Mechanism of Action against Food Spoilage Yeasts and Bioactivity of Tasmannia lanceolata, Backhousia citriodora and Syzygium anisatum Plant Solvent Extracts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Milling
2.3. Solvent Extraction
2.4. Microorganisms
2.5. Antimicrobial
2.6. Yeast Cell Staining and Fluorescence Microscopy
2.7. Total Phenolic Content
2.8. DPPH Radical Scavenging Activity
2.9. Reducing Power
2.10. UHPLC-MS Analysis
2.11. Statistical Analysis
3. Results
3.1. Extraction Yield and Extracts Characteristics
3.2. Total Phenolic Content
3.3. DPPH Radical Scavenging Activity
3.4. Reducing Power
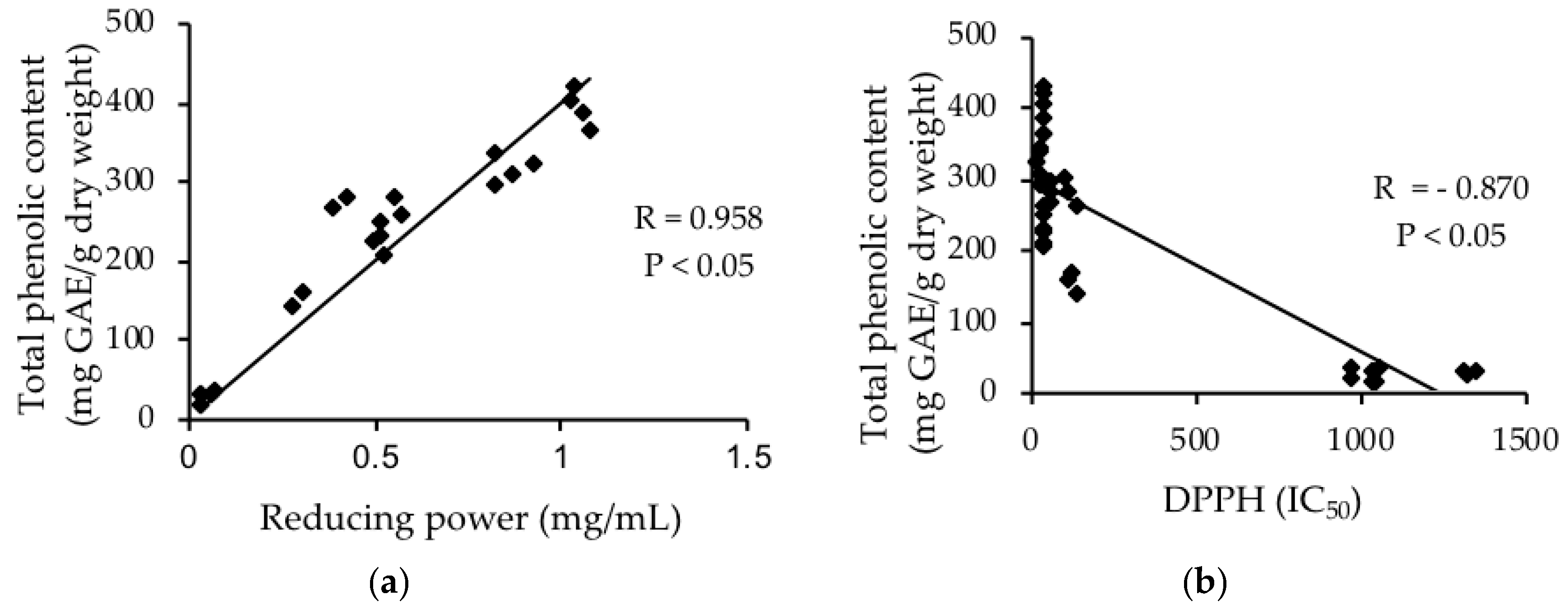
3.5. Relationship between Total Phenolic Content and Antioxidant Capacities
3.6. Antimicrobial Activities
3.7. Mode of Antifungal Action
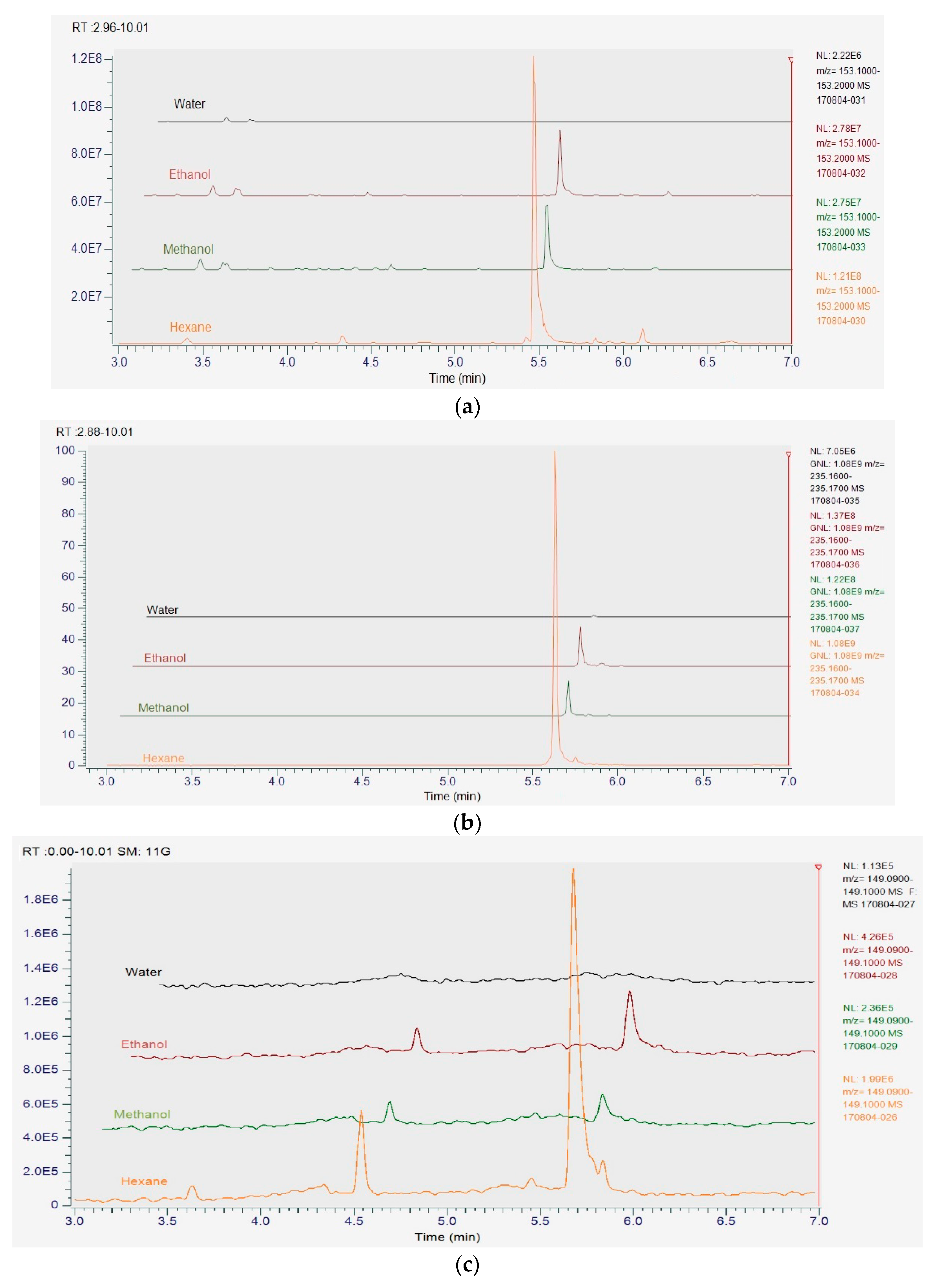
3.8. UHPLC-MS Analysis of Herb Extracts
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wareing, P. Microbiology of soft drinks and fruit juices. In Chemistry and Technology of Soft Drinks and Fruit Juices, 3rd ed.; Ashurst, P.R., Ed.; Wiley-Blackwell: Oxford, UK, 2016; pp. 290–309. [Google Scholar]
- Piper, P.W. Resistance of yeasts to weak organic acid food preservatives. In Advances in Applied Microbiology; Laskin, A.I., Sariaslani, S., Gadd, G.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 77, pp. 97–113. [Google Scholar]
- Hayashi, M.; Naknukool, S.; Hayakawa, S.; Ogawa, M.; Ni’matulah, A.-B.A. Enhancement of antimicrobial activity of a lactoperoxidase system by carrot extract and β-carotene. Food Chem. 2012, 130, 541–546. [Google Scholar] [CrossRef]
- Clarke, M. Australian Native Food Industry Stocktake; RIRDC Publication No. 12/066; Union Offset Printing: Canberra, Australia, 2012. [Google Scholar]
- Pengelly, A. Indigenous and naturalised herbs: Tasmannia lanceolata: Mountain pepper. Aust. J. Med. Herbal. 2002, 14, 71–74. [Google Scholar]
- Sultanbawa, Y. Tasmanian Pepper Leaf (Tasmannia lanceolata) Oils. In Essential Oils in Food Preservation, Flavor and Safety; Preedy, V.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 819–823. [Google Scholar]
- Cock, I.E. The phytochemistry and chemotherapeutic potential of Tasmannia lanceolata (Tasmanian pepper): A review. Pharmacogn. Commun. 2013, 3, 1–13. [Google Scholar] [CrossRef]
- Fujita, K.-I.; Kubo, I. Naturally occurring antifungal agents against Zygosaccharomyces bailii and their synergism. J. Agric. Food Chem. 2005, 53, 5187–5191. [Google Scholar] [CrossRef] [PubMed]
- Kubo, I.; Fujita, K.I.; Lee, S.H.; Ha, T.J. Antibacterial activity of polygodial. Phytother. Res. 2005, 19, 1013–1017. [Google Scholar] [CrossRef] [PubMed]
- Menary, R.C.; Dragar, V.A.; Thomas, S.; Read, C.D. Mountain Pepper Extract, Tasmannia Lanceolata: Quality Stabilisation and Registration: A Report for the Rural Industries Research and Development Corporation; RIRDC: Barton, Australia, 2003. [Google Scholar]
- Guo, Y.; Sakulnarmrat, K.; Konczak, I. Anti-inflammatory potential of native Australian herbs polyphenols. Toxicol. Rep. 2014, 1, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Konczak, I.; Zabaras, D.; Dunstan, M.; Aguas, P. Antioxidant capacity and phenolic compounds in commercially grown native Australian herbs and spices. Food Chem. 2010, 122, 260–266. [Google Scholar] [CrossRef]
- Buchaillot, A.; Caffin, N.; Bhandari, B. Drying of lemon myrtle (Backhousia citriodora) leaves: Retention of volatiles and color. Dry. Technol. 2009, 27, 445–450. [Google Scholar] [CrossRef]
- Horn, T.; Barth, A.; Rühle, M.; Häser, A.; Jürges, G.; Nick, P. Molecular diagnostics of Lemon Myrtle (Backhousia citriodora versus Leptospermum citratum). Eur. Food Res. Technol. 2012, 234, 853–861. [Google Scholar] [CrossRef]
- Forbes-Smith, M.; Paton, J. Innovative Products from Australian Native Foods; Rural Industries Research and Development Corporation (RIRDC): Canberra, Australia, 2002. [Google Scholar]
- Southwell, I.A.; Russell, M.; Smith, R.L.; Archer, D.W. Backhousia citriodora F. Muell. (Myrtaceae), a superior source of citral. J. Essent. Oil Res. 2000, 12, 735–741. [Google Scholar] [CrossRef]
- Scheman, A.; Scheman, N.; Rakowski, E.-M. European directive fragrances in natural products. Dermatitis 2014, 25, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Ress, N.; Hailey, J.; Maronpot, R.; Bucher, J.; Travlos, G.; Haseman, J.; Orzech, D.; Johnson, J.; Hejtmancik, M. Toxicology and carcinogenesis studies of microencapsulated citral in rats and mice. Toxicol. Sci. 2003, 71, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Smyth, H.E.; Sanderson, J.E.; Sultanbawa, Y. Lexicon for the Sensory Description of Australian Native Plant Foods and Ingredients. J. Sens. Stud. 2012, 27, 471–481. [Google Scholar] [CrossRef]
- Pengelly, A. Antimicrobial activity of lemon myrtle and tea tree oils. Aust. J. Med. Herbal. 2003, 15, 9. [Google Scholar]
- Southwell, I.; Russell, M.; Smith, R. Chemical composition of some novel aromatic oils from the Australian flora. In Proceedings of the International Conference on Medicinal and Aromatic Plants (Part II) 597, Budapest, Hungary, 8–11 July 2001; pp. 79–89. [Google Scholar]
- Blewitt, M.; Southwell, I.A. Backhousia anisata Vickery, an alternative source of (E)-anethole. J. Essent. Oil Res. 2000, 12, 445–454. [Google Scholar] [CrossRef]
- Brophy, J.J.; Boland, D.J. The leaf essential oil of two chemotypes of Backhousia anisata Vickery. Flavour Fragr. J. 1991, 6, 187–188. [Google Scholar] [CrossRef]
- Fujita, K.I.; Fujita, T.; Kubo, I. Anethole, a potential antimicrobial synergist, converts a fungistatic dodecanol to a fungicidal agent. Phytother. Res. 2007, 21, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Himejima, M.; Kubo, I. Fungicidal activity of polygodial in combination with anethole and indole against Candida albicans. J. Agric. Food Chem. 1993, 41, 1776–1779. [Google Scholar] [CrossRef]
- Dupont, S.; Caffin, N.; Bhandari, B.; Dykes, G.A. In vitro antibacterial activity of Australian native herb extracts against food-related bacteria. Food Control. 2006, 17, 929–932. [Google Scholar] [CrossRef]
- Sultanbawa, Y. Essential Oils in Food Applications: Australian Aspects. In Essential Oils in Food Preservation, Flavor and Safety; Preedy, V.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 155–160. [Google Scholar]
- Zhao, J.; Agboola, S.O. Functional Properties of Australian Bushfoods: A Report for the Rural Industries Research and Development Corporation; RIRDC: Barton, Australia, 2007. [Google Scholar]
- Tiwari, B.K.; Valdramidis, V.P.; O’Donnell, C.P.; Muthukumarappan, K.; Bourke, P.; Cullen, P.J. Application of natural antimicrobials for food preservation. J. Agric. Food Chem. 2009, 57, 5987. [Google Scholar] [CrossRef] [PubMed]
- Savoia, D. Plant-derived antimicrobial compounds: Alternatives to antibiotics. Future Microbiol. 2012, 7, 979–990. [Google Scholar] [CrossRef] [PubMed]
- Aneja, K.R.; Dhiman, R.; Aggarwal, N.K.; Aneja, A. Emerging preservation techniques for controlling spoilage and pathogenic microorganisms in fruit juices. Int. J. Microbiol. 2014, 2014, 758942. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Ali, A.M.; Israf, D.A.; Ismail, N.H.; Shaari, K.; Lajis, N.H. Antioxidant, radical-scavenging, anti-inflammatory, cytotoxic and antibacterial activities of methanolic extracts of some Hedyotis species. Life Sci. 2005, 76, 1953–1964. [Google Scholar] [CrossRef] [PubMed]
- Shimada, S.; Andou, M.; Naito, N.; Yamada, N.; Osumi, M.; Hayashi, R. Effects of hydrostatic pressure on the ultrastructure and leakage of internal substances in the yeast Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 1993, 40, 123–131. [Google Scholar] [CrossRef]
- Singleton, V.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Nirmal, N.P.; Panichayupakaranant, P. Antioxidant, antibacterial, and anti-inflammatory activities of standardized brazilin-rich Caesalpinia sappan extract. Pharm. Boil. 2015, 53, 1339–1343. [Google Scholar] [CrossRef] [PubMed]
- Karathia, H.; Vilaprinyo, E.; Sorribas, A.; Alves, R. Saccharomyces cerevisiae as a model organism: A comparative study. PLoS ONE 2011, 6, e16015. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Freiesleben, S.; Jager, A. Correlation between Plant Secondary Metabolites and Their Antifungal Mechanisms—A Review. Med. Aromat. Plants 2014, 3, 1–6. [Google Scholar]
- Hayouni, E.A.; Abedrabba, M.; Bouix, M.; Hamdi, M. The effects of solvents and extraction method on the phenolic contents and biological activities in vitro of Tunisian Quercus coccifera L. and Juniperus phoenicea L. fruit extracts. Food Chem. 2007, 105, 1126–1134. [Google Scholar] [CrossRef]
- Zafeiropoulou, T.; Evageliou, V.; Gardeli, C.; Yanniotis, S.; Komaitis, M. Retention of trans-anethole by gelatine and starch matrices. Food Chem. 2010, 123, 364–368. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, J.; Zhou, L.; Wang, J.; Gong, Y.; Chen, X.; Guo, Z.; Wang, Q.; Jiang, W. Antifungal activity of the essential oil of Illicium verum fruit and its main component trans-anethole. Molecules 2010, 15, 7558–7569. [Google Scholar] [CrossRef] [PubMed]
- Kurekci, C.; Padmanabha, J.; Bishop-Hurley, S.L.; Hassan, E.; Al Jassim, R.A.; McSweeney, C.S. Antimicrobial activity of essential oils and five terpenoid compounds against Campylobacter jejuni in pure and mixed culture experiments. Int. J. Food Microbiol. 2013, 166, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Bassolé, I.H.N.; Lamien-Meda, A.; Bayala, B.; Tirogo, S.; Franz, C.; Novak, J.; Nebié, R.C.; Dicko, M.H. Composition and Antimicrobial Activities of Lippia multiflora Moldenke, Mentha x piperita L. and Ocimum basilicum L. Essential Oils and Their Major Monoterpene Alcohols Alone and in Combination. Molecules 2010, 15, 7825. [Google Scholar] [CrossRef] [PubMed]
- Delaquis, P.J.; Stanich, K.; Girard, B.; Mazza, G. Antimicrobial activity of individual and mixed fractions of dill, cilantro, coriander and eucalyptus essential oils. Int. J. Food Microbiol. 2002, 74, 101–109. [Google Scholar] [CrossRef]
- Sultanbawa, Y.; Cusack, A.; Currie, M.; Davis, C. An innovative microplate assay to facilitate the detection of antimicrobial activity in plant extracts. J. Rapid Methods Autom. Microbiol. 2009, 17, 519–534. [Google Scholar] [CrossRef]
- Weerakkody, N.S.; Caffin, N.; Turner, M.S.; Dykes, G.A. In vitro antimicrobial activity of less-utilized spice and herb extracts against selected food-borne bacteria. Food Control 2010, 21, 1408–1414. [Google Scholar] [CrossRef]
- López, V.; Akerreta, S.; Casanova, E.; García-Mina, J.M.; Cavero, R.Y.; Calvo, M.I. In vitro antioxidant and anti-rhizopus activities of Lamiaceae herbal extracts. Plant Foods Hum. Nutr. 2007, 62, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Barchan, A.; Bakkali, M.; Arakrak, A.; Pagán, R.; Laglaoui, A. The effects of solvents polarity on the phenolic contents and antioxidant activity of three Mentha species extracts. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 399–412. [Google Scholar]
- Sakulnarmrat, K.; Konczak, I. Composition of native Australian herbs polyphenolic-rich fractions and in vitro inhibitory activities against key enzymes relevant to metabolic syndrome. Food Chem. 2012, 134, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, B.; Yao, S. Application of ultrasonic technique for extracting chlorogenic acid from Eucommia ulmodies Oliv. (E. ulmodies). Ultrason. Sonochem. 2005, 12, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Du, F.-Y.; Xiao, X.-H.; Luo, X.-J.; Li, G.-K. Application of ionic liquids in the microwave-assisted extraction of polyphenolic compounds from medicinal plants. Talanta 2009, 78, 1177–1184. [Google Scholar] [CrossRef] [PubMed]
- Iloki-Assanga, S.B.; Lewis-Luján, L.M.; Lara-Espinoza, C.L.; Gil-Salido, A.A.; Fernandez-Angulo, D.; Rubio-Pino, J.L.; Haines, D.D. Solvent effects on phytochemical constituent profiles and antioxidant activities, using four different extraction formulations for analysis of Bucida buceras L. and Phoradendron californicum. BMC Res. Notes 2015, 8, 396. [Google Scholar] [CrossRef] [PubMed]
- Brahmi, F.; Mechri, B.; Dabbou, S.; Dhibi, M.; Hammami, M. The efficacy of phenolics compounds with different polarities as antioxidants from olive leaves depending on seasonal variations. Ind. Crops Prod. 2012, 38, 146–152. [Google Scholar] [CrossRef]
- Vlietinck, A.; Van Hoof, L.; Totte, J.; Lasure, A.; Berghe, D.V.; Rwangabo, P.; Mvukiyumwami, J. Screening of hundred Rwandese medicinal plants for antimicrobial and antiviral properties. J. Ethnopharmacol. 1995, 46, 31–47. [Google Scholar] [CrossRef]
- Shan, B.; Cai, Y.-Z.; Brooks, J.D.; Corke, H. The in vitro antibacterial activity of dietary spice and medicinal herb extracts. Int. J. Food Microbiol. 2007, 117, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Ali-Shtayeh, M.; Yaghmour, R.M.-R.; Faidi, Y.; Salem, K.; Al-Nuri, M. Antimicrobial activity of 20 plants used in folkloric medicine in the Palestinian area. J. Ethnopharmacol. 1998, 60, 265–271. [Google Scholar] [CrossRef]
- Chuang, P.-H.; Lee, C.-W.; Chou, J.-Y.; Murugan, M.; Shieh, B.-J.; Chen, H.-M. Anti-fungal activity of crude extracts and essential oil of Moringa oleifera Lam. Bioresour. Technol. 2007, 98, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Ultee, A.; Bennik, M.; Moezelaar, R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 2002, 68, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Santoro, G.F.; das Graças Cardoso, M.; Guimarães, L.G.L.; Salgado, A.P.S.; Menna-Barreto, R.F.; Soares, M.J. Effect of oregano (Origanum vulgare L.) and thyme (Thymus vulgaris L.) essential oils on Trypanosoma cruzi (Protozoa: Kinetoplastida) growth and ultrastructure. Parasitol. Res. 2007, 100, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Prashar, A.; Hili, P.; Veness, R.G.; Evans, C.S. Antimicrobial action of palmarosa oil (Cymbopogon martinii) on Saccharomyces cerevisiae. Phytochemistry 2003, 63, 569–575. [Google Scholar] [CrossRef]
- Lima, I.O.; Pereira, F.D.O.; Oliveira, W.A.D.; Lima, E.D.O.; Menezes, E.A.; Cunha, F.A.; Diniz, M.D.F.F.M. Antifungal activity and mode of action of carvacrol against Candida albicans strains. J. Essent. Oil Res. 2013, 25, 138–142. [Google Scholar] [CrossRef]
- Hoehamer, C.F.; Cummings, E.D.; Hilliard, G.M.; Rogers, P.D. Changes in the proteome of Candida albicans in response to azole, polyene, and echinocandin antifungal agents. Antimicrob. Agents Chemother. 2010, 54, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- Miron, D.; Battisti, F.; Silva, F.K.; Lana, A.D.; Pippi, B.; Casanova, B.; Gnoatto, S.; Fuentefria, A.; Mayorga, P.; Schapoval, E.E. Antifungal activity and mechanism of action of monoterpenes against dermatophytes and yeasts. Rev. Bras. Farmacogn. 2014, 24, 660–667. [Google Scholar] [CrossRef]

| Methanol | Ethanol | Water | Hexane | |||
|---|---|---|---|---|---|---|
| Yields (% w/w) | LM | 22.8 ± 0.4 a | 17.9 ± 0.5 b | 16.3 ± 0.6 c | 6.41 ± 0.2 d | |
| TPL | 28.3 ± 0.3 a | 27.8 ± 0.4 a | 25.8 ± 0.3 b | 8.13 ± 0.5 c | ||
| AM | 21.8 ± 0.4 a | 16.8 ± 0.6 b | 17.5 ± 0.7 b | 3.88 ± 0.3 c | ||
| Total phenolic content (mg GAE/gDW) | LM | 419.3 ± 13.5 a | 373.2 ± 12.6 b | 281.7 ± 21.6 c | 17.5 ± 1.7 d | |
| TPL | 246.3 ± 17.4 a | 215.5 ± 12.8 a | 157.4 ± 14.6 b | 35.7 ± 1.9 d | ||
| AM | 314.2 ± 17.3 a | 310.6 ± 18.3 a | 283.3 ± 16.5 b | 30.5 ± 2.1 c | ||
| DPPH (IC50 µg/mL) | LM | 14.4 ± 0.4 a | 14.3 ± 0.6 a | 31.0 ± 1.1 b | 1678.3 ± 27.9 c | |
| TPL | 36.9 ± 0.6 a | 36.2 ± 0.8 a | 126.4 ± 16.1 b | 1004.7 ± 35.9 c | ||
| AM | 19.1 ± 1.2 a | 21.1 ± 0.1 a | 61.9 ± 0.2 b | 1342.7 ± 22.9 c | ||
| Reducing power (Absorbance 700 nm) | 0.01 mg/mL extracts | LM | 0.59 ± 0.01 a | 0.59 ± 0.02 a | 0.32 ± 0.01 b | 0.03 ± 0.01 c |
| TPL | 0.29 ± 0.01 a | 0.31 ± 0.01 a | 0.14 ± 0.01 b | 0.04 ± 0.02 c | ||
| AM | 0.49 ± 0.02 a | 0.45 ± 0.02 a | 0.25 ± 0.01 b | 0.025 ± 0.01 c | ||
| 0.1 mg/mL extracts | LM | 1.03 ± 0.01 a | 1.07 ± 0.02 a | 0.56 ± 0.02 b | 0.03 ± 0.01 c | |
| TPL | 0.51 ± 0.01 a | 0.52 ± 0.01 a | 0.30 ± 0.02 b | 0.07 ± 0.01 c | ||
| AM | 0.87 ± 0.1 a | 0.84 ± 0.03 a | 0.41 ± 0.03 b | 0.03 ± 0.01 c | ||
| D. anomala | S. pombe | S. cerevisiae | C. albicans | R. mucilaginosa | C. krusei | S. aureus | E. coli | ||
|---|---|---|---|---|---|---|---|---|---|
| TPL | M | 17.6 ± 0.8 b | 13.7 ± 0.3 b | 17.2 ±0.5 b | 14.2 ± 0.3 b | 17.1 ± 0.8 b | 16.4 ± 0.5 c | 12.3 ± 0.4 b | 9.0 ± 0.2 b |
| E | 16.4 ± 0.6 c | 13.0 ± 0.3 b | 17.4 ± 0.4 b | 14.8 ± 0.3 b | 16.7 ± 0.4 b | 15.2 ± 0.7 b | 12.2 ± 0.2 b | 8.3 ± 0.4 b | |
| H | 23.9 ± 0.4 a | 17.0 ± 0.3 a | 20.7 ± 0.4 a | 17.1 ± 0.4 a | 21.4 ± 0.7 a | 19.6 ± 0.5 a | 13.6 ± 0.2 a | 10.9 ± 0.3 a | |
| LM | M | 27.3 ± 0.7 b | 12.1 ± 0.8 b | 11.8 ± 1.2 b | 12.7 ± 1.3 b | 14.7 ± 0.9 b | 11.0 ± 0.8 b | 10.1 ± 0.4 a,b | 0 |
| E | 24.1 ± 0.9 c | 11.1 ± 0.5 b | 10.9 ± 1.0 b | 14.1 ± 0.7 b | 14.1 ± 0.7 b | 10.1 ± 0.7 b | 9.3 ± 0.7 b | 0 | |
| H | 43.3 ± 2.1 a | 35.7 ± 1.2 a | 34.9 ± 1.4 a | 26.8 ± 0.7 a | 21.04 ± 1.8 a | 19.8 ± 1.4 a | 11.5 ± 0.8 a | 8.2 ± 0.7 a | |
| AM | M | 23.4 ± 0.5 b | 13.3 ± 0.7 b | 11.3 ± 0.6 b | 13.1 ± 0.6 b | 14.9 ± 0.3 b | 10.0 ± 0.3 b | 11.4 ± 0.5 a | 0 |
| E | 21.9 ± 0.8 c | 10.5 ± 0.7 c | 9.9 ± 0.7 c | 10.9 ± 0.9 c | 14.2 ± 0.4 b | 8.9 ± 0.3 c | 8.8 ± 0.5 b | 0 | |
| H | 26.9 ± 0.4 a | 14.7 ± 0.4 a | 13.2 ± 0.5 a | 14.7 ± 0.7 a | 16.9 ± 0.6 a | 11.3 ± 0.8 a | 12.5 ± 0.6 a | 0 | |
| Fluconazole | 37.2 ± 4.5 | 11.1 ± 0.3 | 11.4 ± 0.5 | 10.9 ± 0.9 | 9.5 ± 1.5 | 12.7 ± 0.3 | NT | NT | |
| Amphotericin B | 18.4 ± 0.3 | 11.9 ± 0.4 | 0 | 0 | 12.8 ± 0.8 | 0 | NT | NT | |
| Chloramphenicol | NT | NT | NT | NT | NT | NT | 24.0 ± 1.9 | 20.5 ± 0.6 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alderees, F.; Mereddy, R.; Webber, D.; Nirmal, N.; Sultanbawa, Y. Mechanism of Action against Food Spoilage Yeasts and Bioactivity of Tasmannia lanceolata, Backhousia citriodora and Syzygium anisatum Plant Solvent Extracts. Foods 2018, 7, 179. https://doi.org/10.3390/foods7110179
Alderees F, Mereddy R, Webber D, Nirmal N, Sultanbawa Y. Mechanism of Action against Food Spoilage Yeasts and Bioactivity of Tasmannia lanceolata, Backhousia citriodora and Syzygium anisatum Plant Solvent Extracts. Foods. 2018; 7(11):179. https://doi.org/10.3390/foods7110179
Chicago/Turabian StyleAlderees, Fahad, Ram Mereddy, Dennis Webber, Nilesh Nirmal, and Yasmina Sultanbawa. 2018. "Mechanism of Action against Food Spoilage Yeasts and Bioactivity of Tasmannia lanceolata, Backhousia citriodora and Syzygium anisatum Plant Solvent Extracts" Foods 7, no. 11: 179. https://doi.org/10.3390/foods7110179
APA StyleAlderees, F., Mereddy, R., Webber, D., Nirmal, N., & Sultanbawa, Y. (2018). Mechanism of Action against Food Spoilage Yeasts and Bioactivity of Tasmannia lanceolata, Backhousia citriodora and Syzygium anisatum Plant Solvent Extracts. Foods, 7(11), 179. https://doi.org/10.3390/foods7110179






