Prebiotic Effects and Fermentation Kinetics of Wheat Dextrin and Partially Hydrolyzed Guar Gum in an In Vitro Batch Fermentation System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fecal Collection
2.2. Fermentation
2.3. pH Analysis
2.4. Gas Analysis
2.5. SCFA Analysis
2.6. Microbiota Analysis—Quantitative Polymerase Chain Reaction (qPCR)
2.7. Statistical Analysis
3. Results
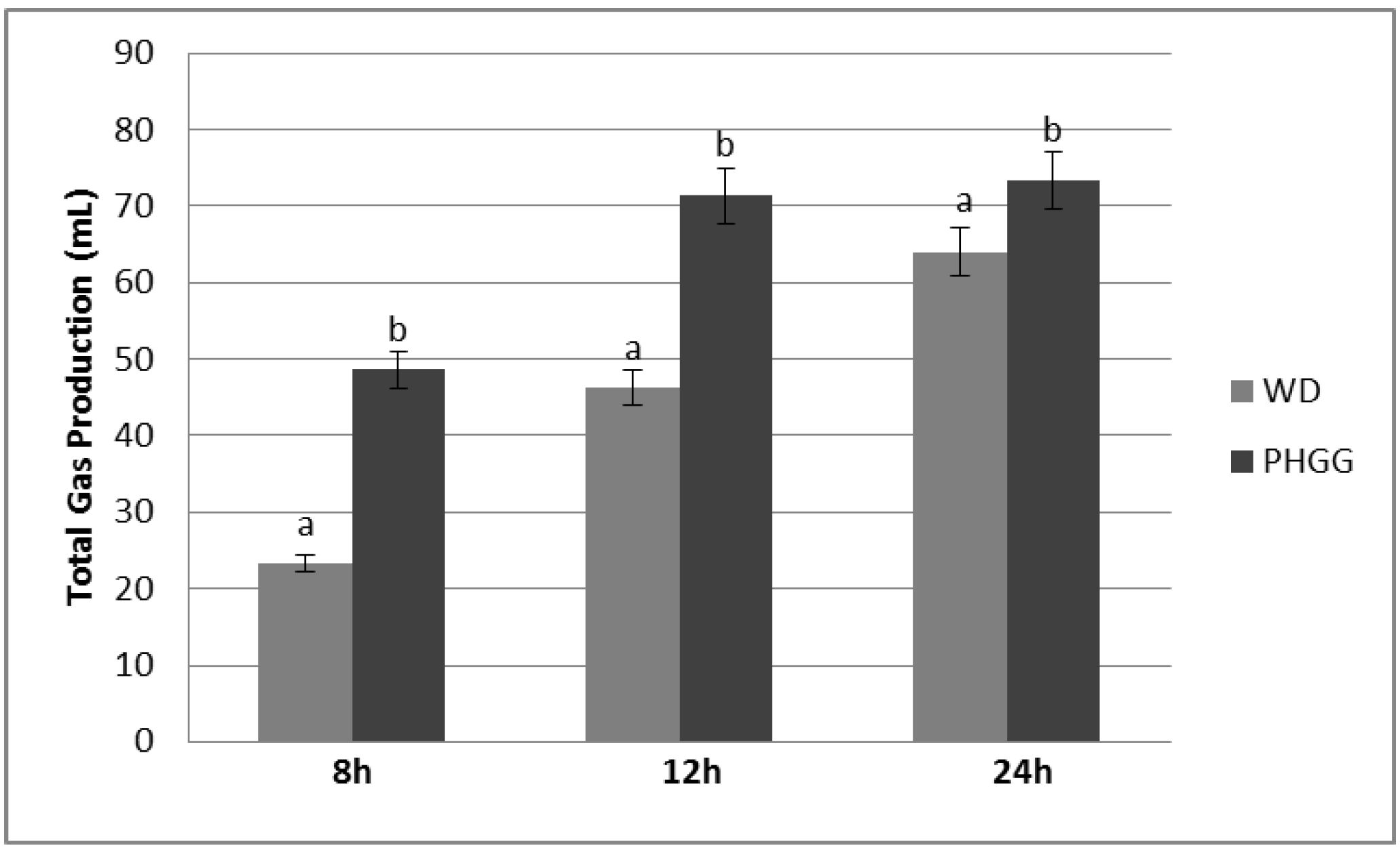
3.1. Gas Production and pH Shift
| Time (h) | pH | ||
|---|---|---|---|
| WD | PHGG | Blank | |
| 0 | 6.96 (0.02) a | 6.97 (0.01) a | 7.06 (0.01) b |
| 4 | 6.51 (0.00) a | 6.59 (0.02) b | 6.96 (0.01) c |
| 8 | 6.34 (0.00) b | 5.93 (0.00) a | 6.92 (0.00) c |
| 12 | 6.07 (0.01) b | 5.90 (0.00) a | 6.92 (0.01) c |
| 24 | 5.78 (0.02) a | 5.86 (0.00) b | 6.92 (0.01) c |
3.2. SCFA Production
| Time (h) | Acetate (μmol/mL) | Propionate (μmol/mL) | Butyrate (μmol/mL) | |||
|---|---|---|---|---|---|---|
| WD | PHGG | WD | PHGG | WD | PHGG | |
| 0 | 1.20 (0.05) | 1.03 (0.09) | 0.69 (0.01) | 0.68 (0.02) | 0.98 (0.03) | 0.95 (0.03) |
| 4 | 5.02 (0.38) | 4.30 (0.14) | 2.45 (0.17) | 2.36 (0.03) | 3.70 (0.29) | 3.22 (0.05) |
| 8 | 7.56 (0.36) | 9.83(0.01) | 7.37 (0.19) | 7.47 (0.29) | 7.77 (0.39) | 10.48 (0.77) |
| 12 | 9.71 (0.53) | 10.85 (0.23) | 8.94 (0.66) | 10.62 (0.10) | 8.35 (0.78) | 10.76 (0.18) |
| 24 | 11.60 (0.71) | 10.74 (0.13) | 10.59 (0.51) | 10.62 (0.44) | 8.32 (0.15) | 9.85 (0.62) |
3.3. Prebiotic Effects
| Time (h) | Bifidobacteria (CFU log10/mL) | Lactobacilli (CFU log10/mL) | ||||
|---|---|---|---|---|---|---|
| WD | PHGG | Blank | WD | PHGG | Blank | |
| 0 | 9.24 (0.03) a | 9.14 (0.07) a | 9.08 (0.00) a | 10.39 (0.04) a | 10.40 (0.09) a | 10.35 (0.02) a |
| 12 | 9.50 (0.03) b | 9.30 (0.04) b | 9.05 (0.06) a | 10.79 (0.04) b | 10.86 (0.07) b | 10.35 (0.02) a |
| 24 | 9.41 (0.07) b | 9.27 (0.04) c | 8.96 (0.07) a | 10.76 (0.09) b | 10.68 (0.04) b | 10.23 (0.03) a |
4. Discussion
Author Contributions
Conflicts of Interest
References
- Slavin, J.L. Position of the American Dietetic Association: Health implications of dietary fiber. J. Am. Diet. Assoc. 2008, 108, 1716–1731. [Google Scholar] [PubMed]
- King, D.E.; Mainous, A.G.; Lambourne, C.A. Trends in dietary fiber intake in the United States, 1999–2008. J. Acad. Nutr. Diet. 2012, 112, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Reicks, M.; Jonnalagadda, S.; Albertson, A.M.; Joshi, N. Total dietary fiber intakes in the US population are related to whole grain consumption: Results from the National Health and Nutrition Examination Survey 2009 to 2010. Nutr. Res. 2014, 34, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J.L. Dietary fiber and body weight. Nutrition 2005, 21, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Vermorel, M.; Coudray, C.; Wils, D.; Sinaud, S.; Tressol, J.C.; Montaurier, C.; Brandolini, M.; Vernet, J.; Bouteloup-Demange, C.; Rayssiguier, Y. Energy value of a low-digestible carbohydrate, NUTRIOSE® FB, and its impact on magnesium, calcium and zinc apparent absorption and retention in healthy young men. Eur. J. Nutr. 2004, 43, 322–349. [Google Scholar] [CrossRef] [PubMed]
- Pasman, W.; Wils, D.; Saniez, M.-H.; Kardinaal, A. Long-term gastrointestinal tolerance of NUTRIOSE FB in healthy men. Eur. J. Clin. Nutr. 2006, 60, 1024–1034. [Google Scholar] [CrossRef] [PubMed]
- Guerin-Deremaux, L.; Li, S.; Pochat, M.; Wils, D.; Mubasher, M.; Reifer, C.; Miller, L.E. Effects of NUTRIOSE® dietary fiber supplementation on body weight, body composition, energy intake, and hunger in overweight men. Int. J. Food Sci. Nutr. 2011, 62, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Guérin-Deremaux, L.; Pochat, M.; Reifer, C.; Wils, D.; Cho, S.; Miller, L.E. The soluble fiber NUTRIOSE induces a dose-dependent beneficial impact on satiety over time in humans. Nutr. Res. 2011, 31, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, E.M.; Westerterp-Plantenga, M.S.; Saris, W.H.; Goossens, I.; Geurten, P.; Brouns, F. The effect of addition of modified guar gum to a low-energy semisolid meal on appetite and body weight loss. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Lavin, J.H.; Read, N.W. The effect on hunger and satiety of slowing the absorption of glucose: Relationship with gastric emptying and postprandial blood glucose and insulin responses. Appetite 1995, 25, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Wisker, E.; Daniel, M.; Rave, G.; Feldheim, W. Fermentation of non-starch polysaccharides in mixed diets and single fibre sources: Comparative studies in human subjects and in vitro. Br. J. Nutr. 1998, 80, 253–261. [Google Scholar] [PubMed]
- Noack, J.; Timm, D.; Hospattankar, A.; Slavin, J. Fermentation profiles of wheat dextrin, inulin and partially hydrolyzed guar gum using an in vitro digestion pretreatment and in vitro batch fermentation system model. Nutrients 2013, 5, 1500–1510. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.M.; Girard-Pipau, F.; Anty, R.; van der Linde, E.G.M.; Philipsen-Geerling, B.J.; Knol, J.; Filippi, J.; Arab, K.; Hébuterne, X. Effects of total enteral nutrition supplemented with a multi-fibre mix on faecal short-chain fatty acids and microbiota. Clin. Nutr. 2006, 25, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Hernot, D.C.; Boileau, T.W.; Bauer, L.L.; Middelbos, I.S.; Murphy, M.R.; Swanson, K.S.; Fahey, G.C. In vitro fermentation profiles, gas production rates, and microbiota modulation as affected by certain fructans, galactooligosaccharides, and polydextrose. J. Agric. Food Chem. 2009, 57, 1354–1361. [Google Scholar] [CrossRef] [PubMed]
- Marco, M.L.; Pavan, S.; Kleerebezem, M. Towards understanding molecular modes of probiotic action. Curr. Opin. Biotechnol. 2006, 17, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Kleerebezem, M.; Vaughan, E.E. Probiotic and gut lactobacilli and bifidobacteria: Molecular approaches to study diversity and activity. Annu. Rev. Microbiol. 2009, 63, 269–290. [Google Scholar] [CrossRef] [PubMed]
- Holscher, H.D.; Caporaso, J.G.; Hooda, S.; Brulc, J.M.; Fahey, G.C., Jr.; Swanson, K.S. Fiber supplementation influences phylogenetic structure and functional capacity of the human intestinal microbiome: Follow-up of a randomized controlled trial 1–4. Am. J. Clin. Nutr. 2015, 101, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Hobden, M.R.; Martin-Morales, A.; Guérin-Deremaux, L.; Wils, D.; Costabile, A.; Walton, G.E.; Rowland, I.; Kennedy, O.B.; Gibson, G.R. In Vitro Fermentation of NUTRIOSE® FB06, a Wheat Dextrin Soluble Fibre, in a Continuous Culture Human Colonic Model System. PLoS ONE 2013, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Scholz-Ahrens, K.E.; Schrezenmeir, J. Inulin, oligofructose and mineral metabolism—Experimental data and mechanism. Br. J. Nutr. 2002, 87, S179–S186. [Google Scholar] [CrossRef] [PubMed]
- Ohta, A.; Ohtsuki, M.T.; Baba, S.; Kimura, T. Effects of fructooligosaccharides on the absorption of iron, calcium and magnesium in iron-deficient anemic rats. J. Nutr. Sci. Vitaminol. 1995, 41, 281–291. [Google Scholar] [CrossRef] [PubMed]
- McNamara, E.A.; Levitt, M.D.; Slavin, J.L. Breath hydrogen and methane: Poor indicators of apparent digestion of soy iber. Am. J. Clin. Nutr. 1986, 43, 898–902. [Google Scholar] [PubMed]
- Hess, J.R.; Birkett, A.M.; Thomas, W.; Slavin, J.L. Effects of short-chain fuctooligosaccharides on satiety response in healthy men and women. Appetite 2011, 56, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Bonnema, A.L.; Kolberg, L.W.; Thomas, W.; Slavin, J.L. Gastrointestinal tolerance of chicory inulin products. J. Am. Diet. Assoc. 2010, 110, 865–868. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.M.W.; de Souza, R.; Kendall, C.W.C.; Emam, A.; Jenkins, D.J.A. Ovid: Colonic Health: Fermentation and Short Chain Fatty Acids. Clin. Gastroenterol. 2006, 40, 235–343. [Google Scholar] [CrossRef]
- Wolever, T.; Spadafora, P.; Eshuis, H. Interaction between colonic acetate and propionate in humans. Am. J. Clin. Nutr. 1991, 53, 681–687. [Google Scholar] [PubMed]
- Velázquez, O.C.; Lederer, H.M.; Rombeau, J.L. Butyrate and the colonocyte. Dig. Dis. Sci. 1996, 41, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Topping, D.L.; Clifton, P.M. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [PubMed]
- Lefranc-Millot, C.; Guérin-Deremaux, L.; Wils, D.; Neut, C.; Miller, L.E.; Saniez-Degrave, M.H. Impact of a resistant dextrin on intestinal ecology: How altering the digestive ecosystem with NUTRIOSE®, a soluble fibre with prebiotic properties, may be beneficial for health. J. Int. Med. Res. 2012, 40, 211–224. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carlson, J.; Hospattankar, A.; Deng, P.; Swanson, K.; Slavin, J. Prebiotic Effects and Fermentation Kinetics of Wheat Dextrin and Partially Hydrolyzed Guar Gum in an In Vitro Batch Fermentation System. Foods 2015, 4, 349-358. https://doi.org/10.3390/foods4030349
Carlson J, Hospattankar A, Deng P, Swanson K, Slavin J. Prebiotic Effects and Fermentation Kinetics of Wheat Dextrin and Partially Hydrolyzed Guar Gum in an In Vitro Batch Fermentation System. Foods. 2015; 4(3):349-358. https://doi.org/10.3390/foods4030349
Chicago/Turabian StyleCarlson, Justin, Ashok Hospattankar, Ping Deng, Kelly Swanson, and Joanne Slavin. 2015. "Prebiotic Effects and Fermentation Kinetics of Wheat Dextrin and Partially Hydrolyzed Guar Gum in an In Vitro Batch Fermentation System" Foods 4, no. 3: 349-358. https://doi.org/10.3390/foods4030349
APA StyleCarlson, J., Hospattankar, A., Deng, P., Swanson, K., & Slavin, J. (2015). Prebiotic Effects and Fermentation Kinetics of Wheat Dextrin and Partially Hydrolyzed Guar Gum in an In Vitro Batch Fermentation System. Foods, 4(3), 349-358. https://doi.org/10.3390/foods4030349





