Mushroom Polysaccharides: Chemistry and Antiobesity, Antidiabetes, Anticancer, and Antibiotic Properties in Cells, Rodents, and Humans
Abstract
:1. Introduction
2. Isolation and Characterization of Mushroom Polysaccharides
2.1. Enhanced Biosynthesis of Mushroom Polysaccharides
2.2. Effect of γ-Radiation on Fractionation of Polysaccharides
3. Antioxidative, Immunostimulating, and Anti-Inflammatory Properties
4. Role of Gut Microbiota in Obesity and Diabetes
4.1. Definitions and Significance of Prebiotics, Probiotics, and Synbiotics
- Prebiotics are post-ingestion fermentable food ingredients such as non-digestible polysaccharides (β-glucans) and fibers that induce changes in the gastrointestinal microbiota that result in improved nutritional and health benefits to the host. Prebiotics of low-molecular-mass oligosaccharides are more rapidly and selectively fermented by Bifidobacteria and lactobacilli than higher molecular weight polysaccharides [110]. Prebiotics including polyphenol-rich apple and grape pomace polyphenols [112,113,114], can beneficially affect disorders of the digestive and immune systems, hypertension, appetite and obesity. Commercial prebiotics include the following non-digestible oligosaccharides: inulin, fructo-oligosaccharides (FOS), and galacto-oligosaccharides (GOS). The fermentation of non-digestible long-chain β-glucans from mushrooms to form short chain fatty acids (SCFAs) provides a potential source of new prebiotics [110]. Because Ganoderma lucidum reduces obesity in mice by modulating microbiota, Delzenne and Bindels [99] suggest that extracts of this mushroom might be considered a new prebiotic to treat obesity.
- Probiotics are preparations containing safe live gut microorganisms, usually Bifidobacteria and Lactobacilli, that are tolerant to the action of gastric and bile actions and which can alter the microflora of the digestive tract resulting in beneficial health effects to the host. Probiotics such as yogurt are reported to ameliorate adverse health syndromes (allergy, asthma, cancer, anxiety, and depression). Multifunctional prebiotics and probiotics have the potential to control obesity by helping regulate gut microbiota, food intake, and body weight. They therefore might be useful in the treatment of obesity, hyperglycemia, and dyslipidemia [115].
- Synbiotics are products containing both prebiotics and probiotics and refer specifically to the utilization of prebiotics by probiotic bacteria that form fermentation products that are more effective in ameliorating adverse health effects via modulation of the intestinal flora than are placebos [116].
4.2. Modulation of Gut Microbiota by Prebiotic Mushroom Polysaccharides
5. Antiobesity Effects
5.1. In Cells and Rodents
5.1.1. Coriolus Versicolor
5.1.2. Tremella Fuciformis
5.1.3. Ganoderma Lucidum
5.1.4. Pleurotus Tuber-Regium
5.2. In Humans
6. Antidiabetic Properties
6.1. Historical Perspective on the Cause and Treatment of Diabetes
6.2. Antidiabetic Activities of Mushroom Polysaccharides
6.3. Mechanisms of Bioactivities of Polysaccharides against Diabetes
6.4. Polysaccharides Accelerate Wound Healing in Diabetic Rats
6.5. Human Clinical Studies on the Efficacy of Mushrooms against Diabetes
7. Anticancer Effects
- Dietary supplementation of Agaricus silvaticus mushrooms reduced glycaemia levels of post-surgery cancer patients [195];
- Oral administration of a polysaccharide from Grifola frondosa stimulated the immune system of breast cancer patients [196];
- Dietary supplementation with Agaricus silvaticus provided metabolic and blood pressure benefits to postsurgical colorectal cancer patients [197];
- The mushroom β-glucan lentinan prolonged the survival of patients with advanced gastric cancer [203];
- Dietary administration of Agaricus silvaticus mushrooms improved nutritional status and reduced abnormal bowel functions and nausea in patients undergoing chemotherapy for breast cancer [204];
- A meta-analysis suggests that dietary mushroom intake seemed to be inversely associated with risk of breast cancer [205];
- Oral consumption of mushrooms glucans seems to be an efficient treatment to prevent colitis-associated cancer through modification of mucosal inflammation and cell proliferation [206];
- Consumption of an Agaricus bisporus mushroom powder by prostate cancer patients beneficially impacted prostate-specific antigen (PSA) levels and modulated the biology of recurrent prostate cancer by decreasing immunosuppressive factors [207].
7.1. Anti-Aromatase Activity against Breast Cancer
7.2. Crohn’s Disease Therapy
8. Antimicrobial Activities
8.1. Antibiotic Effects
8.2. Anti-Quorum Sensing Mushroom Compounds
8.3. Antiviral Activities
9. Additional Health Benefits
9.1. Protection of Mice against Allergic Asthma
9.2. Protection of Mice against Adverse Effects of γ-Radiation
9.3. Effect of UV-B and on Vitamin D2 Content of Mushrooms
10. Conclusions
- Determine whether the antiobesity and antidiabetic properties of pure polysaccharides vary depending on whether they are tested or consumed in their free state or as part of a food.
- Determine the relationship between consumption of mushroom polysaccharides and lower risks of obesity and diabetes.
- Determine whether mushroom polysaccharide metabolites formed in the digestive tract and after absorption into the circulation possess antiobesity and antidiabetic properties.
- Determine whether the polysaccharide content of mushrooms can predict antiobesity and antidiabetic activities.
- Define mitigating effects of conditions associated with the metabolic syndrome of combinations of mushroom polysaccharides with other bioactive natural products and compounds, including apples [255], bioactive potato and eggplant (aubergine) glycoalkaloids and potato calystegine alkaloids [256,257], tomatoes and tomato compounds [256,258,259,260,261,262], rice hull smoke extract [152,263,264,265], bioactive compounds from essential oils and spices [266], bioactive rice bran compounds [267], bioactive tea compounds [268,269], and red wine and winery byproducts [270,271,272].
- Determine bioactivities of mixtures of structurally different polysaccharides isolated from different mushroom species.
- Determine additive and synergistic health properties of combinations of dietary β-glucans derived from mushrooms and cereals.
- Determine additive and synergistic properties of mushroom polysaccharides in combination with antiobesity and antidiabetes drugs.
- Because mushrooms can break down complex plant materials into smaller compounds to produce additional bioactive compounds during bioprocessing (fermentation), determine if this novel approach can be extended to other combinations of mushrooms with polymeric materials present in tree barks and plant cell walls, a largely unexplored area of mushroom research [238].
- Because the preparation of low-sodium meat with improved flavor properties might help reduce hypertension [275], evaluate flavor-enhancing properties of mushrooms added to low-sodium meat-based food [146]. Develop flavor- and health- enhancing mushroom seasonings (powders) that can be added to food [276].
- Because diet-induced obesity in male mice and possibly also in male humans might be associated with reduced fertility caused by acrylamide-induced reproductive toxicity [277,278,279], determine if mushroom-induced reduction in human obesity will concurrently mitigate acrylamide-induced male infertility.
- Because growth hormone receptor deficiency in about 100 Ecuadorian adults seems to be associated with essentially no incidences of lifetime cancers and diabetes among these dwarfs, it might be worthwhile to find out the possible effect of polysaccharides on the growth hormone in the general population [280,281].
- Determine if mushroom polysaccharide-induced stimulation of the human immune system would help protect against susceptible and antibiotic-resistant foodborne (E. coli, Listeria, Salmonella) and medical (Clostridium difficile, Klebsiella, Streptococci) pathogenic bacteria and endotoxemia [31,92,93,282].
- Encourage researchers to create high-polysaccharide mushrooms using plant molecular biology methods and mushroom growers to produce mushrooms with a high content of polysaccharides.
Acknowledgments
Conflicts of Interest
References
- Aida, F.M.N.A.; Shuhaimi, M.; Yazid, M.; Maaruf, A.G. Mushroom as a potential source of prebiotics: A review. Trends Food Sci. Technol. 2009, 20, 567–575. [Google Scholar] [CrossRef]
- Kundaković, T.; Kolundžić, M. Therapeutic properties of mushrooms in managing adverse effects in the metabolic syndrome. Curr. Top. Med. Chem. 2013, 13, 2734–2744. [Google Scholar] [CrossRef] [PubMed]
- Wasser, S.P. Medicinal mushroom science: Current perspectives, advances, evidences, and challenges. Biomed. J. 2014, 37, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Su, C.H.; Lai, M.N.; Lin, C.C.; Ng, L.T. Comparative characterization of physicochemical properties and bioactivities of polysaccharides from selected medicinal mushrooms. Appl. Microbiol. Biotechnol. 2016, 100, 4385–4393. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations, Statistics Division (FAOSTAT). Crops Production. Available online: http://faostat.fao.org (accessed on 5 May 2016).
- U.S. Department of Agriculture, Agricultural Research Service. USDA National Nutrient Database for Standard Reference, Release 28. Available online: http://www.ars.usda.gov/ba/bhnrc/ndl (accessed on 28 April 2016).
- Ooi, V.E.C.; Liu, F. Immunomodulation and anti-cancer activity of polysaccharide-protein complexes. Curr. Med. Chem. 2000, 7, 715–729. [Google Scholar] [CrossRef] [PubMed]
- Tsukagoshi, S.; Hashimoto, Y.; Fujii, G.; Kobayashi, H.; Nomoto, K.; Orita, K. Krestin (PSK). Cancer Treat. Rev. 1984, 11, 131–155. [Google Scholar] [CrossRef]
- Maehara, Y.; Tsujitani, S.; Saeki, H.; Oki, E.; Yoshinaga, K.; Emi, Y.; Morita, M.; Kohnoe, S.; Kakeji, Y.; Yano, T.; et al. Biological mechanism and clinical effect of protein-bound polysaccharide K (KRESTIN®): Review of development and future perspectives. Surg. Today 2012, 42, 8–28. [Google Scholar] [CrossRef] [PubMed]
- Feeney, M.J.; Dwyer, J.; Hasler-Lewis, C.M.; Milner, J.A.; Noakes, M.; Rowe, S.; Wach, M.; Beelman, R.B.; Caldwell, J.; Cantorna, M.T.; et al. Mushrooms and health summit proceedings. J. Nutr. 2014, 144, 1128S–1136S. [Google Scholar] [CrossRef] [PubMed]
- Poddar, K.H.; Ames, M.; Hsin-Jen, C.; Feeney, M.J.; Wang, Y.; Cheskin, L.J. Positive effect of mushrooms substituted for meat on body weight, body composition, and health parameters. A 1-year randomized clinical trial. Appetite 2013, 71, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhou, W.; Lee, D.S.; Shim, S.H.; Kim, Y.C.; Kim, Y.H. Hericirine, a novel anti-inflammatory alkaloid from Hericium erinaceum. Tetrahedron Lett. 2014, 55, 4086–4090. [Google Scholar] [CrossRef]
- Li, W.; Zhou, W.; Kim, E.J.; Shim, S.H.; Kang, H.K.; Kim, Y.H. Isolation and identification of aromatic compounds in Lion’s Mane mushroom and their anticancer activities. Food Chem. 2015, 170, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Beers, M.H. The Merck Manual of Diagnosis and Therapy, 18th ed.; Merck Research Laboratories: Whitehouse Station, NJ, USA, 2006. [Google Scholar]
- Gavin, J.R.; Alberti, K.G.M.M.; Davidson, M.B.; DeFronzo, R.A.; Drash, A.; Gabbe, S.G.; Genuth, S.; Harris, M.I.; Kahn, R.; Keen, H.; et al. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 2003, 26, S5–S20. [Google Scholar]
- CDC. Diabetes Public Health Resource: Self-Reported Risk Factors for Complications. Available online: http://www.cdc.gov/diabetes/statistics/comp/index.htm (accessed on 7 March 2016).
- American Diabetes Foundation. Available online: http://www.diabetes.org (accessed on 9 February 2016).
- Liu, Y.; Sun, J.; Rao, S.; Su, Y.; Yang, Y. Antihyperglycemic, antihyperlipidemic and antioxidant activities of polysaccharides from Catathelasma ventricosum in streptozotocin-induced diabetic mice. Food Chem. Toxicol. 2013, 57, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, R.S.; Pearce, N.; Araujo, A.B.; McKinlay, J.B. The contribution of biogeographical ancestry and socioeconomic status to racial/ethnic disparities in type 2 diabetes mellitus: Results from the Boston Area Community Health Survey. Ann. Epidemiol. 2014, 24, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Rahar, S.; Swami, G.; Nagpal, N.; Nagpal, M.A.; Singh, G.S. Preparation, characterization, and biological properties of β-glucans. J. Adv. Pharm. Technol. Res. 2011, 2, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Elisashvili, V. Submerged cultivation of medicinal mushrooms: Bioprocesses and products (review). Int. J. Med. Mushrooms 2012, 14, 211–239. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Chemistry, nutrition, and health-promoting properties of Hericium erinaceus (Lion’s Mane) mushroom fruiting bodies and mycelia and their bioactive compounds. J. Agric. Food Chem. 2015, 63, 7108–7123. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.T.; Li, W.Z.; Chen, J.; Zhong, Q.X.; Ju, Y.J.; Zhao, J.; Bzhelyansky, A.; Li, S.P. An evaluation system for characterization of polysaccharides from the fruiting body of Hericium erinaceus and identification of its commercial product. Carbohydr. Polym. 2015, 124, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Z.; Wu, D.; Chen, X.; Zhou, S.; Liu, Y.; Yang, Y.; Cui, F. Chemical compositions and macrophage activation of polysaccharides from Lion’s Mane culinary-medicinal mushroom Hericium erinaceus (higher Basidiomycetes) in different maturation stages. Int. J. Med. Mushrooms 2015, 17, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Yong, Y.; Gu, Y.; Wang, Z.; Zhang, S.; Lu, L. Comparison of antioxidant and antiproliferation activities of polysaccharides from eight species of medicinal mushrooms. Int. J. Med. Mushrooms 2015, 17, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chen, Y.; Li, Q.; Zhao, T.; Zhang, M.; Feng, W.; Takase, M.; Wu, X.; Zhou, Z.; Yang, L.; et al. Preparation, characterization, and anti-Helicobacter pylori activity of Bi3+-Hericium erinaceus polysaccharide complex. Carbohydr. Polym. 2014, 110, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Fen, L.; Xuwei, Z.; Nanyi, L.; Puyu, Z.; Shuang, Z.; Xue, Z.; Pengju, L.; Qichao, Z.; Haiping, L. Screening of lignocellulose-degrading superior mushroom strains and determination of their CMCase and laccase activity. Sci. World J. 2014, 2014, 763108. [Google Scholar] [CrossRef] [PubMed]
- Malinowska, E.; Krzyczkowski, W.; Łapienis, G.; Herold, F. Improved simultaneous production of mycelial biomass and polysaccharides by submerged culture of Hericium erinaceum: Optimization using a central composite rotatable design (CCRD). J. Ind. Microbiol. Biotechnol. 2009, 36, 1513–1527. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, Q.; Mao, G.; Zou, Y.; Feng, W.; Zheng, D.; Wang, W.; Zhou, L.; Zhang, T.; Yang, J.; et al. Optimization of enzyme-assisted extraction and characterization of polysaccharides from Hericium erinaceus. Carbohydr. Polym. 2014, 101, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Bak, W.C.; Park, J.H.; Park, Y.A.; Ka, K.H. Determination of glucan contents in the fruiting bodies and mycelia of Lentinula edodes cultivars. Mycobiology 2014, 42, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.P.; Park, S.O.; Lee, S.J.; Nam, S.H.; Friedman, M. A polysaccharide isolated from the liquid culture of Lentinus edodes (Shiitake) mushroom mycelia containing black rice bran protects mice against a Salmonella lipopolysaccharide-induced endotoxemia. J. Agric. Food Chem. 2013, 61, 10987–10994. [Google Scholar] [CrossRef] [PubMed]
- Silveira, M.L.L.; Smiderle, F.R.; Agostini, F.; Pereira, E.M.; Bonatti-Chaves, M.; Wisbeck, E.; Ruthes, A.C.; Sassaki, G.L.; Cipriani, T.R.; Furlan, S.A.; et al. Exopolysaccharide produced by Pleurotus sajor-caju: Its chemical structure and anti-inflammatory activity. Int. J. Biol. Macromol. 2015, 75, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, C.; Luo, X.; Han, G.; Xu, S.; Niu, F.; Hu, X.; Wu, H.; Zhang, H. Characterization of selenium-enriched mycelia of Catathelasma ventricosum and their antihyperglycemic and antioxidant properties. J. Agric. Food Chem. 2015, 63, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, D.; You, Y.; Zeng, S.; Hu, Y.; Duan, X.; Liu, A.; Chen, H.; Hu, X.; Chen, S.; et al. Structural characterization and antidiabetic activity of a glucopyranose-rich heteropolysaccharide from Catathelasma ventricosum. Carbohydr. Polym. 2016, 149, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Li, F.; Jia, S.; Ren, H.; Gong, G.; Wang, Y.; Lv, Z.; Liu, Y. Drying effects on the antioxidant properties of polysaccharides obtained from Agaricus blazei Murrill. Carbohydr. Polym. 2014, 103, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Mao, G.; Feng, W.; Xiao, H.; Zhao, T.; Li, F.; Zou, Y.; Ren, Y.; Zhu, Y.; Yang, L.; Wu, X. Purification, characterization, and antioxidant activities of selenium-containing proteins and polysaccharides in royal sun mushroom, Agaricus brasiliensis (higher Basidiomycetes). Int. J. Med. Mushrooms 2014, 16, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Salvador, C.; Martins, M.R.; Caldeira, A.T. Microanalysis characterization of bioactive protein-bound polysaccharides produced by Amanita ponderosa cultures. Microsc. Microanal. 2015, 21, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.Y.; Zhang, J.Y.; Chen, L.J.; Liu, X.C.; Liu, Y.; Wang, W.X.; Zhang, Y.M. Comparative evaluation of polysaccharides isolated from Astragalus, oyster mushroom, and yacon as inhibitors of α-glucosidase. Chin. J. Nat. Med. 2014, 12, 290–293. [Google Scholar] [CrossRef]
- Zhao, S.; Rong, C.; Liu, Y.; Xu, F.; Wang, S.; Duan, C.; Chen, J.; Wu, X. Extraction of a soluble polysaccharide from Auricularia polytricha and evaluation of its anti-hypercholesterolemic effect in rats. Carbohydr. Polym. 2015, 122, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Sun, S.Q.; Wu, W.Z.; Yang, S.L.; Tan, J.M. Characterization of a water-soluble polysaccharide from Boletus edulis and its antitumor and immunomodulatory activities on renal cancer in mice. Carbohydr. Polym. 2014, 105, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Moreno, R.B.; Ruthes, A.C.; Baggio, C.H.; Vilaplana, F.; Komura, D.L.; Iacomini, M. Structure and antinociceptive effects of β-d-glucans from Cookeina tricholoma. Carbohydr. Polym. 2016, 141, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Zhu, J.; Liu, T.; Bi, S.; Hu, X.; Chen, Z.; Song, L.; Lv, W.; Yu, R. Structural characterization and biological activities of a novel polysaccharide from cultured Cordyceps militaris and its sulfated derivative. J. Agric. Food Chem. 2015, 63, 3464–3471. [Google Scholar] [CrossRef] [PubMed]
- Maity, P.; Sen, I.K.; Maji, P.K.; Paloi, S.; Devi, K.S.P.; Acharya, K.; Maiti, T.K.; Islam, S.S. Structural, immunological, and antioxidant studies of β-glucan from edible mushroom Entoloma lividoalbum. Carbohydr. Polym. 2015, 123, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.F.; Lv, G.Y.; Jiang, X.; Cheng, J.H.; Fan, L.F. Extraction optimization and biological properties of a polysaccharide isolated from Gleoestereum incarnatum. Carbohydr. Polym. 2015, 117, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Liu, G.; Huang, F.; Wu, X.; Yang, H. Improved production, purification and bioactivity of a polysaccharide from submerged cultured Ganoderma lucidum. Arch. Pharm. Res. 2014, 37, 1530–1537. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhou, F.; Chen, Y.; Zhang, Y.; Hou, L.; Cao, X.; Wang, C. A polysaccharide from Grifola frondosa relieves insulin resistance of HepG2 cell by Akt-GSK-3 pathway. Glycoconj. J. 2014, 31, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Mao, G.H.; Ren, Y.; Feng, W.W.; Li, Q.; Wu, H.Y.; Jin, D.; Zhao, T.; Xu, C.Q.; Yang, L.Q.; Wu, X.Y. Antitumor and immunomodulatory activity of a water-soluble polysaccharide from Grifola frondosa. Carbohydr. Polym. 2015, 134, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, L. Effect of extraction method on structure and antioxidant activity of Hohenbuehelia serotina polysaccharides. Int. J. Biol. Macromol. 2016, 83, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.S.; Cao, L.X.; Zhang, B.Z. Efficient purification of antiproliferative polysaccharides from Hypsizigus marmoreus with radial flow chromatography. Biotechnol. Prog. 2014, 30, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Edwards, P.J.; Perera, C.O.; Hemar, Y. Structural features of a novel polysaccharide isolated from a New Zealand Maori mushroom Iliodiction cibarium. Carbohydr. Res. 2015, 406, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Perera, C.; Hemar, Y. Antitumor activity of mushroom polysaccharides: A review. Food Funct. 2012, 3, 1118–1130. [Google Scholar] [CrossRef] [PubMed]
- Villares, A. Isolation and characterization of a glucan-type polysaccharide from the red pine mushroom, Lactarius deliciosus (higher Basidiomycetes). Int. J. Med. Mushrooms 2013, 15, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, C.; Gao, X.; Xu, N.; Lin, L.; Zhao, H.; Jia, S.; Jia, L. Purification, characterization and anti-aging capacity of mycelia zinc polysaccharide by Lentinus edodes SD-08. BMC Complement. Altern. Med. 2015, 15, 111. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.; Nandi, A.K.; Sen, I.K.; Maity, P.; Pattanayak, M.; Devi, K.S.P.; Khatua, S.; Maiti, T.K.; Acharya, K.; Islam, S.S. Studies on antioxidative and immunostimulating fucogalactan of the edible mushroom Macrolepiota dolichaula. Carbohydr. Res. 2015, 413, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.Y.; Liu, J.L.; Yang, W.; Hou, X.; Li, Q.J. Antitumor activity of polysaccharide extracted from Pleurotus ostreatus mycelia against gastric cancer in vitro and in vivo. Mol. Med. Rep. 2015, 12, 2383–2389. [Google Scholar] [PubMed]
- Zhao, C.; Liao, Z.; Wu, X.; Liu, Y.; Liu, X.; Lin, Z.; Huang, Y.; Liu, B. Isolation, purification, and structural features of a polysaccharide from Phellinus linteus and its hypoglycemic effect in alloxan-induced diabetic mice. J. Food Sci. 2014, 79, H1002–H1010. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Jin, Y.; Xing, C.; Hu, J.; Wang, R.; Sun, P. Chemical characterization and in vitro antioxidant activity evaluation of polysaccharides from the fruiting bodies of the red heart mushroom Phellinus pini (higher Basidiomycetes). Int. J. Med. Mushrooms 2015, 17, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Wang, H.; Ng, T.B. Isolation and purification of polysaccharides with anti-tumor activity from Pholiota adiposa (Batsch) P. Kumm. (higher Basidiomycetes). Int. J. Med. Mushrooms 2012, 14, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, N.; Wang, G.; Zhao, H.; Lin, L.; Jia, M.; Jia, L. In vitro and in vivo antioxidant effects of polysaccharides from Nameko medicinal mushroom, Pholiota nameko SW-01 (higher Basidiomycetes). Int. J. Med. Mushrooms 2015, 17, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Yang, W.; Mariga, A.M.; Fang, Y.; Ma, N.; Pei, F.; Hu, Q. Purification, characterization and antitumor activity of polysaccharides from Pleurotus eryngii residue. Carbohydr. Polym. 2014, 114, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, T.; Zhou, H.; Zhang, Y.; Jin, G.; Yang, Y. Antidiabetic effect of polysaccharides from Pleurotus ostreatus in streptozotocin-induced diabetic rats. Int. J. Biol. Macromol. 2016, 83, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Manna, D.K.; Nandi, A.K.; Pattanayak, M.; Maity, P.; Tripathy, S.; Mandal, A.K.; Roy, S.; Tripathy, S.S.; Gupta, N.; Islam, S.S. A water soluble β-glucan of an edible mushroom Termitomyces heimii: Structural and biological investigation. Carbohydr. Polym. 2015, 134, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Jia, Y.; Wang, L.; Liu, X.; Liu, G.; Li, L.; He, C. Isolation and structural elucidation of a novel homogenous polysaccharide from Tricholoma matsutake. Nat. Prod. Res. 2016, 30, 58–64. [Google Scholar] [CrossRef] [PubMed]
- You, Q.; Yin, X.; Zhao, Y.; Jiang, Z. Extraction and purification of polysaccharides from pine medicinal mushroom, Tricholoma matsutake (higher Basidiomycetes) fruit bodies. Int. J. Med. Mushrooms 2014, 16, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; You, Q.; Su, X. A comparison study on extraction of polysaccharides from Tricholoma matsutake by response surface methodology. Carbohydr. Polym. 2014, 102, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.M.; Song, J.H.; Wang, J.; Yang, J.M.; Wang, Z.B.; Liu, Y.H. Optimization of cellulase-assisted extraction process and antioxidant activities of polysaccharides from Tricholoma mongolicum Imai. J. Sci. Food Agric. 2016. [Google Scholar] [CrossRef] [PubMed]
- Chai, R.; Qiu, C.; Liu, D.; Qi, Y.; Gao, Y.; Shen, J.; Qiu, L. β-Glucan synthase gene overexpression and β-glucans overproduction in Pleurotus ostreatus using promoter swapping. PLoS ONE 2013, 8, e61693. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.L.; Liu, R.; Ren, M.F.; Li, H.J.; Xu, J.W. Enhanced production of polysaccharide through the overexpression of homologous uridine diphosphate glucose pyrophosphorylase gene in a submerged culture of Lingzhi or Reishi medicinal mushroom, Ganoderma lucidum (Higher Basidiomycetes). Int. J. Med. Mushrooms 2015, 17, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Liang, H.; Luo, L. Antitumor polysaccharides from mushrooms: A review on the structural characteristics, antitumor mechanisms and immunomodulating activities. Carbohydr. Res. 2016, 424, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.K.; Pei, J.J.; Ma, H.L.; Wang, Z.B.; Liu, Y.S. Advances in antitumor polysaccharides from Phellinus sensu lato: Production, isolation, structure, antitumor activity, and mechanisms. Crit. Rev. Food Sci. Nutr. 2015. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Torres, J.V.; Mata, G.; Martinez-Carrera, D.; Garibay-Orijel, C.; Garibay-Orijel, R. Primers for (1,3)-β-glucan synthase gene amplification and partial characterization of the enzyme in Ganoderma lucidum. Rev. Iberoam. Micol. 2013, 30, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Gani, A.; Shah, A.; Masoodi, F.A.; Hussain, P.R.; Wani, I.A.; Khanday, F.A. Effect of γ-irradiation on structural, functional and antioxidant properties of β-glucan extracted from button mushroom (Agaricus bisporus). Innov. Food Sci. Emerg. Technol. 2015, 31, 123–130. [Google Scholar] [CrossRef]
- Xiao, J.H.; Xiao, D.M.; Chen, D.X.; Xiao, Y.; Liang, Z.Q.; Zhong, J.J. Polysaccharides from the medicinal mushroom Cordyceps taii show antioxidant and immunoenhancing activities in a d-galactose-induced aging mouse model. Evid. Based Complement. Altern. Med. 2012, 2012, 273435. [Google Scholar] [CrossRef] [PubMed]
- Kozarski, M.; Klaus, A.; Jakovljevic, D.; Todorovic, N.; Vunduk, J.; Petrovic, P.; Niksic, M.; Vrvic, M.M.; van Griensven, L. Antioxidants of edible mushrooms. Molecules 2015, 20, 19489–19525. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Hong, E.K. Immunostimulating activity of the polysaccharides isolated from Cordyceps militaris. Int. Immunopharmacol. 2011, 11, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Tang, Q.; Zhou, S.; Liu, Y.; Zhang, Z.; Gao, X.; Wang, S.; Wang, Z. Isolation and purification of a polysaccharide from the caterpillar medicinal mushroom Cordyceps militaris (Ascomycetes) fruit bodies and its immunomodulation of RAW 264.7 macrophages. Int. J. Med. Mushrooms 2014, 16, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.; Zhang, A.; Zhang, W.; Cui, G.; Wang, S.; Duan, J. Antioxidative properties of crude polysaccharides from Inonotus obliquus. Int. J. Mol. Sci. 2012, 13, 9194–9206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lv, G.; Pan, H.; Pandey, A.; He, W.; Fan, L. Antioxidant and hepatoprotective potential of endo-polysaccharides from Hericium erinaceus grown on tofu whey. Int. J. Biol. Macromol. 2012, 51, 1140–1146. [Google Scholar] [CrossRef] [PubMed]
- Nandi, A.K.; Samanta, S.; Maity, S.; Sen, I.K.; Khatua, S.; Devi, K.S.; Acharya, K.; Maiti, T.K.; Islam, S.S. Antioxidant and immunostimulant β-glucan from edible mushroom Russula albonigra (Krombh.) Fr. Carbohydr. Polym. 2014, 99, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.M.; Zhang, J.; Wu, L.H.; Zhao, Y.L.; Li, T.; Li, J.Q.; Wang, Y.Z.; Liu, H.G. A mini-review of chemical composition and nutritional value of edible wild-grown mushroom from China. Food Chem. 2014, 151, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.Y.; Wang, C.L.; Wang, Y.R.; Li, Z.J.; Chen, M.H.; Li, F.J.; Sun, Y.P. Pleurotus nebrodensis polysaccharide (PN-S) enhances the immunity of immunosuppressed mice. Chin. J. Nat. Med. 2015, 13, 760–766. [Google Scholar] [CrossRef]
- Hu, S.H.; Cheung, P.C.; Hung, R.P.; Chen, Y.K.; Wang, J.C.; Chang, S.J. Antitumor and immunomodulating activities of exopolysaccharide produced by Big Cup culinary-medicinal mushroom Clitocybe maxima (Higher Basidiomycetes) in liquid submerged culture. Int. J. Med. Mushrooms 2015, 17, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.H.; Tsai, C.L.; Lien, Y.Y.; Lee, M.S.; Sheu, S.C. High molecular weight of polysaccharides from Hericium erinaceus against amyloid β-induced neurotoxicity. BMC Complement. Altern. Med. 2016, 16, 170. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Zhang, Y.; Xie, J.; Wang, L.; Zhang, H.; Wei, W.; Li, Y. Royal Sun medicinal mushroom, Agaricus brasiliensis (Agaricomycetidae), derived polysaccharides rxert immunomodulatory activities in vitro and in vivo. Int. J. Med. Mushrooms 2016, 18, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; You, Q.; Zhou, X. Complex enzyme-assisted extraction, purification, and antioxidant activity of polysaccharides from the Button Mushroom, Agaricus bisporus (Higher Basidiomycetes). Int. J. Med. Mushrooms 2015, 17, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Alzorqi, I.; Sudheer, S.; Lu, T.J.; Manickam, S. Ultrasonically extracted β-d-glucan from artificially cultivated mushroom, characteristic properties and antioxidant activity. Ultrason. Sonochem. 2016. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.T.; Santos, M.N.; de Souza, L.A.; Pinheiro, T.S.; Paiva, A.A.; Dore, C.M.; Costa, M.S.; Santos, N.D.; Baseia, Y.G.; Araujo, R.M.; et al. Chemical characteristics of a heteropolysaccharide from Tylopilus ballouii mushroom and its antioxidant and anti-inflammatory activities. Carbohydr. Polym. 2016, 144, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Berven, L.; Karppinen, P.; Hetland, G.; Samuelsen, A.B. The polar high molecular weight fraction of the Agaricus blazei Murill extract, AndoSan, reduces the activity of the tumor-associated protease, legumain, in RAW 264.7 cells. J. Med. Food 2015, 18, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Hetland, G.; Johnson, E.; Lyberg, T.; Kvalheim, G. The mushroom Agaricus blazei Murill elicits medicinal effects on tumor, infection, allergy, and inflammation through Its modulation of innate immunity and amelioration of Th1/Th2 imbalance and inflammation. Adv. Pharmacol. Sci. 2011, 2011, 157015. [Google Scholar] [PubMed]
- Tangen, J.M.; Tierens, A.; Caers, J.; Binsfeld, M.; Olstad, O.K.; Troseid, A.M.; Wang, J.; Tjonnfjord, G.E.; Hetland, G. Immunomodulatory effects of the Agaricus blazei Murrill-based mushroom extract AndoSan in patients with multiple myeloma undergoing high dose chemotherapy and autologous stem cell transplantation: A randomized, double blinded clinical study. BioMed Res. Int. 2015, 2015, 718539. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Shao, H. Extract from Agaricus blazei Murill can enhance immune responses elicited by DNA vaccine against foot-and-mouth disease. Vet. Immunol. Immunopathol. 2006, 109, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.P.; Moon, E.; Nam, S.H.; Friedman, M. Hericium erinaceus mushroom extracts protect infected mice against Salmonella Typhimurium-induced liver damage and mortality by stimulation of innate immune cells. J. Agric. Food Chem. 2012, 60, 5590–5596. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.P.; Park, S.O.; Lee, S.J.; Nam, S.H.; Friedman, M. A polysaccharide isolated from the liquid culture of Lentinus edodes (Shiitake) mushroom mycelia containing black rice bran protects mice against salmonellosis through up-regulation of the Th1 immune reaction. J. Agric. Food Chem. 2014, 62, 2384–2391. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.M.; Tan, Y.S.; Chua, K.H.; Sabaratnam, V.; Kuppusamy, U.R. Attenuation of inflammatory mediators (TNF-α and nitric oxide) and up-regulation of IL-10 by wild and domesticated basidiocarps of Amauroderma rugosum (Blume & T. Nees) Torrend in LPS-stimulated RAW264.7 cells. PLoS ONE 2015, 10, e0139593. [Google Scholar]
- Elsayed, E.A.; El Enshasy, H.; Wadaan, M.A.; Aziz, R. Mushrooms: A potential natural source of anti-inflammatory compounds for medical applications. Mediat. Inflamm. 2014, 2014, 805841. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Kang, J.H.; Kim, D.K.; Oh, S.H.; Kim, M.K. Orally administered aqueous extract of Inonotus obliquus ameliorates acute inflammation in dextran sulfate sodium (DSS)-induced colitis in mice. J. Ethnopharmacol. 2012, 143, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Nie, S. The structure of mushroom polysaccharides and their beneficial role in health. Food Funct. 2015, 6, 3205–3217. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.J.; Lin, C.S.; Lu, C.C.; Martel, J.; Ko, Y.F.; Ojcius, D.M.; Tseng, S.F.; Wu, T.R.; Chen, Y.Y.M.; Young, J.D.; et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat. Commun. 2015, 6, 7489. [Google Scholar] [CrossRef] [PubMed]
- Delzenne, N.M.; Bindels, L.B. Gut microbiota: Ganoderma lucidum, a new prebiotic agent to treat obesity? Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 553–554. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yang, J.; Ning, Z.; Zhang, X. Lentinula edodes-derived polysaccharide rejuvenates mice in terms of immune responses and gut microbiota. Food Funct. 2015, 6, 2653–2663. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Lin, C.; Bian, Z.; Xu, B. An insight into anti-inflammatory effects of fungal beta-glucans. Trends Food Sci. Technol. 2015, 41, 49–59. [Google Scholar] [CrossRef]
- Furuncuoğlu, Y.; Tulgar, S.; Dogan, A.N.; Cakar, S.; Tulgar, Y.K.; Cakiroglu, B. How obesity affects the neutrophil/lymphocyte and platelet/lymphocyte ratio, systemic immune-inflammatory index and platelet indices: A retrospective study. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 1300–1306. [Google Scholar] [PubMed]
- Arora, T.; Sharma, R. Fermentation potential of the gut microbiome: Implications for energy homeostasis and weight management. Nutr. Rev. 2011, 69, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Possemiers, S.; van de Wiele, T.; Guiot, Y.; Everard, A.; Rottier, O.; Geurts, L.; Naslain, D.; Neyrinck, A.; Lambert, D.M.; et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009, 58, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Delzenne, N.M.; Cani, P.D. Interaction between obesity and the gut microbiota: Relevance in nutrition. Annu. Rev. Nutr. 2011, 31, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Dutton, R.J.; Turnbaugh, P.J. Taking a metagenomic view of human nutrition. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Gerritsen, J.; Smidt, H.; Rijkers, G.T.; De Vos, W.M. Intestinal microbiota in human health and disease: The impact of probiotics. Genes Nutr. 2011, 6, 209–240. [Google Scholar] [CrossRef] [PubMed]
- Han, J.L.; Lin, H.L. Intestinal microbiota and type 2 diabetes: From mechanism insights to therapeutic perspective. World J. Gastroenterol. 2014, 20, 17737–17745. [Google Scholar] [PubMed]
- Kim, H.; Kim, D.H.; Seo, K.H.; Chon, J.W.; Nah, S.Y.; Bartley, G.E.; Arvik, T.; Lipson, R.; Yokoyama, W. Modulation of the intestinal microbiota is associated with lower plasma cholesterol and weight gain in hamsters fed chardonnay grape seed flour. J. Agric. Food Chem. 2015, 63, 1460–1467. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.L.; Chi-Keung Cheung, P. Non-digestible long chain beta-glucans as novel prebiotics. Bioact. Carbohydr. Diet. Fibre 2013, 2, 45–64. [Google Scholar] [CrossRef]
- Murphy, E.A.; Velazquez, K.T.; Herbert, K.M. Influence of high-fat diet on gut microbiota: A driving force for chronic disease risk. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Espley, R.V.; Butts, C.A.; Laing, W.A.; Martell, S.; Smith, H.; McGhie, T.K.; Zhang, J.; Paturi, G.; Hedderley, D.; Bovy, A.; et al. Dietary flavonoids from modified apple reduce inflammation markers and modulate gut microbiota in mice. J. Nutr. 2014, 144, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Hervert-Hernández, D.; Goñi, I. Dietary polyphenols and human gut microbiota: A review. Food Rev. Int. 2011, 27, 154–169. [Google Scholar] [CrossRef]
- Hervert-Hernández, D.; Pintado, C.; Rotger, R.; Goñi, I. Stimulatory role of grape pomace polyphenols on Lactobacillus acidophilus growth. Int. J. Food Microbiol. 2009, 136, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.; Panahi, S.; Tremblay, A. Childhood obesity: A role for gut microbiota? Int. J. Environ. Res. Public Health 2015, 12, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Dupont, H.L. New approaches for bacteriotherapy: Prebiotics, new-generation probiotics, and synbiotics. Clin. Infect. Dis. 2015, 60, S108–S121. [Google Scholar] [CrossRef] [PubMed]
- Schéle, E.; Grahnemo, L.; Anesten, F.; Halleń, A.; Bac̈khed, F.; Jansson, J.O. The gut microbiota reduces leptin sensitivity and the expression of the obesity-suppressing neuropeptides proglucagon (Gcg) and brain-derived neurotrophic factor (Bdnf) in the central nervous system. Endocrinology 2013, 154, 3643–3651. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.B.; Jeong, H.W.; Cho, D.; Lee, B.J.; Lee, J.H.; Choi, J.Y.; Bae, I.H.; Lee, S.J. Fermented green tea extract alleviates obesity and related complications and alters gut microbiota composition in diet-induced obese mice. J. Med. Food 2015, 18, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Bose, S.; Wang, J.H.; Kim, B.S.; Kim, M.J.; Kim, E.J.; Kim, H.D. Contrasting effects of fresh and fermented kimchi consumption on gut microbiota composition and gene expression related to metabolic syndrome in obese Korean women. Mol. Nutr. Food Res. 2015, 59, 1004–1008. [Google Scholar] [CrossRef] [PubMed]
- Song, M.Y.; Kim, B.S.; Kim, H. Influence of Panax ginseng on obesity and gut microbiota in obese middle-aged Korean women. J. Ginseng Res. 2014, 38, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Nieuwdorp, M.; Gilijamse, P.W.; Pai, N.; Kaplan, L.M. Role of the microbiome in energy regulation and metabolism. Gastroenterology 2014, 146, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.Y.; Korivi, M.; Chaing, Y.Y.; Chien, T.Y.; Tsai, Y.C. Pleurotus tuber-regium polysaccharides attenuate hyperglycemia and oxidative stress in experimental diabetic rats. Evid. Based Complement. Altern. Med. 2012, 2012, 856381. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.Y.; Korivi, M.; Yang, H.T.; Huang, C.C.; Chaing, Y.Y.; Tsai, Y.C. Effect of Pleurotus tuber-regium polysaccharides supplementation on the progression of diabetes complications in obese-diabetic rats. Chin. J. Physiol. 2014, 57, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D. Obesity: Medicinal mushroom reduces obesity by modulating microbiota. Nat. Rev. Endocrinol. 2015, 11, 504. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.J.; Yoon, S.J.; Pyun, Y.R. Polysaccharides from edible mushroom Hinmogi (Tremella fuciformis) inhibit differentiation of 3T3-L1 adipocytes by reducing mRNA expression of PPARγ, C/EBPα, and leptin. Food Sci. Biotechnol. 2008, 17, 267–273. [Google Scholar]
- Thyagarajan-Sahu, A.; Lane, B.; Sliva, D. ReishiMax, mushroom based dietary supplement, inhibits adipocyte differentiation, stimulates glucose uptake and activates AMPK. BMC Complement. Altern. Med. 2011, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; He, A.; Liu, Y.; Xie, B.; Li, Y.; Deng, Y.; Liu, X.; Liu, Q. Development of Lingzhi or Reishi medicinal mushroom, Ganoderma lucidum (higher Basidiomycetes) polysaccharides injection formulation. Int. J. Med. Mushrooms 2014, 16, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, T.; Inatomi, S.; Inazu, A.; Kawahara, E. Hypolipidemic effect of Pleurotus eryngii extract in fat-loaded mice. J. Nutr. Sci. Vitaminol. 2010, 56, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Handayani, D.; Chen, J.; Meyer, B.J.; Huang, X.F. Dietary Shiitake mushroom (Lentinus edodes) prevents fat deposition and lowers triglyceride in rats fed a high-fat diet. J. Obes. 2011, 2011, 258051. [Google Scholar] [CrossRef] [PubMed]
- Inoue, N.; Inafuku, M.; Shirouchi, B.; Nagao, K.; Yanagita, T. Effect of Mukitake mushroom (Panellus serotinus) on the pathogenesis of lipid abnormalities in obese, diabetic ob/ob mice. Lipids Health Dis. 2013, 12. [Google Scholar] [CrossRef] [PubMed]
- Kanagasabapathy, G.; Malek, S.N.; Mahmood, A.A.; Chua, K.H.; Vikineswary, S.; Kuppusamy, U.R. Beta-Glucan-rich extract from Pleurotus sajor-caju (Fr.) Singer prevents obesity and oxidative stress in C57BL/6J mice fed on a high-fat diet. Evid. Based Complement. Alterna Med. 2013, 2013, 185259. [Google Scholar] [CrossRef] [PubMed]
- Vincent, M.; Philippe, E.; Everard, A.; Kassis, N.; Rouch, C.; Denom, J.; Takeda, Y.; Uchiyama, S.; Delzenne, N.M.; Cani, P.D.; et al. Dietary supplementation with Agaricus blazei murill extract prevents diet-induced obesity and insulin resistance in rats. Obesity 2013, 21, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Handayani, D.; Meyer, B.J.; Chen, J.; Brown, S.H.; Mitchell, T.W.; Huang, X.F. A high-dose Shiitake mushroom increases hepatic accumulation of triacylglycerol in rats fed a high-fat diet: Underlying mechanism. Nutrients 2014, 6, 650–662. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Yun, J.; Jang, S.H.; Kang, S.N.; Jeon, B.S.; Ko, Y.G.; Kim, H.D.; Won, C.K.; Kim, G.S.; Cho, J.H. Coprinus comatus cap inhibits adipocyte differentiation via regulation of PPARγ and akt signaling pathway. PLoS ONE 2014, 9, e105809. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zhai, G.; Zhang, J.; Wang, L.; Ma, Z.; Jia, M.; Jia, L. Antihyperlipidemic and hepatoprotective activities of mycelia zinc polysaccharide from Pholiota nameko SW-02. Int. J. Biol. Macromol. 2014, 70, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Iuchi, T.; Hosaka, T.; Shiroishi, M.; Ono, H.; Inukai, K.; Sumita, T.; Sakai, G.; Katayama, S.; Awata, T. Influence of treatment with extracts of Hypsyzigus marmoreus mushroom on body composition during obesity development in KK-A(y) mice. J. Nutr. Sci. Vitaminol. 2015, 61, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Ouchi, K.; Hirasawa, N. The anti-inflammatory effects of Lion’s Mane culinary-medicinal mushroom, Hericium erinaceus (higher Basidiomycetes) in a coculture system of 3T3-L1 adipocytes and RAW264 macrophages. Int. J. Med. Mushrooms 2015, 17, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Reza, M.A.; Hossain, M.A.; Damte, D.; Jo, W.S.; Hsu, W.H.; Park, S.C. Hypolipidemic and hepatic steatosis preventing activities of the wood ear medicinal mushroom Auricularia auricula-judae (higher Basidiomycetes) ethanol extract in vivo and in vitro. Int. J. Med. Mushrooms 2015, 17, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Aoe, S.; Ikenaga, T.; Noguchi, H.; Kohashi, C.; Kakumoto, K.; Kohda, N. Effect of cooked white rice with high β-glucan barley on appetite and energy intake in healthy Japanese subjects: A randomized controlled trial. Plant Foods Hum. Nutr. 2014, 69, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Arena, M.P.; Caggianiello, G.; Fiocco, D.; Russo, P.; Torelli, M.; Spano, G.; Capozzi, V. Barley β-glucans-containing food enhances probiotic performances of beneficial bacteria. Int. J. Mol. Sci. 2014, 15, 3025–3039. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.C.; Huang, C.N.; Yeh, D.M.; Wang, S.J.; Peng, C.H.; Wang, C.J. Oat prevents obesity and abdominal fat distribution, and improves liver function in humans. Plant Foods Hum. Nutr. 2013, 68, 18–23. [Google Scholar] [CrossRef] [PubMed]
- El Khoury, D.; Cuda, C.; Luhovyy, B.L.; Anderson, G.H. Beta glucan: Health benefits in obesity and metabolic syndrome. J. Nutr. Metab. 2012, 2012, 851362. [Google Scholar] [CrossRef] [PubMed]
- Tabesh, F.; Sanei, H.; Jahangiri, M.; Momenizadeh, A.; Tabesh, E.; Pourmohammadi, K.; Sadeghi, M. The effects of beta-glucan rich oat bread on serum nitric oxide and vascular endothelial function in patients with hypercholesterolemia. BioMed Res. Int. 2014, 2014, 481904. [Google Scholar] [CrossRef] [PubMed]
- Cheskin, L.J.; Davis, L.M.; Lipsky, L.M.; Mitola, A.H.; Lycan, T.; Mitchell, V.; Mickle, B.; Adkins, E. Lack of energy compensation over 4 days when white button mushrooms are substituted for beef. Appetite 2008, 51, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Myrdal Miller, A.; Mills, K.; Wong, T.; Drescher, G.; Lee, S.M.; Sirimuangmoon, C.; Schaefer, S.; Langstaff, S.; Minor, B.; Guinard, J.X. Flavor-enhancing properties of mushrooms in meat-based dishes in which sodium has been reduced and meat has been partially substituted with mushrooms. J. Food Sci. 2014, 79, S1795–S1804. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, J.W.; Heo, Y.; Moon, B. Effect of Pleurotus eryngii mushroom beta-glucan on quality characteristics of common wheat pasta. J. Food Sci. 2016, 81, C835–C840. [Google Scholar] [CrossRef] [PubMed]
- Okubo, H.; Sasaki, S.; Murakami, K.; Kim, M.K.; Takahashi, Y.; Hosoi, Y.; Itabashi, M. Three major dietary patterns are all independently related to the risk of obesity among 3760 Japanese women aged 18–20 years. Int. J. Obes. 2008, 32, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J. Sickening Sweet—Relics from a lab hint at centuries spent trying to solve diabetes. Distill. Mag. 2015, 1, 12–15. [Google Scholar]
- Numao, S.; Kawano, H.; Endo, N.; Yamada, Y.; Konishi, M.; Takahashi, M.; Sakamoto, S. Short-term low carbohydrate/high-fat diet intake increases postprandial plasma glucose and glucagon-like peptide-1 levels during an oral glucose tolerance test in healthy men. Eur. J. Clin. Nutr. 2012, 66, 926–931. [Google Scholar] [CrossRef] [PubMed]
- Numao, S.; Kawano, H.; Endo, N.; Yamada, Y.; Konishi, M.; Takahashi, M.; Sakamoto, S. Effects of a single bout of aerobic exercise on short-term low-carbohydrate/high-fat intake-induced postprandial glucose metabolism during an oral glucose tolerance test. Metabolism 2013, 62, 1406–1415. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.J.; Hwang, H.J.; Kim, S.W.; Oh, J.Y.; Baek, Y.M.; Choi, J.W.; Bae, S.H.; Yun, J.W. Hypoglycemic effects of exopolysaccharides produced by mycelial cultures of two different mushrooms Tremella fuciformis and Phellinus baumii in ob/ob mice. Appl. Microbiol. Biotechnol. 2007, 75, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.; Moon, E.; Nam, S.H.; Friedman, M. Antidiabetic effects of rice hull smoke extract on glucose-regulating mechanism in type 2 diabetic mice. J. Agric. Food Chem. 2012, 60, 7442–7449. [Google Scholar] [CrossRef] [PubMed]
- Lo, H.C.; Wasser, S.P. Medicinal mushrooms for glycemic control in diabetes mellitus: History, current status, future perspectives, and unsolved problems (review). Int. J. Med. Mushrooms 2011, 13, 401–426. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Han, C.; Sun, Y.; Qi, X.; Shi, Y.; Gao, X.; Zhang, C. The agaricoglyceride of royal sun medicinal mushroom, Agaricus brasiliensis (higher Basidiomycetes) is anti-inflammatory and reverses diabetic glycemia in the liver of mice. Int. J. Med. Mushrooms 2013, 15, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.R.; Lee, Y.P.; Kim, D.W.; Song, H.Y.; Yoo, K.Y.; Won, M.H.; Kang, T.C.; Lee, K.J.; Kim, K.H.; Joo, J.H.; et al. Amelioration of streptozotocin-induced diabetes by Agrocybe chaxingu polysaccharide. Mol. Cells 2010, 29, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Guo, S.; Han, J.; Wang, Q.; Zhang, X.; Wu, W. Hypoglycemic and hypolipidemic activities of MT-α-glucan and its effect on immune function of diabetic mice. Carbohydr. Polym. 2012, 89, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.R.; Ng, T.B.; Li, L.; Fang, J.C.; Jiang, Y.; Wen, T.Y.; Qiao, W.T.; Li, N.; Liu, F. Isolation of a polysaccharide with antiproliferative, hypoglycemic, antioxidant and HIV-1 reverse transcriptase inhibitory activities from the fruiting bodies of the abalone mushroom Pleurotus abalonus. J. Pharm. Pharmacol. 2011, 63, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Li, J.P.; Lei, Y.L.; Zhan, H. The effects of the king oyster mushroom Pleurotus eryngii (higher Basidiomycetes) on glycemic control in alloxan-induced diabetic mice. Int. J. Med. Mushrooms 2014, 16, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Ganeshpurkar, A.; Kohli, S.; Rai, G. Antidiabetic potential of polysaccharides from the white oyster culinary-medicinal mushroom Pleurotus florida (higher Basidiomycetes). Int. J. Med. Mushrooms 2014, 16, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Kanagasabapathy, G.; Chua, K.H.; Malek, S.N.; Vikineswary, S.; Kuppusamy, U.R. AMP-activated protein kinase mediates insulin-like and lipo-mobilising effects of β-glucan-rich polysaccharides isolated from Pleurotus sajor-caju (Fr.), Singer mushroom, in 3T3-L1 cells. Food Chem. 2014, 145, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.G.; Phan, C.W.; Sabaratnam, V.; Abdullah, N.; Abdulla, M.A.; Kuppusamy, U.R. Polysaccharides-rich extract of Ganoderma lucidum (M.A. Curtis:Fr.) P. Karst accelerates wound healing in Streptozotocin-Induced diabetic rats. Evid. Based Complement. Altern. Med. 2013, 2013, 671252. [Google Scholar] [CrossRef] [PubMed]
- Yamac, M.; Kanbak, G.; Zeytinoglu, M.; Bayramoglu, G.; Senturk, H.; Uyanoglu, M. Hypoglycemic effect of Lentinus strigosus (Schwein.) Fr. crude exopolysaccharide in streptozotocin-induced diabetic rats. J. Med. Food 2008, 11, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Kwon, A.H.; Qiu, Z.; Hashimoto, M.; Yamamoto, K.; Kimura, T. Effects of medicinal mushroom (Sparassis crispa) on wound healing in streptozotocin-induced diabetic rats. Am. J. Surg. 2009, 197, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.C.; Lian, B.; Huang, D.M.; Cui, F.J. Compare activities on regulating lipid-metabolism and reducing oxidative stress of diabetic rats of Tremella aurantialba broth’s extract (TBE) with its mycelia polysaccharides (TMP). J. Food Sci. 2009, 74, H15–H21. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Zhang, J.; Lv, Z.; Ye, L.; Yang, Y.; Tang, Q. Chemical modification of an acidic polysaccharide (TAPA1) from Tremella aurantialba and potential biological activities. Food Chem. 2014, 143, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Tang, Q.J.; Zhang, Z.; Li, C.H.; Cao, H.; Yang, Y.; Zhang, J.S. Nutritional composition of three domesticated culinary-medicinal mushrooms: Oudemansiella sudmusida, Lentinus squarrosulus, and Tremella aurantialba. Int. J. Med. Mushrooms 2015, 17, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Jayasuriya, W.J.; Wanigatunge, C.A.; Fernando, G.H.; Abeytunga, D.T.; Suresh, T.S. Hypoglycaemic activity of culinary Pleurotus ostreatus and P. cystidiosus mushrooms in healthy volunteers and type 2 diabetic patients on diet control and the possible mechanisms of action. Phytother. Res. 2015, 29, 303–309. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, Y.C.; O’Brien, N.M.; Kenny, O.; Harrington, T.; Brunton, N.; Smyth, T.J. Anti-inflammatory effects of wild Irish mushroom extracts in RAW264.7 mouse macrophage cells. J. Med. Food 2015, 18, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Zhang, M.; Wang, Q.; Guo, S.; Han, J.; Sun, H.; Wu, W. MT-α-glucan from the fruit body of the maitake medicinal mushroom Grifola frondosa (higher Basidiomyetes) shows protective effects for hypoglycemic pancreatic β-cells. Int. J. Med. Mushrooms 2013, 15, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Duobin, M.; Yuping, M.; Lujing, G.; Aijing, Z.; Jianqiang, Z.; Chunping, X. Fermentation characteristics in stirred-tank reactor of exopolysaccharides with hypolipidemic activity produced by Pleurotus geesteranus 5#. An. Acad. Bras. Cienc. 2013, 85, 1473–1481. [Google Scholar] [PubMed]
- Kanagasabapathy, G.; Kuppusamy, U.R.; Abd Malek, S.N.; Abdulla, M.A.; Chua, K.H.; Sabaratnam, V. Glucan-rich polysaccharides from Pleurotus sajor-caju (Fr.) Singer prevents glucose intolerance, insulin resistance and inflammation in C57BL/6J mice fed a high-fat diet. BMC Complement. Altern. Med. 2012, 12, 261. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Xun, M.; Wutong, W. Anti-diabetic effect of an alpha-glucan from fruit body of maitake (Grifola frondosa) on KK-Ay mice. J. Pharm. Pharmacol. 2007, 59, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Chen, H.; Dong, P.; Fu, L.; Zhang, X. Phytochemical characteristics and hypoglycaemic activity of fraction from mushroom Inonotus obliquus. J. Sci. Food Agric. 2010, 90, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.H.; Hu, T.; Li, Z.Y.; Huang, Z.L.; Jiang, J.G. In vitro antioxidant activities of the polysaccharides from Pleurotus tuber-regium (Fr.) Sing. Food Chem. 2014, 148, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Zhao, Y.; Nie, Y.; Lu, X.; Sun, Y.; Yang, X. Chemical composition of Pleurotus eryngii polysaccharides and their inhibitory effects on high-fructose diet-induced insulin resistance and oxidative stress in mice. Food Funct. 2014, 5, 2609–2620. [Google Scholar] [CrossRef] [PubMed]
- Karumuthil-Melethil, S.; Gudi, R.; Johnson, B.M.; Perez, N.; Vasu, C. Fungal β-glucan, a Dectin-1 ligand, promotes protection from type 1 diabetes by inducing regulatory innate immune response. J. Immunol. 2014, 193, 3308–3321. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Kim, H.W. Innate immunity induced by fungal β-glucans via dectin-1 signaling pathway. Int. J. Med. Mushrooms 2014, 16, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Lee, C.H.; Hsu, T.H.; Lo, H.C. Submerged-culture mycelia and broth of the maitake medicinal mushroom Grifola frondosa (Higher basidiomycetes) alleviate type 2 diabetes-induced alterations in immunocytic function. Int. J. Med. Mushrooms 2015, 17, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Calvo, M.S.; Mehrotra, A.; Beelman, R.B.; Nadkarni, G.; Wang, L.; Cai, W.; Goh, B.C.; Kalaras, M.D.; Uribarri, J. A retrospective study in adults with metabolic syndrome: Diabetic risk factor response to daily consumption of Agaricus bisporus (white button mushrooms). Plant Foods Hum. Nutr. 2016, 71, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.C.; Jeong, Y.T.; Yang, B.K.; Islam, R.; Koyyalamudi, S.R.; Pang, G.; Cho, K.Y.; Song, C.H. White button mushroom (Agaricus bisporus) lowers blood glucose and cholesterol levels in diabetic and hypercholesterolemic rats. Nutr. Res. 2010, 30, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.; Wang, H.; Li, C.; Qi, P.; Bao, J. Agaricus bisporus lectins mediates islet beta-cell proliferation through regulation of cell cycle proteins. Exp. Biol. Med. 2012, 237, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Klupp, N.L.; Chang, D.; Hawke, F.; Kiat, H.; Grant, S.J.; Bensoussan, A. Ganoderma lucidum for the treatment of cardiovascular risk factors. Cochrane Database Syst. Rev. 2008, 2008, CD007259. [Google Scholar]
- Kahl, K.G.; Schweiger, U.; Correll, C.; Müller, C.; Busch, M.L.; Bauer, M.; Schwarz, P. Depression, anxiety disorders, and metabolic syndrome in a population at risk for type 2 diabetes mellitus. Brain Behav. 2015, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Nanri, A. Nutritional epidemiology of type 2 diabetes and depressive symptoms. J. Epidemiol. 2013, 23, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Kong, F.; Zhang, D.; Cui, J. Anti-glycated and antiradical activities in vitro of polysaccharides from Ganoderma capense. Pharmacogn. Mag. 2013, 9, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.P.; Su, C.H.; Lu, T.M.; Lai, M.N.; Ng, L.T. Effects of Grifola frondosa non-polar bioactive components on high-fat diet fed and streptozotocin-induced hyperglycemic mice. Pharm. Biol. 2015, 53, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, T. The extraction and development of antitumor-active polysaccharides from medicinal mushrooms in Japan (review). Int. J. Med. Mushrooms 1999, 1, 9–29. [Google Scholar] [CrossRef]
- Oba, K.; Teramukai, S.; Kobayashi, M.; Matsui, T.; Kodera, Y.; Sakamoto, J. Efficacy of adjuvant immunochemotherapy with polysaccharide K for patients with curative resections of gastric cancer. Cancer Immunol. Immunother. 2007, 56, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Oba, K.; Kobayashi, M.; Matsui, T.; Kodera, Y.; Sakamoto, J. Individual patient based meta-analysis of lentinan for unresectable/recurrent gastric cancer. Anticancer Res. 2009, 29, 2739–2745. [Google Scholar] [PubMed]
- Wang, K.P.; Zhang, Q.L.; Liu, Y.; Wang, J.; Cheng, Y.; Zhang, Y. Structure and inducing tumor cell apoptosis activity of polysaccharides isolated from Lentinus edodes. J. Agric. Food Chem. 2013, 61, 9849–9858. [Google Scholar] [CrossRef] [PubMed]
- Gaullier, J.M.; Sleboda, J.; Øfjord, E.S.; Ulvestad, E.; Nurminiemi, M.; Moe, C.; Tor, A.; Gudmundsen, O. Supplementation with a soluble-glucan exported from Shiitake medicinal mushroom, Lentinus edodes (Berk.) singer mycelium: A crossover, placebo-controlled study in healthy elderly. Int. J. Med. Mushrooms 2011, 13, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Durgo, K.; Koncar, M.; Komes, D.; Belscak-Cvitanovic, A.; Franekic, J.; Jakopovich, I.; Jakopovich, N.; Jakopovich, B. Cytotoxicity of blended versus single medicinal mushroom extracts on human cancer cell lines: contribution of polyphenol and polysaccharide content. Int. J. Med. Mushrooms 2013, 15, 435–448. [Google Scholar] [CrossRef] [PubMed]
- Jakopovich, I. New dietary supplements from medicinal mushrooms: Dr Myko San—A registration report. Int. J. Med. Mushrooms 2011, 13, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Nandan, C.K.; Sarkar, R.; Bhanja, S.K.; Sikdar, S.R.; Islam, S.S. Isolation and characterization of polysaccharides of a hybrid mushroom (backcross mating between PfloVv12 and Volvariella volvacea). Carbohydr. Res. 2011, 346, 2451–2456. [Google Scholar] [CrossRef] [PubMed]
- Fortes, R.C.; Recôva, V.L.; Melo, A.L.; Novaes, M.R.C.G. Effects of dietary supplementation with medicinal fungus in fasting glycemia levels of patients with colorectal cancer: A randomized, double-blind, placebo-controlled clinical study. Nutr. Hosp. 2008, 23, 591–598. [Google Scholar] [PubMed]
- Deng, G.; Lin, H.; Seidman, A.; Fornier, M.; D’Andrea, G.; Wesa, K.; Yeung, S.; Cunningham-Rundles, S.; Vickers, A.J.; Cassileth, B. A phase I/II trial of a polysaccharide extract from Grifola frondosa (Maitake mushroom) in breast cancer patients: Immunological effects. J. Cancer Res. Clin. Oncol. 2009, 135, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Costa Fortes, R.; Carvalho Garbi Novaes, M.R. The effects of Agaricus sylvaticus fungi dietary supplementation on the metabolism and blood pressure of patients with colorectal cancer during post surgical phase. Nutr. Hosp. 2011, 26, 176–186. [Google Scholar] [PubMed]
- Okuno, K.; Uno, K. Efficacy of orally administered Lentinula edodes mycelia extract for advanced gastrointestinal cancer patients undergoing cancer chemotherapy: A pilot study. Asian Pac. J. Cancer Prev. 2011, 12, 1671–1674. [Google Scholar] [PubMed]
- Tanigawa, K.; Ito, Y.; Sakai, M.; Kobayashi, Y. Evaluation of quality of life and immune function in cancer patients receiving combined immunotherapy and oral administration of Lentinula edodes mycelia extract. Gan Kagaku Ryoho 2012, 39, 1779–1781. [Google Scholar]
- Yamaguchi, Y.; Miyahara, E.; Hihara, J. Efficacy and safety of orally administered Lentinula edodes mycelia extract for patients undergoing cancer chemotherapy: A pilot study. Am. J. Chin. Med. 2011, 39, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Takimoto, Y.; Suzuki, R.; Arai, T.; Uebaba, K.; Nakai, M.; Strong, J.M.; Tokuda, H. Efficacy of oral administration of Lentinula eododes mycelia extract for breast cancer patients undergoing postoperative hormone therapy. Asian Pac. J. Cancer Prev. 2013, 14, 3469–3472. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, Y.; Maeda, N.; Yamamoto, S.; Yoshino, S.; Oka, M. Evaluation of host quality of life and immune function in breast cancer patients treated with combination of adjuvant chemotherapy and oral administration of Lentinula edodes mycelia extract. Onco Targets Ther. 2013, 6, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Ina, K.; Kataoka, T.; Ando, T. The use of lentinan for treating gastric cancer. Anticancer Agents Med. Chem. 2013, 13, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Valadares, F.; Novaes, M.R.C.G.; Cañete, R. Effect of Agaricus sylvaticus supplementation on nutritional status and adverse events of chemotherapy of breast cancer: A randomized, placebo-controlled, double-blind clinical trial. Indian J. Pharmacol. 2013, 45, 217–222. [Google Scholar] [PubMed]
- Li, J.; Zou, L.; Chen, W.; Zhu, B.; Shen, N.; Ke, J.; Lou, J.; Song, R.; Zhong, R.; Miao, X. Dietary mushroom intake may reduce the risk of breast cancer: Evidence from a meta-analysis of observational studies. PLoS ONE 2014, 9, e93437. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, B.; Hadar, Y. Possible mechanisms of action of mushroom-derived glucans on inflammatory bowel disease and associated cancer. Ann. Transl. Med. 2014, 2, 19. [Google Scholar] [CrossRef] [PubMed]
- Twardowski, P.; Kanaya, N.; Frankel, P.; Synold, T.; Ruel, C.; Pal, S.K.; Junqueira, M.; Prajapati, M.; Moore, T.; Tryon, P.; et al. A phase I trial of mushroom powder in patients with biochemically recurrent prostate cancer: Roles of cytokines and myeloid-derived suppressor cells for Agaricus bisporus-induced prostate-specific antigen responses. Cancer 2015, 121, 2942–2950. [Google Scholar] [CrossRef] [PubMed]
- Masuda, Y.; Nawa, D.; Nakayama, Y.; Konishi, M.; Nanba, H. Soluble β-glucan from Grifola frondosa induces tumor regression in synergy with TLR9 agonist via dendritic cell-mediated immunity. J. Leukoc. Biol. 2015, 98, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Han, Y.; Huang, J.; Yu, X.; Jiao, C.; Yang, X.; Dhaliwal, P.; Xie, Y.; Yang, B.B. Purification and identification of a polysaccharide from medicinal mushroom Amauroderma rude with immunomodulatory activity and inhibitory effect on tumor growth. Oncotarget 2015, 6, 17777–17791. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.S.; Kuo, H.P.; Chang, K.L.; Kong, Z.L. Apoptosis of hepatocellular carcinoma cells induced by nanoencapsulated polysaccharides extracted from Antrodia camphorata. PLoS ONE 2015, 10, e0136782. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.P.; Nam, S.H.; Friedman, M. Hericium erinaceus (Lion’s Mane) mushroom extracts inhibit metastasis of cancer cells to the lung in CT-26 colon cancer-transplanted mice. J. Agric. Food Chem. 2013, 61, 4898–4904. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Niu, Y.B.; Sun, Y.; Zhang, F.; Liu, C.X.; Fan, L.; Mei, Q.B. Role of phytochemicals in colorectal cancer prevention. World J. Gastroenterol. 2015, 21, 9262–9272. [Google Scholar] [CrossRef] [PubMed]
- Santen, R.J.; Yue, W.; Naftolin, F.; Mor, G.; Berstein, L. The potential of aromatase inhibitors in breast cancer prevention. Endocr. Relat. Cancer 1999, 6, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Grube, B.J.; Eng, E.T.; Kao, Y.C.; Kwon, A.; Chen, S. White button mushroom phytochemicals inhibit aromatase activity and breast cancer cell proliferation. J. Nutr. 2001, 131, 3288–3293. [Google Scholar] [PubMed]
- Chen, S.; Oh, S.R.; Phung, S.; Hur, G.; Ye, J.J.; Kwok, S.L.; Shrode, G.E.; Belury, M.; Adams, L.S.; Williams, D. Anti-aromatase activity of phytochemicals in white button mushrooms (Agaricus bisporus). Cancer Res. 2006, 66, 12026–12034. [Google Scholar] [CrossRef] [PubMed]
- Hovde, Ø.; Høivik, M.L.; Henriksen, M.; Solberg, I.C.; Småstuen, M.C.; Moum, B.A. Malignancies in patients with inflammatory bowel disease: Results from 20 years of follow-up in the IBSEN study. J. Crohns Colitis 2016. [Google Scholar] [CrossRef] [PubMed]
- Therkelsen, S.P.; Hetland, G.; Lyberg, T.; Lygren, I.; Johnson, E. Cytokine levels after consumption of a medicinal Agaricus blazei Murill-based mushroom extract, AndoSan™, in patients with Crohn’s disease and ulcerative colitis in a randomized single-blinded placebo controlled study. Scand. J. Immunol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Førland, D.T.; Johnson, E.; Sætre, L.; Lyberg, T.; Lygren, I.; Hetland, G. Effect of an extract based on the medicinal mushroom Agaricus blazei Murill on expression of cytokines and calprotectin in patients with ulcerative colitis and Crohn’s disease. Scand. J. Immunol. 2011, 73, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.C.; Liu, J.C.; Zhao, X.M.; Su, F.Q.; Cui, H.X. Immunostimulatory activities of a low molecular weight antitumoral polysaccharide isolated from Agaricus blazei Murill (LMPAB) in Sarcoma 180 ascitic tumor-bearing mice. Pharmazie 2009, 64, 472–476. [Google Scholar] [PubMed]
- Cui, L.; Sun, Y.; Xu, H.; Xu, H.; Cong, H.; Liu, J. A polysaccharide isolated from Agaricus blazei Murill (ABP-AW1) as a potential Th1 immunity-stimulating adjuvant. Oncol. Lett. 2013, 6, 1039–1044. [Google Scholar] [PubMed]
- Bergendiova, K.; Tibenska, E.; Majtan, J. Pleuran (β-glucan from Pleurotus ostreatus) supplementation, cellular immune response and respiratory tract infections in athletes. Eur. J. Appl. Physiol. 2011, 111, 2033–2040. [Google Scholar] [CrossRef] [PubMed]
- Sen, I.K.; Mandal, A.K.; Chakraborti, S.; Dey, B.; Chakraborty, R.; Islam, S.S. Green synthesis of silver nanoparticles using glucan from mushroom and study of antibacterial activity. Int. J. Biol. Macromol. 2013, 62, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Signoretto, C.; Marchi, A.; Bertoncelli, A.; Burlacchini, G.; Papetti, A.; Pruzzo, C.; Zaura, E.; Lingström, P.; Ofek, I.; Pratten, J.; et al. The anti-adhesive mode of action of a purified mushroom (Lentinus edodes) extract with anticaries and antigingivitis properties in two oral bacterial phatogens. BMC Complement. Altern. Med. 2014, 14, 75. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Lin, Y.; Luo, Y.L.; Liang, H.H.; Sun, P.L. Extraction, antimicrobial, and antioxidant activities of crude polysaccharides from the Wood Ear medicinal mushroom Auricularia auricula-judae (higher Basidiomycetes). Int. J. Med. Mushrooms 2015, 17, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Shah, N.P. Antioxidant and antibacterial activities of sulphated polysaccharides from Pleurotus eryngii and Streptococcus thermophilus ASCC 1275. Food Chem. 2014, 165, 262–270. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, M.P.; Gulotta, G.; do Amaral, M.W.; Lunsdorf, H.; Sasse, F.; Abraham, W.R. Coprinuslactone protects the edible mushroom Coprinus comatus against biofilm infections by blocking both quorum-sensing and MurA. Environ. Microbiol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Soković, M.; Ćirić, A.; Glamočlija, J.; Nikolić, M.; van Griensven, L. Agaricus blazei hot water extract shows anti quorum sensing activity in the nosocomial human pathogen Pseudomonas aeruginosa. Molecules 2014, 19, 4189–4199. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Liu, W.; Tian, B.; Liu, H.; Ning, S. Inhibition of quorum sensing in the opportunistic pathogenic bacterium Chromobacterium violaceum by an extract from fruiting bodies of Lingzhi or Reishi medicinal mushroom, Ganoderma lucidum (W.Curt.:Fr.) P. Karst. (higher Basidiomycetes). Int. J. Med. Mushrooms 2011, 13, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-P.; Lee, S.-J.; Nam, S.-H.; Friedman, M. Turmeric (Curcuma longa) bioprocessed with mycelia of shiitake (Lentinus edodes) mushrooms: Composition and mechanism of protection against salmonellosis in mice. Int. J. Med. Mushrooms 2016. submitted. [Google Scholar]
- Tochikura, T.S.; Nakashima, H.; Yamamoto, N. Antiviral agents with activity against human retro viruses. JAIDS J. Acquir. Immune Defic. Syndr. 1989, 2, 441–447. [Google Scholar]
- Seniuk, O.F.; Gorovoj, L.F.; Beketova, G.V.; Savichuk, H.O.; Rytik, P.G.; Kucherov, I.I.; Prilutskay, A.B.; Prilutsky, A.I. Anti-infective properties of the melanin-glucan complex obtained from medicinal tinder bracket mushroom, Fomes fomentarius (L.: Fr.) Fr. (Aphyllophoromycetideae). Int. J. Med. Mushrooms 2011, 13, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Minari, M.C.; Rincão, V.P.; Soares, S.A.; Ricardo, N.M.P.S.; Nozawa, C.; Linhares, R.E.C. Antiviral properties of polysaccharides from Agaricus brasiliensis in the replication of bovine herpesvirus 1. Acta Virol. 2011, 55, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, F.T.; Camelini, C.M.; Leal, P.C.; Kratz, J.M.; Nunes, R.J.; Mendonca, M.M.; Simoes, C.M. Antiherpetic mechanism of a sulfated derivative of Agaricus brasiliensis fruiting bodies polysaccharide. Intervirology 2014, 57, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.A.; Galhardi, L.C.F.; Rincão, V.P.; Soares, S.D.A.; Vieira, T.G.P.; Ricardo, N.M.P.S.; Nozawa, C.; Linhares, R.E.C. Antiherpetic activity of an Agaricus brasiliensis polysaccharide, its sulfated derivative and fractions. Int. J. Biol. Macromol. 2013, 52, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Rincão, V.P.; Yamamoto, K.A.; Ricardo, N.M.; Soares, S.A.; Meirelles, L.D.; Nozawa, C.; Linhares, R.E. Polysaccharide and extracts from Lentinula edodes: Structural features and antiviral activity. Virol. J. 2012, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Razumov, I.A.; Kazachinskaia, E.I.; Puchkova, L.I.; Kosogorova, T.A.; Gorbunova, I.A.; Loktev, V.B.; Tepliakova, T.V. Protective activity of aqueous extracts from higher mushrooms against Herpes simplex virus type-2 on albino mice model. Antibiot. Khimioter. 2013, 58, 8–12. [Google Scholar] [PubMed]
- Wang, J.; Yu, G.; Li, Y.; Shen, L.; Qian, Y.; Yang, J.; Wang, F. Inhibitory effects of sulfated lentinan with different degree of sulfation against tobacco mosaic virus (TMV) in tobacco seedlings. Pestic. Biochem. Physiol. 2015, 122, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-P.; Lee, S.-J.; Nam, S.-H.; Friedman, M. Elm tree (Ulmus parvifolia) bark bioprocessed with mycelia of Shiitake (Lentinus edodes) mushrooms in liquid culture: Composition and mechanism of protection against allergic asthma in mice. J. Agric. Food Chem. 2016, 64, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.; Panicker, S.N.; Janardhanan, K.K. Protective effect of polysaccharide-protein complex from a polypore mushroom, Phellinus rimosus against radiation-induced oxidative stress. Redox Rep. 2012, 17, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, L.; Wang, Z. Radioprotective activity of neutral polysaccharides isolated from the fruiting bodies of Hohenbuehelia serotina. Phys. Med. 2015, 31, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Pillai, T.G.; Uma Devi, P. Mushroom beta glucan: Potential candidate for post irradiation protection. Mutat. Res. 2013, 751, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Fritz, H.; Kennedy, D.A.; Ishii, M.; Fergusson, D.; Fernandes, R.; Cooley, K.; Seely, D. Polysaccharide K and Coriolus versicolor Extracts for Lung Cancer: A Systematic Review. Integr. Cancer Ther. 2015, 14, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.S.; Teichert, A.; McHugh, T.H. Vitamin D2 formation from post-harvest UV-B treatment of mushrooms (Agaricus bisporus) and retention during storage. J. Agric. Food Chem. 2008, 56, 4541–4544. [Google Scholar] [CrossRef] [PubMed]
- Kalaras, M.D.; Beelman, R.B.; Elias, R.J. Effects of postharvest pulsed UV light treatment of white button mushrooms (Agaricus bisporus) on vitamin D2 content and quality attributes. J. Agric. Food Chem. 2012, 60, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.Y.; Mau, J.L.; Huang, S.J. Enhancement of antioxidant properties and increase of content of vitamin D2 and non-volatile components in fresh button mushroom, Agaricus bisporus (higher Basidiomycetes) by γ-irradiation. Int. J. Med. Mushrooms 2014, 16, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, Â.; Antonio, A.L.; Oliveira, M.B.P.P.; Martins, A.; Ferreira, I.C.F.R. Effect of gamma and electron beam irradiation on the physico-chemical and nutritional properties of mushrooms: A review. Food Chem. 2012, 135, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Sławińska, A.; Fornal, E.; Radzki, W.; Skrzypczak, K.; Zalewska-Korona, M.; Michalak-Majewska, M.; Parfieniuk, E.; Stachniuk, A. Study on Vitamin D2 stability in dried mushrooms during drying and storage. Food Chem. 2016, 199, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, A.; Calvo, M.S.; Beelman, R.B.; Levy, E.; Siuty, J.; Kalaras, M.D.; Uribarri, J. Bioavailability of vitamin D2 from enriched mushrooms in prediabetic adults: A randomized controlled trial. Eur. J. Clin. Nutr. 2014, 68, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Calvo, M.S.; Babu, U.S.; Garthoff, L.H.; Woods, T.O.; Dreher, M.; Hill, G.; Nagaraja, S. Vitamin D2 from light-exposed edible mushrooms is safe, bioavailable and effectively supports bone growth in rats. Osteoporosis Int. 2013, 24, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Yu, H.T.; Kao, J.P.; Yang, C.C.; Chiang, S.S.; Mishchuk, D.O.; Mau, J.L.; Slupsky, C.M. Consumption of vitamin D2 enhanced mushrooms is associated with improved bone health. J. Nutr. Biochem. 2015, 26, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Simon, R.R.; Borzelleca, J.F.; DeLuca, H.F.; Weaver, C.M. Safety assessment of the post-harvest treatment of button mushrooms (Agaricus bisporus) using ultraviolet light. Food Chem. Toxicol. 2013, 56, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.J.; Lin, C.P.; Mau, J.L.; Li, Y.S.; Tsai, S.Y. Effect of UV-B irradiation on physiologically active substance content and antioxidant properties of the medicinal caterpillar fungus Cordyceps militaris (Ascomycetes). Int. J. Med. Mushrooms 2015, 17, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Rajapakse, N.W.; Karim, F.; Straznicky, N.E.; Fernandez, S.; Evans, R.G.; Head, G.A.; Kaye, D.M. Augmented endothelial-specific l-arginine transport prevents obesity-induced hypertension. Acta Physiol. 2014, 212, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.C.; Feng, R.N.; Hou, Y.; Li, K.; Kang, Z.; Wang, J.; Sun, C.H.; Li, Y. Histidine and arginine are associated with inflammation and oxidative stress in obese women. Br. J. Nutr. 2012, 108, 57–61. [Google Scholar] [CrossRef] [PubMed]
- O'Neil, C.E.; Nicklas, T.A.; Fulgoni, V.L. Consumption of apples is associated with a better diet quality and reduced risk of obesity in children: National Health and Nutrition Examination Survey (NHANES) 2003–2010. Nutr. J. 2015, 14, 48. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Chemistry and anticarcinogenic mechanisms of glycoalkaloids produced by eggplants, potatoes, and tomatoes. J. Agric. Food Chem. 2015, 63, 3323–3337. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Levin, C.E. Glycoalkaloids and calystegine alkaloids in potatoes. In Advances in Potato Chemistry and Technology, 2nd ed.; Singh, J., Kaur, L., Eds.; Elsevier: Oxford, UK, 2016; pp. 167–194. [Google Scholar]
- Shao, D.; Bartley, G.E.; Yokoyama, W.; Pan, Z.; Zhang, H.; Zhang, A. Plasma and hepatic cholesterol-lowering effects of tomato pomace, tomato seed oil and defatted tomato seed in hamsters fed with high-fat diets. Food Chem. 2013, 139, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Ghavipour, M.; Saedisomeolia, A.; Djalali, M.; Sotoudeh, G.; Eshraghyan, M.R.; Moghadam, A.M.; Wood, L.G. Tomato juice consumption reduces systemic inflammation in overweight and obese females. Br. J. Nutr. 2013, 109, 2031–2035. [Google Scholar] [CrossRef] [PubMed]
- Ghavipour, M.; Sotoudeh, G.; Ghorbani, M. Tomato juice consumption improves blood antioxidative biomarkers in overweight and obese females. Clin. Nutr. 2015, 34, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Vinha, A.F.; Barreira, S.V.P.; Costa, A.S.G.; Alves, R.C.; Oliveira, M.B.P.P. Pre-meal tomato (Lycopersicon esculentum) intake can have anti-obesity effects in young women? Int. J. Food Sci. Nutr. 2014, 65, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Anticarcinogenic, cardioprotective, and other health benefits of tomato compounds lycopene, α-tomatine, and tomatidine in pure form and in fresh and processed tomatoes. J. Agric. Food Chem. 2013, 61, 9534–9550. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.P.; Nam, S.H.; Friedman, M. Rice hull smoke extract protects mice against a Salmonella lipopolysaccharide-induced endotoxemia. J. Agric. Food Chem. 2014, 62, 7753–7759. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.P.; Yang, J.Y.; Kang, M.Y.; Park, J.C.; Nam, S.H.; Friedman, M. Composition of liquid rice hull smoke and anti-inflammatory effects in mice. J. Agric. Food Chem. 2011, 59, 4570–4581. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.; Kang, M.Y.; Nam, S.H.; Friedman, M. Antidiabetic effects of rice hull smoke extract in alloxan-induced diabetic mice. J. Agric. Food Chem. 2012, 60, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Chemistry and multi-beneficial bioactivities of carvacrol (4-isopropyl-2-methylphenol), a component of essential oils produced by aromatic plants and spices. J. Agric. Food Chem. 2014, 62, 7652–7670. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Rice brans, rice bran oils, and rice hulls: Composition, food and industrial uses, and bioactivities in humans, animals, and cells. J. Agric. Food Chem. 2013, 61, 10626–10641. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Mackey, B.E.; Kim, H.-J.; Lee, I.-S.; Lee, K.-R.; Lee, S.-U.; Kozukue, E.; Kozukue, N. Structure-activity relationships of tea compounds against human cancer cells. J. Agric. Food Chem. 2007, 55, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Overview of antibacterial, antitoxin, antiviral, and antifungal activities of tea flavonoids and teas. Mol. Nutr. Food Res. 2007, 51, 116–134. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Antibacterial, antiviral, and antifungal properties of wines and winery byproducts in relation to their flavonoid content. J. Agric. Food Chem. 2014, 62, 6025–6042. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Bartley, G.E.; Arvik, T.; Lipson, R.; Nah, S.Y.; Seo, K.; Yokoyama, W. Dietary supplementation of chardonnay grape seed flour reduces plasma cholesterol concentration, hepatic steatosis, and abdominal fat content in high-fat diet-induced obese hamsters. J. Agric. Food Chem. 2014, 62, 1919–1925. [Google Scholar] [CrossRef] [PubMed]
- Tresserra-Rimbau, A.; Medina-Remón, A.; Lamuela-Raventós, R.M.; Bulló, M.; Salas-Salvadó, J.; Corella, D.; Fitó, M.; Gea, A.; Gómez-Gracia, E.; Lapetra, J.; et al. Moderate red wine consumption is associated with a lower prevalence of the metabolic syndrome in the PREDIMED population. Br. J. Nutr. 2015, 113, S121–S130. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.T.; Ciou, J.Y.; Chiang, C.J.; Chao, Y.P.; Yu, B. Effect of Pleurotus eryngii stalk residue on the oxidative status and meat quality of broiler chickens. J. Agric. Food Chem. 2012, 60, 11157–11163. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.P.; Marcante, R.C.; Santana, T.T.; Tanaka, H.S.; Funari, P., Jr.; Alberton, L.R.; Faria, E.V.; Valle, J.S.; Colauto, N.B.; Linde, G.A. Oyster culinary-medicinal mushroom, Pleurotus ostreatus (higher Basidiomycetes), growth in grain-based diet improves broiler chicken production. Int. J. Med. Mushrooms 2015, 17, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Juneja, V.K.; Altuntas, E.G.; Ayhan, K.; Hwang, C.-A.; Sheen, S.; Friedman, M. Predictive model for the reduction of heat resistance of Listeria monocytogenes in ground beef by the combined effect of sodium chloride and apple polyphenols. Int. J. Food Microbiol. 2013, 164, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Grosshauser, S.; Schieberle, P. Characterization of the key odorants in pan-fried white mushrooms (Agaricus bisporus L.) by means of molecular sensory science: Comparison with the raw mushroom tissue. J. Agric. Food Chem. 2013, 61, 3804–3813. [Google Scholar] [CrossRef] [PubMed]
- Ghanayem, B.I.; Bai, R.; Kissling, G.E.; Travlos, G.; Hoffler, U. Diet-induced obesity in male mice is associated with reduced fertility and potentiation of acrylamide-induced reproductive toxicity. Biol. Reprod. 2010, 82, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Chambers, T.J.; Richard, R.A. The impact of obesity on male fertility. Hormones (Athens) 2015, 14, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Acrylamide: Inhibition of formation in processed food and mitigation of toxicity in cells, animals, and humans. Food Funct. 2015, 6, 1752–1772. [Google Scholar] [CrossRef] [PubMed]
- Guevara-Aguirre, J.; Rosenbloom, A.L. Obesity, diabetes and cancer: Insight into the relationship from a cohort with growth hormone receptor deficiency. Diabetologia 2015, 58, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Guevara-Aguirre, J.; Rosenbloom, A.L.; Balasubramanian, P.; Teran, E.; Guevara-Aguirre, M.; Guevara, C.; Procel, P.; Alfaras, I.; de Cabo, R.; di Biase, S.; et al. GH receptor deficiency in Ecuadorian adults is associated with obesity and enhanced insulin sensitivity. J. Clin. Endocrinol. Metab. 2015, 100, 2589–2596. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Antibiotic-resistant bacteria: prevalence in food and inactivation by food-compatible compounds and plant extracts. J. Agric. Food Chem. 2015, 63, 3805–3822. [Google Scholar] [CrossRef] [PubMed]
- Hennechart-Collette, C.; Martin-Latil, S.; Guillier, L.; Perelle, S. Determination of which virus to use as a process control when testing for the presence of hepatitis A virus and norovirus in food and water. Int. J. Food Microbiol. 2015, 202, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.E.; Staples, J.E.; Meaney-Delman, D.; Fischer, M.; Ellington, S.R.; Callaghan, W.M.; Jamieson, D.J. Interim guidelines for pregnant women during a Zika virus outbreak—United States, 2016. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Rasooly, R. Review of the inhibition of biological activities of food-related selected toxins by natural compounds. Toxins 2013, 5, 743–775. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Bhattacharya, S.; Palaniswamy, M.; Angayarkanni, J. Biodegradation of aflatoxin B1 in contaminated rice straw by Pleurotus ostreatus MTCC 142 and Pleurotus ostreatus GHBBF10 in the presence of metal salts and surfactants. World J. Microbiol. Biotechnol. 2014, 30, 2315–2324. [Google Scholar] [CrossRef] [PubMed]
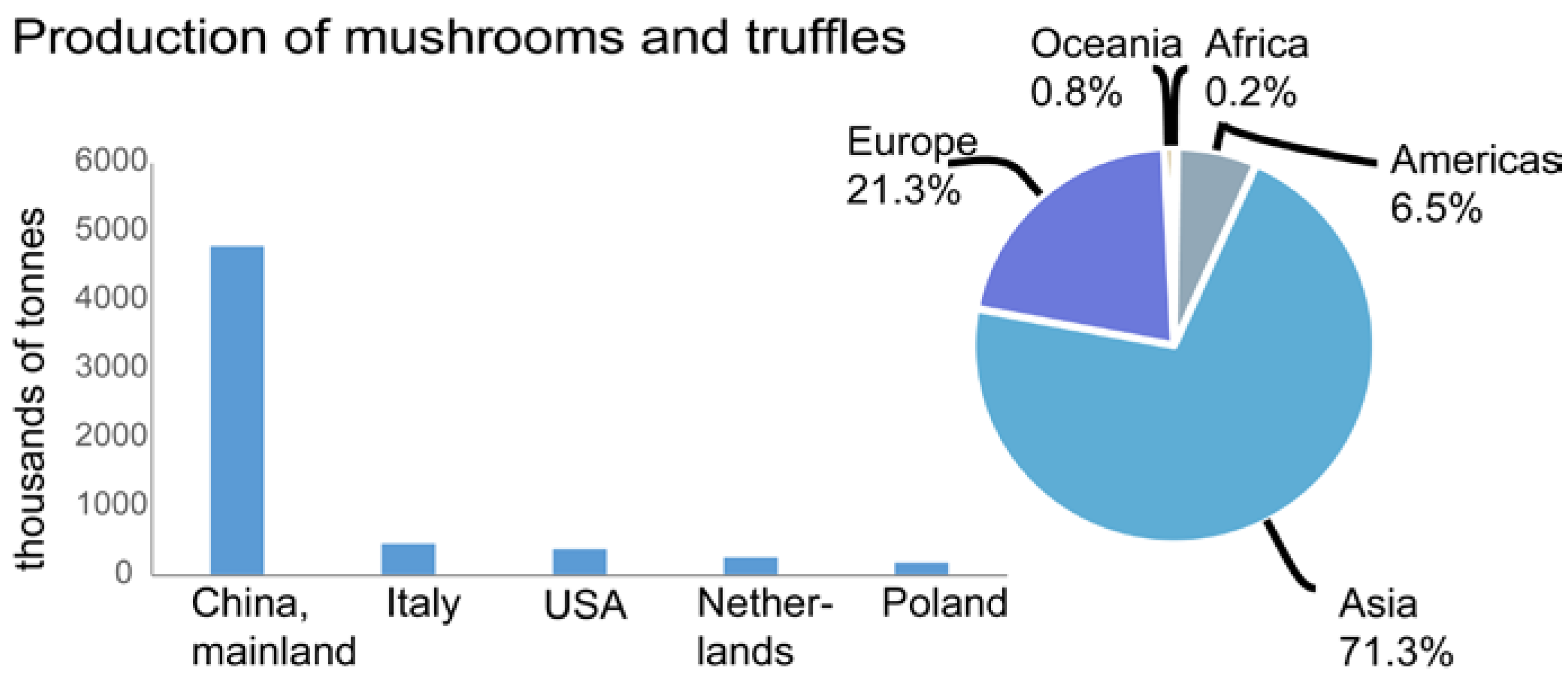
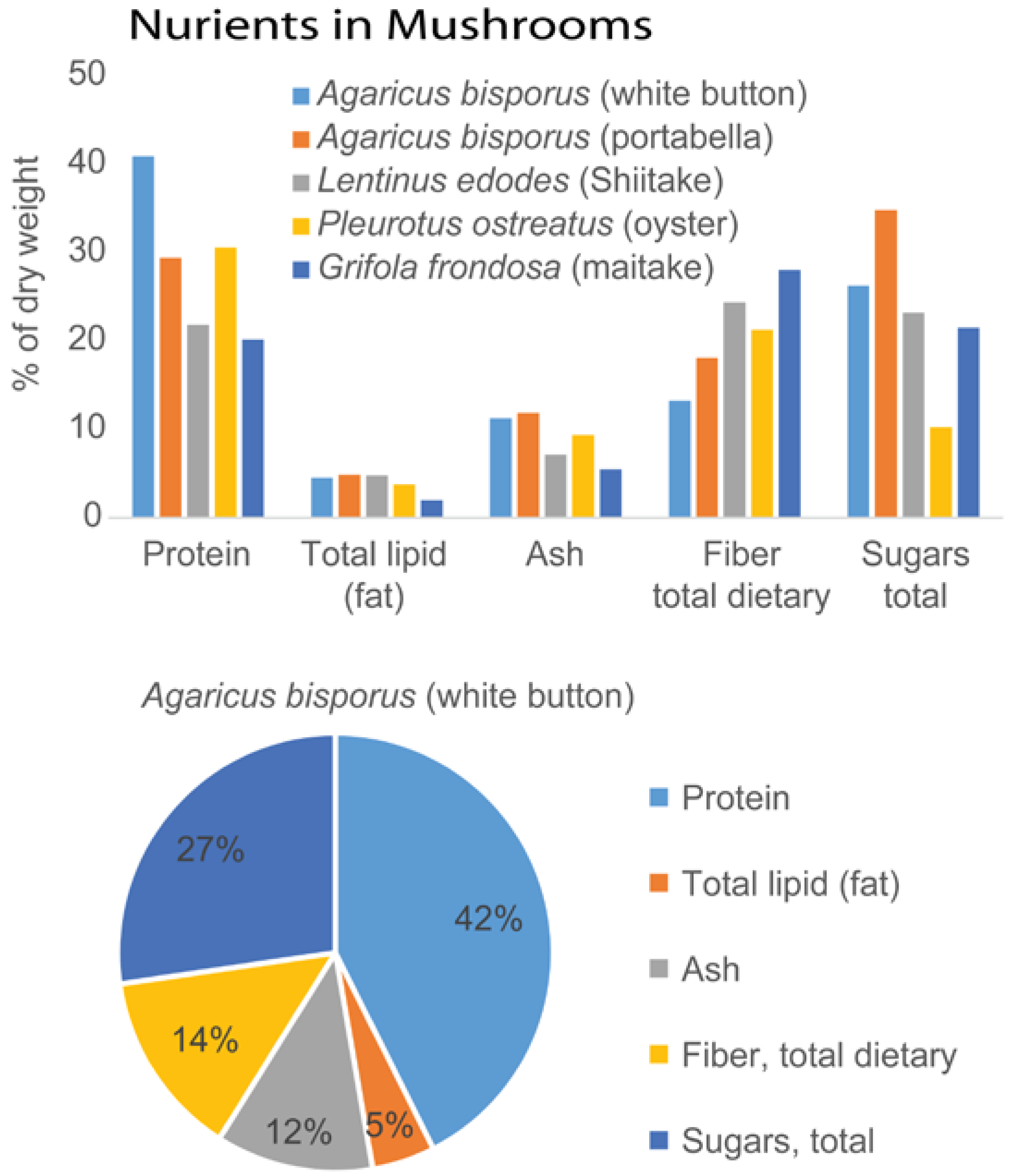

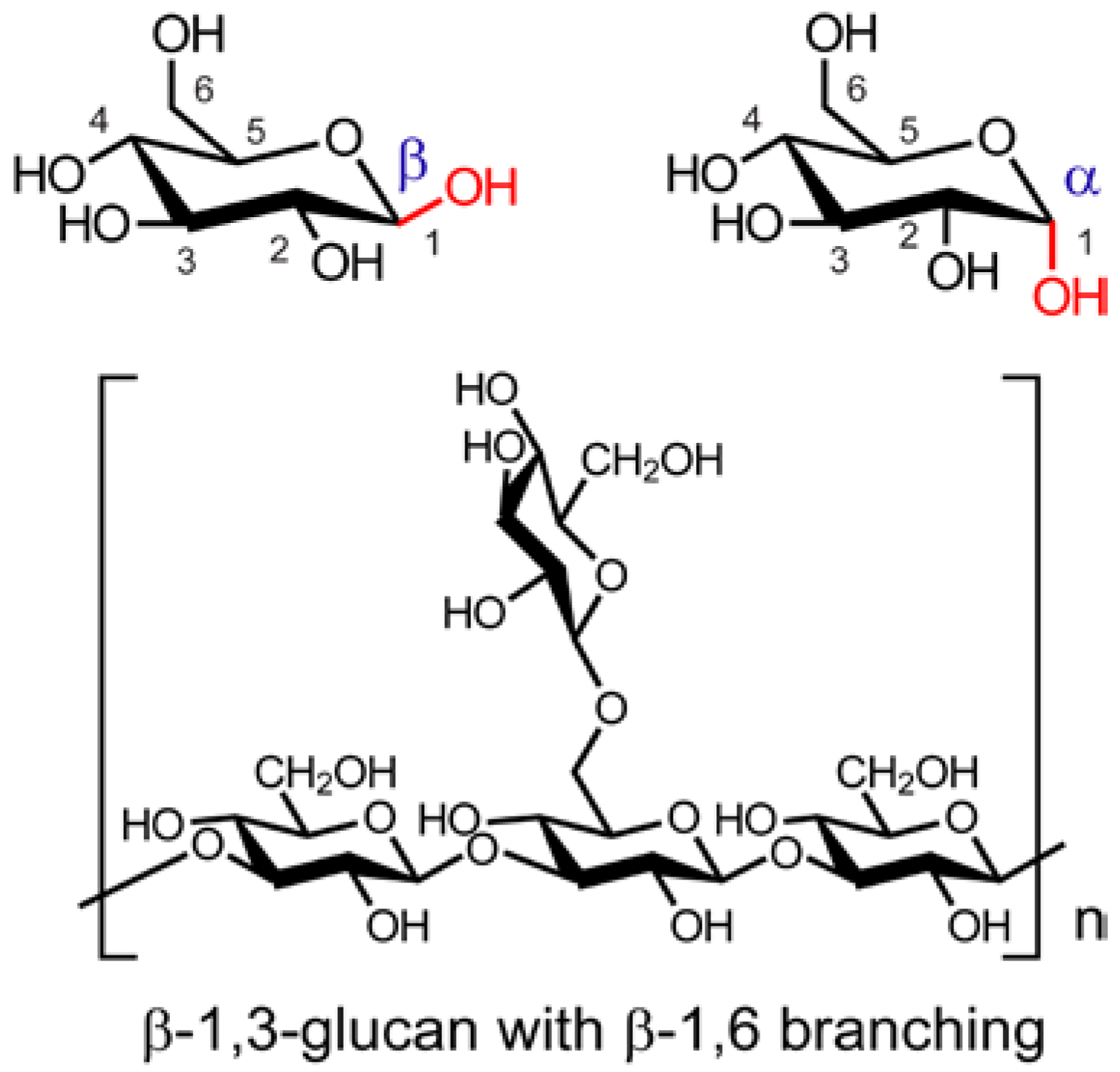
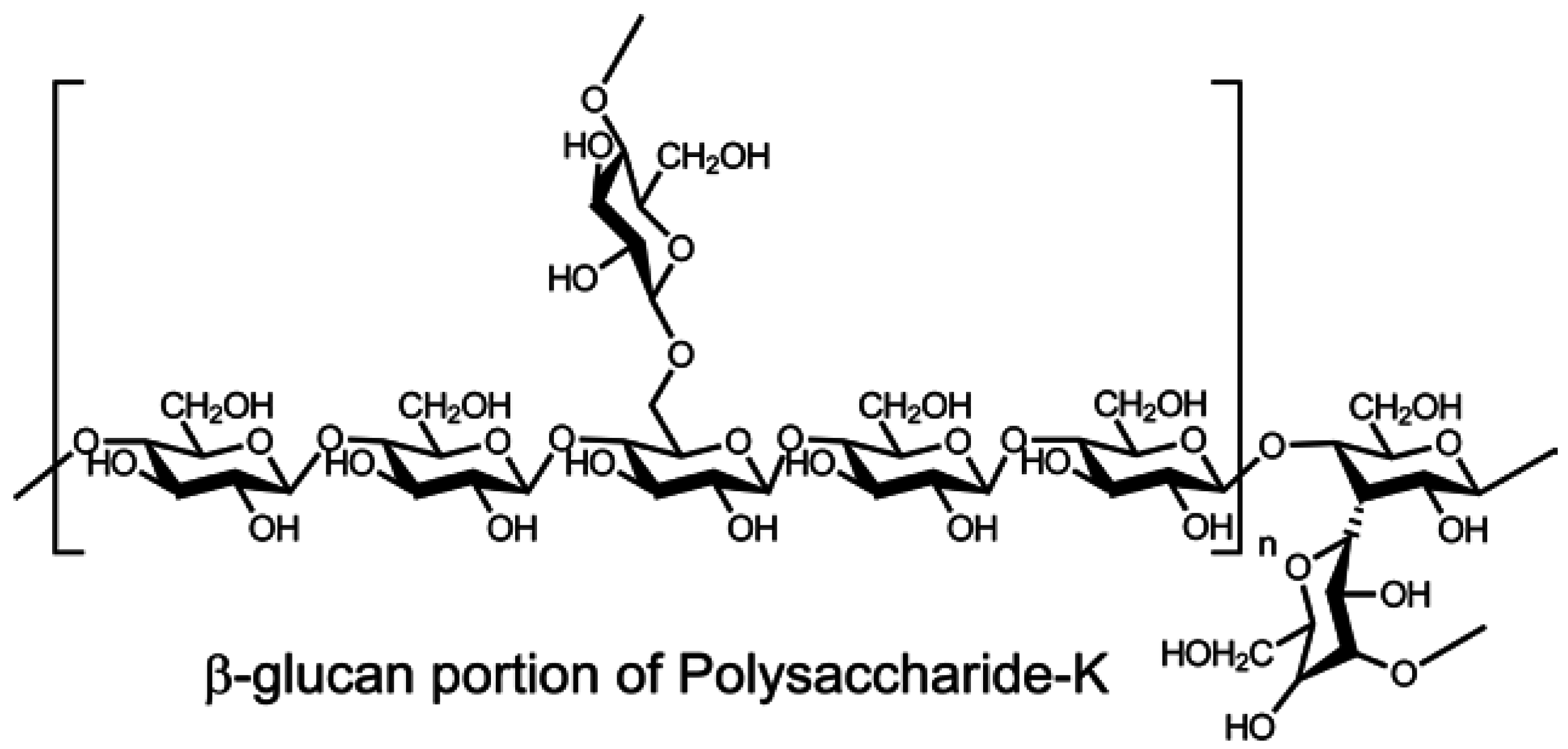
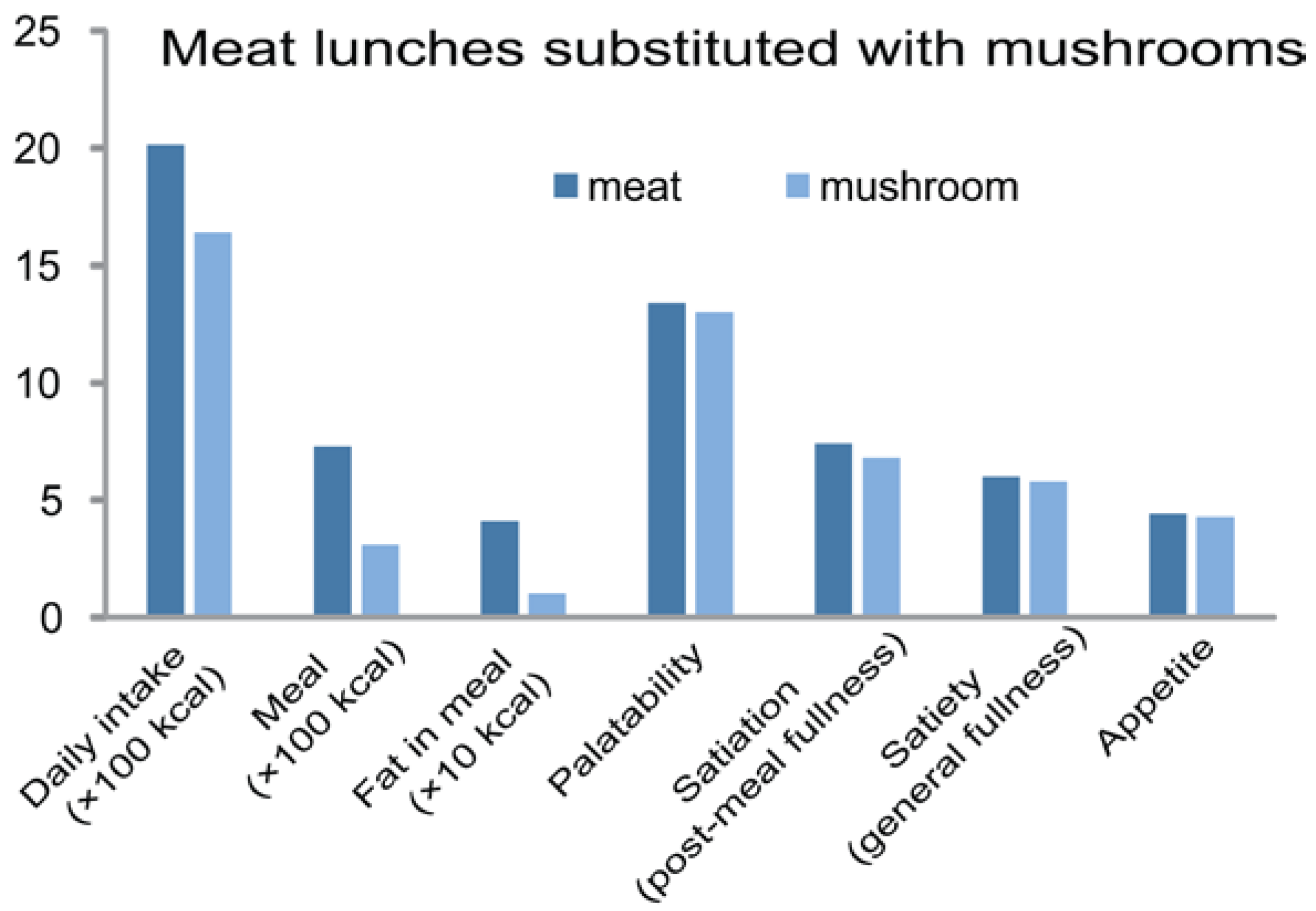
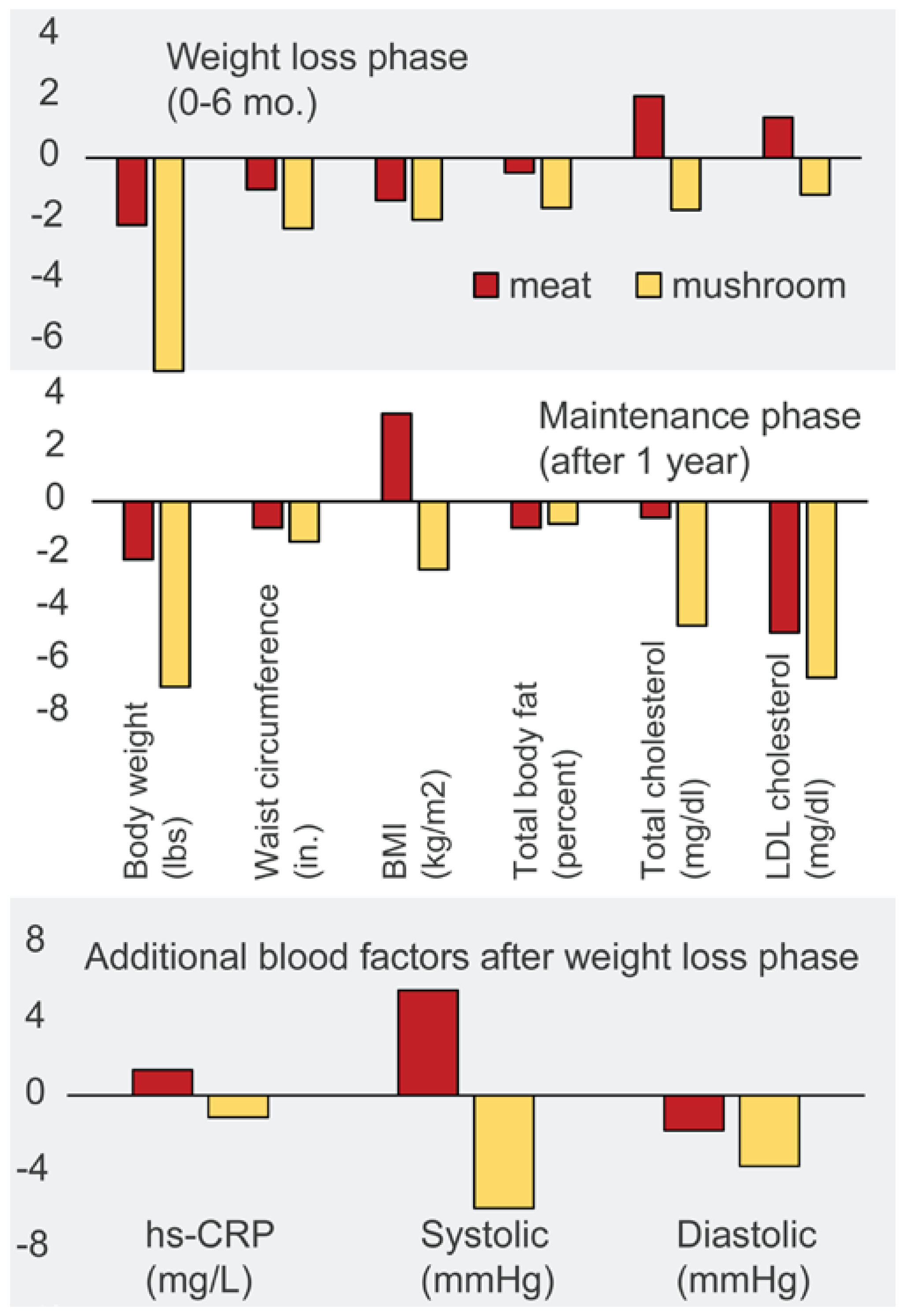
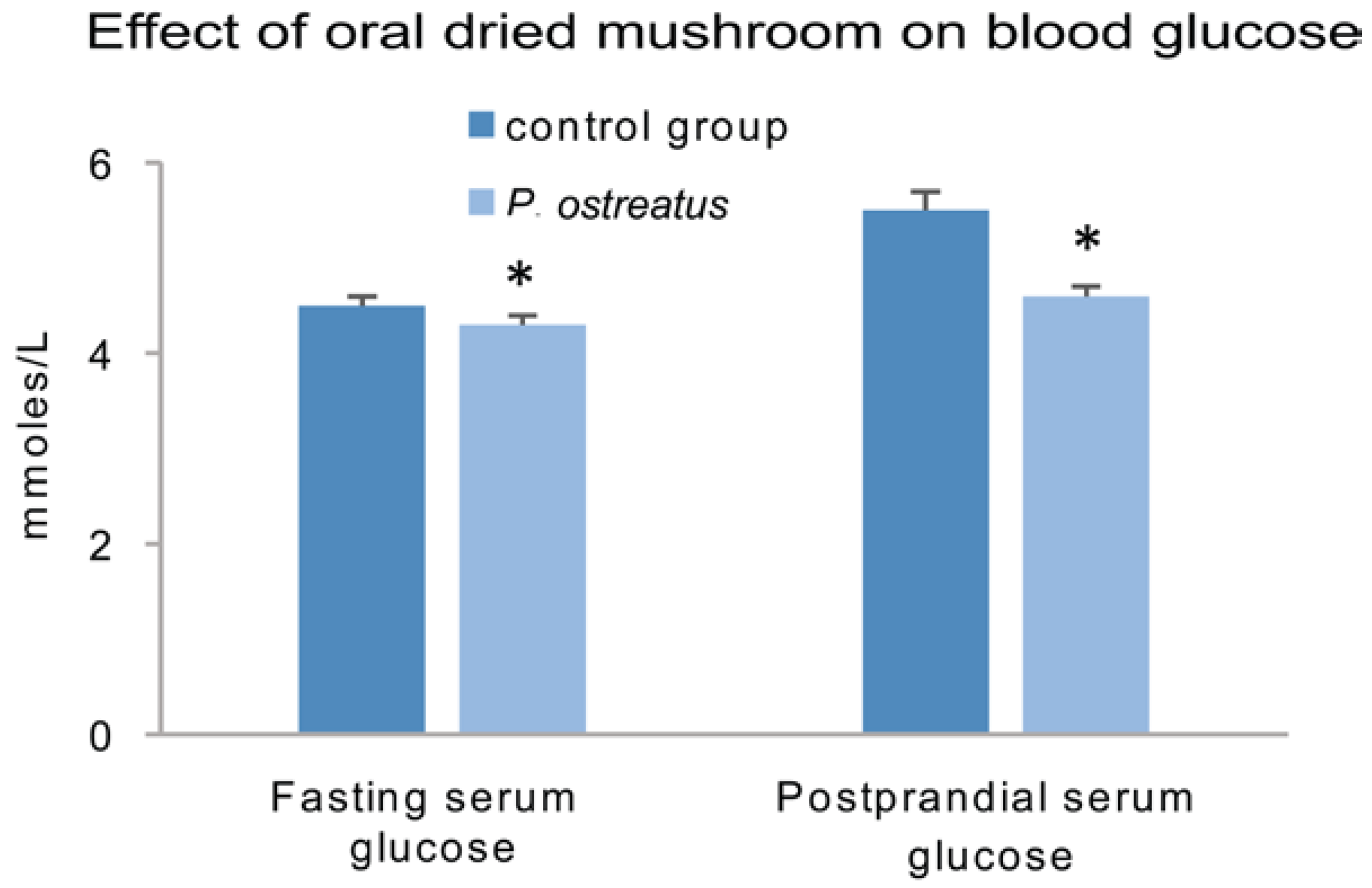
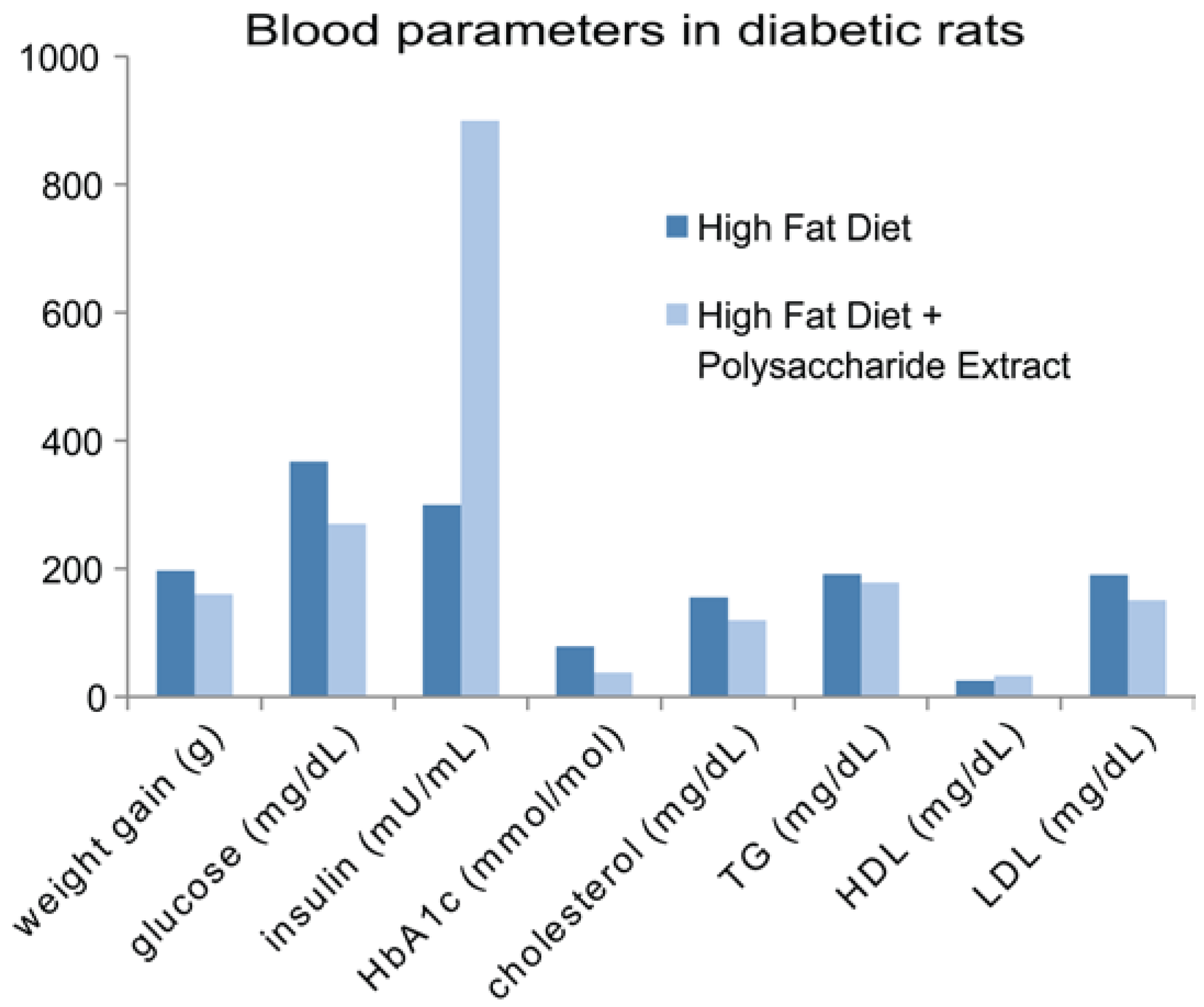
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Friedman, M. Mushroom Polysaccharides: Chemistry and Antiobesity, Antidiabetes, Anticancer, and Antibiotic Properties in Cells, Rodents, and Humans. Foods 2016, 5, 80. https://doi.org/10.3390/foods5040080
Friedman M. Mushroom Polysaccharides: Chemistry and Antiobesity, Antidiabetes, Anticancer, and Antibiotic Properties in Cells, Rodents, and Humans. Foods. 2016; 5(4):80. https://doi.org/10.3390/foods5040080
Chicago/Turabian StyleFriedman, Mendel. 2016. "Mushroom Polysaccharides: Chemistry and Antiobesity, Antidiabetes, Anticancer, and Antibiotic Properties in Cells, Rodents, and Humans" Foods 5, no. 4: 80. https://doi.org/10.3390/foods5040080
APA StyleFriedman, M. (2016). Mushroom Polysaccharides: Chemistry and Antiobesity, Antidiabetes, Anticancer, and Antibiotic Properties in Cells, Rodents, and Humans. Foods, 5(4), 80. https://doi.org/10.3390/foods5040080