72.3 a | ||||
| Q-3-O-caf-sophtr | broad-banded UV | 0.063 A | ± | 0.017 | 108.5 a | 89 a | |||
| narrow-banded UVB | 0.065 A | ± | 0.011 | 96.1 b | 99.7 a | ||||
| Quercetin glycosides | broad-banded UV | 3.772 A | ± | 0.362 | 101.7 a | 93.3 a | |||
| narrow-banded UVB | 3.697 A | ± | 0.261 | 80.3 a | 95.8 b | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klopsch, R.; Baldermann, S.; Voss, A.; Rohn, S.; Schreiner, M.; Neugart, S. Narrow-Banded UVB Affects the Stability of Secondary Plant Metabolites in Kale (Brassica oleracea var. sabellica) and Pea (Pisum sativum) Leaves Being Added to Lentil Flour Fortified Bread: A Novel Approach for Producing Functional Foods. Foods 2019, 8, 427. https://doi.org/10.3390/foods8100427
Klopsch R, Baldermann S, Voss A, Rohn S, Schreiner M, Neugart S. Narrow-Banded UVB Affects the Stability of Secondary Plant Metabolites in Kale (Brassica oleracea var. sabellica) and Pea (Pisum sativum) Leaves Being Added to Lentil Flour Fortified Bread: A Novel Approach for Producing Functional Foods. Foods. 2019; 8(10):427. https://doi.org/10.3390/foods8100427
Chicago/Turabian StyleKlopsch, Rebecca, Susanne Baldermann, Alexander Voss, Sascha Rohn, Monika Schreiner, and Susanne Neugart. 2019. "Narrow-Banded UVB Affects the Stability of Secondary Plant Metabolites in Kale (Brassica oleracea var. sabellica) and Pea (Pisum sativum) Leaves Being Added to Lentil Flour Fortified Bread: A Novel Approach for Producing Functional Foods" Foods 8, no. 10: 427. https://doi.org/10.3390/foods8100427
APA StyleKlopsch, R., Baldermann, S., Voss, A., Rohn, S., Schreiner, M., & Neugart, S. (2019). Narrow-Banded UVB Affects the Stability of Secondary Plant Metabolites in Kale (Brassica oleracea var. sabellica) and Pea (Pisum sativum) Leaves Being Added to Lentil Flour Fortified Bread: A Novel Approach for Producing Functional Foods. Foods, 8(10), 427. https://doi.org/10.3390/foods8100427