Prospects for the Use of Amaranth Grain in the Production of Functional and Specialized Food Products
Abstract
1. Introduction
2. Chemical Composition
2.1. Proteins, Essential Amino Acids, and Bioactive Peptides
2.2. Fats
2.3. Carbohydrates and Dietary Fibers
2.4. Minerals and Vitamins
2.5. Other Bioactive Components
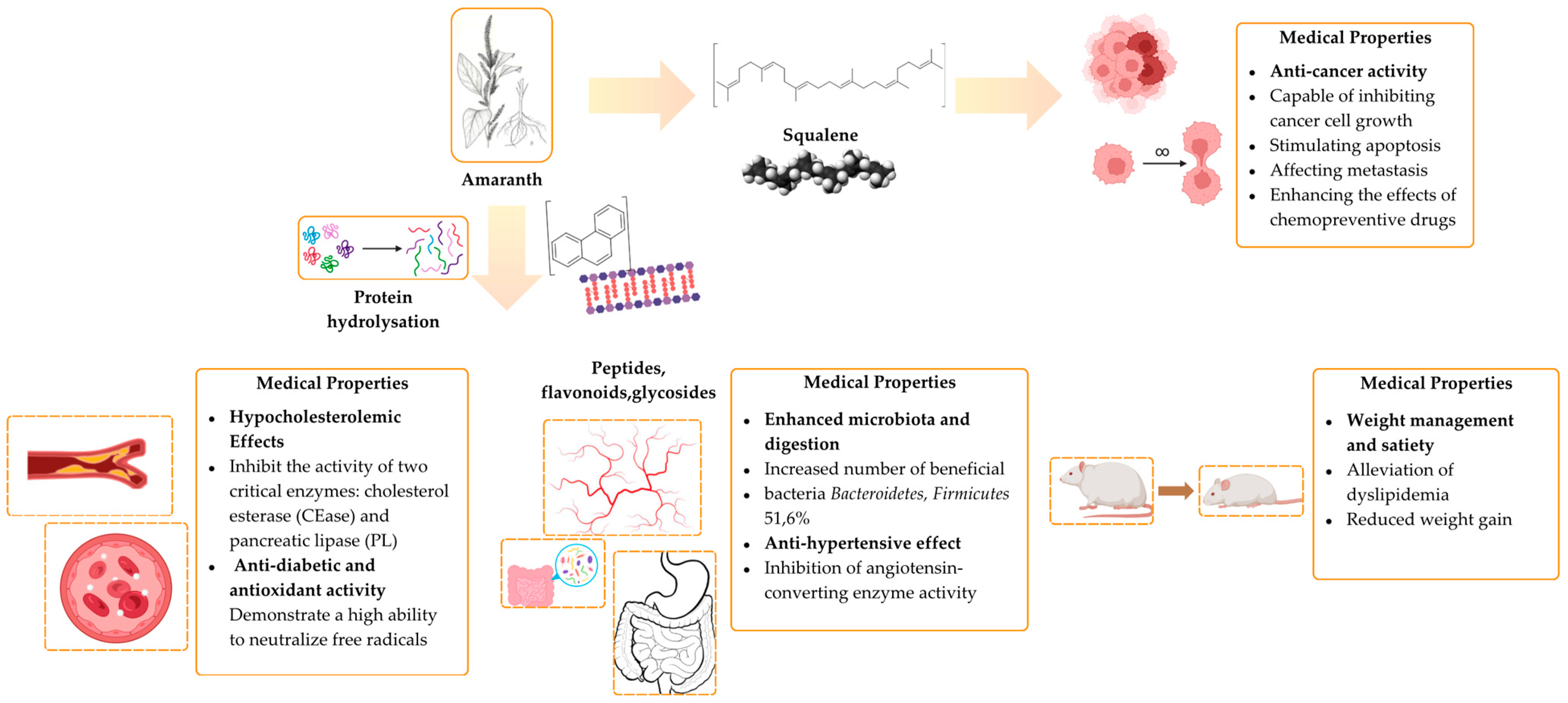
3. Therapeutic and Preventative Properties of Amaranth
3.1. Enhancing Gut Microbiota and Digestion
3.2. Antioxidant Activity of Amaranth
3.3. Antidiabetic Activty
3.4. Anticancer Activity
3.5. Antihypertensive Effect
3.6. Antimicrobial Properties
3.7. Immunoregulatory Properties
3.8. Hypocholesterolemic Effect
3.9. Weight Management and Satiety (Anti-Obesity)
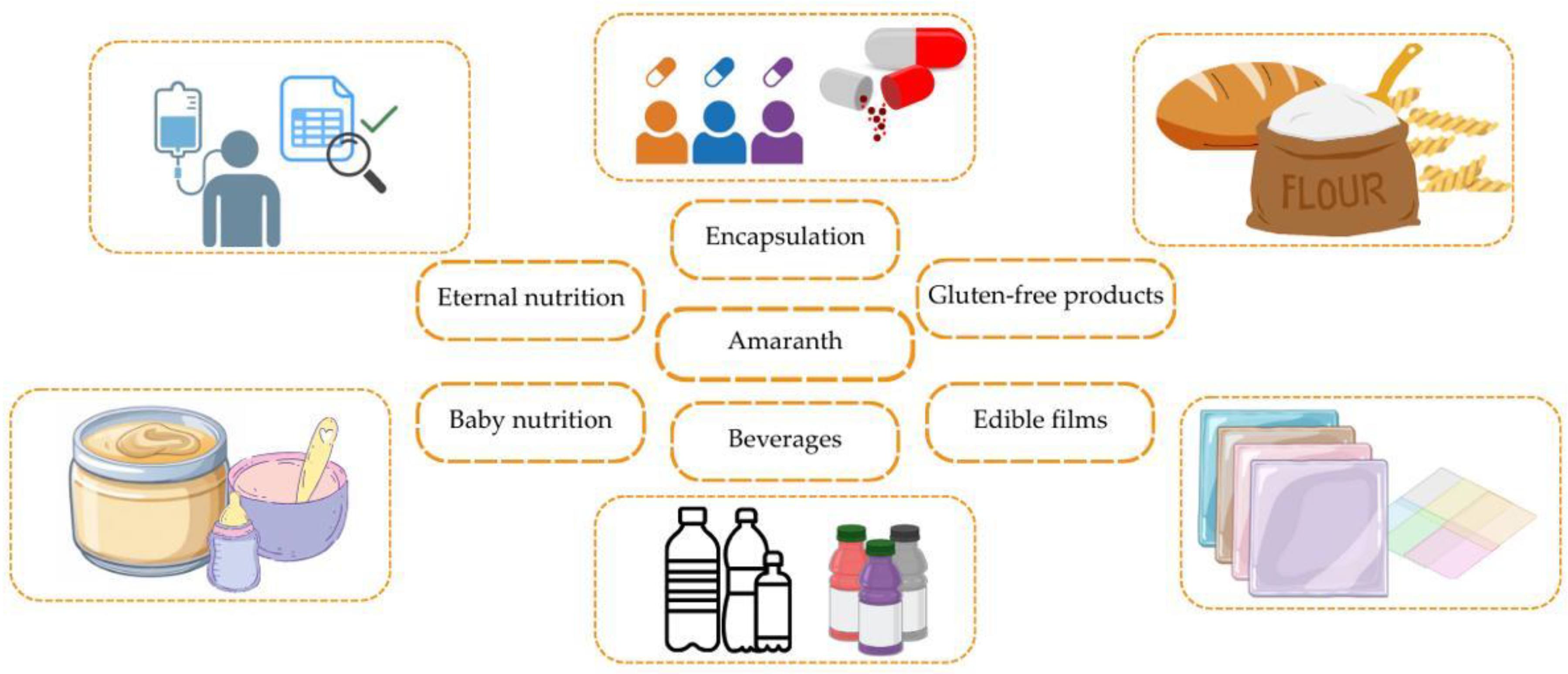
4. Innovative Applications of Amaranth in Functional Foods and Nutraceuticals
4.1. Gluten-Free Products
4.2. Baby Nutrition
4.3. Nutraceuticals
4.4. Amaranth-Based Beverages
4.5. Encapsulation of Bioactive Compounds
4.6. Edible Coating or Active Films for Food Preservation
4.7. Potential Application in Enteral Nutrition
4.8. Limitations of Amaranth Grain Utilization
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| TAG | Triacylglycerol |
| LEA | Late embryogenesis abundant |
| GBSSI | Granule bound starch synthase I |
| SSPs | Seed storage proteins |
| LDL | Low-density lipoprotein |
| HDL | High-density lipoprotein |
| IBS | Irritable bowel syndrome |
| SCFA | Short-chain fatty acid |
| AG | Amaranth hydrolysate |
| DPP-IV | Dipeptidyl peptidase—IV |
| AP | Amaranth peptides |
| ACE | Angiotensin-I-converting enzyme |
| AhLun | Lunasin-like peptide |
| NIH-3T3 | Mouse embryonic fibroblast |
| 3MC | 3-methylcholanthrene |
| ASP-HD | Amaranth seed protein—heat-denatured |
| AKR1C3 | Aldo-keto reductase family 1 member C3 |
| GAF | Germinated amaranth flour |
| SGD | Stimulated gastrointestinal digestion |
| iNOS | Inducible nitric oxide synthase |
| LPS | Lipopolysaccharide |
| GAD90 | 90 min digestion fraction |
| COX-2 | Cyclooxygenase-2 |
| PL | Pancreatic lipase |
| APH | Amaranth protein hydrolysate |
| HMG-CoA | 3-hydroxy-3methyl-glutaryl-CoA reductase |
| FAS | Fatty acid synthase |
| PPAR | Peroxisome proliferator-activated receptor |
References
- Thakur, P.; Kumar, K.; Dhaliwal, H.S. Nutritional facts, bio-active components and processing aspects of pseudocereals: A comprehensive review. Food Biosci. 2021, 42, 101170. [Google Scholar] [CrossRef]
- Martinez-Lopez, A.; Millan-Linares, M.C.; Rodriguez-Martin, N.M.; Millan, F.; Montserrat-de la Paz, S. Nutraceutical value of kiwicha (Amaranthus caudatus L.). J. Funct. Foods 2020, 65, 103735. [Google Scholar] [CrossRef]
- Constantino, A.B.T.; Garcia-Rojas, E.E. Proteins from pseudocereal seeds: Solubility, extraction, and modifications of the physicochemical and techno-functional properties. J. Sci. Food Agric. 2022, 102, 2630–2639. [Google Scholar] [PubMed]
- Escudero, N.L. Triglycerides Metabolism. Diet. Protein Res. Trends 2007, 251. [Google Scholar]
- Balakrishnan, G.; Schneider, R.G. The role of amaranth, quinoa, and millets for the development of healthy, sustainable food products—A concise review. Foods 2022, 11, 2442. [Google Scholar] [CrossRef]
- Rodríguez, M.; Tironi, V.A. Polyphenols in amaranth (A. manteggazianus) flour and protein isolate: Interaction with other components and effect of the gastrointestinal digestion. Food Res. Int. 2020, 137, 109524. [Google Scholar]
- Popoola, O.O. Phenolic compounds composition and in vitro antioxidant activity of Nigerian Amaranthus viridis seed as affected by autoclaving and germination. Meas. Food 2022, 6, 100028. [Google Scholar] [CrossRef]
- Council, N.R. Amaranth: Modern Prospects for an Ancient Crop; National Academic Press: Washington, DC, USA, 1930. [Google Scholar]
- Bojórquez-Velázquez, E.; Barrera-Pacheco, A.; Espitia-Rangel, E.; Herrera-Estrella, A.; Barba de la Rosa, A.P. Protein analysis reveals differential accumulation of late embryogenesis abundant and storage proteins in seeds of wild and cultivated amaranth species. BMC Plant Biol. 2019, 19, 59. [Google Scholar] [CrossRef]
- Estrada, Y.; Fernández-Ojeda, A.; Morales, B.; Egea-Fernández, J.M.; Flores, F.B.; Bolarín, M.C.; Egea, I. Unraveling the strategies used by the underexploited amaranth species to confront salt stress: Similarities and differences with quinoa species. Front. Plant Sci. 2021, 12, 604481. [Google Scholar]
- Dabija, A.; Ciocan, M.E.; Chetrariu, A.; Codină, G.G. Buckwheat and amaranth as raw materials for brewing, a review. Plants 2022, 11, 756. [Google Scholar] [CrossRef]
- Condés, M.C.; Añón, M.C.; Dufresne, A.; Mauri, A.N. Composite and nanocomposite films based on amaranth biopolymers. Food Hydrocoll. 2018, 74, 159–167. [Google Scholar]
- Joshi, D.C.; Sood, S.; Hosahatti, R.; Kant, L.; Pattanayak, A.; Kumar, A.; Yadav, D.; Stetter, M.G. From zero to hero: The past, present and future of grain amaranth breeding. Theor. Appl. Genet. 2018, 131, 1807–1823. [Google Scholar] [CrossRef] [PubMed]
- Bressani, R. The proteins of grain amaranth. Food Rev. Int. 1989, 5, 13–38. [Google Scholar]
- Aguilar, E.G.; Peiretti, E.G.; Uñates, M.A.; Marchevsky, E.J.; Escudero, N.L.; Camiña, J.M. Amaranth seed varieties. A chemometric approach. J. Food Meas. Charact. 2013, 7, 199–206. [Google Scholar] [CrossRef]
- Quiroga, A.V.; Aphalo, P.; Ventureira, J.L.; Martínez, E.N.; Añón, M.C. Physicochemical, functional and angiotensin converting enzyme inhibitory properties of amaranth (Amaranthus hypochondriacus) 7S globulin. J. Sci. Food Agric. 2012, 92, 397–403. [Google Scholar]
- Ayala-Niño, A.; Rodríguez-Serrano, G.M.; González-Olivares, L.G.; Contreras-López, E.; Regal-López, P.; Cepeda-Saez, A. Sequence identification of bioactive peptides from amaranth seed proteins (Amaranthus hypochondriacus spp.). Molecules 2019, 24, 3033. [Google Scholar] [CrossRef]
- Shen, W.; Yang, J.; Wang, Z.; Liu, B. Structural characterization and physicochemical properties of grain amaranth starch. Food Chem. X 2024, 23, 101723. [Google Scholar]
- Kong, X.; Bao, J.; Corke, H. Physical properties of Amaranthus starch. Food Chem. 2009, 113, 371–376. [Google Scholar]
- Tapia-Blácido, D.; Mauri, A.N.; Menegalli, F.C.; Sobral, P.J.d.A.; Añón, M.C. Contribution of the starch, protein, and lipid fractions to the physical, thermal, and structural properties of amaranth (Amaranthus caudatus) flour films. J. Food Sci. 2007, 72, E293–E300. [Google Scholar]
- Li, W.; Liu, Y.; Liu, M.; Zheng, Q.; Li, B.; Li, Z.; Li, H. Sugar accumulation is associated with leaf senescence induced by long-term high light in wheat. Plant Sci. 2019, 287, 110169. [Google Scholar]
- Venskutonis, P.R.; Kraujalis, P. Nutritional components of amaranth seeds and vegetables: A review on composition, properties, and uses. Compr. Rev. Food Sci. Food Saf. 2013, 12, 381–412. [Google Scholar] [CrossRef] [PubMed]
- Arendt, E.K.; Zannini, E. Cereal Grains for the Food and Beverage Industries; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Coelho, L.M.; Silva, P.M.; Martins, J.T.; Pinheiro, A.C.; Vicente, A.A. Emerging opportunities in exploring the nutritional/functional value of amaranth. Food Funct. 2018, 9, 5499–5512. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Guleria, S.; Kimta, N.; Dhalaria, R.; Nepovimova, E.; Dhanjal, D.S.; Alomar, S.Y.; Kuca, K. Amaranth and buckwheat grains: Nutritional profile, development of functional foods, their pre-clinical cum clinical aspects and enrichment in feed. Curr. Res. Food Sci. 2024, 9, 100836. [Google Scholar] [CrossRef] [PubMed]
- Akinoso, R.; Tanimola, A.R.; Abereola, A.E. Effects of roasting conditions on the properties of Amaranth (Amaranthus cruentus L.) grain using response surface methodology. Food Humanit. 2024, 2, 100298. [Google Scholar] [CrossRef]
- Chauhan, A.; Kumari, N.; Saxena, D.; Singh, S. Effect of germination on fatty acid profile, amino acid profile and minerals of amaranth (Amaranthus spp.) grain. J. Food Meas. Charact. 2022, 16, 1777–1786. [Google Scholar] [CrossRef]
- Schmidt, D.; Verruma-Bernardi, M.R.; Forti, V.A.; Borges, M.T.M.R. Quinoa and amaranth as functional foods: A review. Food Rev. Int. 2023, 39, 2277–2296. [Google Scholar] [CrossRef]
- Aderibigbe, O.; Ezekiel, O.; Owolade, S.; Korese, J.; Sturm, B.; Hensel, O. Exploring the potentials of underutilized grain amaranth (Amaranthus spp.) along the value chain for food and nutrition security: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 656–669. [Google Scholar] [CrossRef]
- Janssen, F.; Pauly, A.; Rombouts, I.; Jansens, K.J.; Deleu, L.J.; Delcour, J.A. Proteins of amaranth (Amaranthus spp.), buckwheat (Fagopyrum spp.), and quinoa (Chenopodium spp.): A food science and technology perspective. Compr. Rev. Food Sci. Food Saf. 2017, 16, 39–58. [Google Scholar] [CrossRef]
- Zhang, Z.-s.; Kang, Y.-j.; Che, L. Composition and thermal characteristics of seed oil obtained from Chinese amaranth. LWT 2019, 111, 39–45. [Google Scholar] [CrossRef]
- Amare, E.; Grigoletto, L.; Corich, V.; Giacomini, A.; Lante, A. Fatty acid profile, lipid quality and squalene content of teff (Eragrostis teff (Zucc.) Trotter) and Amaranth (Amaranthus caudatus L.) varieties from Ethiopia. Appl. Sci. 2021, 11, 3590. [Google Scholar] [CrossRef]
- Gamel, T.H.; Linssen, J.P.; Mesallam, A.S.; Damir, A.A.; Shekib, L.A. Effect of seed treatments on the chemical composition of two amaranth species: Oil, sugars, fibres, minerals and vitamins. J. Sci. Food Agric. 2006, 86, 82–89. [Google Scholar] [CrossRef]
- Burisová, A.; Tomaskova, B.; Sasinková, V.; Ebringerová, A. Isolation and characterization of the non-starch polysaccharides of amaranth seeds. Chem. Pap. Slovak Acad. Sci. 2001, 55, 254–260. [Google Scholar]
- Wei, H.; Rui, J.; Yan, X.; Xu, R.; Chen, S.; Zhang, B.; Wang, L.; Zhang, Z.; Zhu, C.; Ma, M. Plant polyphenols as natural bioactives for alleviating lipid metabolism disorder: Mechanisms and application challenges. Food Res. Int. 2025, 203, 115682. [Google Scholar] [CrossRef] [PubMed]
- Gorinstein, S.; Pawelzik, E.; Delgado-Licon, E.; Haruenkit, R.; Weisz, M.; Trakhtenberg, S. Characterisation of pseudocereal and cereal proteins by protein and amino acid analyses. J. Sci. Food Agric. 2002, 82, 886–891. [Google Scholar] [CrossRef]
- Malik, M.; Sindhu, R.; Dhull, S.B.; Bou-Mitri, C.; Singh, Y.; Panwar, S.; Khatkar, B.S. Nutritional composition, functionality, and processing technologies for amaranth. J. Food Process. Preserv. 2023, 2023, 1753029. [Google Scholar] [CrossRef]
- Ventureira, J.L.; Bolontrade, A.J.; Speroni, F.; David-Briand, E.; Scilingo, A.A.; Ropers, M.-H.; Boury, F.; Añón, M.C.; Anton, M. Interfacial and emulsifying properties of amaranth (Amaranthus hypochondriacus) protein isolates under different conditions of pH. LWT-Food Sci. Technol. 2012, 45, 1–7. [Google Scholar] [CrossRef]
- Zheleznov, A.V.; Solonenko, L.; Zheleznova, N. Seed proteins of the wild and the cultivated Amaranthus species. Euphytica 1997, 97, 177–182. [Google Scholar] [CrossRef]
- Kadoshnikov, S.I.; Kadoshnikova, I.G.; Kulikov, Y.A.; Martirosyan, D.M. Researches of fractional composition of protein of amaranth. Curr. Nutr. Food Sci. 2008, 4, 196–205. [Google Scholar] [CrossRef]
- Kristinsson, H.G.; Rasco, B.A. Fish protein hydrolysates: Production, biochemical, and functional properties. Crit. Rev. Food Sci. Nutr. 2000, 40, 43–81. [Google Scholar] [CrossRef]
- Bressani, R.; Garcia-Vela, L.A. Protein fractions in amaranth grain and their chemical characterization. J. Agric. Food Chem. 1990, 38, 1205–1209. [Google Scholar] [CrossRef]
- Búcaro Segura, M.E.; Bressani, R. Distribución de la proteína en fracciones físicas de la molienda y tamizado del grano de amaranto. Arch. Latinoam. De Nutr. 2002, 52, 167–171. [Google Scholar]
- Maldonado-Cervantes, E.; Huerta-Ocampo, J.A.; Montero-Morán, G.M.; Barrera-Pacheco, A.; Espitia-Rangel, E.; de la Rosa, A.P.B. Characterization of Amaranthus cruentus L. seed proteins by 2-DE and LC/MS–MS: Identification and cloning of a novel late embryogenesis-abundant protein. J. Cereal Sci. 2014, 60, 172–178. [Google Scholar] [CrossRef]
- Huerta-Ocampo, J.A.; Barrera-Pacheco, A.; Mendoza-Hernández, C.S.; Espitia-Rangel, E.; Mock, H.-P.; Barba de la Rosa, A.P. Salt stress-induced alterations in the root proteome of Amaranthus cruentus L. J. Proteome Res. 2014, 13, 3607–3627. [Google Scholar] [CrossRef] [PubMed]
- Klubicová, K.; Szabová, M.; Skultety, L.; Libiaková, G.; Hricová, A. Revealing the seed proteome of the health benefitting grain amaranth (Amaranthus cruentus L.). Chem. Pap. 2016, 70, 1322–1335. [Google Scholar] [CrossRef]
- Nardo, A.E.; Suárez, S.; Quiroga, A.V.; Añón, M.C. Amaranth as a source of antihypertensive peptides. Front. Plant Sci. 2020, 11, 578631. [Google Scholar] [CrossRef]
- Bojórquez-Velázquez, E.; Zamora-Briseño, J.A.; Barrera-Pacheco, A.; Espitia-Rangel, E.; Herrera-Estrella, A.; Barba de la Rosa, A.P. Comparative Proteomic Analysis of Wild and Cultivated Amaranth Species Seeds by 2-DE and ESI-MS/MS. Plants 2024, 13, 2728. [Google Scholar] [CrossRef]
- Velarde-Salcedo, A.J.; Regalado-Rentería, E.; Velarde-Salcedo, R.; Juárez-Flores, B.I.; Barrera-Pacheco, A.; González de Mejía, E.; Barba De La Rosa, A.P. Consumption of amaranth induces the accumulation of the antioxidant protein paraoxonase/arylesterase 1 and modulates dipeptidyl peptidase iv activity in plasma of streptozotocin-induced hyperglycemic rats. J. Nutr. Nutr. 2018, 10, 181–193. [Google Scholar] [CrossRef]
- Bressani, R.; Gonzales, J.; Zuniga, J.; Breuner, M.; Elias, L. Yield, selected chemical composition and nutritive value of 14 selections of amaranth grain representing four species. J. Sci. Food Agric. 1987, 38, 347–356. [Google Scholar] [CrossRef]
- Sá, A.G.A.; Moreno, Y.M.F.; Carciofi, B.A.M. Plant proteins as high-quality nutritional source for human diet. Trends Food Sci. Technol. 2020, 97, 170–184. [Google Scholar] [CrossRef]
- Amare, E.; Mouquet-Rivier, C.; Servent, A.; Morel, G.; Adish, A.; Desse Haki, G. Protein quality of amaranth grains cultivated in Ethiopia as affected by popping and fermentation. Food Nutr. Sci. 2015, 6, 38–48. [Google Scholar] [CrossRef]
- Awasthi, T.; Singh, N.; Nishinari, K. Ultrasonic-assisted enhancement of malt characteristics in amaranth: A study on physicochemical, amino acid, sugar profile, rheological properties, and muffin making. Cereal Chem. 2025, 102, 34–52. [Google Scholar] [CrossRef]
- Motta, C.; Castanheira, I.; Gonzales, G.B.; Delgado, I.; Torres, D.; Santos, M.; Matos, A.S. Impact of cooking methods and malting on amino acids content in amaranth, buckwheat and quinoa. J. Food Compos. Anal. 2019, 76, 58–65. [Google Scholar] [CrossRef]
- Procopet, O.; Oroian, M. Amaranth seed polyphenol, fatty acid and amino acid profile. Appl. Sci. 2022, 12, 2181. [Google Scholar] [CrossRef]
- Tömösközi, S.; Baracskai, I.; Schönlechner, R.; Berghofer, E.; Läsztity, R. Comparative study of composition and technological quality of amaranth: I. Gross chemical composition, amino acid and mineral content. Acta Aliment. 2009, 38, 341–347. [Google Scholar] [CrossRef]
- Narwade, S.; Pinto, S. Amaranth—A functional food. Concepts Dairy Vet. Sci 2018, 1. [Google Scholar]
- Galili, G.; Amir, R. Fortifying plants with the essential amino acids lysine and methionine to improve nutritional quality. Plant Biotechnol. J. 2013, 11, 211–222. [Google Scholar] [CrossRef]
- Lahiri, A.; Rastogi, K.; Datta, A.; Septiningsih, E.M. Bayesian network analysis of lysine biosynthesis pathway in rice. Inventions 2021, 6, 37. [Google Scholar] [CrossRef]
- Berghofer, E.; Schoenlechner, R. Grain amaranth. In Pseudocereals Less Common Cereals; Springer: Berlin/Heidelberg, Germany, 2002; Volume 219. [Google Scholar]
- Rivero Meza, S.L.; Hirsch Ramos, A.; Cañizares, L.; Raphaelli, C.d.O.; Bueno Peres, B.; Gaioso, C.A.; Egea, I.; Estrada, Y.; Flores, F.B.; de Oliveira, M. A review on amaranth protein: Composition, digestibility, health benefits and food industry utilisation. Int. J. Food Sci. Technol. 2023, 58, 1564–1574. [Google Scholar] [CrossRef]
- Rastogi, A.; Shukla, S. Amaranth: A new millennium crop of nutraceutical values. Crit. Rev. Food Sci. Nutr. 2013, 53, 109–125. [Google Scholar] [CrossRef]
- Valcárcel-Yamani, B.; Lannes, S.d.S. Applications of quinoa (Chenopodium quinoa Willd.) and amaranth (Amaranthus spp.) and their influence in the nutritional value of cereal based foods. Food Public Health 2012, 2, 265–275. [Google Scholar]
- Ruales, J.; Nair, B.M. Nutritional quality of the protein in quinoa (Chenopodium quinoa, Willd) seeds. Plant Foods Hum. Nutr. 1992, 42, 1–11. [Google Scholar] [CrossRef]
- Pedersen, B.; Kalinowski, L.; Eggum, B. The nutritive value of amaranth grain (Amaranthus caudatus) 1. Protein and minerals of raw and processed grain. Plant Foods Hum. Nutr. 1987, 36, 309–324. [Google Scholar] [CrossRef]
- Sidorova, Y.S.; Petrov, N.A.; Perova, I.B.; Kolobanov, A.I.; Zorin, S.N. Physical and chemical characterization and bioavailability evaluation in vivo of Amaranth protein concentrate. Foods 2023, 12, 1728. [Google Scholar] [CrossRef]
- Tovar-Pérez, E.G.; Guerrero-Legarreta, I.; Farrés-González, A.; Soriano-Santos, J. Angiotensin I-converting enzyme-inhibitory peptide fractions from albumin 1 and globulin as obtained of amaranth grain. Food Chem. 2009, 116, 437–444. [Google Scholar] [CrossRef]
- Kamal, H.; Mudgil, P.; Bhaskar, B.; Fisayo, A.F.; Gan, C.-Y.; Maqsood, S. Amaranth proteins as potential source of bioactive peptides with enhanced inhibition of enzymatic markers linked with hypertension and diabetes. J. Cereal Sci. 2021, 101, 103308. [Google Scholar] [CrossRef]
- Velarde-Salcedo, A.J.; Barrera-Pacheco, A.; Lara-González, S.; Montero-Morán, G.M.; Díaz-Gois, A.; De Mejia, E.G.; De La Rosa, A.P.B. In vitro inhibition of dipeptidyl peptidase IV by peptides derived from the hydrolysis of amaranth (Amaranthus hypochondriacus L.) proteins. Food Chem. 2013, 136, 758–764. [Google Scholar] [CrossRef]
- Akin-Idowu, P.E.; Ademoyegun, O.T.; Olagunju, Y.O.; Aduloju, A.O.; Adebo, U.G. Phytochemical content and antioxidant activity of five grain amaranth species. Am. J. Food Sci. Technol 2017, 5, 249–255. [Google Scholar]
- Jahaniaval, F.; Kakuda, Y.; Marcone, M. Fatty acid and triacylglycerol compositions of seed oils of five Amaranthus accessions and their comparison to other oils. J. Am. Oil Chem. Soc. 2000, 77, 847–852. [Google Scholar] [CrossRef]
- Szabóová, M.; Záhorský, M.; Gažo, J.; Geuens, J.; Vermoesen, A.; D’Hondt, E.; Hricová, A. Differences in seed weight, amino acid, fatty acid, oil, and squalene content in γ-irradiation-developed and commercial amaranth varieties (Amaranthus spp.). Plants 2020, 9, 1412. [Google Scholar] [CrossRef]
- Wirkowska-Wojdyła, M.; Ostrowska-Ligęza, E.; Górska, A.; Bryś, J. Application of chromatographic and thermal methods to study fatty acids composition and positional distribution, oxidation kinetic parameters and melting profile as important factors characterizing amaranth and quinoa oils. Appl. Sci. 2022, 12, 2166. [Google Scholar] [CrossRef]
- Symoniuk, E.; Ratusz, K.; Krygier, K. Comparison of the oxidative stability of linseed (Linum usitatissimum L.) oil by pressure differential scanning calorimetry and Rancimat measurements. J. Food Sci. Technol. 2016, 53, 3986–3995. [Google Scholar] [CrossRef]
- Kraujalis, P.; Venskutonis, P.R. Supercritical carbon dioxide extraction of squalene and tocopherols from amaranth and assessment of extracts antioxidant activity. J. Supercrit. Fluids 2013, 80, 78–85. [Google Scholar] [CrossRef]
- Czaplicki, S.; Ogrodowska, D.; Zadernowski, R.; Derewiaka, D. Characteristics of biologically-active substances of amaranth oil obtained by various techniques. Pol. J. Food Nutr. Sci. 2012, 62, 235–239. [Google Scholar] [CrossRef]
- Mondor, M.; Melgar-Lalanne, G.; Hernández-Álvarez, A.-J. Cold pressed amaranth (Amaranthus tricolor) oil. In Cold Pressed Oils; Elsevier: Amsterdam, The Netherlands, 2020; pp. 113–127. [Google Scholar]
- Bozorov, S.S.; Berdiev, N.S.; Ishimov, U.J.; Olimjonov, S.S.; Ziyavitdinov, J.F.; Asrorov, A.M.; Salikhov, S.I. Chemical composition and biological activity of seed oil of amaranth varieties. Nova Biotechnol. Chim. 2018, 17, 66–73. [Google Scholar] [CrossRef]
- Becker, R.; Wheeler, E.; Lorenz, K.; Stafford, A.; Grosjean, O.; Betschart, A.; Saunders, R. A compositional study of amaranth grain. J. Food Sci. 1981, 46, 1175–1180. [Google Scholar] [CrossRef]
- Opute, F.I. Seed lipids of the grain amaranths. J. Exp. Bot. 1979, 30, 601–606. [Google Scholar] [CrossRef]
- Petkova, Z.Y.; Antova, G.; Angelova-Romova, M.; Vaseva, I.C. A comparative study on chemical and lipid composition of amaranth seeds with different origin. Bulg. Chem. Commun. 2019, 51, 262–267. [Google Scholar]
- Bialek, A.; Bialek, M.; Jelinska, M.; Tokarz, A. Fatty acid profile of new promising unconventional plant oils for cosmetic use. Int. J. Cosmet. Sci. 2016, 38, 382–388. [Google Scholar] [CrossRef]
- Villacrés, E.; Pástor, G.; Quelal, M.B.; Zambrano Villavicencio, I.; Morales, S. Effect of processing on the content of fatty acids, tocopherols and sterols in the oils of quinoa (Chenopodium quinoa Willd), lupine (Lupinus mutabilis Sweet), amaranth (Amaranthus caudatus L.) and sangorache (Amaranthus quitensis L.). Glob. Adv. Res. J. Food Sci. Tecnnol. 2013, 2, 44–53. [Google Scholar]
- Gómez, M.J.R.; Prieto, J.M.; Sobrado, V.C.; Magro, P.C. Nutritional characterization of six quinoa (Chenopodium quinoa Willd) varieties cultivated in Southern Europe. J. Food Compos. Anal. 2021, 99, 103876. [Google Scholar] [CrossRef]
- De Bock, P.; Daelemans, L.; Selis, L.; Raes, K.; Vermeir, P.; Eeckhout, M.; Van Bockstaele, F. Comparison of the chemical and technological characteristics of wholemeal flours obtained from amaranth (Amaranthus sp.), quinoa (Chenopodium quinoa) and buckwheat (Fagopyrum sp.) seeds. Foods 2021, 10, 651. [Google Scholar] [CrossRef]
- Radosavljević, M. Comparison of Amaranthus cruentus and Zea mays L. stach characteristics. Genetika 2006, 38, 31–36. [Google Scholar] [CrossRef]
- Inouchi, N.; Nishi, K.; Tanaka, S.; Asai, M.; Kawase, Y.; Hata, Y.; Konishi, Y.; Yue, S.; Fuwa, H. Characterization of amaranth and quinoa starches. J. Appl. Glycosci. 1999, 46, 233–240. [Google Scholar] [CrossRef]
- Januszewska-Jozwiak, K.; Synowiecki, J. Characteristics and suitability of amaranth components in food biotechnology. Biotechnologia 2008, 3, 89–102. [Google Scholar]
- Wolosik, K.; Markowska, A. Amaranthus cruentus taxonomy, botanical description, and review of its seed chemical composition. Nat. Prod. Commun. 2019, 14, 1934578X19844141. [Google Scholar] [CrossRef]
- Capriles, V.; Coelho, K.; Guerra-Matias, A.; Arêas, J. Effects of processing methods on amaranth starch digestibility and predicted glycemic index. J. Food Sci. 2008, 73, H160–H164. [Google Scholar] [CrossRef]
- Srichuwong, S.; Curti, D.; Austin, S.; King, R.; Lamothe, L.; Gloria-Hernandez, H. Physicochemical properties and starch digestibility of whole grain sorghums, millet, quinoa and amaranth flours, as affected by starch and non-starch constituents. Food Chem. 2017, 233, 1–10. [Google Scholar] [CrossRef]
- Repo-Carrasco-Valencia, R.A.-M.; Serna, L.A. Quinoa (Chenopodium quinoa, Willd.) as a source of dietary fiber and other functional components. Food Sci. Technol. 2011, 31, 225–230. [Google Scholar] [CrossRef]
- Joye, I.J. Dietary fibre from whole grains and their benefits on metabolic health. Nutrients 2020, 12, 3045. [Google Scholar] [CrossRef]
- Lamothe, L.M.; Srichuwong, S.; Reuhs, B.L.; Hamaker, B.R. Quinoa (Chenopodium quinoa W.) and amaranth (Amaranthus caudatus L.) provide dietary fibres high in pectic substances and xyloglucans. Food Chem. 2015, 167, 490–496. [Google Scholar] [CrossRef]
- Vidal Torres, E.; Valencia, E.; Simsek, S.; Ramírez, A.M.L. Amaranth: Multipurpose Agroindustrial Crop. Agronomy 2024, 14, 2323. [Google Scholar] [CrossRef]
- HozoVá, B.; Kuniak, L.; Moravcikova, P.; Gajdosova, A. Determination of water-insoluble beta-D-glucan in the whole-grain cereals and pseudocereals. Czech J. Food Sci. 2007, 25, 316. [Google Scholar] [CrossRef]
- Budin, J.T.; Breene, W.M.; Putnam, D.H. Some compositional properties of seeds and oils of eight Amaranthus species. J. Am. Oil Chem. Soc. 1996, 73, 475–481. [Google Scholar] [CrossRef]
- Havrlentová, M.; Petruláková, Z.; Burgárová, A.; Gago, F.; Hlinková, A.; Šturdík, E. β-glucans and their significance for the preparation of functional foods-a review. Czech J. Food Sci. 2011, 29, 1–14. [Google Scholar]
- Kraic, D. Natural sources of health-promoting starch. J. Food Nutr. Res. 2006, 45, 69–76. [Google Scholar]
- Liu, X.; Xu, F.; Yong, H.; Chen, D.; Tang, C.; Kan, J.; Liu, J. Recent advances in chitosan-based active and intelligent packaging films incorporated with flavonoids. Food Chem. X 2025, 25, 102200. [Google Scholar] [CrossRef]
- Bhat, A.; Satpathy, G.; Gupta, R.K. Evaluation of Nutraceutical properties of Amaranthus hypochondriacus L. grains and formulation of value added cookies. J. Pharmacogn. Phytochem. 2015, 3, 51–54. [Google Scholar]
- Nascimento, A.C.; Mota, C.; Coelho, I.; Gueifão, S.; Santos, M.; Matos, A.S.; Gimenez, A.; Lobo, M.; Samman, N.; Castanheira, I. Characterisation of nutrient profile of quinoa (Chenopodium quinoa), amaranth (Amaranthus caudatus), and purple corn (Zea mays L.) consumed in the North of Argentina: Proximates, minerals and trace elements. Food Chem. 2014, 148, 420–426. [Google Scholar] [CrossRef]
- Oh, Y.; Kim, J.; Cao, D.; Kim, C.; Boo, K. Analysis of the water-soluble vitamins B 2 and B 6 of crops in the Amaranthaceae family by HPLC-FLD. Int. Food Res. J. 2021, 28, 503–507. [Google Scholar] [CrossRef]
- Ogrodowska, D.; Tanska, M.; Brandt, W.; Czaplicki, S.; Skrajda, M.; Dabrowski, G. Effect of the drying process on the physicochemical characteristics and oxidative stability of microencapsulated amaranth oil. Riv. Ital. Delle Sostanze Grasse 2017, 94, 257–264. [Google Scholar]
- Lehmann, J.W.; Putnam, D.H.; Qureshi, A.A. Vitamin E isomers in grain amaranths (Amaranthus spp.). Lipids 1994, 29, 177–181. [Google Scholar] [CrossRef]
- Jiang, Q. Natural forms of vitamin E: Metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic. Biol. Med. 2014, 72, 76–90. [Google Scholar] [CrossRef]
- Xia, W.; Mo, H. Potential of tocotrienols in the prevention and therapy of Alzheimer’s disease. J. Nutr. Biochem. 2016, 31, 1–9. [Google Scholar] [CrossRef]
- Klimczak, I.; Małecka, M.; Pachołek, B. Antioxidant activity of ethanolic extracts of amaranth seeds. Food/Nahr. 2002, 46, 184–186. [Google Scholar] [CrossRef]
- Paśko, P.; Sajewicz, M.; Gorinstein, S.; Zachwieja, Z. Analysis of selected phenolic acids and flavonoids in Amaranthus cruentus and Chenopodium quinoa seeds and sprouts by HPLC. Acta Chromatogr. 2008, 20, 661–672. [Google Scholar]
- Steffensen, S.K.; Rinnan, Å.; Mortensen, A.G.; Laursen, B.; de Troiani, R.M.; Noellemeyer, E.J.; Janovska, D.; Dusek, K.; Délano-Frier, J.; Taberner, A. Variations in the polyphenol content of seeds of field grown Amaranthus genotypes. Food Chem. 2011, 129, 131–138. [Google Scholar] [CrossRef]
- Bunzel, M.; Ralph, J.; Steinhart, H. Association of non-starch polysaccharides and ferulic acid in grain amaranth (Amaranthus caudatus L.) dietary fiber. Mol. Nutr. Food Res. 2005, 49, 551–559. [Google Scholar] [CrossRef]
- Kalinova, J.; Dadakova, E. Rutin and total quercetin content in amaranth (Amaranthus spp.). Plant Foods Hum. Nutr. 2009, 64, 68–74. [Google Scholar] [CrossRef]
- Manyelo, T.G.; Sebola, N.A.; Hassan, Z.M.; Mabelebele, M. Characterization of the phenolic compounds in different plant parts of Amaranthus cruentus grown under cultivated conditions. Molecules 2020, 25, 4273. [Google Scholar] [CrossRef]
- Vollmannova, A.; Margitanova, E.; Tóth, T.; Timoracka, M.; Urminska, D.; Bojňanská, T.; Čičová, I. Cultivar Influence on Total Polyphenol and Rutin Contents and Total Antioxidant Capacity in Buckwheat, Amaranth, and Quinoa Seeds. Czech J. Food Sci. 2013, 31, 589–595. [Google Scholar] [CrossRef]
- Sala, M.; Berardi, S.; Bondioli, P. Amaranth seed: The potentialities. Riv. Ital. Delle Sostanze Grasse 1998, 75, 503. [Google Scholar]
- Kalač, P.; Moudrý, J. Composition and nutritional value of amaranth seeds. Czech J. Food Sci. 2000, 18, 201. [Google Scholar] [CrossRef]
- Alvarez-Jubete, L.; Arendt, E.K.; Gallagher, E. Nutritive value of pseudocereals and their increasing use as functional gluten-free ingredients. Trends Food Sci. Technol. 2010, 21, 106–113. [Google Scholar] [CrossRef]
- Taia, W.K.; Shehata, A.A.; Elshamy, E.M.; Ibrahim, M.M. Biosystematic studies for some Egyptian Amaranthus L. taxa and their significance in their identification. Taeckholmia 2020, 40, 85–99. [Google Scholar] [CrossRef]
- Ibrahim, N.I.; Fairus, S.; Zulfarina, M.S.; Naina Mohamed, I. The efficacy of squalene in cardiovascular disease risk-a systematic review. Nutrients 2020, 12, 414. [Google Scholar] [CrossRef]
- Ravi Kumar, S.; Narayan, B.; Sawada, Y.; Hosokawa, M.; Miyashita, K. Combined effect of astaxanthin and squalene on oxidative stress in vivo. Mol. Cell. Biochem. 2016, 417, 57–65. [Google Scholar] [CrossRef]
- Nergiz, C.; Çelikkale, D. The effect of consecutive steps of refining on squalene content of vegetable oils. J. Food Sci. Technol. 2011, 48, 382–385. [Google Scholar] [CrossRef]
- Lozano-Grande, M.A.; Gorinstein, S.; Espitia-Rangel, E.; Dávila-Ortiz, G.; Martínez-Ayala, A.L. Plant sources, extraction methods, and uses of squalene. Int. J. Agron. 2018, 2018, 1829160. [Google Scholar] [CrossRef]
- León-Camacho, M.; García-González, D.L.; Aparicio, R. A detailed and comprehensive study of amaranth (Amaranthus cruentus L.) oil fatty profile. Eur. Food Res. Technol. 2001, 213, 349–355. [Google Scholar] [CrossRef]
- Ciecierska, M.; Obiedziński, M. Polycyclic aromatic hydrocarbons in vegetable oils from unconventional sources. Food Control 2013, 30, 556–562. [Google Scholar] [CrossRef]
- Hussain, A.N.; Geuens, J.; Vermoesen, A.; Munir, M.; Iamonico, D.; Marzio, P.D.; Fortini, P. Characterization of seed oil from six in situ collected wild Amaranthus species. Diversity 2023, 15, 237. [Google Scholar] [CrossRef]
- Tang, Y.; Li, X.; Chen, P.X.; Zhang, B.; Liu, R.; Hernandez, M.; Draves, J.; Marcone, M.F.; Tsao, R. Assessing the fatty acid, carotenoid, and tocopherol compositions of amaranth and quinoa seeds grown in Ontario and their overall contribution to nutritional quality. J. Agric. Food Chem. 2016, 64, 1103–1110. [Google Scholar] [CrossRef]
- Magwele, M.; Satekge, T.; Mpai, S.; Beta, T.; Van Staden, J.; Ndhlala, A. Effects of Moringa-based phytostim biostimulant on the growth, nutritional composition, and antioxidant bioactivity of two amaranth species. South Afr. J. Bot. 2024, 173, 430–440. [Google Scholar] [CrossRef]
- Zhu, F. Amaranth proteins and peptides: Biological properties and food uses. Food Res. Int. 2023, 164, 112405. [Google Scholar] [CrossRef]
- Yang, Y.; Fukui, R.; Jia, H.; Kato, H. Amaranth supplementation improves hepatic lipid dysmetabolism and modulates gut microbiota in mice fed a high-fat diet. Foods 2021, 10, 1259. [Google Scholar] [CrossRef]
- Olagunju, A.I.; Arigbede, T.I.; Makanjuola, S.A.; Oyebode, E.T. Nutritional compositions, bioactive properties, and in-vivo glycemic indices of amaranth-based optimized multigrain snack bar products. Meas. Food 2022, 7, 100039. [Google Scholar] [CrossRef]
- Sabbione, A.C.; Ogutu, F.O.; Scilingo, A.; Zhang, M.; Añón, M.C.; Mu, T.-H. Antiproliferative effect of amaranth proteins and peptides on HT-29 human colon tumor cell line. Plant Foods Hum. Nutr. 2019, 74, 107–114. [Google Scholar] [CrossRef]
- Mazorra-Carrillo, J.L.; De León-Rodríguez, A.; Huerta-Ocampo, J.A.; Velarde-Salcedo, A.J.; de Mejía, E.G.; de la Rosa, A.P.B. Proteomic analysis of chemically transformed NIH-3T3 cells reveals novel mechanisms of action of amaranth lunasin-like peptide. Food Res. Int. 2022, 157, 111374. [Google Scholar] [CrossRef]
- Taniya, M.; Reshma, M.; Shanimol, P.; Krishnan, G.; Priya, S. Bioactive peptides from amaranth seed protein hydrolysates induced apoptosis and antimigratory effects in breast cancer cells. Food Biosci. 2020, 35, 100588. [Google Scholar] [CrossRef]
- Suárez, S.E.; Rabesona, H.; Ménard, O.; Jardin, J.; Anton, M.; Añón, M.C. Dynamic digestion of a high protein beverage based on amaranth: Structural changes and antihypertensive activity. Food Res. Int. 2024, 187, 114416. [Google Scholar] [CrossRef]
- Cruz-Casas, D.E.; Ramos-González, R.; Prado-Barragán, L.A.; Iliná, A.; Aguilar, C.N.; Rodríguez-Herrera, R.; Tsopmo, A.; Flores-Gallegos, A.C. Protein hydrolysates with ACE-I inhibitory activity from amaranth seeds fermented with Enterococcus faecium-LR9: Identification of peptides and molecular docking. Food Chem. 2025, 464, 141598. [Google Scholar] [CrossRef] [PubMed]
- Ajayi, F.F.; Mudgil, P.; Gan, C.-Y.; Maqsood, S. Identification and characterization of cholesterol esterase and lipase inhibitory peptides from amaranth protein hydrolysates. Food Chem. X 2021, 12, 100165. [Google Scholar]
- Wu, T.; Gao, Y.; Hao, J.; Yin, J.; Li, W.; Geng, J.; Liu, R.; Sui, W.; Zhang, M. Lycopene, amaranth, and sorghum red pigments counteract obesity and modulate the gut microbiota in high-fat diet fed C57BL/6 mice. J. Funct. Foods 2019, 60, 103437. [Google Scholar] [CrossRef]
- Calva-Cruz, O.d.J.; Ovando-Vázquez, C.; De León-Rodríguez, A.; Veana, F.; Espitia-Rangel, E.; Treviño, S.; Barba-de la Rosa, A.P. Dietary supplementation with popped amaranth modulates the gut microbiota in low height-for-age children: A nonrandomized pilot trial. Foods 2023, 12, 2760. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Sheikh, M.A.; Mir, N.A. Exploring the potential of amaranth proteins: Composition, functional characteristics, modifications, bioactive peptides and possible applications in food and packaging industries. J. Food Compos. Anal. 2024, 139, 107146. [Google Scholar] [CrossRef]
- Olagunju, A.I.; Arigbede, T.I.; Oyeleye, I.S.; Makanjuola, S.A.; Oyebode, E.T.; Enikuomehin, A.C. High-protein, low glycemic index snack from optimized blend of three wholegrains exhibits nutraceutical quality and elicits low glycemic response in diabetic human subjects. Food Prod. Process. Nutr. 2024, 6, 32. [Google Scholar] [CrossRef]
- Tarhan, İ. A new and rapid analysis method for the most important herbal squalene source: Comparison of UV–visible, fluorescence, and FTIR techniques for the quantification of squalene in amaranth seed oil. Microchem. J. 2021, 168, 106446. [Google Scholar] [CrossRef]
- Wejnerowska, G.; Heinrich, P.; Gaca, J. Separation of squalene and oil from Amaranthus seeds by supercritical carbon dioxide. Sep. Purif. Technol. 2013, 110, 39–43. [Google Scholar] [CrossRef]
- Suárez, S.; Aphalo, P.; Rinaldi, G.; Añón, M.C.; Quiroga, A. Effect of amaranth proteins on the RAS system. In vitro, in vivo and ex vivo assays. Food Chem. 2020, 308, 125601. [Google Scholar] [CrossRef]
- Ontiveros, N.; López-Teros, V.; de Jesús Vergara-Jiménez, M.; Islas-Rubio, A.R.; Cárdenas-Torres, F.I.; Cuevas-Rodríguez, E.-O.; Reyes-Moreno, C.; Granda-Restrepo, D.M.; Lopera-Cardona, S.; Ramírez-Torres, G.I. Amaranth-hydrolyzate enriched cookies reduce the systolic blood pressure in spontaneously hypertensive rats. J. Funct. Foods 2020, 64, 103613. [Google Scholar] [CrossRef]
- Shafreen, R.B.; Seema, S.; Martinez-Ayala, A.L.; Lozano-Grande, M.A.; Robles-Sánchez, M.; Szterk, A.; Grishko, M.; Hanuka, E.; Katrich, E.; Gorinstein, S. Binding and potential antibiofilm activities of Amaranthus proteins against Candida albicans. Colloids Surf. B Biointerfaces 2019, 183, 110479. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Sicairos, E.S.; Milán-Noris, A.K.; Luna-Vital, D.A.; Milán-Carrillo, J.; Montoya-Rodríguez, A. Anti-inflammatory and antioxidant effects of peptides released from germinated amaranth during in vitro simulated gastrointestinal digestion. Food Chem. 2021, 343, 128394. [Google Scholar] [CrossRef] [PubMed]
- Moronta, J.; Smaldini, P.L.; Docena, G.H.; Añón, M.C. Peptides of amaranth were targeted as containing sequences with potential anti-inflammatory properties. J. Funct. Foods 2016, 21, 463–473. [Google Scholar] [CrossRef]
- Zhao, Q.; Fan, Y.; Zhao, L.; Zhu, Y.; Jiang, Y.; Gu, J.; Xue, Y.; Hao, Z.; Shen, Q. Identification and molecular binding mechanism of novel pancreatic lipase and cholesterol esterase inhibitory peptides from heat-treated adzuki bean protein hydrolysates. Food Chem. 2024, 439, 138129. [Google Scholar] [CrossRef]
- Mendonça, S.; Saldiva, P.H.; Cruz, R.J.; Arêas, J.A. Amaranth protein presents cholesterol-lowering effect. Food Chem. 2009, 116, 738–742. [Google Scholar] [CrossRef]
- Soares, R.A.M.; Mendonça, S.; De Castro, L.Í.A.; Menezes, A.C.C.C.C.; Arêas, J.A.G. Major peptides from amaranth (Amaranthus cruentus) protein inhibit HMG-CoA reductase activity. Int. J. Mol. Sci. 2015, 16, 4150–4160. [Google Scholar] [CrossRef]
- Bhuiya, S.; Kaushik, S.; Prathiviraj, R.; Selvin, J.; Kiran, G.S. Dietary additives interloping with the epigenetic network and human health: Implications in gut microbiota and endocrine toxicity. Food Biosci. 2025, 64, 105903. [Google Scholar] [CrossRef]
- Mishima, E.; Fukuda, S.; Mukawa, C.; Yuri, A.; Kanemitsu, Y.; Matsumoto, Y.; Akiyama, Y.; Fukuda, N.N.; Tsukamoto, H.; Asaji, K. Evaluation of the impact of gut microbiota on uremic solute accumulation by a CE-TOFMS–based metabolomics approach. Kidney Int. 2017, 92, 634–645. [Google Scholar] [CrossRef]
- Sabbione, A.C.; Bengoa, A.A.; Garrote, G.L.; Scilingo, A.; Añón, M.C.; Abraham, A.G. Amaranth fiber acts as fermentable substrate for children’s fecal microbiota. Bioact. Carbohydr. Diet. Fibre 2024, 32, 100447. [Google Scholar] [CrossRef]
- Zeyneb, H.; Pei, H.; Cao, X.; Wang, Y.; Win, Y.; Gong, L. In vitro study of the effect of quinoa and quinoa polysaccharides on human gut microbiota. Food Sci. Nutr. 2021, 9, 5735–5745. [Google Scholar] [CrossRef]
- Dias-Martins, A.M.; Pessanha, K.L.F.; Pacheco, S.; Rodrigues, J.A.S.; Carvalho, C.W.P. Potential use of pearl millet (Pennisetum glaucum (L.) R. Br.) in Brazil: Food security, processing, health benefits and nutritional products. Food Res. Int. 2018, 109, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Escudero, N.; De Arellano, M.; Luco, J.; Giménez, M.; Mucciarelli, S. Comparison of the chemical composition and nutritional value of Amaranthus cruentus flour and its protein concentrate. Plant Foods Hum. Nutr. 2004, 59, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Laisk, A.; Edwards, G.E. Oxygen and electron flow in C 4 photosynthesis: Mehler reaction, photorespiration and CO2 concentration in the bundle sheath. Planta 1998, 205, 632–645. [Google Scholar] [CrossRef]
- Roučková, J.; Trčková, M.; Herzig, I. The use of amaranth grain in diets for broiler chickens and its effect on performance and selected biochemical indicators. Czech J. Anim. Sci. 2004, 49, 532–541. [Google Scholar] [CrossRef]
- Schnetzler, K.A. Food uses and amaranth product research: A comprehensive review. In Amaranth Biology, Chemistry and Technology; CRC Press: Boca Raton, FL, USA, 2018; pp. 155–184. [Google Scholar]
- Sanna, S.; van Zuydam, N.R.; Mahajan, A.; Kurilshikov, A.; Vich Vila, A.; Võsa, U.; Mujagic, Z.; Masclee, A.A.; Jonkers, D.M.; Oosting, M. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat. Genet. 2019, 51, 600–605. [Google Scholar] [CrossRef]
- Kumar, J.; Rani, K.; Datt, C. Molecular link between dietary fibre, gut microbiota and health. Mol. Biol. Rep. 2020, 47, 6229–6237. [Google Scholar] [CrossRef]
- Mudgil, P.; Omar, L.S.; Kamal, H.; Kilari, B.P.; Maqsood, S. Multi-functional bioactive properties of intact and enzymatically hydrolysed quinoa and amaranth proteins. LWT 2019, 110, 207–213. [Google Scholar] [CrossRef]
- Sayed-Ahmad, B.; Urrutigoïty, M.; Hijazi, A.; Saad, Z.; Cerny, M.; Evon, P.; Talou, T.; Merah, O. Amaranth oilseed composition and cosmetic applications. Separations 2022, 9, 181. [Google Scholar] [CrossRef]
- Zamaratskaia, G.; Omar, N.A.M.; Brunius, C.; Hallmans, G.; Johansson, J.-E.; Andersson, S.-O.; Larsson, A.; Åman, P.; Landberg, R. Consumption of whole grain/bran rye instead of refined wheat decrease concentrations of TNF-R2, e-selectin, and endostatin in an exploratory study in men with prostate cancer. Clin. Nutr. 2020, 39, 159–165. [Google Scholar] [CrossRef]
- Maldonado-Cervantes, E.; Jeong, H.J.; León-Galván, F.; Barrera-Pacheco, A.; De León-Rodríguez, A.; De Mejia, E.G.; De Lumen, B.O.; De La Rosa, A.P.B. Amaranth lunasin-like peptide internalizes into the cell nucleus and inhibits chemical carcinogen-induced transformation of NIH-3T3 cells. Peptides 2010, 31, 1635–1642. [Google Scholar] [CrossRef]
- Silva-Sánchez, C.; De La Rosa, A.B.; León-Galván, M.F.; de Lumen, B.O.; de León-Rodríguez, A.; De Mejía, E.G. Bioactive peptides in amaranth (Amaranthus hypochondriacus) seed. J. Agric. Food Chem. 2008, 56, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Rajamani, K.; Thirugnanasambandan, S.S.; Natesan, C.; Subramaniam, S.; Thangavel, B.; Aravindan, N. Squalene deters drivers of RCC disease progression beyond VHL status. Cell Biol. Toxicol. 2021, 37, 611–631. [Google Scholar] [CrossRef] [PubMed]
- Pili, B.; Bourgaux, C.; Amenitsch, H.; Keller, G.; Lepêtre-Mouelhi, S.; Desmaële, D.; Couvreur, P.; Ollivon, M. Interaction of a new anticancer prodrug, gemcitabine–squalene, with a model membrane: Coupled DSC and XRD study. Biochim. Biophys. Acta (BBA)-Biomembr. 2010, 1798, 1522–1532. [Google Scholar] [CrossRef] [PubMed]
- Tatewaki, N.; Konishi, T.; Nakajima, Y.; Nishida, M.; Saito, M.; Eitsuka, T.; Sakamaki, T.; Ikekawa, N.; Nishida, H. Squalene inhibits ATM-dependent signaling in γIR-induced DNA damage response through induction of Wip1 phosphatase. PLoS ONE 2016, 11, e0147570. [Google Scholar] [CrossRef]
- Pandey, D.; Bharti, M.; Rana, A.; Prabhakaran, S.; Chauhan, R. Molecular Docking of Phytomolecules of Grain Amaranth (Amaranthus hypochondriacus) with AKR1C3Protein Involved in Prostate Cancer in Human Beings. Lett. Org. Chem. 2024, 21, 677–686. [Google Scholar] [CrossRef]
- Aljuraiban, G.S.; Gibson, R.; Chan, D.S.; Van Horn, L.; Chan, Q. The role of diet in the prevention of hypertension and management of blood pressure: An umbrella review of meta-analyses of interventional and observational studies. Adv. Nutr. 2024, 15, 100123. [Google Scholar] [CrossRef]
- Gómez-Cardona, E.E.; Hernández-Domínguez, E.E.; Huerta-Ocampo, J.Á.; Jiménez-Islas, H.; Díaz-Gois, A.; Velarde-Salcedo, A.J.; Barrera-Pacheco, A.; Goñi-Ochoa, A.; de la Rosa, A.P.B. Effect of amaranth consumption on diabetes-related biomarkers in patients with diabetes. Diabetes Obes. Metab. Disord. 2017, 3, 5–10. [Google Scholar]
- Daskaya-Dikmen, C.; Yucetepe, A.; Karbancioglu-Guler, F.; Daskaya, H.; Ozcelik, B. Angiotensin-I-converting enzyme (ACE)-inhibitory peptides from plants. Nutrients 2017, 9, 316. [Google Scholar] [CrossRef]
- Diao, M.; Li, Z.; Zhou, R.; Yan, X.; Zhang, T. The combined antimicrobial activity of α-lactalbumin and thymol against Escherichia coli and Staphylococcus aureus. Food Chem. 2025, 473, 143048. [Google Scholar] [CrossRef]
- Jahan, F.; Bhuiyan, M.N.H.; Islam, M.J.; Ahmed, S.; Hasan, M.S.; Al Bashera, M.; Waliullah, M.; Chowdhury, A.N.; Islam, M.B.; Saha, B.K. Amaranthus tricolor (red amaranth), an indigenous source of nutrients, minerals, amino acids, phytochemicals, and assessment of its antibacterial activity. J. Agric. Food Res. 2022, 10, 100419. [Google Scholar] [CrossRef]
- Choudhary, R.; Kumar, P.; Shukla, S.K.; Bhagat, A.; Anal, J.M.H.; Kour, G.; Ahmed, Z. Synthesis and potential anti-inflammatory response of indole and amide derivatives of ursolic acid in LPS-induced RAW 264.7 cells and systemic inflammation mice model: Insights into iNOS, COX2 and NF-κB. Bioorganic Chem. 2025, 155, 108091. [Google Scholar]
- Lamichhane, G.; Pandeya, P.R.; Lamichhane, R.; Yun, H.D.; Shrivastava, A.K.; Cheon, J.-y.; Sapkota, B.; Devkota, H.P.; Jung, H.-J. Evaluation of anti-inflammatory potential of extract, fractions and major compounds of Ponciri Fructus in LPS-induced RAW 264.7 cells. Curr. Res. Biotechnol. 2023, 6, 100138. [Google Scholar]
- Soliman, G.A. Dietary fiber, atherosclerosis, and cardiovascular disease. Nutrients 2019, 11, 1155. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.; Monnard, I.; Dionisi, F.; Gumy, D.; Hayes, K.; Lambelet, P. Cholesterol-lowering properties of amaranth flakes, crude and refined oils in hamsters. Food Chem. 2003, 81, 119–124. [Google Scholar]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.-P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P. Obesity and cardiovascular disease: A scientific statement from the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef]
- Yannakoulia, M.; Poulimeneas, D.; Mamalaki, E.; Anastasiou, C.A. Dietary modifications for weight loss and weight loss maintenance. Metabolism 2019, 92, 153–162. [Google Scholar] [CrossRef]
- Patil, N.D.; Bains, A.; Chawla, P. Amaranth. Cereals and Nutraceuticals; Springer: Berlin/Heidelberg, Germany, 2024; pp. 251–284. [Google Scholar]
- Borges-Martínez, J.E.; del Rosario Moguel-Concha, D.; Cid-Gallegos, M.S.; Gómez-Gómez, A.L.; Téllez-Medina, D.I.; Jiménez-Martínez, C. Amaranth grain: Nutritional composition, bioactive compounds, processing, and applications. In Improving Health and Nutrition Through Functional Foods; Elsevier: Amsterdam, The Netherlands, 2025; pp. 67–81. [Google Scholar]
- Woo, S.-H.; Park, J.; Sung, J.M.; Choi, E.-J.; Park, J.-D.; Park, E.Y. Effect of Amaranth Protein Isolate and Fermentation Time on Buckwheat Sourdough Bread. LWT 2025, 217, 117325, Available at SSRN 4969998. [Google Scholar] [CrossRef]
- Vici, G.; Belli, L.; Biondi, M.; Polzonetti, V. Gluten free diet and nutrient deficiencies: A review. Clin. Nutr. 2016, 35, 1236–1241. [Google Scholar]
- López-Alonso, W.M.; Gallegos-Martínez, J.; Reyes-Hernández, J. Impact of a nutritional intervention based on amaranth flour consumption to recovery undernourished children. Curr. Res. Nutr. Food Sci. J. 2021, 9, 222–232. [Google Scholar] [CrossRef]
- Okoth, J.K. Impact of an amaranth-sorghum grains product on nutrient intake of children aged 6–23 months in Kiandutu Slums, Thika, Kenya. J. Food Nutr. Sci. Res. (JFNSR) 2020, 2, 64–93. [Google Scholar]
- Jiménez, D.; Lobo, M.; Irigaray, B.; Grompone, M.A.; Sammán, N. Oxidative stability of baby dehydrated purees formulated with different oils and germinated grain flours of quinoa and amaranth. LWT 2020, 127, 109229. [Google Scholar] [CrossRef]
- Jan, N.; Hussain, S.Z.; Naseer, B.; Bhat, T.A. Amaranth and quinoa as potential nutraceuticals: A review of anti-nutritional factors, health benefits and their applications in food, medicinal and cosmetic sectors. Food Chem. X 2023, 18, 100687. [Google Scholar] [CrossRef] [PubMed]
- Shahbaz, M.; Raza, N.; Islam, M.; Imran, M.; Ahmad, I.; Meyyazhagan, A.; Pushparaj, K.; Balasubramanian, B.; Park, S.; Rengasamy, K.R. The nutraceutical properties and health benefits of pseudocereals: A comprehensive treatise. Crit. Rev. Food Sci. Nutr. 2023, 63, 10217–10229. [Google Scholar] [CrossRef] [PubMed]
- Añón, M.C.; Quiroga, A.V.; Scilingo, A.A.; Tironi, V.A.; Sabbione, A.C.; Nardo, A.E.; Suárez, S.E.; Fillería, S.F.G. Action of amaranth peptides on the cardiovascular system. In Native Crops in Latin America; CRC Press: Boca Raton, FL, USA, 2022; pp. 209–236. [Google Scholar]
- Manassero, C.A.; Añón, M.C.; Speroni, F. Development of a high protein beverage based on amaranth. Plant Foods Hum. Nutr. 2020, 75, 599–607. [Google Scholar] [CrossRef]
- Rodríguez, M.; Tironi, V.A. Chemical and cell antioxidant activity of amaranth flour and beverage after simulated gastrointestinal digestion. Role of peptides. Food Res. Int. 2023, 173, 113410. [Google Scholar] [CrossRef]
- Peñalver, R.; Nieto, G. Developing a functional gluten-free sourdough bread by incorporating quinoa, amaranth, rice and spirulina. LWT 2024, 201, 116162. [Google Scholar] [CrossRef]
- Poshadri, A.; Deshpande, H.; Machewad, G.; Kshirsagar, R.; Gadhe, K.; Kadam, S. Functional properties of selected composite gluten-free pseudocereals flour. Food Humanit. 2023, 1, 1200–1205. [Google Scholar] [CrossRef]
- Habib, H.; Kumar, A.; Amin, T.; Bhat, T.A.; Aziz, N.; Rasane, P.; Ercisli, S.; Singh, J. Process optimization, growth kinetics, and antioxidant activity of germinated buckwheat and amaranth-based yogurt mimic. Food Chem. 2024, 457, 140138. [Google Scholar] [CrossRef]
- Lux, T.; Spillmann, F.; Reimold, F.; Erdös, A.; Lochny, A.; Flöter, E. Physical quality of gluten-free doughs and fresh pasta made of amaranth. Food Sci. Nutr. 2023, 11, 3213–3223. [Google Scholar]
- Ayeni, F.A.; Malomo, A.A.; Ikujenlola, A.V. Physicochemical Characteristics of Biscuits Produced from Gluten-Free Amaranth Seed and Tiger Nut Composite Flour. Acta Univ. Cinbinesis Ser. E Food Technol. 2024, 28, 93–104. [Google Scholar] [CrossRef]
- Omoba, O.S.; Olagunju, A.I.; Oluwajuyitan, T.D.; Akinrinlola, O.F. Functional extruded snacks from amaranth, soycake and shallot flours: Nutritional composition, physicochemical and antioxidant properties. Meas. Food 2024, 15, 100194. [Google Scholar] [CrossRef]
- UNICEF. The Extension of the 2025 Maternal, Infant and Young Child Nutrition Targets to 2030; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Bochnak-Niedźwiecka, J.; Szymanowska, U.; Świeca, M. The Protein-Rich Powdered Beverages Stabilized with Flax Seeds Gum—Antioxidant and Antiproliferative Properties of the Potentially Bioaccessible Fraction. Appl. Sci. 2022, 12, 7159. [Google Scholar] [CrossRef]
- Cruz-Casas, D.E.; Aguilar, C.N.; Ascacio-Valdés, J.A.; Rodríguez-Herrera, R.; Chávez-González, M.L.; Flores-Gallegos, A.C. Bioactive protein hydrolysates obtained from amaranth by fermentation with lactic acid bacteria and Bacillus species. Heliyon 2023, 9, 7159. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Zhang, Y. Biopolymer-based encapsulation of anthocyanins as reinforced natural colorants for food applications. J. Agric. Food Res. 2023, 11, 100488. [Google Scholar] [CrossRef]
- Li, S.; Hao, Y.; Gao, Q. Emulsion gels stabilized by cyclodextrin inclusion/chitosan complexes with varying OSA substitution degree for co-encapsulation of bioactive compounds. Food Chem. 2025, 474, 143086. [Google Scholar] [CrossRef]
- Atma, Y.; Sadeghpour, A.; Murray, B.S.; Goycoolea, F.M. Chitosan-alginate polyelectrolyte complexes for encapsulation of low molecular weight fish bioactive peptides. Food Hydrocoll. 2025, 160, 110789. [Google Scholar] [CrossRef]
- De Kruif, C.G.; Weinbreck, F.; de Vries, R. Complex coacervation of proteins and anionic polysaccharides. Curr. Opin. Colloid Interface Sci. 2004, 9, 340–349. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Akanbi, T.O.; Khalid, N.; Adhikari, B.; Barrow, C.J. Complex coacervation: Principles, mechanisms and applications in microencapsulation. Int. J. Biol. Macromol. 2019, 121, 1276–1286. [Google Scholar] [CrossRef]
- Delaporte, A.; Paraskevopoulou, A.; Grisel, M.; Gore, E. Animal-free coacervates: The combination of fungal chitosan-gum Arabic for the encapsulation of lipophilic compounds. Int. J. Biol. Macromol. 2025, 299, 140003. [Google Scholar] [CrossRef]
- Mao, Z.; Li, F.; Qiao, X.; Zhou, Q.; Yang, L.; Liu, Y.; Wang, X.; Xu, J.; Xue, C. Chitosan/octenyl succinic anhydride starch complex particles stabilize Pickering emulsion for astaxanthin encapsulation. Int. J. Biol. Macromol. 2025, 299, 140056. [Google Scholar] [CrossRef]
- Coelho, L.M.; Gonçalves, I.; Ferreira, P.; Pinheiro, A.C.; Vicente, A.A.; Martins, J.T. Exploring the performance of amaranth grain starch and protein microcapsules as β-carotene carrier systems for food applications. Food Struct. 2022, 33, 100287. [Google Scholar] [CrossRef]
- Constantino, A.B.T.; Garcia-Rojas, E.E. Microencapsulation of betanin by complex coacervation of carboxymethylcellulose and amaranth protein isolate for application in edible gelatin films. Food Hydrocoll. 2022, 133, 107956. [Google Scholar] [CrossRef]
- Constantino, A.B.T.; Garcia-Rojas, E.E. Microencapsulation of beta-carotene by complex coacervation using amaranth carboxymethyl starch and lactoferrin for application in gummy candies. Food Hydrocoll. 2023, 139, 108488. [Google Scholar] [CrossRef]
- Constantino, A.B.T.; Garcia-Rojas, E.E. Vitamin D3 microcapsules formed by heteroprotein complexes obtained from amaranth protein isolates and lactoferrin: Formation, characterization, and bread fortification. Food Hydrocoll. 2022, 129, 107636. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, L.; Hu, Z.; Guan, X.; Chen, Y.; Xu, M.; Chen, X.; Bu, N.; Duan, J.; Liu, W. Konjac glucomannan/zein active film loaded with tea polyphenol–ferric nanoparticles for strawberry preservation. Int. J. Biol. Macromol. 2025, 299, 139905. [Google Scholar] [CrossRef]
- Condés, M.C.; Añón, M.C.; Mauri, A.N. Amaranth protein films from thermally treated proteins. J. Food Eng. 2013, 119, 573–579. [Google Scholar] [CrossRef]
- Tapia-Blacido, D.; Sobral, P.; Menegalli, F.C. Effects of drying temperature and relative humidity on the mechanical properties of amaranth flour films plasticized with glycerol. Braz. J. Chem. Eng. 2005, 22, 249–256. [Google Scholar] [CrossRef]
- Chandla, N.K.; Saxena, D.C.; Singh, S. Amaranth (Amaranthus spp.) starch isolation, characterization, and utilization in development of clear edible films. J. Food Process. Preserv. 2017, 41, e13217. [Google Scholar] [CrossRef]
- Morales-Olán, G.; Ríos-Corripio, M.A.; Hernández-Cázares, A.S.; Zaca-Morán, P.; Luna-Suárez, S.; Rojas-López, M. Effect of chitosan nanoparticles incorporating antioxidants from Salvia hispanica L. on the amaranth flour films. Food Technol. Biotechnol. 2022, 60, 52–66. [Google Scholar] [CrossRef]
- Coelho, L.M.; Faria, C.; Madalena, D.; Genisheva, Z.; Martins, J.T.; Vicente, A.A.; Pinheiro, A.C. Valorization of amaranth (Amaranthus cruentus) grain extracts for the development of alginate-based active films. Molecules 2022, 27, 5798. [Google Scholar] [CrossRef]
- Yadav, P.; Gautam, S.; Bosco, S.J.D. Amaranthus paniculatus (Rajgeera) a non-conventional source of starch: Effect of oxidation and heat moisture treatment and its application in edible film. Biomass Convers. Biorefinery 2024, 14, 23733–23741. [Google Scholar] [CrossRef]
- Menezes, I.M.R.; Nascimento, P.d.A.; Peixoto, R.R.; Oliveira, A. Nutritional profile and risk assessment of inorganic elements in enteral and parenteral nutrition formulas. J. Trace Elem. Med. Biol. 2024, 84, 127442. [Google Scholar]
- Yeh, A.; Conners, E.M.; Ramos-Jimenez, R.G.; Firek, B.; Novak, E.A.; Rogers, M.B.; Cheek, R.; Ozolek, J.; Mollen, K.P.; Morowitz, M.J. Plant-based enteral nutrition modifies the gut microbiota and improves outcomes in murine models of colitis. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 872–874.e876. [Google Scholar] [CrossRef] [PubMed]
- Hertzler, S.; Cassady, B.A.; Patil, S.; Haselberger, P. Protein Quality Estimations for Plant-Based Enteral Formulas. Curr. Dev. Nutr. 2024, 8, 102346. [Google Scholar] [CrossRef]
- McClanahan, D.; Yeh, A.; Firek, B.; Zettle, S.; Rogers, M.; Cheek, R.; Nguyen, M.V.; Gayer, C.P.; Wendell, S.G.; Mullett, S.J. Pilot study of the effect of plant-based enteral nutrition on the gut microbiota in chronically ill tube-fed children. J. Parenter. Enter. Nutr. 2019, 43, 899–911. [Google Scholar] [CrossRef]
- van Eck, E.B.; Hofman, Z.; van Eijnatten, E.J.; Knol, J.; Renes, I.B.; Abrahamse, E. Plant protein dominant enteral nutrition, containing soy and pea, is non-coagulating after gastric digestion in contrast to casein dominant enteral nutrition. Food Res. Int. 2024, 197, 115162. [Google Scholar] [CrossRef]
- Varela-Ortega, C.; Blanco-Gutiérrez, I.; Manners, R.; Detzel, A. Life cycle assessment of animal-based foods and plant-based protein-rich alternatives: A socio-economic perspective. J. Sci. Food Agric. 2022, 102, 5111–5120. [Google Scholar] [CrossRef]
- Force, A.o.D.a.C.T. Guidelines for the use of parenteral and enteral nutrition in adult and pediatric patients. JPEN J. Parenter Enter. Nutr 2002, 26, 1SA–138SA. [Google Scholar]
- Mirtallo, J.; Johnson, D.; Kumpf, V. Safe practices for parenteral nutrition. JPEN J. Parenter. Enter. Nutr. 2004, 28, S39. [Google Scholar] [CrossRef]
- McClave, S.A.; Martindale, R.G.; Vanek, V.W.; McCarthy, M.; Roberts, P.; Taylor, B.; Ochoa, J.B.; Napolitano, L.; Cresci, G.; Directors, A.B.o. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN). J. Parenter. Enter. Nutr. 2009, 33, 277–316. [Google Scholar] [CrossRef]
- Goelen, N.; Janssen, P.; Ripken, D.; van Horssen, P.; Byloos, K.; Ghysels, S.; Putzeys, G.; Hofman, Z.; Vandecaveye, V.; Tack, J. Effect of protein composition of enteral formula on gastric content volume during continuous feeding: A randomized controlled cross-over study in healthy adults. Clin. Nutr. 2021, 40, 2663–2672. [Google Scholar] [CrossRef] [PubMed]
- Kuyumcu, S.; Menne, D.; Curcic, J.; Goetze, O.; Klebach, M.; Abrahamse, E.; Hofman, Z.; Fried, M.; Schwizer, W.; Steingoetter, A. Noncoagulating enteral formula can empty faster from the stomach: A double-blind, randomized crossover trial using magnetic resonance imaging. J. Parenter. Enter. Nutr. 2015, 39, 544–551. [Google Scholar] [CrossRef]
- Karamać, M.; Gai, F.; Longato, E.; Meineri, G.; Janiak, M.A.; Amarowicz, R.; Peiretti, P.G. Antioxidant activity and phenolic composition of amaranth (Amaranthus caudatus) during plant growth. Antioxidants 2019, 8, 173. [Google Scholar] [CrossRef] [PubMed]
- Pavlik, V. The revival of amaranth as a third-millennium food. Neuroendocrinol. Lett. 2012, 33, 3. [Google Scholar] [PubMed]
- Correa, A.D.; Jokl, L.; Carlsson, R. Amino acid composition of some Amaranthus sp. grain proteins and of its fractions. Arch. Latinoam. Nutr. 1986, 36, 466–476. [Google Scholar]
- Kim, H.K.; Kim, M.-J.; Shin, D.-H. Improvement of lipid profile by amaranth (Amaranthus esculantus) supplementation in streptozotocin-induced diabetic rats. Ann. Nutr. Metab. 2006, 50, 277–281. [Google Scholar] [CrossRef]
- Bailey, M.; Ray, S.; Narayanaswamy, D.; Homer, M. Plant-based oral nutrition supplementation promotes weight gain in patient status-post burn injury. Curr. Dev. Nutr. 2022, 6, 736. [Google Scholar] [CrossRef]
- Withrow, N.A.; Al-Tawil, Y.; Patterson, P.; Wilson, M.; Ryan, E.; Millovich, V.; Valentine, C.J. Retrospective Cohort Study Demonstrates Tolerance and Adherence to Pea-Based Complete Enteral Formula When Transitioned from a Previous Hypoallergenic Product. Nutrients 2024, 16, 3365. [Google Scholar] [CrossRef]
- Nurrohima, D.; Rahman, N.; Lutfiyah, F. Nutritional and Organoleptic Value in the Formula Enteral of Growol and Germinated Mung Bean Flour as an Alternative Enteral Type 2 Diabetes Mellitus. Amerta Nutr. 2024, 8, 424–432. [Google Scholar] [CrossRef]
- Zula, A.T.; Ayele, D.A.; Egigayhu, W.A. Proximate, antinutritional, microbial, and sensory acceptability of bread formulated from wheat (Triticum aestivum) and amaranth (Amaranthus caudatus). Int. J. Food Sci. 2020, 2020, 9429584. [Google Scholar] [CrossRef]
- Adebo, O.A.; Molelekoa, T.; Makhuvele, R.; Adebiyi, J.A.; Oyedeji, A.B.; Gbashi, S.; Adefisoye, M.A.; Ogundele, O.M.; Njobeh, P.B. A review on novel non-thermal food processing techniques for mycotoxin reduction. Int. J. Food Sci. Technol. 2021, 56, 13–27. [Google Scholar] [CrossRef]
- Nasab, S.S.; Tahmouzi, S.; Feizollahi, E.; Mollakhalili-Meybodi, N. Impacts of novel non-thermal processing (NTP) on anti-nutritional compounds of food grains and seeds. Food Control 2024, 162, 110469. [Google Scholar] [CrossRef]

| Composition (mg/g) | ||||
|---|---|---|---|---|
| Name of the Essential Amino Acid | [5] | [53] | [54] | [55] |
| Leucine | 6.20 | 7.11 | 7.31 | 7.85 |
| Valine | 5.90 | 4.62 | 4.53 | 3.11 |
| Lysine | 5.70 | 8.97 | 5.59 | 10.10 |
| Phenylalanine | 5.40 | 6.55 | 6.84 | 3.80 |
| Threonine | 5.10 | 4.40 | 4.52 | 3.05 |
| Methionine | 4.60 | 3.03 | 3.35 | 4.69 |
| Isoleucine | 3.90 | 4.38 | 4.06 | 2.87 |
| Histidine | 3.00 | 4.08 | 3.98 | 6.83 |
| Name of the Illness | Part or Product of Amaranth Used in Clinical Study | Effect of Treatment | References |
|---|---|---|---|
| Intestinal dysbiosis, inflammation and colitis | Amaranth popcorn | Decreased numbers of Alistipes putredinis, Bacteroides coprocola, and Bacteroides stercoris Increased numbers of Akkermansia muciniphila, and Streptococcus thermophilus bacteria | [138] |
| Oxidative stress | Protein fractions of amaranth | Antioxidant activity aids in neutralizing free radicals | [139] |
| Diabetes | Amaranth hydrolysates and peptides | Inhibition of the enzyme dipeptidyl peptidase IV, α-glucosidase, and α-amylase | [128] |
| Low glycemic amaranth-based multigrain bars | Antioxidant activity neutralizes free radicals and binds metals, preventing sharp fluctuations in blood glucose levels | [130] | |
| A low glycemic index, decreased postprandial blood glucose levels, and enhanced activity of antioxidant enzymes in serum, such as catalase, superoxide dismutase, and glutathione peroxidase, along with increased glutathione levels | [140] | ||
| Cancer, tumor | Amaranth lunasin-like peptide | Reduces the accumulation of tropomyosin, decreases the formation of anizokaryosis, controls cell shape, and regulates the movement of organelles | [132] |
| Squalene | Stimulates apoptosis, affects metastasis, and enhances the action of chemotherapeutic agents | [141,142] | |
| Breast cancer | Thermally denatured hydrolysates from amaranth seed protein | Inhibition of cancer cell growth, antimetastatic activity | [133] |
| High blood pressure | Amaranth powder hydrolysate | Inhibition of angiotensin-converting enzyme activity | [134,143] |
| Amaranth cookies | [144] | ||
| Pathogenic microorganism C. albicans | Protein isolates from amaranth grain | Prevention of yeast cell attachment due to the high content of polyphenols and flavonoids | [145] |
| Inflammatory illnesses | Sprouted amaranth powder | Reduction in nitric oxide production, exerting an anti-inflammatory effect | [146] |
| Amaranth bioactive peptides | Modulation of the inflammatory response by decreasing the production of chemokine (CCL20) and blocking NF-kB activation. | [147] | |
| Hypercholesterolemia, dyslipidemia | Hydrolysate of amaranth protein | Inhibition of cholesterol esterase and pancreatic lipase enzymes | [136,148,149] |
| Inhibition of 3-hydroxy-3-methylglutaryl-CoA-reductase | [150] | ||
| Obesity | Amaranth compounds: lutein, saponin, and anthocyanin | Improvement in metabolic parameters, reduction in triglyceride and cholesterol levels | [137] |
| Application Area | Product Type | Functionality | Authors |
|---|---|---|---|
| Functional food products | Amaranth-based products (flour, cereals, mixtures) | Nutrient enrichment, health improvement | [25,182,183] |
| Gluten-free products | Bread, pasta, cookies, gluten-free mixes | Alternative to gluten-containing products, improving digestion | [139,184,185] |
| Baby food | Baby formulas, purees, cereals based on amaranth | Supports growth and development, source of proteins and microelements | [186,187,188] |
| Nutraceuticals | Amaranth supplements, capsules, powders | Antioxidant properties, support for the cardiovascular system | [189,190,191] |
| Amaranth-based beverages | Fermented drinks, protein shakes | Reduced hypertension, improved digestion | [134,192,193] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toimbayeva, D.; Saduakhasova, S.; Kamanova, S.; Kiykbay, A.; Tazhina, S.; Temirova, I.; Muratkhan, M.; Shaimenova, B.; Murat, L.; Khamitova, D.; et al. Prospects for the Use of Amaranth Grain in the Production of Functional and Specialized Food Products. Foods 2025, 14, 1603. https://doi.org/10.3390/foods14091603
Toimbayeva D, Saduakhasova S, Kamanova S, Kiykbay A, Tazhina S, Temirova I, Muratkhan M, Shaimenova B, Murat L, Khamitova D, et al. Prospects for the Use of Amaranth Grain in the Production of Functional and Specialized Food Products. Foods. 2025; 14(9):1603. https://doi.org/10.3390/foods14091603
Chicago/Turabian StyleToimbayeva, Dana, Saule Saduakhasova, Svetlana Kamanova, Amirsana Kiykbay, Sayagul Tazhina, Indira Temirova, Marat Muratkhan, Bakhyt Shaimenova, Linara Murat, Dina Khamitova, and et al. 2025. "Prospects for the Use of Amaranth Grain in the Production of Functional and Specialized Food Products" Foods 14, no. 9: 1603. https://doi.org/10.3390/foods14091603
APA StyleToimbayeva, D., Saduakhasova, S., Kamanova, S., Kiykbay, A., Tazhina, S., Temirova, I., Muratkhan, M., Shaimenova, B., Murat, L., Khamitova, D., & Ospankulova, G. (2025). Prospects for the Use of Amaranth Grain in the Production of Functional and Specialized Food Products. Foods, 14(9), 1603. https://doi.org/10.3390/foods14091603






