Analysis of Growth Inhibition and Metabolism of Hydroxycinnamic Acids by Brewing and Spoilage Strains of Brettanomyces Yeast
Abstract
:1. Introduction
2. Experimental Section
2.1. Strains
| Strain | Species | Source * | Brewing Suitability |
|---|---|---|---|
| 1 | B. bruxellensis | Environmental (Bc02) | No |
| 2 | B. bruxellensis | Environmental (Bc07) | No |
| 3 | B. bruxellensis | Environmental (Bc11) | No |
| 4 | B. anomalus | Environmental (Cs01) | Yes |
| 5 | B. anomalus | Environmental (Cu02) | Yes |
| 6 | B. anomalus | Environmental (Ej02) | Yes |
| 7 | B. anomalus | Environmental (Rs01) | Yes |
| 8 | B. anomalus | Environmental (Vc01) | No |
| 9 | B. anomalus | Commercial (WLP645) | Yes |
| 10 | B. bruxellensis | Commercial (WLP650) | Yes |
| 11 | B. bruxellensis | Commercial (WLP653) | Yes |
| 12 | B. anomalus | Commercial (WY5151) | Yes |
2.2. Minimum Inhibitory Concentration
2.3. Growth Characteristics in HCAs
2.4. Spectral Scan Peak Absorbance
2.5. Metabolism of HCAs
3. Results
3.1. Minimum Inhibitory Concentration
| HCA | Strain | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| ferulic acid | 10 | 10 | 8 | 14 | 8 | 12 | 12 | 16 | 12 | 12 | 12 | 8 |
| p-coumaric acid | 25 | 25 | 10 | 12 | 8 | 14 | 25 | 25 | 25 | 25 | 25 | 8 |
| caffeic acid | 25 | 25 | 25 | 25 | 25 | 25 | 30 | 25 | 25 | 25 | 30 | 25 |
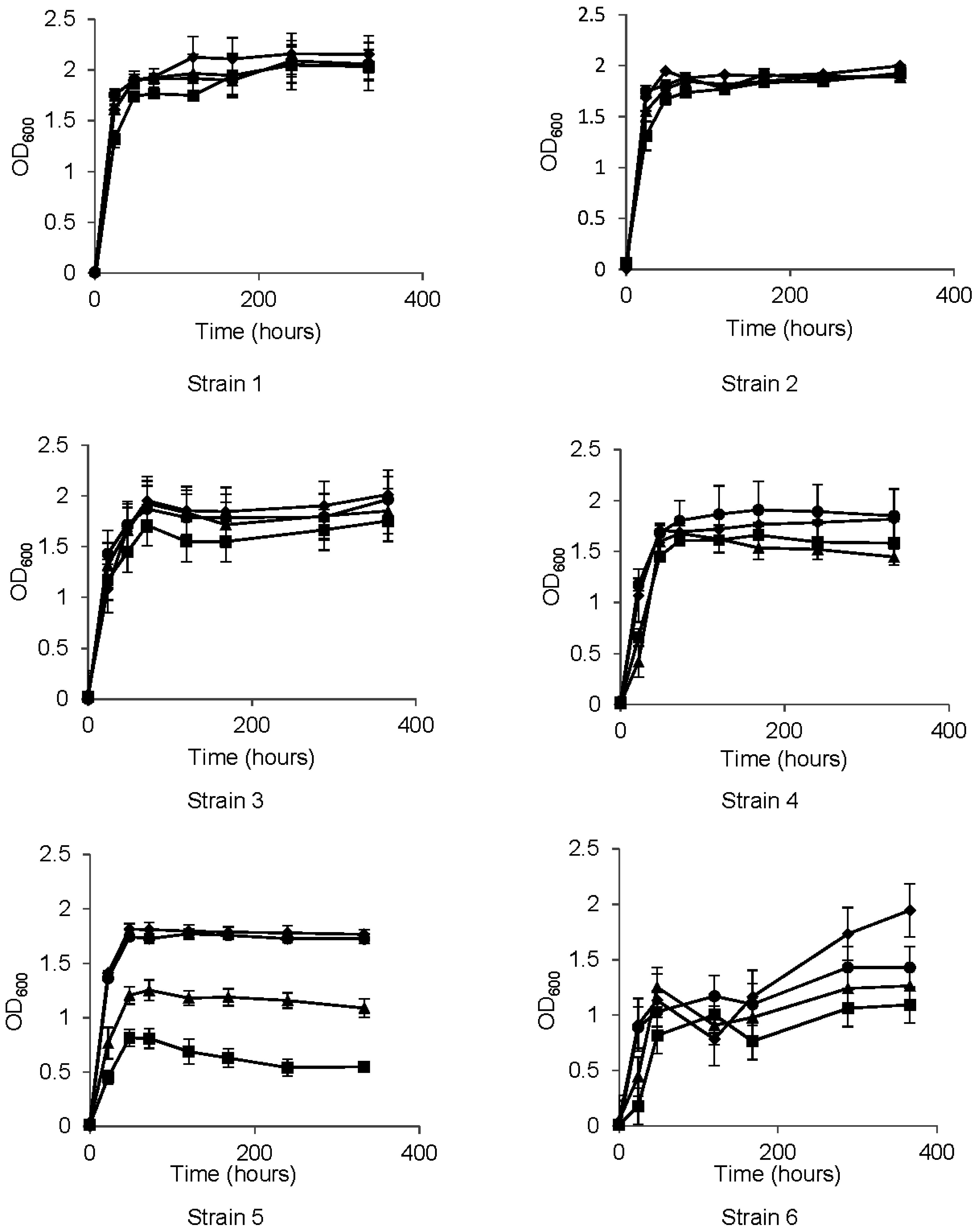
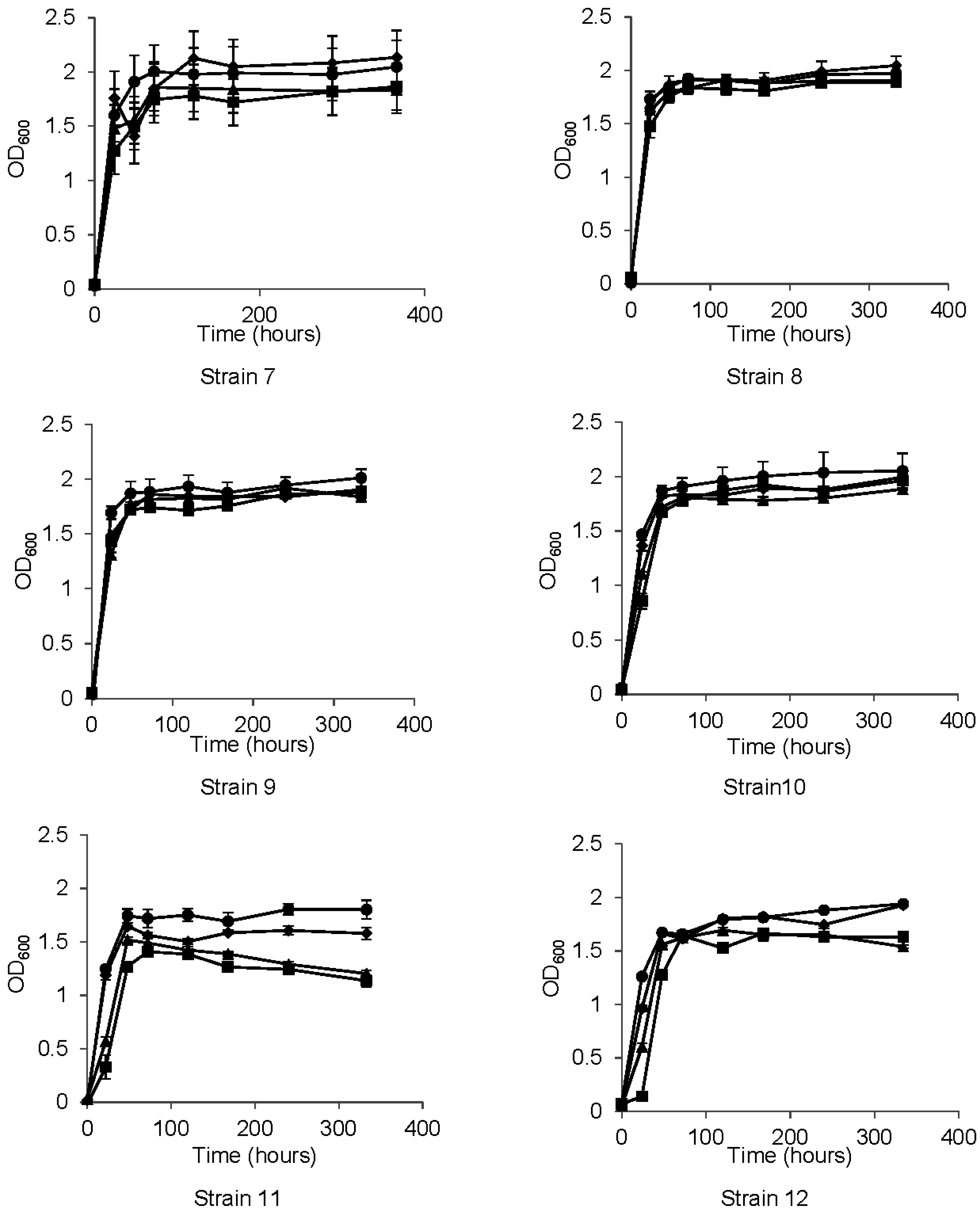
3.2. Growth Properties
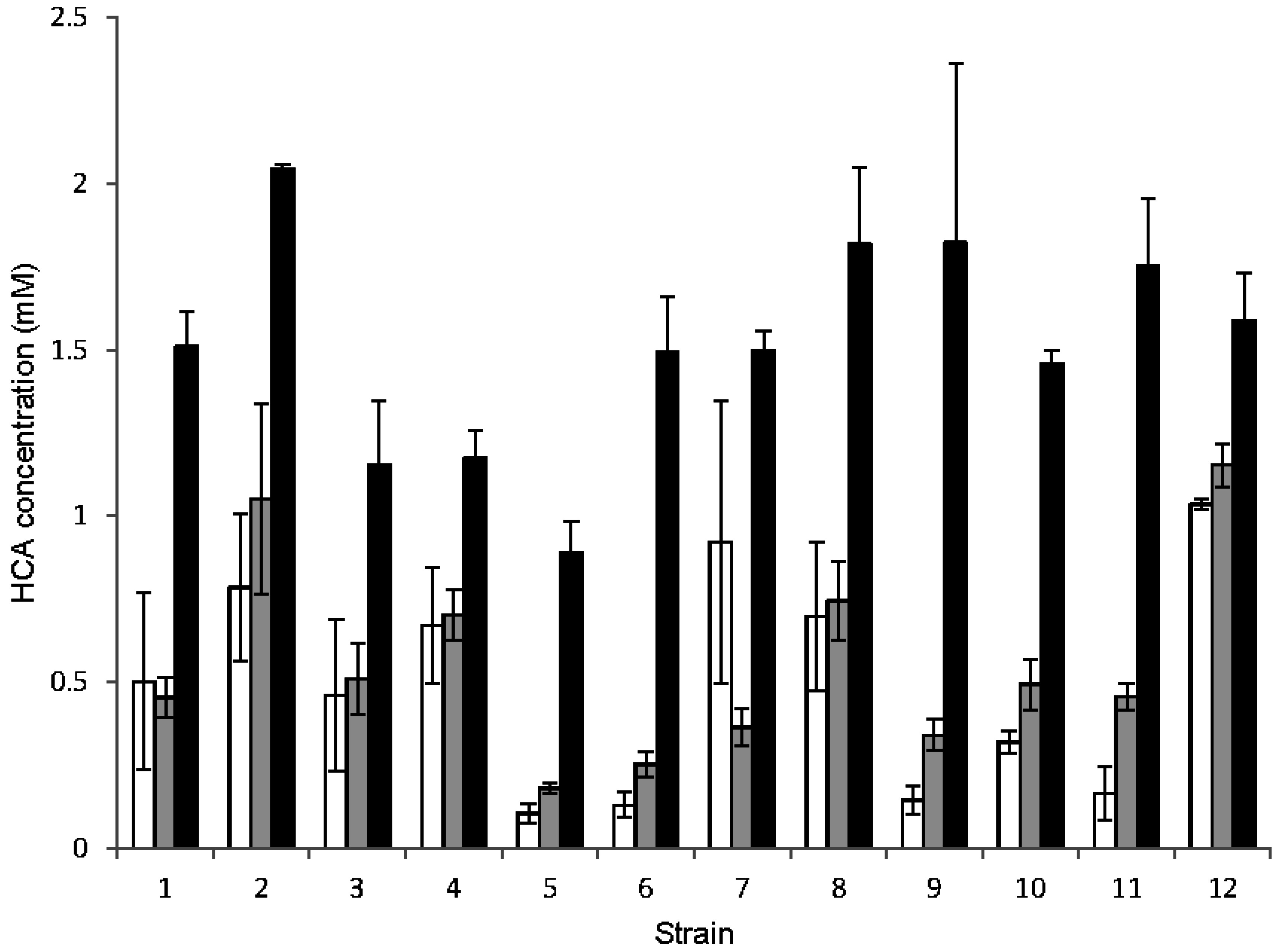
3.3. Metabolism of HCAs
| Strain | Greater inhibition by* | Metabolism of HCAs* | Suitability | ||||
|---|---|---|---|---|---|---|---|
| FA | pCA | Same | FA > pCA | pCA > FA | FA = pCA | For Brewing | |
| 1 | X | X | No | ||||
| 2 | X | X | No | ||||
| 3 | X | X | No | ||||
| 4 | X | X | Yes | ||||
| 5 | X | ||||||
| 6 | X | ||||||
| 7 | X | X | Yes | ||||
| 8 | X | X | No | ||||
| 9 | X | ||||||
| 10 | X | ||||||
| 11 | X | ||||||
| 12 | X | ||||||
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schifferdecker, A.J.; Dashko, S.; Ishchuk, O.P.; Piškur, J. The wine and beer yeast Dekkera bruxellensis. Yeast 2014, 31, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Wedral, D.; Shewfelt, R.; Frank, J. The challenge of Brettanomyces in wine. LWT—Food Sci. Technol. 2010, 43, 1474–1479. [Google Scholar] [CrossRef]
- Zuehlke, J.M.; Petrova, B.; Edwards, C.G. Advances in the Control of Wine Spoilage by Zygosaccharomyces and Dekkera/Brettanomyces. Ann. Rev. Food Sci. Technol. 2013, 4, 57–78. [Google Scholar] [CrossRef] [PubMed]
- Suarez, R.; Suarez-Lepe, J.; Morata, A.; Calderon, F. The production of ethylphenols in wine by yeasts of the genera Brettanomyces and Dekkera: A review. Food Chem. 2007, 102, 10–21. [Google Scholar] [CrossRef]
- Garijo, P.; Gonzalez-Arenzana, L.; Lopez-Alfaro, I.; Garde-Cerda, T.; Lopez, R.; Santamaria, P.; Gutierrez, A.R. Analysis of grapes and the first stages of the vinification process in wine contamination with Brettanomyces bruxellensis. Eur. J. Food Res. Technol. 2015, 240. [Google Scholar] [CrossRef]
- Loureiro, V.; Malfeito-Ferreira, M. Spoilage yeasts in the wine industry. Int. J. Food Microbiol. 2003, 86, 23–50. [Google Scholar] [CrossRef]
- Silva, P.; Cardoso, H.; Geros, H. Studies on the Wine Spoilage Capacity of Brettanomyces/Dekkera spp. Am. J. Enol. Vitic. 2004, 55, 65–72. [Google Scholar]
- Chatonnet, P.; Dubourdieu, D.; Boidron, J.N.; Pons, M. The origin of ethylphenols in wines. J. Sci. Food Agric. 1992, 60, 165–178. [Google Scholar] [CrossRef]
- Heresztyn, T. Metabolism of volatile phenolic compounds from hydroxycinnamic acids by Brettanomyces yeast. Arch. Microbiol. 1986, 146, 96–98. [Google Scholar] [CrossRef]
- Bidlack, J.; Malone, M.; Benson, R. Molecular Structure and Component Integration of Secondary Cell Walls in Plants. Proc. Okla Acad. Sci. 1992, 72, 51–56. [Google Scholar]
- Faulds, C.B.; Williamson, G. The role of hydroxycinnamates in the plant cell wall. J. Sci. Food Agric. 1999, 79, 393–395. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, Y.; Lu, J. Phenolic contents and compositions in skins of red wine grape cultivars among various genetic backgrounds and originations. Int. J. Mol. Sci. 2012, 13, 3492–3510. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Vasanthan, T.; Temelli, F. Analysis of phenolic acids in barley by high-performance liquid chromatography. J. Agric. Food Chem. 2001, 49, 4352–4358. [Google Scholar] [CrossRef] [PubMed]
- Pinelo, M.; Arnous, A.; Meyer, A.S. Upgrading of grape skins: Significance of plant cell-wall structural components and extraction techniques for phenol release. Trends Food Sci. Technol. 2006, 17, 579–590. [Google Scholar] [CrossRef]
- Vanbeneden, N.; Van Roey, T.; Willems, F.; Delvaux, F.; Delvaux, F.R. Release of phenolic flavour precursors during wort production: Influence of process parameters and grist composition on ferulic acid release. Food Chem. 2008, 111, 83–91. [Google Scholar] [CrossRef]
- Harris, V.; Jiranek, V.; Ford, C.; Grbin, P. Inhibitory effect of hydroxycinnamic acids on Dekkera spp. Appl. Microbiol. Biotechnol. 2010, 86, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Stead, D. The effect of hydroxycinnamic acids and potassium sorbate on the growth of 11 strain of spoilage yeast. J. Appl. Bacteriol. 1995, 78, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Pastorkova, E.; Zakova, T.; Landa, P.; Novakova, J.; Vadlejch, J.; Kokoska, L. Growth inhibitory effect of grape phenolics against wine spoilage yeasts and acetic acid bacteria. Int. J. Food Microbiol. 2013, 161, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Rosazza, J.P.N.; Huang, Z.; Dostal, L.; Volm, T.; Rousseau, B. Review: Biocatalytic transformations of ferulic acid: An abundant aromatic natural product. J. Ind. Microbiol. 1995, 15, 457–471. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, T.; Kubodera, S.; Yanagida, F. Distribution of phenolic yeasts and production of phenolic off-flavors in wine fermentation. J. Biosci. Bioeng. 2000, 90, 90–97. [Google Scholar] [CrossRef]
- Edlin, D.A.N.; Narbad, A.; Dickinson, J.R.; Lloyd, D. The biotransformation of simple phenolic compounds by Brettanomyces anomalus. FEMS Microbiol. Lett. 1995, 125, 311–316. [Google Scholar] [CrossRef]
- Weaver, K. Report: The Fastest Growing Beer Styles, on RateBeer. Available online: http://us2.campaign-archive1.com/?u=927a170c4a7bc5ac2c11d7a5f&id=ae8ff00c56 (accessed on 6 May 2015).
- Curtin, C.D.; Pretorius, I.S. Genomic insights into the evolution of industrial yeast species Brettanomyces bruxellensis. FEMS Yeast Res. 2014, 14, 997–1005. [Google Scholar] [PubMed]
- Daenen, L.; Saison, D.; Sterckx, F.; Delvaux, F.R.; Verachtert, H.; Derdelinckx, G. Screening and evaluation of the glucoside hydrolase activity in Saccharomyces and Brettanomyces brewing yeasts. J. Appl. Microbiol. 2008, 104, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Vanbeneden, N.; Gils, F.; Delvaux, F.; Delvaux, F.R. Formation of 4-vinyl and 4-ethyl derivatives from hydroxycinnamic acids: Occurrence of volatile phenolic flavour compounds in beer and distribution of Pad1-activity among brewing yeasts. Food Chem. 2008, 107, 221–230. [Google Scholar] [CrossRef]
- Lentz, M.; Putzke, T.; Hessler, R.; Luman, E. Genetic and physiological characterization of yeast isolated from ripe fruit and analysis of fermentation and brewing potential. J. Inst. Brew. 2014, 120, 559–564. [Google Scholar] [CrossRef]
- Harris, V.; Ford, C.; Jiranek, V.; Grbin, P. Survey of enzyme activity responsible for phenolic off-flavour production by Dekkera and Brettanomyces yeast. Appl. Microbiol. Biotechnol. 2009, 81, 1117–1127. [Google Scholar] [CrossRef] [PubMed]
- Harris, V.; Ford, C.; Jiranek, V.; Grbin, P. Dekkera and Brettanomyces growth and utilisation of hydroxycinnamic acids in synthetic media. Appl. Microbiol. Biotechnol. 2008, 78, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Humberstone, F.J.; Briggs, D.E. Extraction and assay of ferulic acid esterase from malted barley. J. Inst. Brew. 2000, 106, 21–29. [Google Scholar] [CrossRef]
- Szwajgier, D.; Wasko, A.; Targonski, Z. Influence of pH and temperature on ferulic acid esterase and acetic acid esterase activities during malting and mashing. Pol. J. Food Nutr. Sci. 2006, 15, 183–191. [Google Scholar]
- Borneman, A.R.; Zeppel, R.; Chambers, P.J.; Curtin, C.D. Insights into the Dekkera bruxellensis genomic landscape: comparative genomics reveals variations in ploidy and nutrient utilisation potential amongst wine isolates. PLoS Genet. 2014, 10, e1004161. [Google Scholar] [CrossRef] [PubMed]
- Curtin, C.D.; Borneman, A.R.; Chambers, P.J.; Pretorius, I.S. De-novo assembly and analysis of the heterozygous triploid genome of the wine spoilage yeast Dekkera bruxellensis AWRI1499. PLoS ONE 2012, 7, e33840. [Google Scholar] [CrossRef] [PubMed]
- Piskur, J.; Ling, Z.; Marcet-Houben, M.; Ishchuk, O.; Aerts, A.; LaButti, K.; Copeland, A.; Lindquist, E.; Barry, K.; Compagno, C.; et al. The genome of wine yeast Dekkera bruxellensis provides a tool to explore its food-related properties. Int. J. Food Microbiol. 2012, 157, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Woolfit, M.; Rozpedowska, E.; Piskur, J.; Wolfe, K.H. Genome survey sequencing of the wine spoilage yeast Dekkera (Brettanomyces) bruxellensis. Eukaryot. Cell 2007, 6, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Lentz, M.; University of North Florida, Jacksonville, FL, USA. Unpublished work. 2015.
- Campolongo, S.; Siegumfeldt, H.; Aabo, T.; Cocolin, L.; Arneborg, N. The effects of extracellular pH and hydroxycinnamic acids influence the intracellular pH of Brettanomyces bruxellensis DSM 7001. LWT Food Sci. Technol. 2014, 59, 1088–1092. [Google Scholar] [CrossRef]
- Brul, S.; Coote, P. Preservative agents in foods: Mode of action and microbial resistance mechanisms. Int. J. Food Microbiol. 1999, 50, 1–17. [Google Scholar] [CrossRef]
- Casal, M.; Paiva, S.; Queiros, O.; Soares-Silva, I. Transport of carboxylic acids in yeast. FEMS Microbiol. Rev. 2008, 32, 974–994. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lentz, M.; Harris, C. Analysis of Growth Inhibition and Metabolism of Hydroxycinnamic Acids by Brewing and Spoilage Strains of Brettanomyces Yeast. Foods 2015, 4, 581-593. https://doi.org/10.3390/foods4040581
Lentz M, Harris C. Analysis of Growth Inhibition and Metabolism of Hydroxycinnamic Acids by Brewing and Spoilage Strains of Brettanomyces Yeast. Foods. 2015; 4(4):581-593. https://doi.org/10.3390/foods4040581
Chicago/Turabian StyleLentz, Michael, and Chad Harris. 2015. "Analysis of Growth Inhibition and Metabolism of Hydroxycinnamic Acids by Brewing and Spoilage Strains of Brettanomyces Yeast" Foods 4, no. 4: 581-593. https://doi.org/10.3390/foods4040581
APA StyleLentz, M., & Harris, C. (2015). Analysis of Growth Inhibition and Metabolism of Hydroxycinnamic Acids by Brewing and Spoilage Strains of Brettanomyces Yeast. Foods, 4(4), 581-593. https://doi.org/10.3390/foods4040581





