The gelled systems formed by plant proteins and polysaccharides present with intrinsically complex characteristics, often resulting from the synergistic interactions between the components. This complexity makes the gels highly sensitive to a wide range of experimental variables, which require rigorous control during the development and analysis of these systems.
Among the factors that directly influence the properties of the gels are the choice of protein source (PE), the polysaccharide used, the concentrations of the biopolymers, the type and charge of ions present (monovalent or divalent), the use of crosslinking agents (such as enzymes), and the pH, in addition to the process conditions, such as the temperature, preparation time, and the method for the addition of the ingredients. Polysaccharides have distinct gelation and protein interaction properties, depending upon their chemical structure and the degree of acylation or methoxylation, directly influencing the texture, opacity, and stability of the systems formed. The addition of NaCl or CaCl2 was explored here as a strategy to enhance or modify the molecular interactions, aiming to expand the applicability of these systems in industrial contexts, such as in the manufacture of food and biomaterials.
Due to the diversity of the objectives associated with these systems, building an initial model entirely based on the literature is not always possible, especially when focusing on specific and innovative applications. In the present study, the formulation of a meat analog was the main driver in selecting the components and experimental conditions.
3.1. Pure Polysaccharide Gels
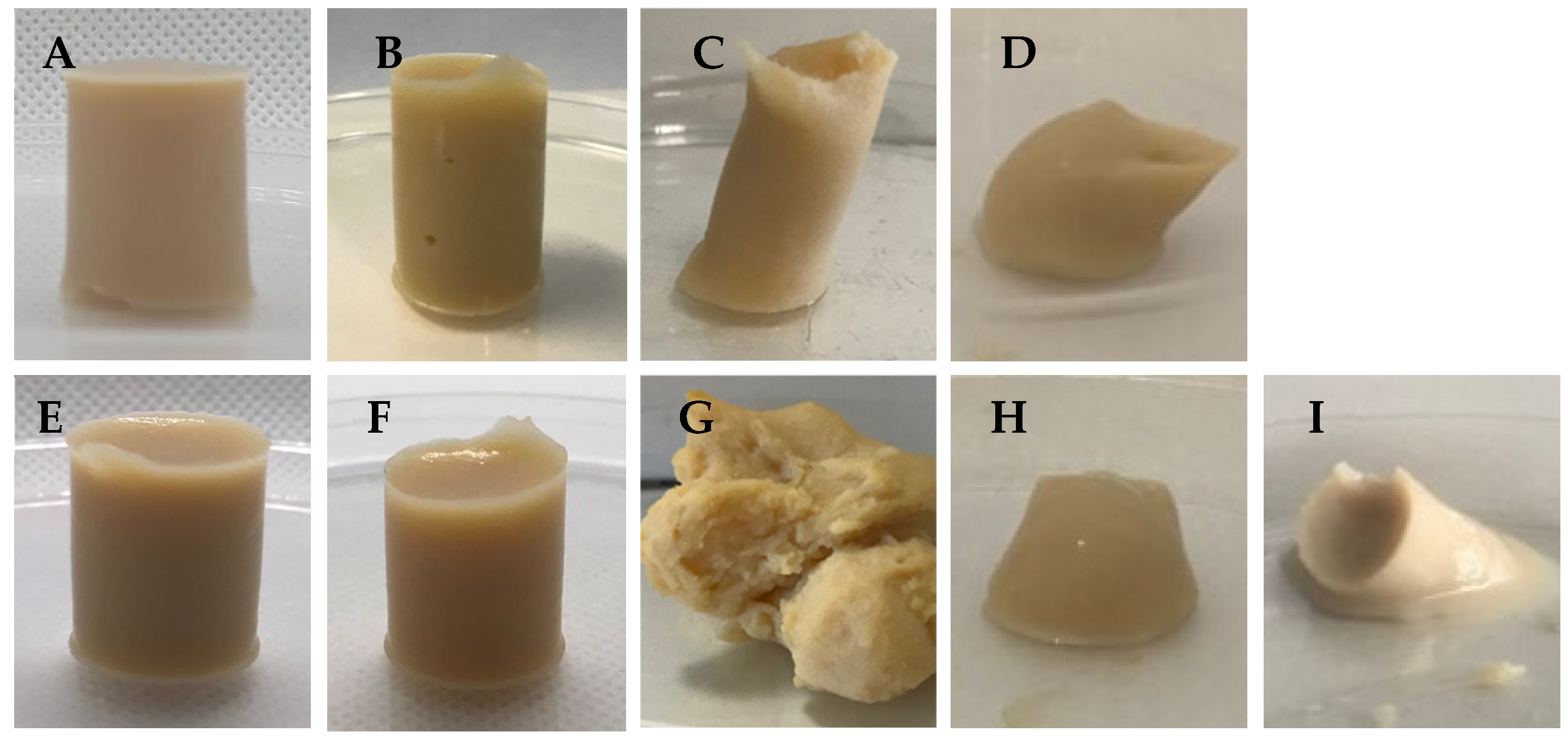
All the manufactured samples are shown in
Figure 1, which illustrates the different gelation behaviors of the different combinations of gelling agents. The samples composed of pure polysaccharides presented different behaviors when forming gels. The formulations containing 1% CA and 1% GGLA, represented in
Figure 1A,B, formed self-supporting gels characterized by the absence of visible pores. The gel obtained with 1% GGHA (
Figure 1C) presented a three-dimensional structure with a low support capacity. Both forms of gellan gum were able to promote gelation. However, the native GGHA produced translucent elastic gels, while the GGLA, the most common version known for its high gelling power, produced rigid gels [
15]. The sample with 1% PEC (
Figure 1D) could not form a self-supporting structure under the conditions evaluated, indicating the need for strategies to improve its gel-forming capacity, considering the combination assessed in the present work.
Adding NaCl visually optimized the gel formation. Monovalent ions, such as K+, increased the interactions between the proteins and polysaccharides that helped increase the gel’s hardness and firmness [
11]. In
Figure 1E,F, it is possible to see that the structure with the sodium ions remained sustainable. In contrast, the samples GGHA_Na and PEC_Na, in
Figure 1G,H, exhibited little support capacity.
In the specific case of the samples containing PEC, which initially did not form self-sustaining gels, the addition of CaCl
2 was tested. The addition of CaCl
2 promoted the formation of weak gels through interactions with the carboxylic groups of pectin (
Figure 1I). In low-methoxylated pectins, gelation occurs through the formation of ionic bonds mediated by calcium ions (Ca
2+), resulting in the formation of a continuous three-dimensional network [
16,
17,
18].
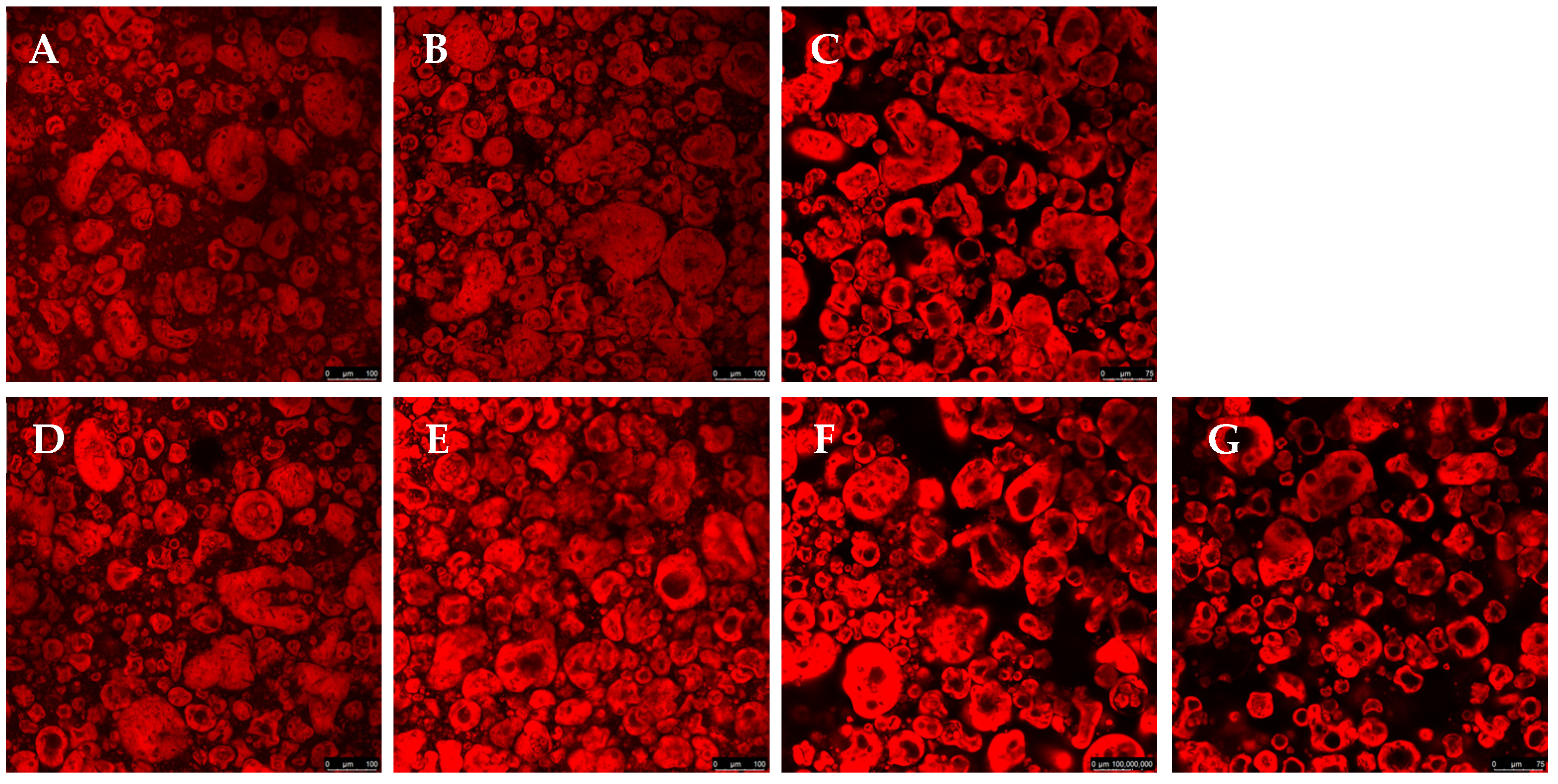
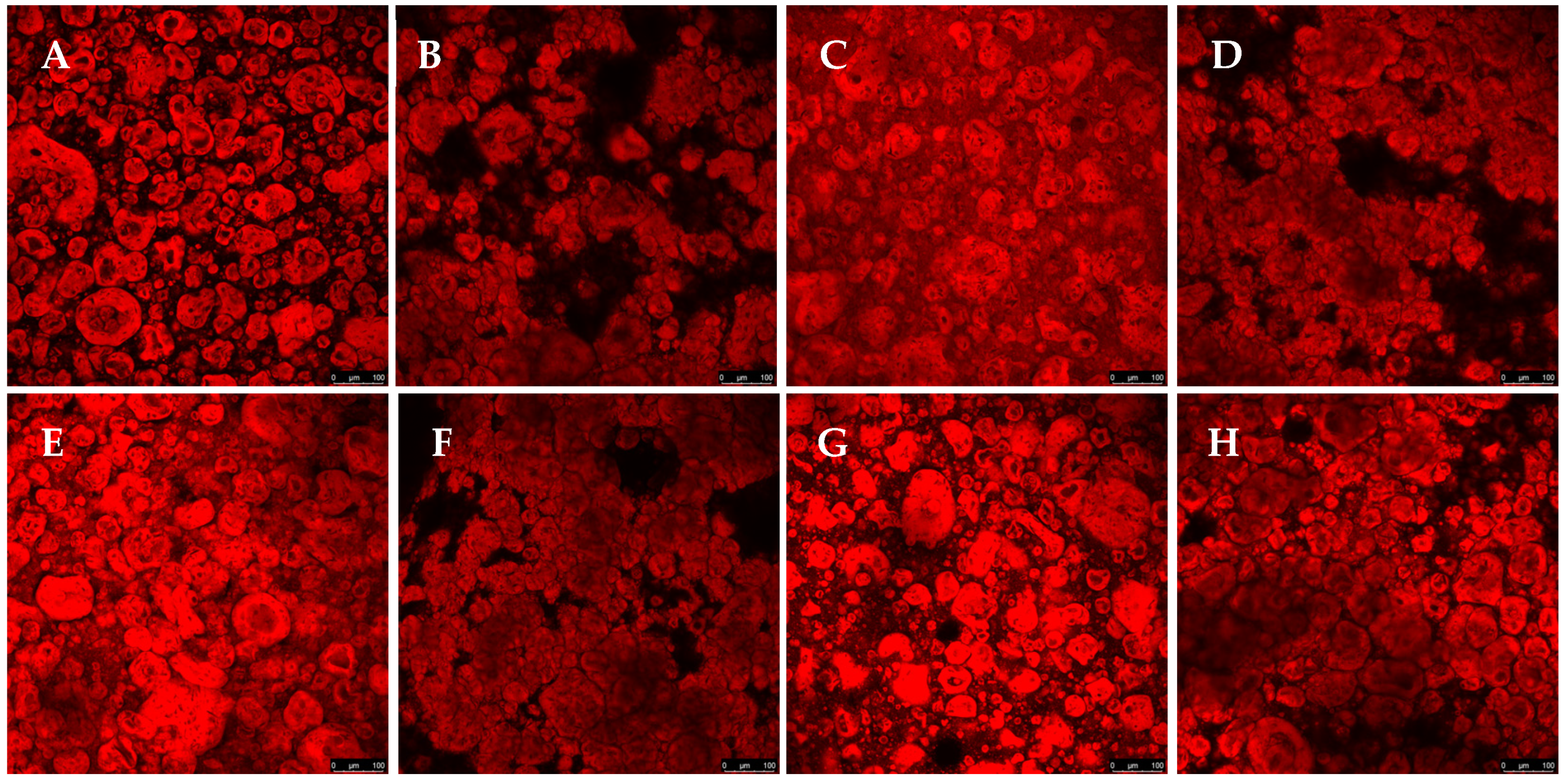
Confocal microscopy allows for high-resolution three-dimensional images and is widely used to characterize complex structures in biological materials and polymers. The present study used confocal microscopy to evaluate the microstructure, phase distribution, and interaction between the components (
Figure 2). The red coloration, resulting from the interaction of Rhodamine B with proteins, indicates regions of protein aggregation. At the same time, the dark areas represent polysaccharides, which do not interact with the fluorescent dye. A detailed analysis of these images allows us to understand how each component influences the structural organization of the gel. Confocal microscopy testing was not performed for the GGHA samples due to their rapid gelation.
In
Figure 2A–C, for the samples containing only pea protein and polysaccharides, a relatively homogeneous distribution of the red regions is observed. This suggests that, in the absence of salt or crosslinking agents, the proteins present in the system remained more dispersed without forming significant aggregates. The morphology obtained reflects a lower interaction between the proteins and polysaccharides, resulting in a less structured network.
The addition of NaCl or CaCl
2 to the formulations significantly altered the morphology of the gels. In
Figure 2D,E, an increase in protein aggregation is observed, forming more intensely red regions. This effect can be attributed to the ability of NaCl to modify the electrostatic interactions between proteins, promoting the aggregation and stabilization of the gel. Some studies have indicated that adding NaCl before heat treatment can facilitate protein unfolding, strengthening hydrophobic interactions and leading to the formation of protein aggregates [
19].
The confocal microscopy images also revealed that the composition of the gel directly affected its morphology, with significant impacts from the type of polysaccharide on the structural organization of the gels. While the gelled systems with CA and GGLA showed a more homogeneous structure, the gels with PEC showed a micro-phase separation, with a clear formation of protein-rich domains dispersed on a continuous polysaccharide phase [
20]. This phenomenon was observed even with salt addition. Phase separation is a common phenomenon that occurs in mixtures of proteins and polysaccharides due to the thermodynamic incompatibility and repulsion between biopolymers with the same charge. However, when the gelation process is fast, it immobilizes the structure, resulting in a micro-phase separation in the gel [
21,
22].
Based on these results, only the formulations containing CA and GGLA, with or without NaCl, were selected for evaluation by uniaxial compression, syneresis, and rheological analyses, as they were the only samples that formed self-supporting structures.
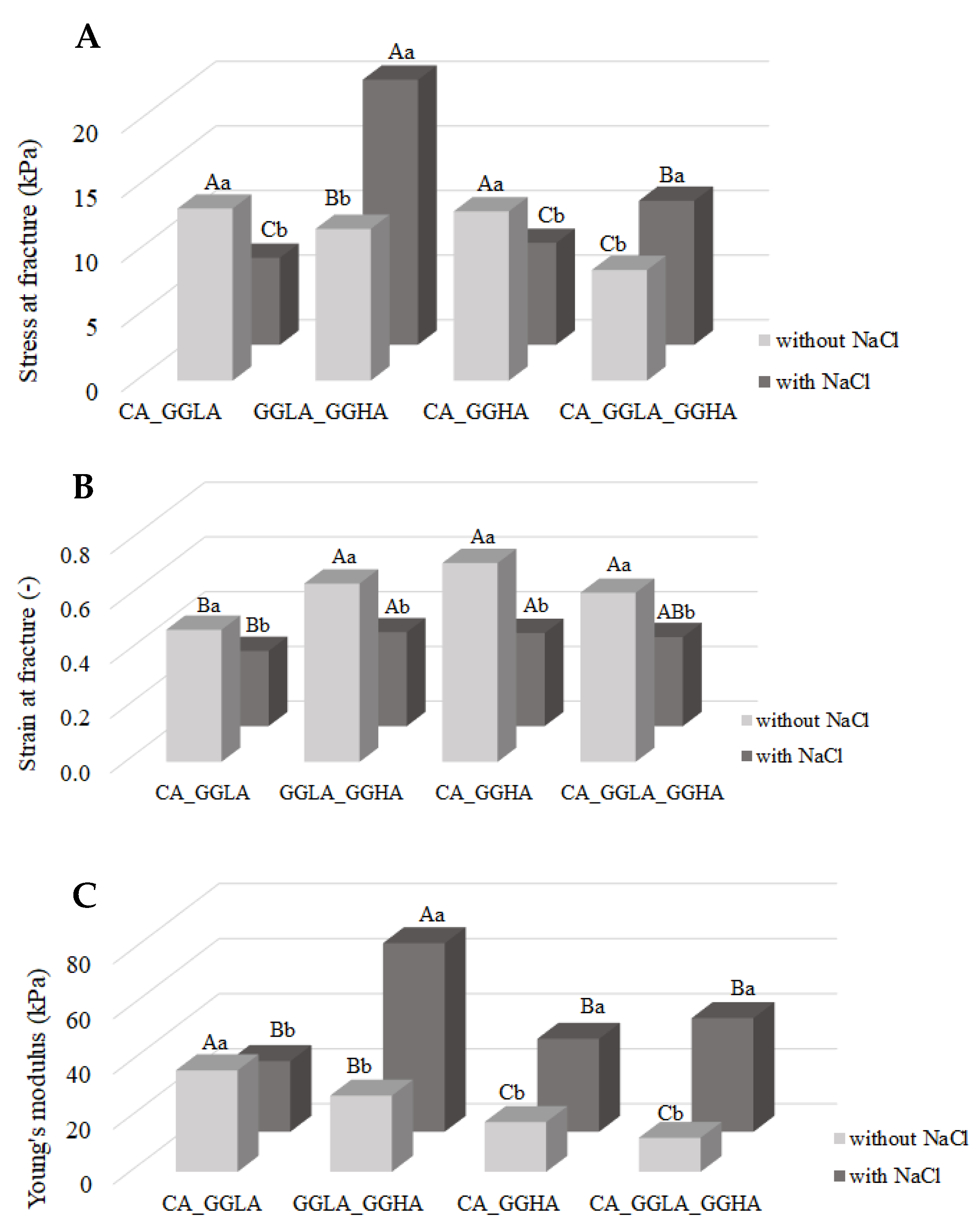
To respond to the panoramic texture behavior of the produced gels, the analysis of the mechanical behavior during uniaxial compression was evaluated through the stress at fracture (
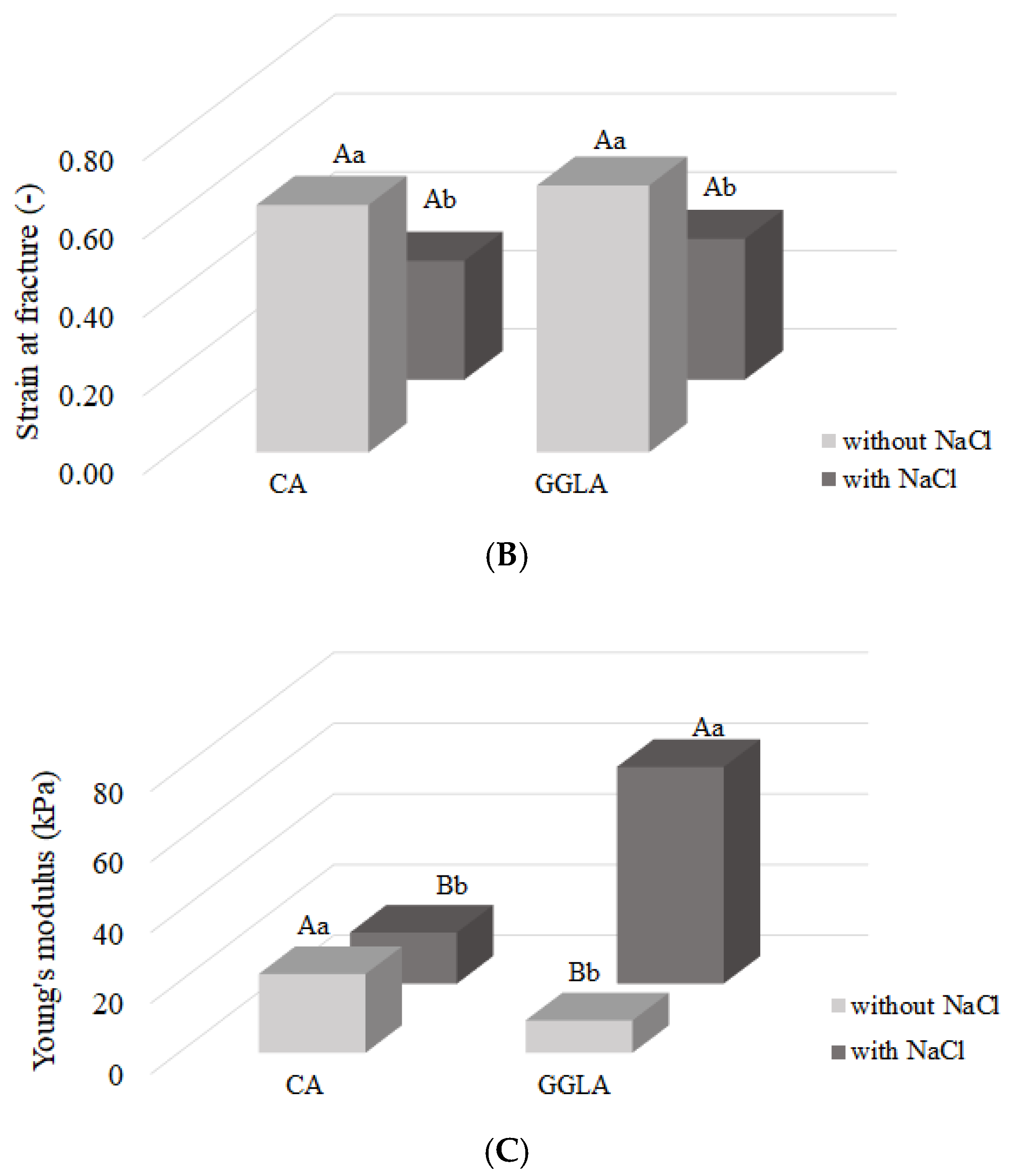
Figure 3A), strain at fracture (
Figure 3B), and Young’s modulus (
Figure 3C). All the experimental conditions in the graphs refer to the concentrations described in
Table 2, ensuring consistency between the data presented and the parameters established during the research.
From
Figure 3A, it is possible to observe distinct behaviors among the analyzed gels with different polysaccharides (CA or GGLA) regarding the fracture stress in the presence or absence of NaCl. For CA, it is observed that the lack of sodium ions resulted in a significant increase in the fracture stress, reaching 19.68 ± 1.78 kPa, compared to the sample containing NaCl, which reached 3.39 ± 0.68 kPa. This difference of 5.81 times shows that CA displays a better mechanical performance without salt. Therefore, the addition of sodium ions can negatively interfere with the formation and stabilization of the gel network. The same behavior was observed by Ipsen [
23], who investigated systems composed of pea protein isolate (PEI) or soy protein isolate (PSI) in combination with κ-carrageenan in the presence of calcium ions or sodium chloride. The study demonstrated that adding NaCl significantly reduced the strength and stiffness of the gels, regardless of the protein source, which was attributed to the low solubility of CA in the presence of salt, not achieving the formation of an optimal gel network. In addition, CA is found in the coil state at room temperature in the presence of Na
+, making its gelation difficult [
24].
On the other hand, GGLA showed the opposite behavior. The presence of NaCl resulted in a higher rupture voltage, reaching 11.50 ± 1.01 kPa, compared to the sample without salt, which reached 4.24 ± 0.60 kPa. This 2.71-fold increase indicates that sodium ions favor the formation of a harder gel network. Sodium ions act by facilitating interactions between polymer chains, promoting the greater organization and resistance of a gel network. This occurs because monovalent cations act as a shield against the electrostatic repulsion between gellan chains, facilitating the gelling of gellan gum [
25].
When analyzing
Figure 3B, it is observed that both CA and GGLA present with a higher fracture strain in the absence of sodium ions, respectively, of 0.63 ± 0.07 and 0.68 ± 0.09 (
p > 0.05). This behavior can be explained by the influence of monovalent cations, such as Na
+, in the structuring of the gels. Both CA and GGLA have similar gelation mechanisms, with a coil-to-helix transition, followed by the self-assembling of helices to produce the gels [
13,
26]. However, while Na
+ ions make the coil-to-helix transition difficult for CA [
24], they increase the assembling of GGLA helices [
27]. As a result, the addition of NaCl results in weaker and less deformable gels for CA, due to the fragility of the gel network, and harder and less deformable gels for GGLA, associated with the strengthening of the structure of the junction zones.
It is known that Young’s modulus reflects a material’s resistance to small deformations and indicates the firmness of the gel. On the other hand, the fracture stress is associated with the hardness of a material [
28]. In many cases, Young’s modulus and the stress at fracture exhibit similar behaviors, especially when the variation in a variable, such as the concentration, is analyzed. However, this trend is not a rule. In
Figure 3C, the Young’s modulus results show a behavior similar to that observed for the fracture stress, a pattern that, as reported by Silva et al. [
11], is common in gels. The CA without salt showed an increase of 1.55 kPa compared to the sample containing salt, while the GGLA without salt demonstrated a more impressive increase of 52.16 kPa.
Syneresis is often considered an undesirable characteristic, which negatively affects the quality of food products [
29]. Therefore, a gel should have a good water retention capacity, contributing to its greater stability over time and during the processes it goes through, such as cooking. In addition, water retention also has a positive impact on the juiciness of the gelled product [
11].
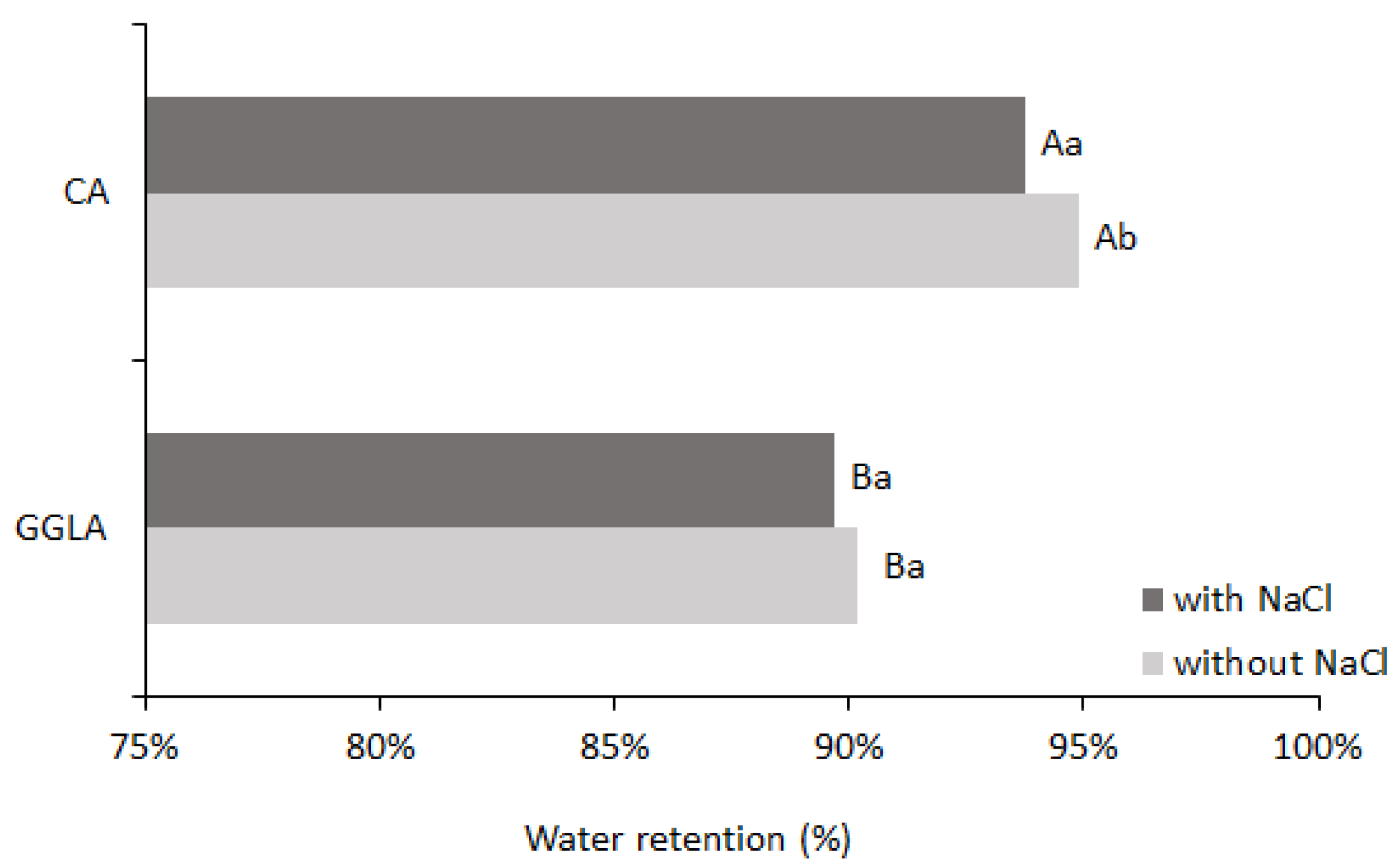
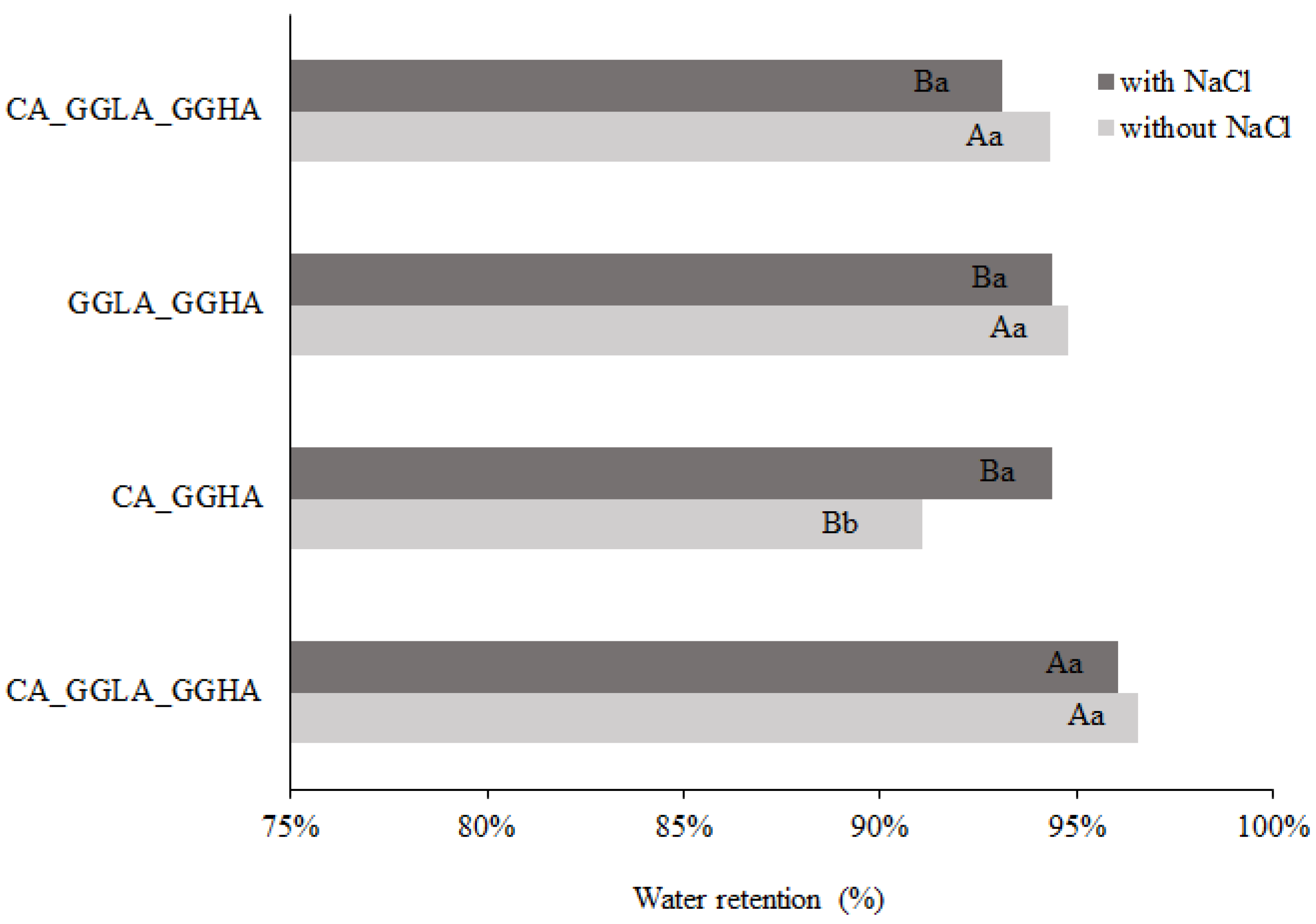
Figure 4 shows the percentage of water retention after the syneresis analysis.
Analyzing
Figure 4, the sample with CA presented with higher water retention values (94.87% without sodium ions and 93.77% with sodium ions) than the gels with GGLA (90.16% without sodium ions and 89.66% with sodium ions). The excellent water-holding capacity of CA gels has already been reported in the literature [
30,
31], and may be attributed to its chemical structure, which is rich in sulfated groups, favoring electrostatic interactions and increasing its affinity for water. For both gels, the addition of NaCl reduced the water retention efficiency. This occurred because the sodium ions interfered in the interactions between the biopolymers and water, competing for water molecules and reducing the cohesion of the three-dimensional networks [
32]. Therefore, the gels with NaCl had lower water retention than those without salt; this may be related to a salting-out effect, with the approximation of the biopolymers and the exclusion of water.
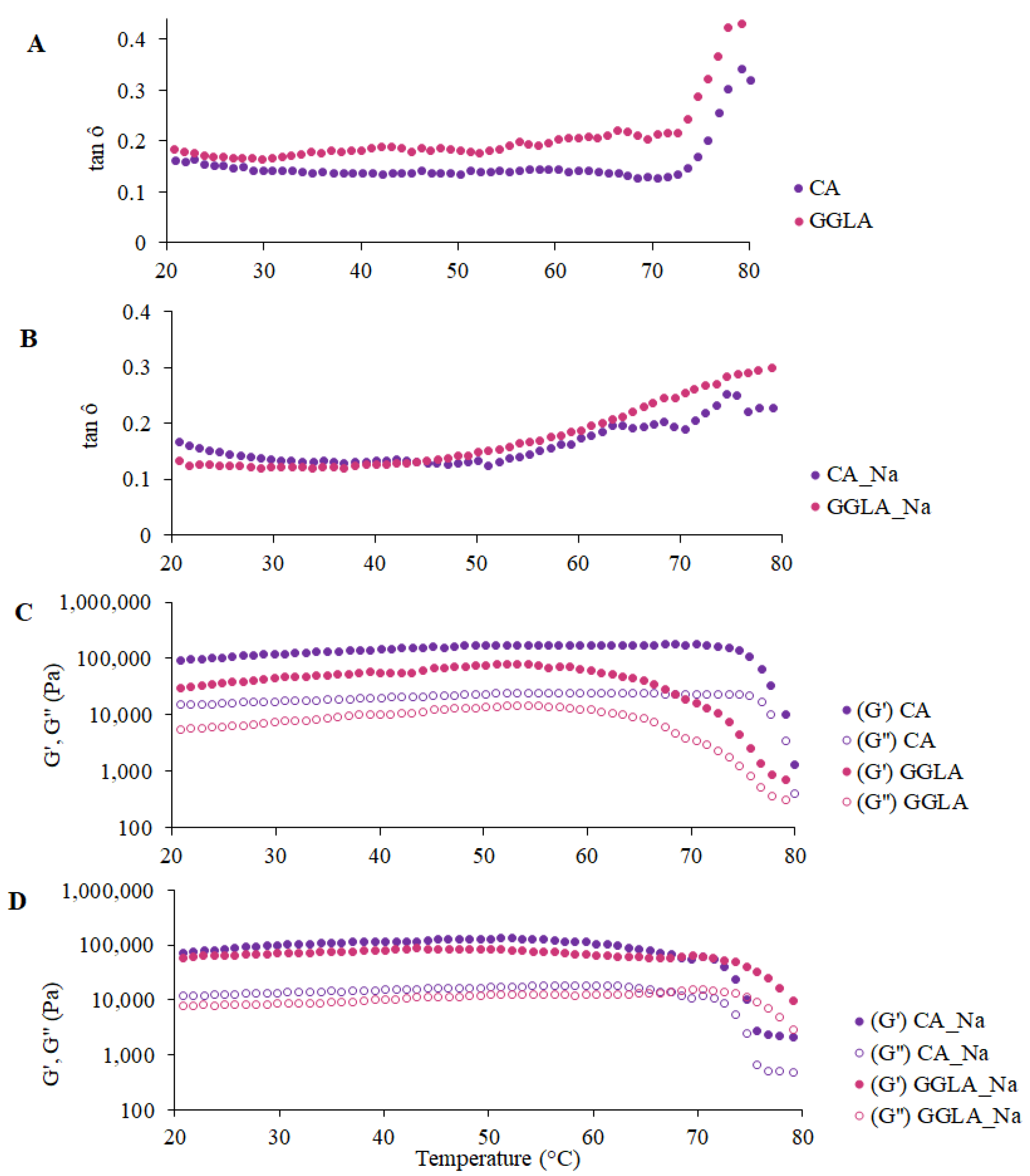
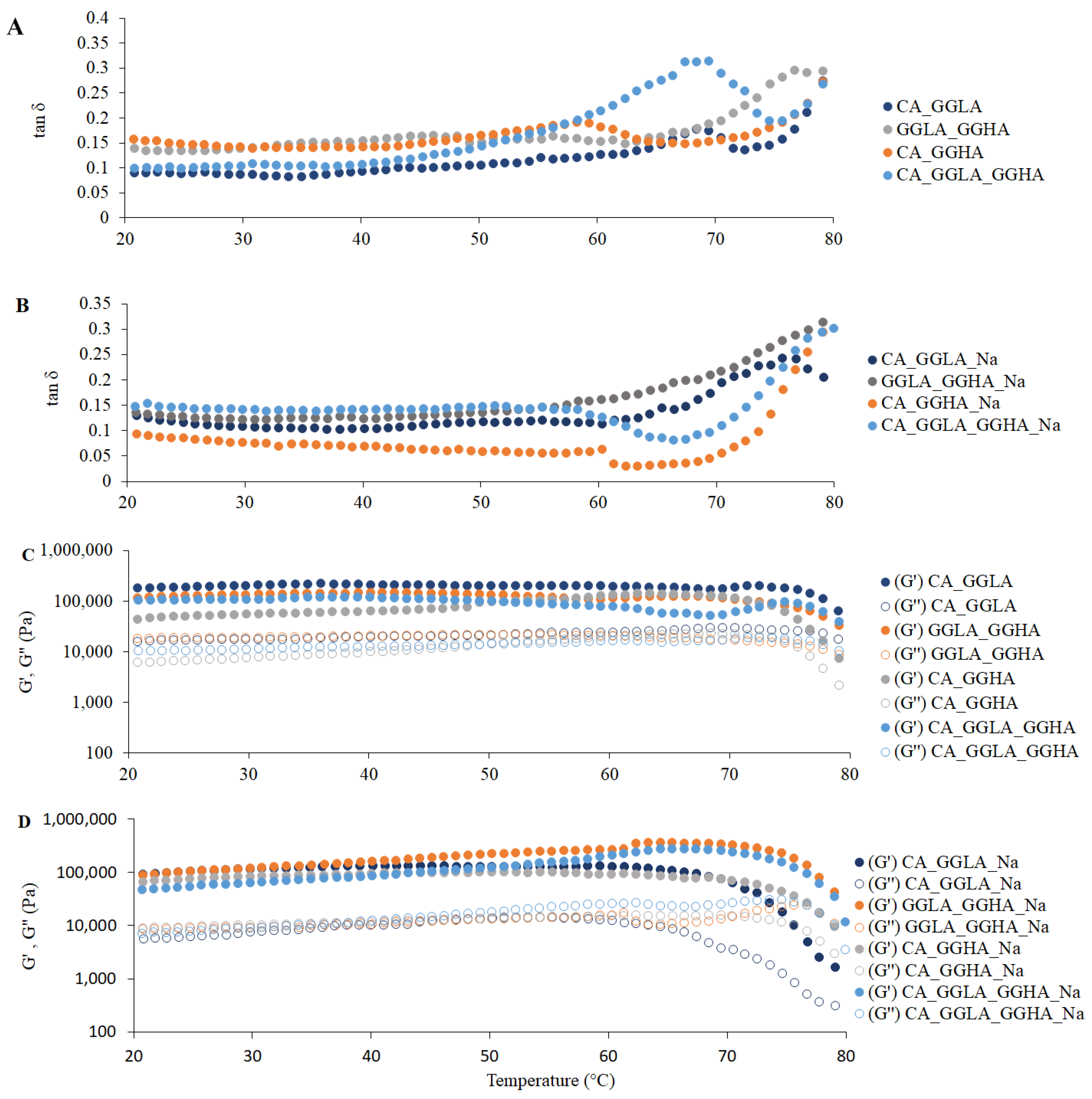
In terms of the rheological measurements,
Figure 5 presents the tan δ and the G′ and G″ moduli for the gels with pea protein and pure polysaccharides CA and GGLA, with the presence or absence of NaCl.
The tan δ results are presented in
Figure 5A,B, indicating that, since the first recording at 80 °C, all the samples presented with tan δ values lower than 1. A progressive drop in the tan δ values was observed with a reduction in the temperature, showing that the gels became firmer. The tan δ parameter is defined as the quotient between the viscous (G″) and elastic (G′) moduli and is indicative of the structural nature of a material: tan δ values < 1 reflect predominantly solid behavior, while tan δ > 1 characterizes a viscous or liquid structure [
33,
34]. Hence, the results confirm that gel structures had already formed in all the formulations analyzed, since the G′ was higher than the G″ (i.e., tan δ < 1) throughout the temperature range evaluated.
In
Figure 5A,B, it can be seen that the gel composed of GGLA presented with the lowest tan δ values, suggesting greater structural rigidity. In addition, there was a more pronounced reduction in this parameter up to approximately 70 °C, followed by stabilization for all the samples. Previous studies, such as that of Picone and Cunha [
13], evaluated gellan gum gels (1.5%
w/w) at pH 7.0 and identified that the sol–gel transition occurred at 34.2 ± 0.6 °C. Thus, in the present study, the protein content and the addition of monovalent salt may have influenced the gelation temperature of the gellan gum, as discussed by Ozorio et al. [
35].
The behavior of the elastic (G′) and viscous (G″) moduli, illustrated in
Figure 5C,D, corroborates the trends observed for the tan δ. In all the samples, the G′ was consistently greater than the G″, indicating the formation of a well-structured three-dimensional network characteristic of solid gels. Gels have a semi-solid structure due to their polymer network that retains the liquid phase. Their rheological behaviors are crucial in the formulation and development of products, directly influencing the choice of the appropriate system according to the desired application [
36].
Figure 5C,D also show the differences between CA and GGLA during the gelation process. In the absence of salt (
Figure 5C), the gels with CA showed a fast increase in the G′ modulus, while this variable slowly increased in the GGLA gels. On the other hand, the opposite tendency was observed for the gels with NaCl. This result corroborates the results discussed earlier, which showed that Na
+ ions hindered the formation of CA helices, while they can reinforce the self-assembly of GGLA helices during the gelation process.
In order to assess the influence of freezing as a possible process of conservation, the gels made of pea protein with pure GGLA and CA polysaccharides were subjected to subsequent performance evaluation analyses after deep freezing and thawing. The comparison of the mechanical properties (
Table 2) showed that the gels became weaker and less rigid after the freezing–thawing cycle, with exception of sample GGLA that became more rigid. It is interesting to note that freezing and thawing may have caused greater weakening of the stronger gels (samples CA and GGLA_Na). In addition, the gels without NaCl were less deformable after the freezing–thawing cycle, while the strain at fracture did not change for the samples with NaCl.
Table 2 also shows the water retention results for the GGLA and CA gels, with and without adding NaCl, after freezing and thawing. This test was essential to evaluate the ability of the gels to retain water and resist the phenomenon of syneresis after freezing (loss of water through the gel network). The addition of NaCl led to a negative effect on the water retention for both GGLA and CA. This may have occurred because the Na
+ ions competed with the water molecules for interactions with the polymer chains, reducing the capacity of the gel network to retain water [
32].
Freezing impacts the gel structure because freezing can cause the formation of large ice crystals, which rupture the gel network, resulting in the weakening of the structure and greater water release during thawing [
37].
3.2. Mixture of Polysaccharides Gels
Since different polysaccharides can confer various properties to gels, especially in terms of texture, it was decided to analyze some mixtures of these biopolymers (
Table 1) and compare the parameters evaluated for the gels of pea protein with those of pure polysaccharides. It is important to note that the concentration of GGHA was limited to 0.05%, as higher concentrations resulted in excessively high viscosity, which hindered the proper filling of the molds.
Figure 6 illustrates the appearance of the protein gels with polysaccharide mixtures. The visual analysis of these samples allowed for identifying the color, translucency, shape, and structural integrity variations, which may be related to their composition and experimental conditions.
In general, the polysaccharide mixtures used allowed for the formation of self-supporting and similar gels. Compared to the pure polysaccharide samples, the mixed gels also showed visual similarity with the self-supporting protein-pure polysaccharide gels.
Figure 7 shows that the interaction between different polysaccharides influenced the protein phase distribution and each gel’s structural organization. In the samples without salt, it was observed that the protein aggregation varied according to the composition of the gel. When there was a predominance of gellan gum (samples GGLA_GGHA and CA_GGLA_GGHA), the protein aggregation increased, resulting in more concentrated and defined red areas with a micro-phase separation (
Figure 7B,D). In the samples with CA (samples CA_GGLA and CA_GGHA), the protein appeared more soluble and homogeneously distributed (
Figure 7E,G).
With the addition of NaCl, there was an increase in protein aggregation in all the samples, regardless of the polysaccharide. This occurred because the monovalent ion Na
+ reduced the electrostatic repulsion between the proteins and polysaccharides, favoring their association and forming more aggregated structures [
11]. Compared to
Figure 2, CA kept the protein relatively more dispersed, even in salt’s presence, while GGLA intensified aggregation, mainly with the addition of GGHA.
The analysis of the mechanical behavior during uniaxial compression was also evaluated for the mixture samples (
Figure 8) through the stress at fracture (
Figure 8A), strain at fracture (
Figure 8B), and Young’s modulus (
Figure 8C). For the gels without salt, samples CA_GGLA and CA_GGHA showed the highest values of stress at fracture. However, the addition of salt (NaCl) reduced the fracture stress of these formulations and increased the hardness of samples GGLA_GGHA_Na and CA_GGLA_GGHA_Na. These results suggest that the influence of NaCl is directly linked to the composition and proportion of the polysaccharides in a formulation, determining the structural network’s capacity for formation and resistance. Thus, for the samples CA_GGLA and CA_GGHA, the behavior was determined by the CA, whose gels weaken in the presence of NaCl [
24]. On the contrary, for the formulations GGLA_GGHA and CA_GGLA_GGHA, the behavior was determined by the gellan gum, whose gels are reinforced in the presence of monovalent ions [
25], as observed in
Figure 3.
It is interesting to note that the gels with a mixture of polysaccharides (
Figure 3) were harder than those containing pure polysaccharides, indicating a synergistic effect among the polysaccharides in the structuring the gel network. The harder gel was observed for the sample with the higher concentration of GGLA in the presence of NaCl (GGLA_GGHA_Na), with a fracture stress reaching 20.51 kPa. This result may be related to the interaction between gellan gum and NaCl, as discussed earlier.
As shown in
Figure 8B, which presents the data for the strain at fracture, the absence of sodium ions resulted in more deformable gels in all formulations. This behavior was also identified in isolated polysaccharides (
Figure 3) and can be attributed to the absence of ionic interactions promoted by NaCl. This led to a less rigid and, consequently, more elastic polymer network. The presence of NaCl favored electrostatic interactions between the polymer chains, especially in the presence of gellan gum, resulting in more structured and less deformable gels. These results reinforce the role of Na
+ ions as structuring agents, restricting the mobility of polymer chains and conferring greater rigidity to gels. On the other hand, the absence of NaCl provides greater flexibility to a polymer matrix, allowing gels to withstand a greater deformation before rupture [
35,
38].
This comparison of the different polysaccharide compositions shows that the samples containing GGHA presented with a greater strain at fracture when compared to the formulations without this polysaccharide. It is well known that the gels of GGHA have a lower hardness and much higher deformability than GGLA [
39]. The results of the present work show that even a low concentration of GGHA (0.05%) is sufficient to increase the elasticity of gels.
Finally, in
Figure 8C, it can be observed that Young’s modulus did not follow the same trend as the stress at rupture. Samples GGLA_GGHA_Na, CA_GGHA_Na, and CA_GGLA_GGHA_Na presented with higher Young’s modulus values, indicating a greater rigidity of the gels. This behavior can be attributed to the formation of stronger ionic bonds between the polysaccharides and the sodium ions, resulting in a more compact gel network resistant to deformation. The effect appears to be associated with the presence of GGHA in the samples GGLA_GGHA_Na, CA_GGHA_Na and CA_GGLA_GGHA_Na. On the other hand, the sample CA_GGLA_Na showed a behavior similar to that observed in pure polysaccharide gels, maintaining the tendency of stress at fracture. The sample GGLA_GGHA_Na showed the most significant increase in Young’s modulus (68.48 kPa) with salt addition, a result that was also reflected in the stress at fracture. This substantial increase can be attributed to the better packing of the polymer chains provided by the interaction among NaCl, GGLA, and GGHA, which intensified the intermolecular interactions and conferred greater mechanical strength to the gel.
Figure 9 shows the results for water retention of systems composed of pea protein and a mixture of polysaccharides, with and without NaCl. In general, the systems did not present significant differences between the polysaccharides with and without salt. An exception was the sample rich in CA, which presented with greater water retention in the presence of NaCl. This trend was opposite to that observed for the protein-pure polysaccharide gels, which can be explained by the presence of GGHA in the formulation.
The formulation that combined all the polysaccharides (CA_GGLA_GGHA) showed the highest water retention, both in the presence and absence of salt, reaching a retention index of approximately 96%. This superior performance, compared to gels formed exclusively by protein and pure polysaccharides, can be attributed to the synergistic effect among the components, which enhanced water retention. In addition, it is possible that the specific contribution of GGHA played a relevant role, positively influencing the structure of the gel network, as previously observed by Buldo et al. [
40].
On the other hand, the sample CA_GGHA showed the lowest performance when evaluated without salt, registering a water retention of only 90.69%. This behavior can be explained by the high interaction between the biopolymers, as observed in
Figure 7, expelling the water from the network structure. In the presence of NaCl, this gel is weakened (
Figure 8), i.e., the interactions between the biopolymers are not so strong, leading to an increase in the water retention.
This difference in performance highlights the importance of both the composition of the polysaccharides and the presence of salt for controlling the water retention capacity, evidencing how structural and interactional factors directly affect the functional properties of gel networks.
The results of the rheological measurements for the mixed polysaccharides (CA, GGLA, and GGHA) and pea protein with the presence or absence of NaCl are presented in
Figure 10. For the samples without NaCl (
Figure 10A), the tan δ was consistently lower than one throughout the temperature range (80–20 °C). This indicates that the gel structures were already formed, with predominantly solid behavior (G′ > G″). The differences among the samples CA_GGLA, GGLA_GGHA, CA_GGHA, and CA_GGLA_GGHA were associated with the concentrations of and interactions among the biopolymers, with the sample CA_GGLA presenting with the lowest tan δ value, suggesting greater structural rigidity. In the samples containing NaCl (
Figure 10B), the tan δ was also less than one. Still, there was a more pronounced reduction in this parameter up to 60 °C, followed by stabilization for all the samples, and the tan δ values were initially higher at 80 °C. This indicates that the formulations with salt were initially more liquid at 80 °C, which may have facilitated the incorporation of these samples into the molds. The sample CA_GGHA_Na presented with the lowest value for this parameter, indicating that the combination of a higher concentration of CA and NaCl and a low temperature led to a more rigid gel.
In
Figure 10C,D, the behavior of the elastic (G′) and viscous (G″) moduli reinforces the observations for the tan δ. The elastic modulus (G′) was consistently higher than the viscous modulus (G″), indicating the formation of a well-structured three-dimensional network characteristic of solid gels. This trend was more pronounced at room temperature in the samples without NaCl, which appeared more structured than those with NaCl, except for sample P.