Exploring the Potential of Torulaspora delbrueckii, Starmerella bacillaris, and Saccharomyces cerevisiae as a Probiotic Starter for Craft Beer Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Yeast Strains and Growth Conditions
2.2. Molecular Identification
2.3. Probiotic Properties Characterization
2.3.1. Resistance to Simulated Gastrointestinal Conditions
2.3.2. Antibacterial Activity
2.3.3. Antibiotic Susceptibility
2.3.4. Hemolytic Assay
2.3.5. DPPH Free Radical Scavenging Activity
2.3.6. Preservation of Yeast Cells
2.4. Technological and Functional Yeast Screening
2.4.1. Carbon Assimilation, Osmotic Tolerance, and CO2 Production
2.4.2. Ethanol and Low pH Tolerance
2.4.3. Acetic Acid Production
2.4.4. H2S Production
2.4.5. Growth at Various Temperatures
2.4.6. Enzymatic Activity
2.4.7. Killer Toxin Assay
2.5. Pilot-Scale Fermentations
2.5.1. Inoculum Preparation
2.5.2. Beer Wort Preparation and Fermentation Conditions
2.5.3. Physico-Chemical Analyses of Wort and Beer
Ethanol Content and Original Extract of Beer
Bitterness of Beer
Total Acidity
Color of Beer
Soluble Dry Matter in Wort and Sugar Determination in Wort and Beer
Real Degree of Fermentation of Beer
- A is the alcohol % (m/m);
- ER is the real extract, % Plato.
Evaluation of the Total Polyphenol Content
DPPH Free Radical Scavenging Activity of Beer Samples
2.5.4. Assessment of Yeast Cell Survival in Beer Samples Following a 2-Month Storage Duration
2.5.5. Sensorial Analysis
2.6. Statistical Analysis
3. Results
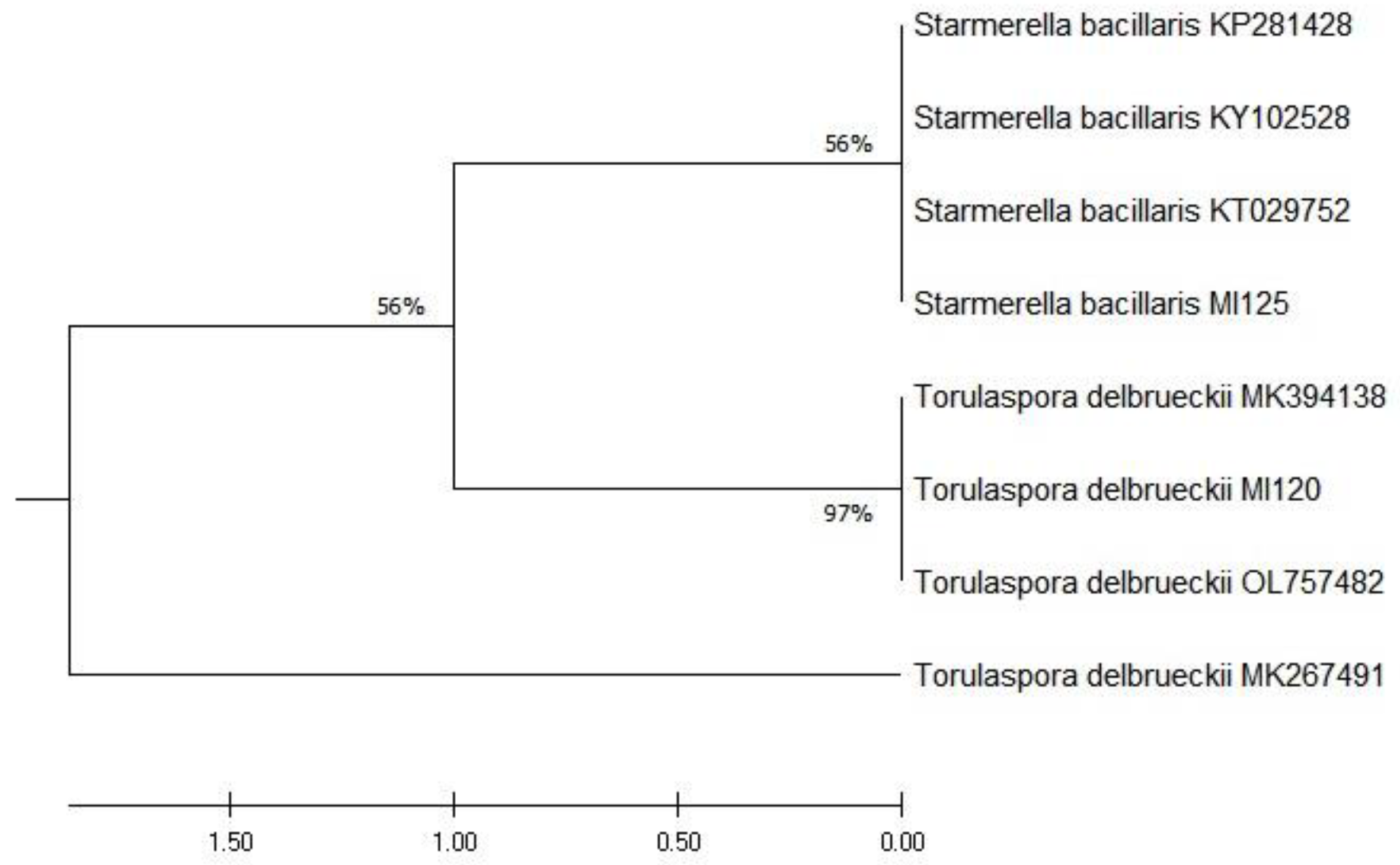
3.1. Molecular Identification of Yeast Strains Based on ITS rDNA Sequencing
3.2. Assessment of Yeast Strains as Potential Probiotics
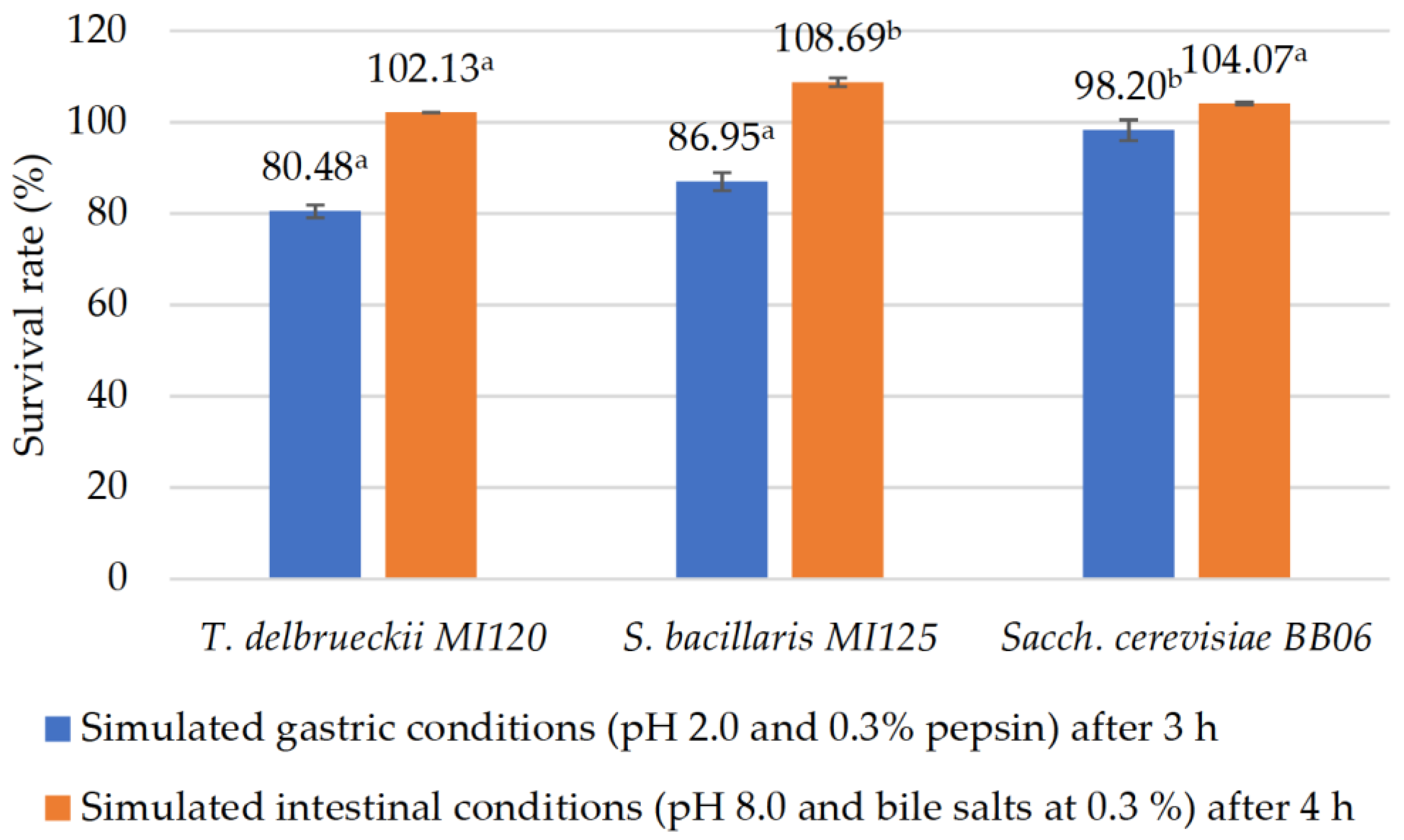
3.2.1. Resistance in Simulated Digestive Conditions
3.2.2. Antibacterial Properties
3.2.3. Assessment of the Antibiotic Sensitivity of Yeast Strains
3.2.4. Hemolysis Assay
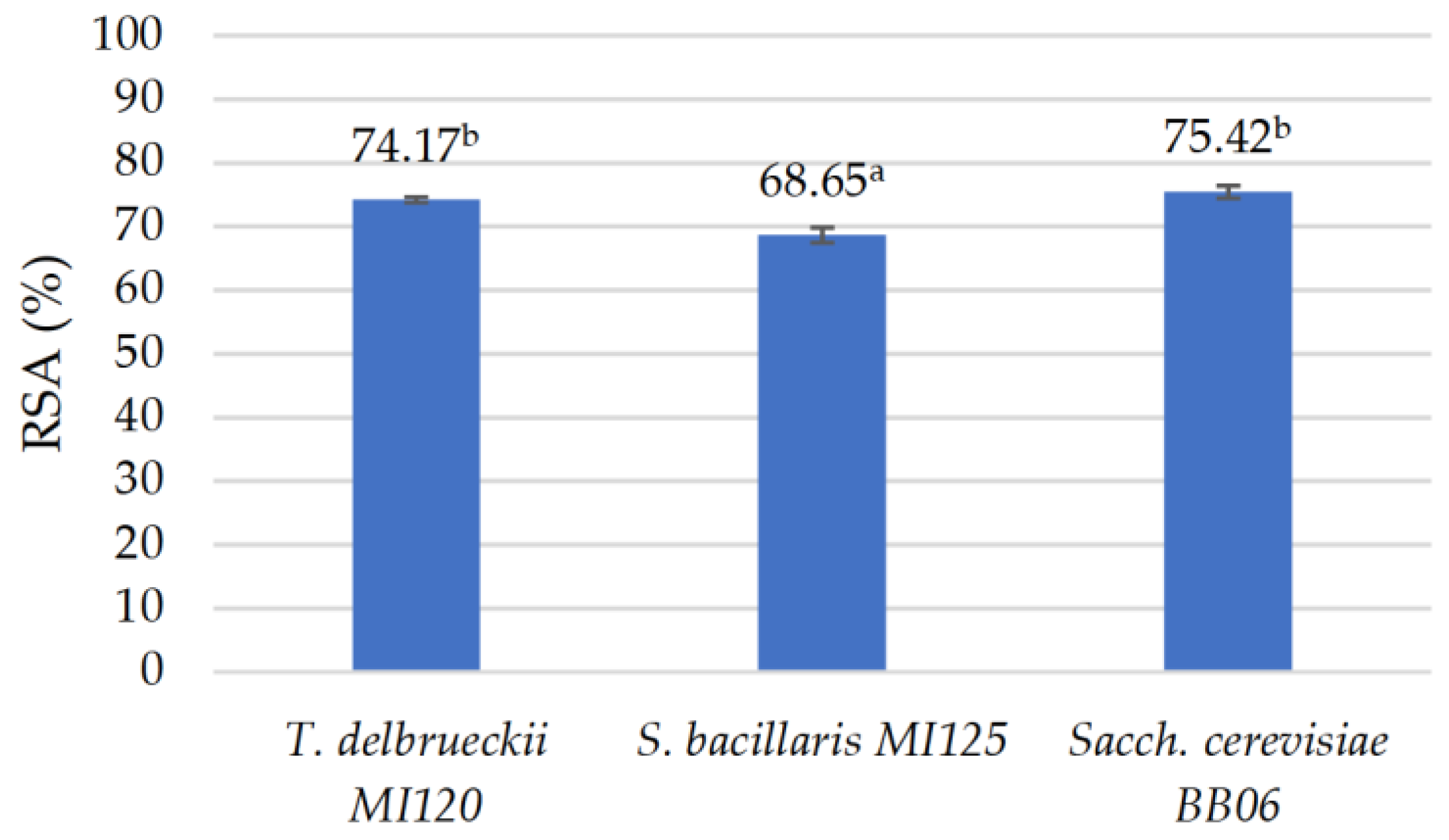
3.2.5. Antioxidant Properties
3.2.6. Preservation of Probiotic Yeast Strains via Freeze-Drying Method
3.3. In Vitro Assessment of Yeast Strains for Brewing Potential
3.4. Characterization of Beer Samples
3.4.1. Physico-Chemical and Technological Characterization of Beer Fermented with Selected Yeast Strains
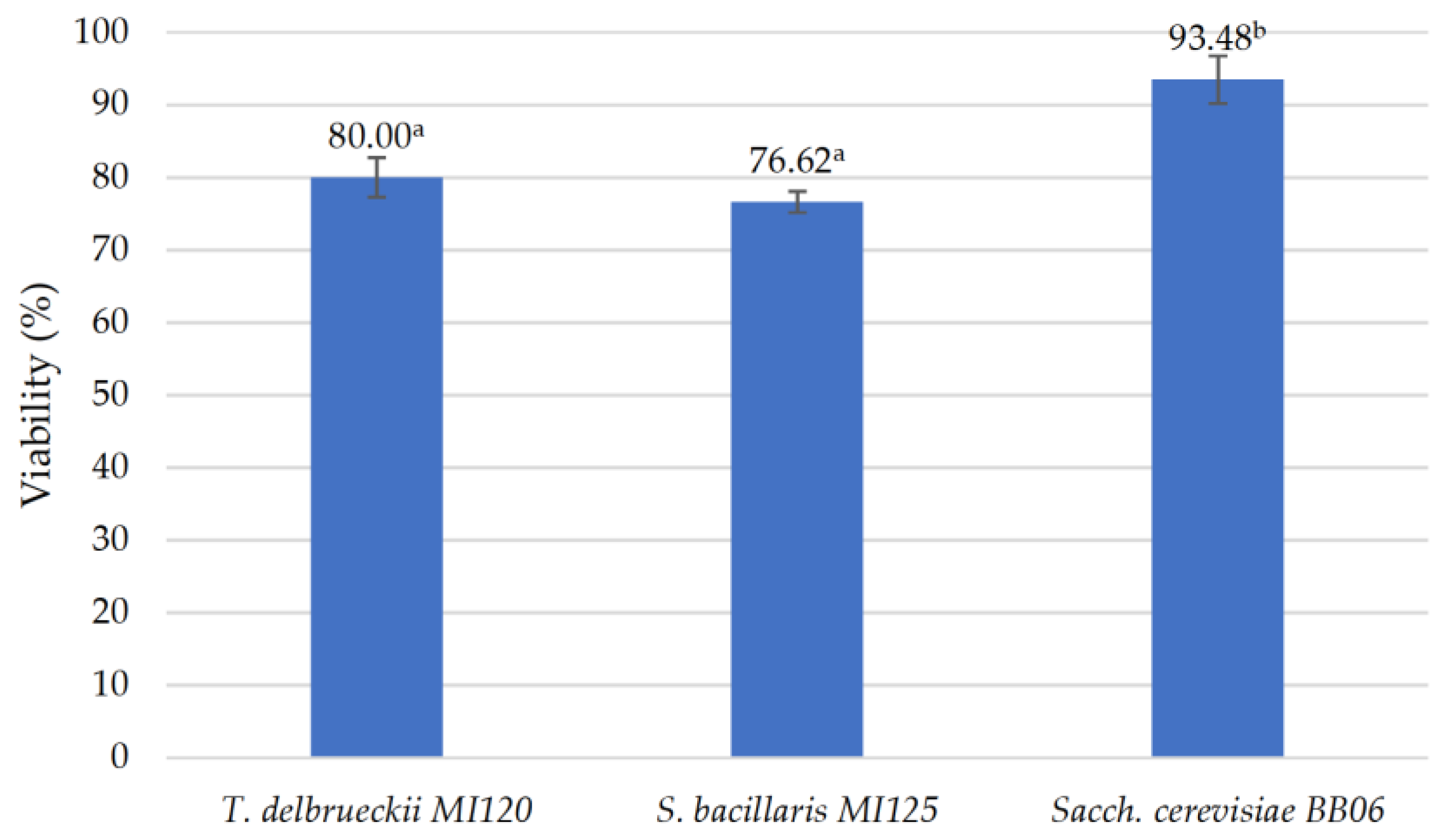
3.4.2. Assessment of Yeast Cell Viability in Craft Beer Following 2 Months of Storage
3.4.3. Sensorial Analysis of Beer Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Statista Beer Production by Country Worldwide 2023. Available online: https://www.statista.com/statistics/270275/worldwide-beer-production/ (accessed on 3 March 2025).
- BARTHHAAS REPORT 2023/24. Available online: https://www.barthhaas.com/fileadmin/user_upload/01-barthhaas-2022/Resources/BarthHaas_Report/2024/en/WEB_BH_Bericht_2023_24_EN.pdf (accessed on 3 March 2025).
- The Brewers of Europe European Beer Trends. Available online: https://brewersofeurope.eu/wp-content/uploads/2024/12/eu-beer-trends-2024-web.pdf (accessed on 3 March 2025).
- Number of Active Beer Microbreweries in Romania From 2008 to 2023. Available online: https://www.statista.com/statistics/447661/romania-number-beer-microbreweries (accessed on 3 March 2025).
- Constantin, D.L.; Popescu, I.A. Traditions and entrepreneurial spirit: The evolving geography of craft brewing in Romania and dynamic interactions with the local development environment. Appl. Geogr. 2025, 176, 103543. [Google Scholar] [CrossRef]
- De Simone, N.; Russo, P.; Tufariello, M.; Fragasso, M.; Solimando, M.; Capozzi, V.; Grieco, F.; Spano, G. Autochthonous Biological Resources for the Production of Regional Craft Beers: Exploring Possible Contributions of Cereals, Hops, Microbes, and Other Ingredients. Foods 2021, 10, 1831. [Google Scholar] [CrossRef]
- Villacreces, S.; Blanco, C.A.; Caballero, I. Developments and characteristics of craft beer production processes. Food Biosci. 2022, 45, 101495. [Google Scholar] [CrossRef]
- Vrînceanu, C.R.; Bărbulescu, I.D.; Matei, F.; Begea, M.; Tudor, V.; Frîncu, M.; Teodorescu, R.I. Review: Actual approaches for the craft beer fermentations. Sci. Pap. Ser. B Hortic. 2022, 66, 944–949. [Google Scholar]
- Salanță, L.C.; Coldea, T.E.; Ignat, M.V.; Pop, C.R.; Tofană, M.; Mudura, E.; Borșa, A.; Pasqualone, A.; Zhao, H. Non-Alcoholic and Craft Beer Production and Challenges. Processes 2020, 8, 1382. [Google Scholar] [CrossRef]
- Ciont, C.; Epuran, A.; Kerezsi, A.D.; Coldea, T.E.; Mudura, E.; Pasqualone, A.; Zhao, H.; Suharoschi, R.; Vriesekoop, F.; Pop, O.L. Beer Safety: New Challenges and Future Trends within Craft and Large-Scale Production. Foods 2022, 11, 2693. [Google Scholar] [CrossRef]
- Zeng, Y.; Ahmed, H.G.M.-D.; Li, X.; Yang, L.; Pu, X.; Yang, X.; Yang, T.; Yang, J. Physiological Mechanisms by Which the Functional Ingredients in Beer Impact Human Health. Molecules 2024, 29, 3110. [Google Scholar] [CrossRef] [PubMed]
- Karabín, M.; Jelínek, L.; Kotrba, P.; Cejnar, R.; Dostálek, P. Enhancing the performance of brewing yeasts. Biotech. Adv. 2018, 36, 691–706. [Google Scholar] [CrossRef]
- Marongiu, A.; Zara, G.; Legras, J.L.; Del Caro, A.; Mascia, I.; Fadda, C.; Budroni, M. Novel starters for old processes: Use of Saccharomyces cerevisiae strains isolated from artisanal sourdough for craft beer production at a brewery scale. J. Ind. Microbiol. Biotechnol. 2015, 42, 85–92. [Google Scholar] [CrossRef]
- Budroni, M.; Zara, G.; Ciani, M.; Comitini, F. Saccharomyces and non-Saccharomyces starter yeast. In Brewing Technology; Kanauchi, M., Ed.; Intech: Rijeka, Croatia, 2017; pp. 81–100. [Google Scholar]
- Capece, A.; Romaniello, R.; Siesto, G.; Romano, P. Conventional and Non-Conventional Yeasts in Beer Production. Fermentation 2018, 4, 38. [Google Scholar] [CrossRef]
- Shopska, V.; Denkova-Kostova, R.; Kostov, G. Modeling in Brewing—A Review. Processes 2022, 10, 267. [Google Scholar] [CrossRef]
- Calderon, N.; Jiang, G.Z.; Gibney, P.A.; Dando, R. A Consumer Assessment of Fermented Green Coffee Beans with Common Beer/Wine Yeast Strains for Novel Flavor Properties. Fermentation 2023, 9, 865. [Google Scholar] [CrossRef]
- Yabaci Karaoglan, S.; Jung, R.; Gauthier, M.; Kinčl, T.; Dostálek, P. Maltose-Negative Yeast in Non-Alcoholic and Low-Alcoholic Beer Production. Fermentation 2022, 8, 273. [Google Scholar] [CrossRef]
- Telini, B.d.P.; Villa, L.C.; Vainstein, M.H.; Lopes, F.C. From Vineyard to Brewery: A Review of Grape Pomace Characterization and Its Potential Use to Produce Low-Alcohol Beverages. Fermentation 2025, 11, 57. [Google Scholar] [CrossRef]
- Bruner, J.; Fox, G. Novel Non-Cerevisiae Saccharomyces Yeast Species Used in Beer and Alcoholic Beverage Fermentations. Fermentation 2020, 6, 116. [Google Scholar] [CrossRef]
- Holt, S.; Mukherjee, V.; Lievens, B.; Verstrepen, K.J.; Thevelein, J.M. Bioflavoring by non-conventional yeasts in sequential beer fermentations. Food Microbiol. 2018, 72, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Sannino, C.; Mezzasoma, A.; Buzzini, P.; Turchetti, B. Non-conventional Yeasts for Producing Alternative Beers. In Non-Conventional Yeasts: From Basic Research to Application; Sibirny, A., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 361–388. ISBN 978-3-030-21110-3. [Google Scholar]
- Miguel, G.A.; Carlsen, S.; Arneborg, N.; Saerens, S.M.G.; Laulund, S.; Knudsen, G.M. Non-Saccharomyces yeasts for beer production: Insights into safety aspects and considerations. Int. J. Food Microbiol. 2022, 383, 109951. [Google Scholar] [CrossRef]
- Nasuti, C.; Solieri, L. Yeast Bioflavoring in Beer: Complexity Decoded and Built up Again. Fermentation 2024, 10, 183. [Google Scholar] [CrossRef]
- Qin, X.; Li, F.; Bo, W.; Li, W.; Dong, X. The non-Saccharomyces yeasts as novel starter cultures to innovate beer brewing. CyTA J. Food 2025, 23, 2459819. [Google Scholar] [CrossRef]
- Methner, Y.; Hutzler, M.; Matoulková, D.; Jacob, F.; Michel, M. Screening for the Brewing Ability of Different Non-Saccharomyces Yeasts. Fermentation 2019, 5, 101. [Google Scholar] [CrossRef]
- Mestre Furlani, M.V.; Vargas Perucca, M.F.; Petrignani, D.B.; Vergara, S.C.; Leiva-Alaniz, M.J.; Maturano, Y.P.; Vazquez, F.; Dellacassa, E. Enhancing Flavor Complexity in Craft Beer: Sequential Inoculation with Indigenous Non-Saccharomyces and Commercial Saccharomyces Yeasts. Fermentation 2024, 10, 657. [Google Scholar] [CrossRef]
- Rădoi-Encea, R.-Ș.; Pădureanu, V.; Diguță, C.F.; Ion, M.; Brîndușe, E.; Matei, F. Achievements of Autochthonous Wine Yeast Isolation and Selection in Romania—A Review. Fermentation 2023, 9, 407. [Google Scholar] [CrossRef]
- Bellut, K.; Michel, M.; Zarnkow, M.; Hutzler, M.; Jacob, F.; De Schutter, D.P.; Daenen, L.; Lynch, K.M.; Zannini, E.; Arendt, E.K. Application of Non-Saccharomyces Yeasts Isolated from Kombucha in the Production of Alcohol-Free Beer. Fermentation 2018, 4, 66. [Google Scholar] [CrossRef]
- Canonico, L.; Agarbati, A.; Zannini, E.; Ciani, M.; Comitini, F. Lentil Fortification and Non-Conventional Yeasts as Strategy to Enhance Functionality and Aroma Profile of Craft Beer. Foods 2022, 11, 2787. [Google Scholar] [CrossRef] [PubMed]
- Postigo, V.; Sánchez, A.; Cabellos, J.M.; Arroyo, T. New Approaches for the Fermentation of Beer: Non-Saccharomyces Yeasts from Wine. Fermentation 2022, 8, 280. [Google Scholar] [CrossRef]
- Bărbulescu, I.D.; Ghica, M.V.; Begea, M.; Albu Kaya, M.G.; Teodorescu, R.I.; Popa, L.; Mărculescu, S.I.; Cîrîc, A.I.; Dumitrache, C.; Lupuliasa, D.; et al. Optimization of the Fermentation Conditions for Brewing Yeast Biomass Production Using the Response Surface Methodology and Taguchi Technique. Agriculture 2021, 11, 1237. [Google Scholar] [CrossRef]
- Satora, P.; Pater, A. The Influence of Different Non-Conventional Yeasts on the Odour-Active Compounds of Produced Beers. Appl. Sci. 2023, 13, 2872. [Google Scholar] [CrossRef]
- Saerens, S.; Swiegers, J.H. Production of Low-alcohol or Alcohol-free Beer With Pichia Kluyveri Yeast Strains. W.O. Patent 2014/135673 A2, 7 March 2014. [Google Scholar]
- King, A.; Dickinson, J.R. Biotransformation of monoterpene alcohols by Saccharomyces cerevisiae, Torulaspora delbrueckii and Kluyveromyces lactis. Yeast 2000, 16, 499–506. [Google Scholar] [CrossRef]
- Tataridis, P.; Kanelis, A.; Logotetis, S.; Nerancis, E. Use of non-Saccharomyces Torulaspora delbrueckii yeast strains in winemaking and brewing. Zb. Matice Srp. Prir. Nauke 2013, 124, 415–426. [Google Scholar] [CrossRef]
- Michel, M.; Kopecká, J.; Meier-Dörnberg, T.; Zarnkow, M.; Jacob, F.; Hutzler, M. Screening for new brewing yeasts in the non-Saccharomyces sector with Torulaspora delbrueckii as model. Yeast 2016, 33, 129–144. [Google Scholar] [CrossRef]
- Canonico, L.; Agarbati, A.; Comitini, F.; Ciani, M. Torulaspora delbrueckii in the brewing process: A new approach to enhance bioflavour and to reduce ethanol content. Food Microbial. 2016, 56, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Silva-Sousa, F.; Fernandes, T.; Pereira, F.; Rodrigues, D.; Rito, T.; Camarasa, C.; Franco-Duarte, R.; Sousa, M.J. Torulaspora delbrueckii Phenotypic and Metabolic Profiling towards Its Biotechnological Exploitation. J. Fungi 2022, 8, 569. [Google Scholar] [CrossRef]
- Toh, D.W.K.; Chua, J.Y.; Lu, Y.; Liu, S.Q. Evaluation of the Potential of Commercial Non-Saccharomyces Yeast Strains of Torulaspora delbrueckii and Lachancea thermotolerans in Beer Fermentation. Int. J. Food Sci. Technol. 2020, 55, 2049–2059. [Google Scholar] [CrossRef]
- Kayadelen, F.; Agirman, B.; Jolly, N.P.; Erten, H. The influence of Torulaspora delbrueckii on beer fermentation. FEMS Yeast Res. 2023, 23, foad006. [Google Scholar] [CrossRef]
- Postigo, V.; Esteban, S.; Arroyo, T. Lachancea thermotolerans, an Innovative Alternative for Sour Beer Production. Beverages 2023, 9, 20. [Google Scholar] [CrossRef]
- Domizio, P.; House, J.F.; Joseph, C.M.L.; Bisson, L.F.; Bamforth, C.W. Lachancea thermotolerans as an alternative yeast for the production of beer. J. Inst. Brew. 2016, 122, 599–604. [Google Scholar] [CrossRef]
- De Francesco, G.; Turchetti, B.; Sileoni, V.; Marconi, O.; Perretti, G. Screening of new strains of Saccharomycodes ludwigii and Zygosaccharomyces rouxii to produce low-alcohol beer. J. Inst. Brew. 2015, 121, 113–121. [Google Scholar] [CrossRef]
- Bourbon-Melo, N.; Palma, M.; Rocha, M.P.; Ferreira, A.; Bronze, M.R.; Elias, H.; Sá-Correia, I. Use of Hanseniaspora guilliermondii and Hanseniaspora opuntiae to Enhance the Aromatic Profile of Beer in Mixed-Culture Fermentation with Saccharomyces cerevisiae. Food Microbiol. 2021, 95, 103678. [Google Scholar] [CrossRef]
- Englezos, V.; Torchio, F.; Cravero, F.; Marengo, F.; Giacosa, S.; Gerbi, V.; Rantsiou, K.; Rolle, L.; Cocolin, L. Aroma profile and composition of Barbera wines obtained by mixed fermentations of Starmerella bacillaris (synonym Candida zemplinina) and Saccharomyces cerevisiae. LWT Food Sci. Technol. 2016, 73, 567–575. [Google Scholar] [CrossRef]
- Estela-Escalante, W.D.; Rosales-Mendoza, S.; Moscosa-Santillán, M.; González-Ramírez, J.E. Evaluation of the Fermentative Potential of Candida zemplinina Yeasts for Craft Beer Fermentation. J. Inst. Brew. 2016, 122, 530–535. [Google Scholar] [CrossRef]
- Karaulli, J.; Xhaferaj, N.; Coppola, F.; Testa, B.; Letizia, F.; Kyçyk, O.; Kongoli, R.; Ruci, M.; Lamçe, F.; Sulaj, K.; et al. Bioprospecting of Metschnikowia pulcherrima Strains, Isolated from a Vineyard Ecosystem, as Novel Starter Cultures for Craft Beer Production. Fermentation 2024, 10, 513. [Google Scholar] [CrossRef]
- Crauwels, S.; Steensels, J.; Aerts, G.; Willems, K.; Verstrepen, K.; Lievens, B. Brettanomyces bruxellensis, essential contributor in spontaneous beer fermentations providing novel opportunities for the brewing industry. Brew. Sci. 2015, 68, 110–121. [Google Scholar]
- Tullio, V. Probiotic Yeasts: A Developing Reality? J. Fungi 2024, 10, 489. [Google Scholar] [CrossRef] [PubMed]
- Corbu, V.; Vassu, T.; Csutak, O. Up-to-date knowledge on yeasts for food industry. Sci. Bulletin. Ser. F Biotechnol. 2020, 24, 101–111. [Google Scholar]
- Agarbati, A.; Canonico, L.; Marini, E.; Zannini, E.; Ciani, M.; Comitini, F. Potential Probiotic Yeasts Sourced from Natural Environmental and Spontaneous Processed Foods. Foods 2020, 9, 287. [Google Scholar] [CrossRef]
- Abid, R.; Waseem, H.; Ali, J.; Ghazanfar, S.; Muhammad Ali, G.; Elasbali, A.M.; Alharethi, S.H. Probiotic Yeast Saccharomyces: Back to Nature to Improve Human Health. J. Fungi 2022, 8, 444. [Google Scholar] [CrossRef]
- Shruthi, B.; Deepa, N.; Somashekaraiah, R.; Adithi, G.; Divyashree, S.; Sreenivasa, M.Y. Exploring biotechnological and functional characteristics of probiotic yeasts: A review. Biotechnol. Rep. 2022, 34, e00716. [Google Scholar] [CrossRef]
- Kunyeit, L.; Rao, R.P.; Anu-Appaiah, K.A. Yeasts originating from fermented foods, their potential as probiotics and therapeutic implication for human health and disease. Crit. Rev. Food Sci. Nutr. 2023, 64, 1–12. [Google Scholar] [CrossRef]
- Kanak, E.K.; Öztürk Yılmaz, S. Determination of the Probiotic and Functional Properties of Yeasts Isolated from Different Dairy Products. Fermentation 2025, 11, 104. [Google Scholar] [CrossRef]
- Staniszewski, A.; Kordowska-Wiater, M. Probiotic and potentially probiotic yeasts—Characteristics and food application. Foods. 2021, 10, 1306. [Google Scholar] [CrossRef]
- Diguță, C.F.; Mihai, C.; Toma, R.C.; Cîmpeanu, C.; Matei, F. In Vitro Assessment of Yeasts Strains with Probiotic Attributes for Aquaculture Use. Foods 2023, 12, 124. [Google Scholar] [CrossRef] [PubMed]
- Mogmenga, I.; Somda, M.K.; Ouattara, C.A.T.; Keita, I.; Dabiré, Y.; Diguță, C.F.; Toma, R.C.; Ezeogu, L.I.; Ugwuanyi, J.O.; Ouattara, A.S.; et al. Promising Probiotic Properties of the Yeasts Isolated from Rabilé, a Traditionally Fermented Beer Produced in Burkina Faso. Microorganisms 2023, 11, 802. [Google Scholar] [CrossRef]
- FAO/WHO. Guidelines for the Evaluation of Probiotics in Food; Working Group Report; Food and Agriculture Organization of the United Nations and World Health Organization: London, ON, Canada, 2002. [Google Scholar]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The International Scientific Association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Gil-Rodríguez, A.M.; Carrascosa, A.V.; Requena, T. Yeasts in foods and beverages: In vitro characterisation of probiotic traits. LWT-Food Sci. Technol. 2015, 64, 1156–1162. [Google Scholar] [CrossRef]
- Rodriguez, P.F.-P.; Arévalo-Villena, M.; Rosa, I.Z.; Perez, A.B. Selection of potential non-Saccharomyces probiotic yeasts from food origin by a step-by-step approach. Food Res. Int. 2018, 112, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Pacheco, P.; Ramos Monge, I.M.; Fernández-González, M.; Poveda Colado, J.M.; Arévalo-Villena, M. Safety Evaluation of Yeasts With Probiotic Potential. Front. Nutr. 2021, 8, 659328. [Google Scholar] [CrossRef]
- Fernández-Pacheco, P.; Pintado, C.; Briones Pérez, A.; Arévalo-Villena, M. Potential Probiotic Strains of Saccharomyces and Non-Saccharomyces: Functional and Biotechnological Characteristics. J. Fungi 2021, 7, 177. [Google Scholar] [CrossRef] [PubMed]
- Alkalbani, N.S.; Osaili, T.M.; Al-Nabulsi, A.A.; Olaimat, A.N.; Liu, S.-Q.; Shah, N.P.; Apostolopoulos, V.; Ayyash, M.M. Assessment of yeasts as potential probiotics: A review of gastrointestinal tract conditions and investigation methods. J. Fungi 2022, 8, 365. [Google Scholar] [CrossRef]
- Pais, P.; Almeida, V.; Yılmaz, M.; Teixeira, M.C. Saccharomyces boulardii: What makes it tick as successful probiotic? J. Fungi 2020, 6, 78. [Google Scholar] [CrossRef]
- Fu, J.; Liu, J.; Wen, X.; Zhang, G.; Cai, J.; Qiao, Z.; An, Z.; Zheng, J.; Li, L. Unique probiotic properties and bioactive metabolites of Saccharomyces boulardii. Probiotics Antimicrob. Proteins 2022, 15, 1–16. [Google Scholar] [CrossRef]
- Barbulescu, I.D.; Dumitrache, C.; Diguta, C.F.; Matei, P.M.; Frincu, M.; Marculescu, S.I.; Ciric, A.I.; Tudor, V.; Boroiu, E.M.; Teodorescu, R. Evolution at the microfermenter level of the growth dynamics of Saccharomyces cerevisiae and Starmella bacillaris yeasts with potential for use in winemaking at the Pietroasa winery. AgroLife Sci. J. 2022, 11, 9–16. [Google Scholar] [CrossRef]
- Shen, Y.; Bai, X.; Zhang, Y.; Gao, Q.; Bu, X.; Xu, Y.; Guo, N. Evaluation of the Potential Probiotic Yeast Characteristics with Anti-MRSA Abilities. Probiotics Antimicrob. Proteins 2022, 14, 727–740. [Google Scholar] [CrossRef]
- Corbu, V.; Vassu, T.; Csutak, O. Pichia (Kodamaea) ohmeri CMGB-ST19-A new strain with complex biotechnological properties. AgroLife Sci. J. 2019, 8, 77–86. [Google Scholar]
- Zendeboodi, F.; Gholian, M.M.; Khanniri, E.; Sohrabvandi, S.; Mortazavian, A.M. Beer as a Vehicle for Probiotics. Appl. Food Biotechnol. 2021, 8, 329–337. [Google Scholar]
- Canonico, L.; Zannini, E.; Ciani, M.; Comitini, F. Assessment of non-conventional yeasts with potential probiotic for protein-fortified craft beer production. LWT 2021, 145, 111361. [Google Scholar] [CrossRef]
- Hinojosa-Avila, C.R. Exploring the Potential of Probiotic-Enriched Beer: Microorganisms, Fermentation Strategies, Sensory Attributes, and Health Implications. Food Res. Int. 2024, 175, 113717. [Google Scholar] [CrossRef] [PubMed]
- Ramanan, M.; Fox, G.P.; Marco, M.L. Beer for Live Microbe Delivery. J. Funct. Foods 2024, 113, 105987. [Google Scholar] [CrossRef]
- Santos, D.; Barreiros, L.; Jesus, Â.; Silva, A.L.; Martins, J.P.; Oliveira, A.I.; Pinho, C. Beer with Probiotics: Benefits and Challenges of Their Incorporation. Beverages 2024, 10, 109. [Google Scholar] [CrossRef]
- Pereira de Paula, B.; de Souza Lago, H.; Firmino, L.; Fernandes Lemos Júnior, W.J.; Ferreira Dutra Corrêa, M.; Fioravante Guerra, A.; Signori Pereira, K.; Zarur Coelho, M.A. Technological features of Saccharomyces cerevisiae var. boulardii for potential probiotic wheat beer development. LWT 2021, 135, 110233. [Google Scholar]
- Capece, A.; Romaniello, R.; Pietrafesa, A.; Siesto, G.; Pietrafesa, R.; Zambuto, M.; Romano, P. Use of Saccharomyces cerevisiae var. boulardii in co-fermentations with S. cerevisiae for the production of craft beers with potential healthy value-added. Int. J. Food Microbiol. 2018, 284, 22–30. [Google Scholar] [CrossRef]
- Mulero-Cerezo, J.; Briz-Redón, Á.; Serrano-Aroca, Á. Saccharomyces cerevisiae var. boulardii: Valuable Probiotic Starter for Craft Beer Production. Appl. Sci. 2019, 9, 3250. [Google Scholar]
- Senkarcinova, B.; Graça Dias, I.A.; Nespor, J.; Branyik, T. Probiotic alcohol-free beer made with Saccharomyces cerevisiae var. boulardii. LWT 2019, 100, 362–367. [Google Scholar] [CrossRef]
- Ramírez-Cota, G.Y.; López-Villegas, E.O.; Jiménez-Aparicio, A.R.; Hernández-Sánchez, H. Modeling the Ethanol Tolerance of the Probiotic Yeast Saccharomyces cerevisiae var. boulardii CNCM I-745 for its Possible Use in a Functional Beer. Probiotics Antimicrob. Proteins 2021, 13, 187–194. [Google Scholar]
- Dumitrache, C.; Ghica, M.V.; Frîncu, M.; Bărbulescu, I.D.; Begea, M.; Diguță, C.F.; Baniță, C.; Cotea, V.V.; Israel-Roming, F.; Teodorescu, R.I. Bioprocess Optimization by Taguchi Design and Response Surface Analysis for Obtaining Active Yeast Used in Vinification. Fermentation 2024, 10, 413. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Tamura, K.N.M.; Kumar, S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc. Natl. Acad. Sci. USA 2004, 101, 11030–11035. [Google Scholar] [CrossRef] [PubMed]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- CLSI. CLSI Supplement M100S. Performance Standards for Antimicrobial Susceptibility Testing, 26th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2016. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Sidari, R.; Ženišová, K.; Tobolková, B.; Belajová, E.; Cabicarová, T.; Bučková, M.; Puškárová, A.; Planý, M.; Kuchta, T.; Pangallo, D. Wine Yeasts Selection: Laboratory Characterization and Protocol Review. Microorganisms 2021, 9, 2223. [Google Scholar] [CrossRef]
- Cordente, A.G.; Heinrich, A.; Pretorius, I.S.; Swiegers, J.H. Isolation of sulfite reductase variants of a commercial wine yeast with significantly reduced hydrogen sulfide production. FEMS Yeast Res. 2009, 9, 446–459. [Google Scholar] [CrossRef]
- Găgeanu, A.; Câmpeanu, G.; Diguță, C.; Matei, F. Isolation and Identification of Local Wine Yeast Strains from Dealurile Bujorului Vineyard. Sci. Bulletin. Ser. F Biotechnol. 2012, 16, 22–25. [Google Scholar]
- European Brewery Convention Analytica EBC. Section 9 Original, Real an Apparent Extract and Original Gravity of Beer Method 9.4. In EBC Methods of Analysis; Fachverlag Hans Carl: Nürnberg, Germany, 2004. [Google Scholar]
- Popescu, V.; Soceanu, A.; Dobrinas, S.; Stanicu, G. A study of beer bitterness loss during the various stages of the Romanian beer production process. J. Inst. Brew. 2013, 119, 111–115. [Google Scholar] [CrossRef]
- SR 13355-6:2000; Beer. Analysis Methods. Determination of Total Acidity. American Society of Brewing Chemists: St. Paul, MN, USA, 2020.
- European Brewery Convention Analytica EBC. Section 8 Colour of Wort: Spectrophotometric Method Method 8.5. In EBC Methods of Analysis; Fachverlag Hans Carl: Nürnberg, Germany, 2000. [Google Scholar]
- European Brewery Convention Analytica EBC. Section 9 Colour of Beer: Spectrophotometric Method Method 9.6. In EBC Methods of Analysis; Fachverlag Hans Carl: Nürnberg, Germany, 2000. [Google Scholar]
- European Brewery Convention Analytica EBC. Section 9 Real Degree of Fermentation of Beer Method 9.5. In EBC Methods of Analysis; Fachverlag Hans Carl: Nürnberg, Germany, 1997. [Google Scholar]
- European Brewery Convention Analytica EBC. Section 13 Sensory Analysis Method 13.10. In EBC Methods of Analysis; Fachverlag Hans Carl: Nürnberg, Germany, 1997. [Google Scholar]
- Helmy, E.A.; Abdel-Fadeel, R.H.; Yosri, M.; Hassan, E. Does Torulaspora delbrueckii has some probiotic capabilities? In vitro and in vivo assessment. Nutrire 2024, 49, 15. [Google Scholar] [CrossRef]
- Staniszewski, A.; Kordowska-Wiater, M. Probiotic Yeasts and How to Find Them—Polish Wines of Spontaneous Fermentation as Source for Potentially Probiotic Yeasts. Foods 2023, 12, 3392. [Google Scholar] [CrossRef] [PubMed]
- Nicolae, G.; Dopcea, I.; Nicolae, A.-M.; Stelian, P.; Matei, F. Conservation methods used for yeast isolated from vineyards—Lyophilisation advantage. Sci. Bull. Biotechnol. 2010, 14, 31–36. [Google Scholar]
- Canonico, L.; Ciani, E.; Galli, E.; Comitini, F.; Ciani, M. Evolution of Aromatic Profile of Torulaspora delbrueckii Mixed Fermentation at Microbrewery Plant. Fermentation 2020, 6, 7. [Google Scholar] [CrossRef]
- Kucharczyk, K.; Tuszyński, T. The Effect of Temperature on Fermentation and Beer Volatiles at an Industrial Scale. J. Inst. Brew. 2018, 124, 230–235. [Google Scholar] [CrossRef]
- Du, Q.; Ye, D.; Zang, X.; Nan, H.; Liu, Y. Effect of Low Temperature on the Shaping of Yeast-Derived Metabolite Compositions during Wine Fermentation. Food Res. Int. 2022, 162, 112016. [Google Scholar] [CrossRef] [PubMed]
- Buiatti, S.; Tat, L.; Natolino, A.; Passaghe, P. Biotransformations Performed by Yeasts on Aromatic Compounds Provided by Hop—A Review. Fermentation 2023, 9, 327. [Google Scholar] [CrossRef]
- SR 4230; Beer. Romanian Standard. Asro: Turku, Finland, 2004.
- Callejo, M.J.; Tesfaye, W.; González, M.C.; Morata, A. Craft beers: Current situation and future trends. In New Advances on Fermentation Processes; Martínez-Espinosa, R.M., Ed.; IntechOpen: London, UK, 2019; pp. 1–18. [Google Scholar]
- Callejo, M.J.; González, C.; Morata, A. Use of non-Saccharomyces yeasts in bottle fermentation of aged beers. In Brewing Technology; Kanauchi, M., Ed.; IntechOpen: London, UK, 2017; Available online: https://www.intechopen.com/books/brewing-technology/use-of-non-saccharomyces-yeasts-in-bottle-fermentation-of-aged-beers (accessed on 3 March 2025).
- Da Costa Jardim, C.; De Souza, D.; Cristina Kasper Machado, I.; Massochin Nunes Pinto, L.; De Souza Ramos, R.C.; Garavaglia, J. Sensory Profile, Consumer Preference and Chemical Composition of Craft Beers from Brazil. Beverages 2018, 4, 106. [Google Scholar] [CrossRef]
- Skendi, A.; Papageorgiou, M. Influence of Kilning Temperature on Chemical Composition of a Greek Barley Malt and Its Wort Properties. Millenium J. Educ. Technol. Health 2018, 7, 49–58. [Google Scholar] [CrossRef]
- He, Y.; Dong, J.; Yin, H.; Zhao, Y.; Chen, R.; Wan, X.; Chen, P.; Hou, X.; Liu, J.; Chen, L. Wort composition and its impact on theflavour-active higher alcohol and esterformation of beer—A review. J. Inst. Brew. 2014, 120, 157–163. [Google Scholar] [CrossRef]
- Bellassai, S.; Mearelli, L. Conversion of sugar and formation of wort density in beer fermentation. BRAUWELT International 4 2024, 4, 217–219. [Google Scholar]
- Bruner, J.; Marcus, A.; Fox, G. Dry-Hop Creep Potential of Various Saccharomyces Yeast Species and Strains. Fermentation 2021, 7, 66. [Google Scholar] [CrossRef]
- Postigo, V.; Mauro, L.; Diaz, T.; Saiz, R.; Arroyo, T.; García, M. Autochthonous Ingredients for Craft Beer Production. Fermentation 2024, 10, 225. [Google Scholar] [CrossRef]
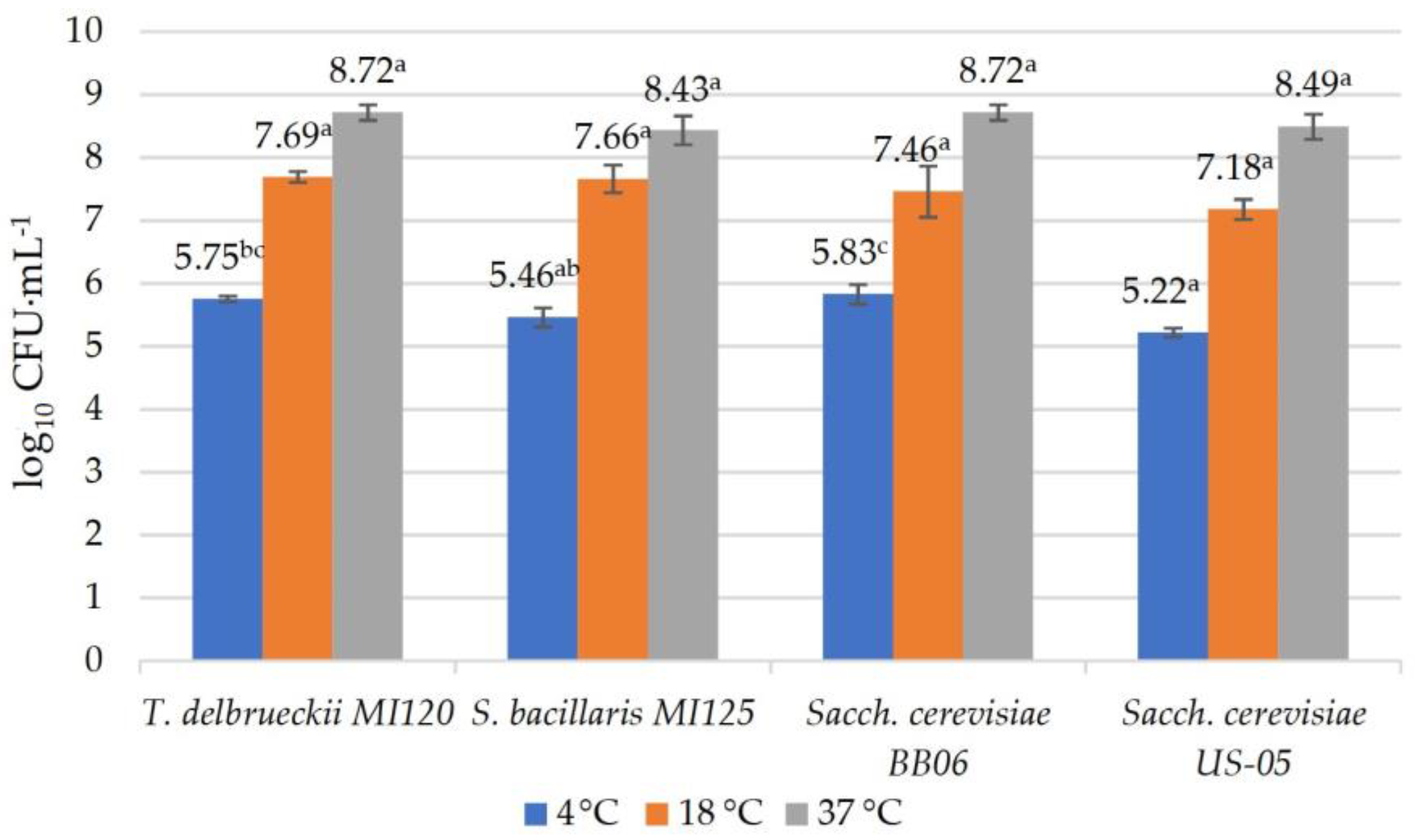
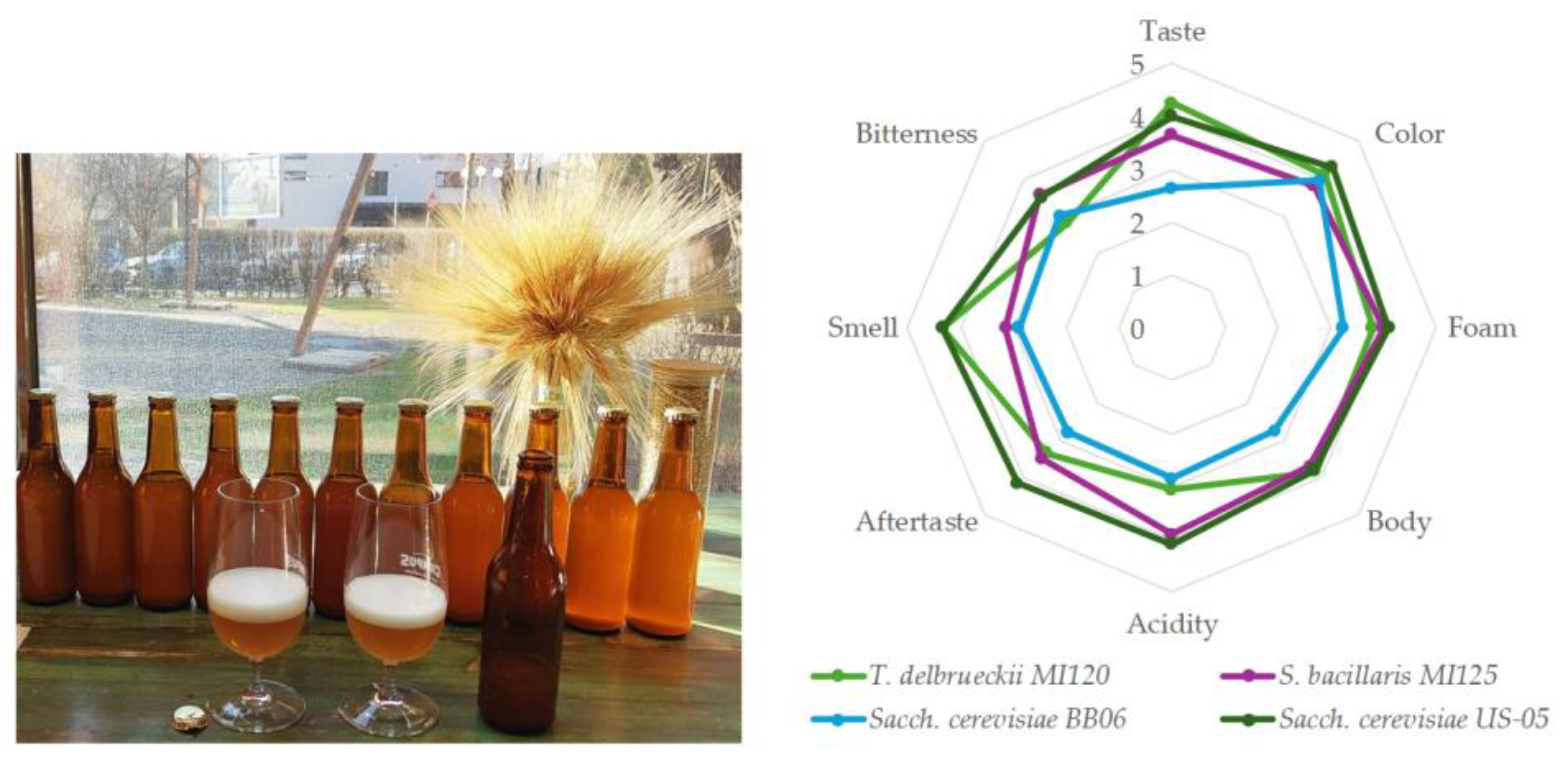
| Pathogenic Bacteria | T. delbrueckii MI120 | S. bacillaris MI125 | Sacch. cerevisiae BB06 |
|---|---|---|---|
| Bacillus cereus ATCC 11778 | 10.83 ± 1.04 a | 25.83 ± 0.85 c | 14.83 ± 0.62 b |
| Listeria monocytogenes ATCC 13932 | 0.00 ± 0.00 a | 25.00 ± 1.63 c | 14.67 ± 0.47 b |
| Staphylococcus aureus ATCC 33592 | 0.00 ± 0.00 a | 18.33 ± 0.47 c | 16.17 ± 0.47 b |
| Pseudomonas aeruginosa ATCC 15442 | 0.00 ± 0.00 a | 25.33 ± 0.47 c | 18.67 ± 0.94 b |
| Salmonella enterica subsp. enterica serovar Typhimurium ATCC 14028 | 0.00 ± 0.00 a | 26.67 ± 0.94 c | 16.67 ± 1.25 b |
| Salmonella enterica subsp. enterica serovar Enteritidis ATCC 13076 | 0.00 ± 0.00 a | 24.33 ± 0.47 c | 18.50 ± 1.47 b |
| Escherichia coli ATCC 8739 | 0.00 ± 0.00 a | 24.33 ± 1.25 c | 15.50 ± 1.08 b |
| Serratia marcescens ATCC 14756 | 7.83 ± 0.76 a | 22.00 ± 1.41 c | 16.83 ± 1.03 b |
| Yeast Strains | Antibiotics Susceptibility | Hemolytic Activity | ||||||
|---|---|---|---|---|---|---|---|---|
| FLU-25 | ITR-10 | KCA-10 | MCL-10 | AMP-10 | C-30 | E-15 | ||
| T. delbrueckii MI120 | S | R | S | S | R | R | R | γ |
| S. bacillaris MI125 | R | R | R | R | R | R | R | γ |
| Sacch. cerevisiae BB06 | R | R | R | R | R | R | R | γ |
| Parameters | Yeast Strains | ||||
|---|---|---|---|---|---|
| T. delbrueckii MI 120 | S. bacillaris MI125 | Sacch. cerevisiae BB06 | Sacch. cerevisiae US-05 | ||
| Carbon assimilation a | 2% Glucose | + | + | + | + |
| 2% Fructose | + | + | + | + | |
| 2% Maltose | + | − | + | + | |
| 2% Sucrose | + | + | + | + | |
| Osmotic stress a | 30% Glucose | + | + | + | + |
| 10% Malt extract tolerance with gas production b | - | − | + | + | |
| Ethanol tolerance a | 0% | + | + | + | + |
| 2.5% | + | + | + | + | |
| 5% | + | + | + | + | |
| 7.5% | + | − | + | + | |
| Low pH tolerance c | +++ | +++ | +++ | +++ | |
| Acetic acid production d | + | + | + | + | |
| H2S production e | brown | brown | brown | brown | |
| Killer phenotype f | Sensitive | − | − | − | + |
| Killer | − | − | − | − | |
| Enzyme | Substrate | T. delbrueckii MI 120 | S. bacillaris MI125 | Sacch. cerevisiae BB06 | Sacch. cerevisiae US-05 |
|---|---|---|---|---|---|
| Alkaline phosphatase | 2-Naphthyl phosphate | 1 | 1 | 1 | |
| Esterase (C4) | 2-Naphthyl butyrate | 4 | 3 | 2 | 3 |
| Lipase esterase (C8) | 2-Naphthyl caprylate | 3 | 2 | 2 | 2 |
| Leucine arylamidase | L-Leucyl-2-naphthylamide | 4 | 3 | 3 | |
| Valine arylamidase | L-Valyl-2-naphthylamide | 3 | 2 | 2 | 2 |
| Cystine arylamidase | L-Cisteyl-2-naphthylamide | 2 | 1 | 2 | 2 |
| Acid phosphatase | 2-Naphthyl phosphate | 2 | 2 | 2 | 2 |
| Naphthol-AS-BI-phosphohydrolase | Naphthol-AS-BI-phosphate | 2 | 2 | 2 | 1 |
| α-Galactosidase | 6-Br-2-naphthyl-DD-galactopyranoside | 2 | 1 | ||
| β-Galactosidase | 2-Naphthyl-ßD-galactopyranoside | 1 | |||
| β-Glucuronidase | Naphthol-AS-BI-ßD-glucuronide | 1 | 1 | 1 | |
| α-Glucosidase | 2-Naphthyl-DD-glucopyranoside | 3 | |||
| β-Glucosidase | 6-Br-2-naphthyl-ßD-glucopyranoside | 2 |
| Beer Samples | Ep, %P | Alcohol, % v/v | Bitterness, BU | Colour, EBC | Total Acidity, mL NaOH 1 N at 100 mL Beer | RDF, % |
|---|---|---|---|---|---|---|
| Beer fermented with T. delbrueckii MI120 | 11.08 ± 0.08 a | 4.21 ± 0.02 b | 28.00 ± 2.27 c | 9.00 ± 0.51 a | 1.60 ± 0.01 a | 59.17 ± 0.16 d |
| Beer fermented with S. bacillaris MI125 | 10.90 ± 0.05 a | 3.36 ± 0.01 a | 28.00 ± 1.67 c | 9.19 ± 0.04 a | 2.23 ± 0.01 b | 48.27 ± 0.05 a |
| Beer fermented with Sacch. cerevisiae BB06 | 10.97 ± 0.05 a | 3.50 ± 0.04 a | 26.00 ± 1.14 a | 9.78 ± 0.34 a | 2.00 ± 0.01 b | 49.76 ± 0.24 b |
| Beer fermented with Sacch. cerevisiae US-05 | 11.37 ± 0.17 a | 4.26 ± 0.14 b | 27.00 ± 1.68 b | 9.69 ± 0.27 a | 1.80 ± 0.01 a | 58.38 ± 0.99 c |
| Beer Samples | Spectrum of Sugars, g/100 mL |
|---|---|
| Brewing wort used for the production of all beer samples | Glucose: 0.67 Sucrose: 0.54 Maltose: 2.81 Maltotriose: 0.89 Fructose: 0.06 Total sugars: 4.97 |
| Beer fermented with T. delbrueckii MI120 | Maltotriose: 0.043 Maltose: 0.019 Total sugars: 0.062 |
| Beer fermented with S. bacillaris MI125 | Maltose: 0.046 Fructose: 0.012 Total sugars: 0.058 |
| Beer fermented with Sacch. cerevisiae BB06 | Fructose: 0.010 Maltose: 0.021 Total sugars: 0.031 |
| Beer fermented with Sacch. cerevisiae US-05 | Fructose: 0.010 Total sugars: 0.010 |
| Beer Samples | TPC μg GAE·mL−1 | Antioxidant Activity % RSA |
|---|---|---|
| Beer fermented with T. delbrueckii MI120 | 96.02 ± 4.57 b | 90.43 + 0.21 b |
| Beer fermented with S. bacillaris MI125 | 76.59 ± 3.15 a | 90.53 + 0.37 b |
| Beer fermented with Sacch. cerevisiae BB06 | 74.97 ± 1.69 a | 89.27 + 0.52 a |
| Beer fermented with Sacch. cerevisiae US-05 | 93.65 ± 2.55 b | 91.05 + 0.31 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vrînceanu, C.-R.; Diguță, F.C.; Cudalbeanu, M.D.; Ortan, A.; Mihai, C.; Bărbulescu, I.D.; Frîncu, M.; Begea, M.; Matei, F.; Teodorescu, R.I. Exploring the Potential of Torulaspora delbrueckii, Starmerella bacillaris, and Saccharomyces cerevisiae as a Probiotic Starter for Craft Beer Production. Foods 2025, 14, 1608. https://doi.org/10.3390/foods14091608
Vrînceanu C-R, Diguță FC, Cudalbeanu MD, Ortan A, Mihai C, Bărbulescu ID, Frîncu M, Begea M, Matei F, Teodorescu RI. Exploring the Potential of Torulaspora delbrueckii, Starmerella bacillaris, and Saccharomyces cerevisiae as a Probiotic Starter for Craft Beer Production. Foods. 2025; 14(9):1608. https://doi.org/10.3390/foods14091608
Chicago/Turabian StyleVrînceanu, Carmen-Rodica, Filofteia Camelia Diguță, Mihaela Dragoi Cudalbeanu, Alina Ortan, Constanța Mihai, Iuliana Diana Bărbulescu, Mihai Frîncu, Mihaela Begea, Florentina Matei, and Răzvan Ionuț Teodorescu. 2025. "Exploring the Potential of Torulaspora delbrueckii, Starmerella bacillaris, and Saccharomyces cerevisiae as a Probiotic Starter for Craft Beer Production" Foods 14, no. 9: 1608. https://doi.org/10.3390/foods14091608
APA StyleVrînceanu, C.-R., Diguță, F. C., Cudalbeanu, M. D., Ortan, A., Mihai, C., Bărbulescu, I. D., Frîncu, M., Begea, M., Matei, F., & Teodorescu, R. I. (2025). Exploring the Potential of Torulaspora delbrueckii, Starmerella bacillaris, and Saccharomyces cerevisiae as a Probiotic Starter for Craft Beer Production. Foods, 14(9), 1608. https://doi.org/10.3390/foods14091608









