A Review of Novel Antioxidant Ergothioneine: Biosynthesis Pathways, Production, Function and Food Applications
Abstract
1. Introduction
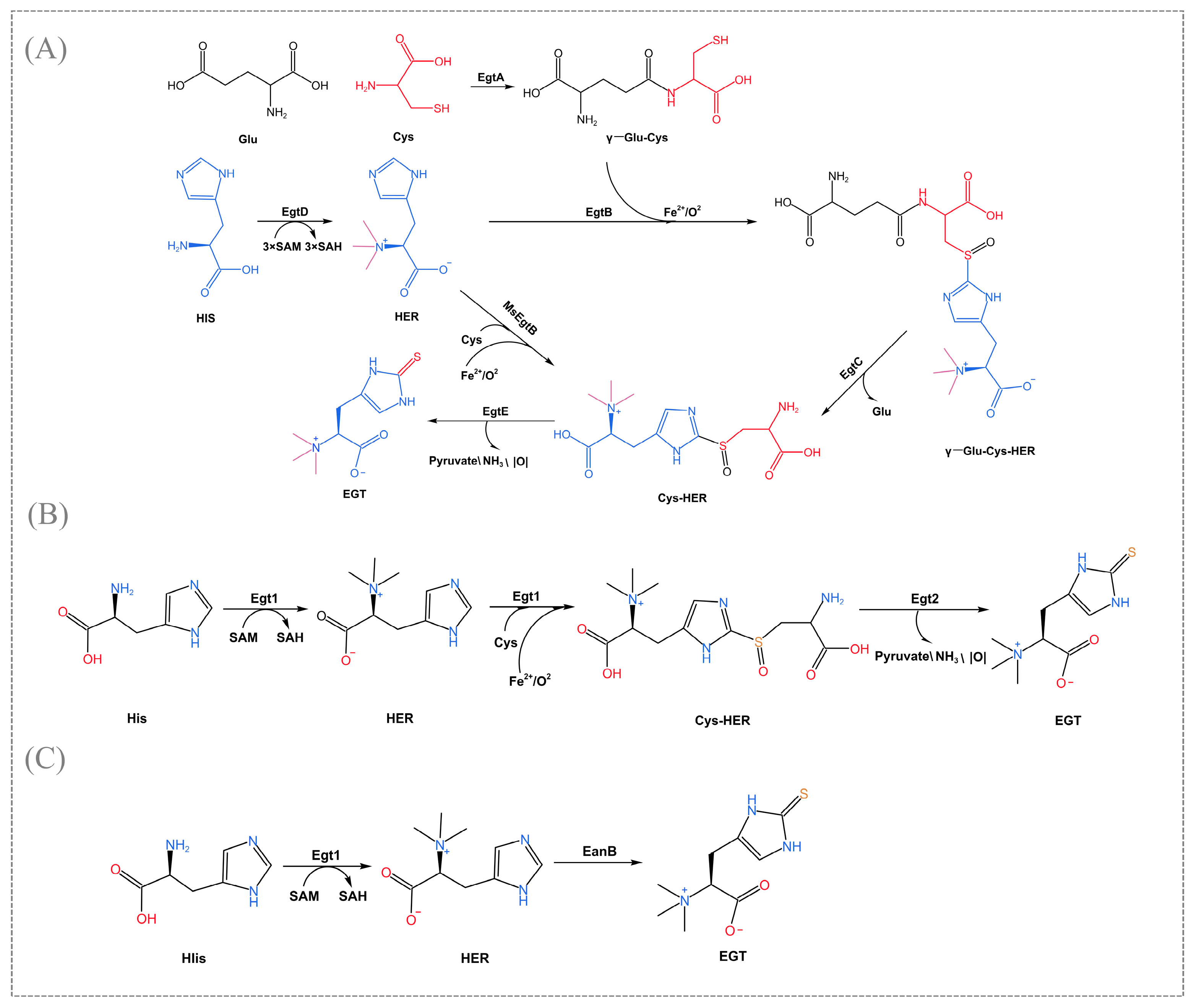
2. Biosynthetic Pathway of EGT
| Types | Microbial Species | Enzyme | References |
|---|---|---|---|
| Type Ⅰ | Mycobacterium smegmatis | MsmEgtB | [5] |
| Mycobacterium thermoresistibile | MthEgtB | [33] | |
| Type Ⅱ | C. thermophilum | CthEgtB | [28] |
| Methylobacterium | MsEgtB | [27] | |
| Type Ⅲ | Neurospora crassa | NcrEgt1 | [29] |
| Type Ⅳ | Microcystis aeruginosa | MaeEgtB | [24] |
| Type Ⅴ | Thermosynechococcus elongate | TelEgtB | [27] |
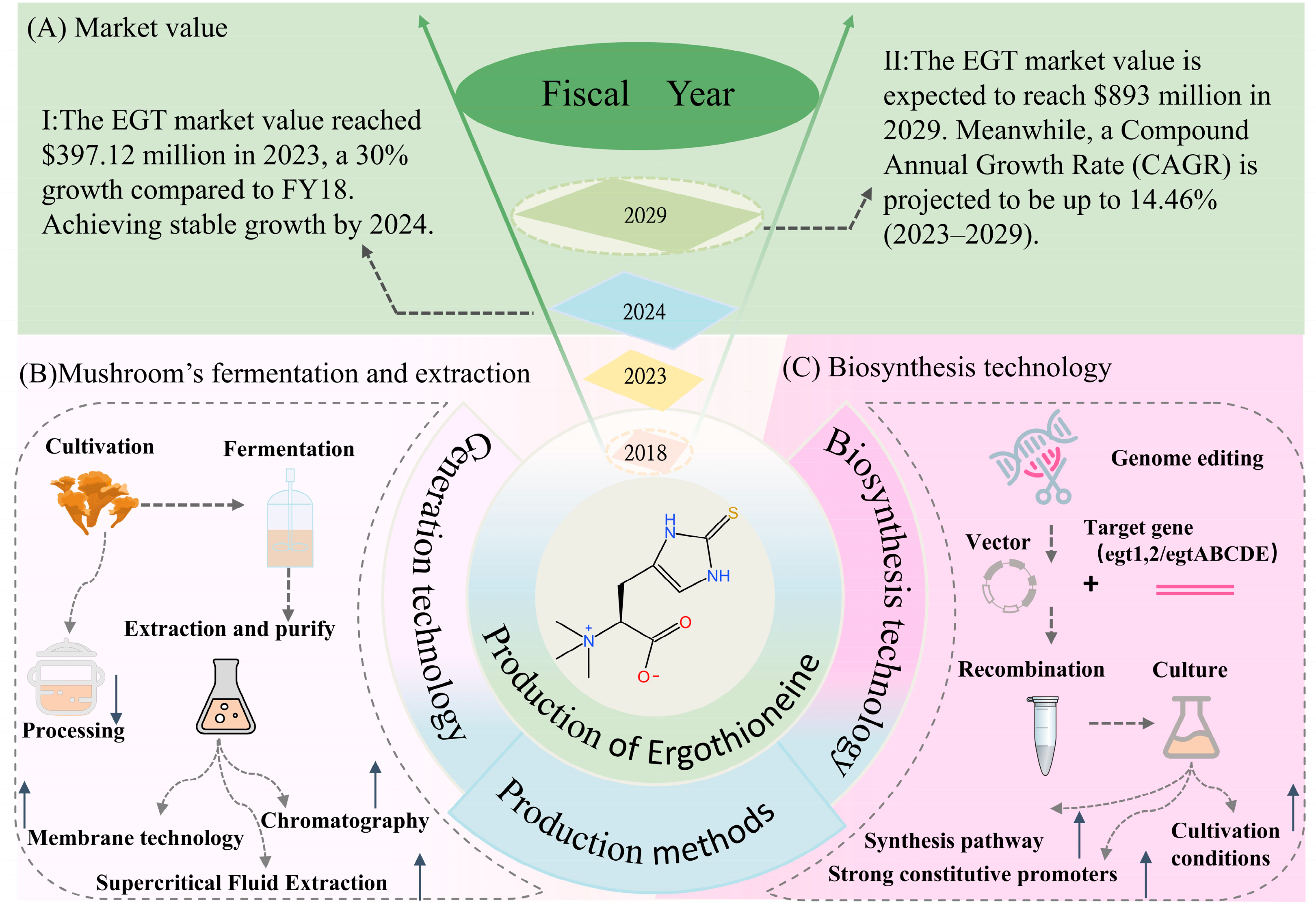
3. Production of EGT
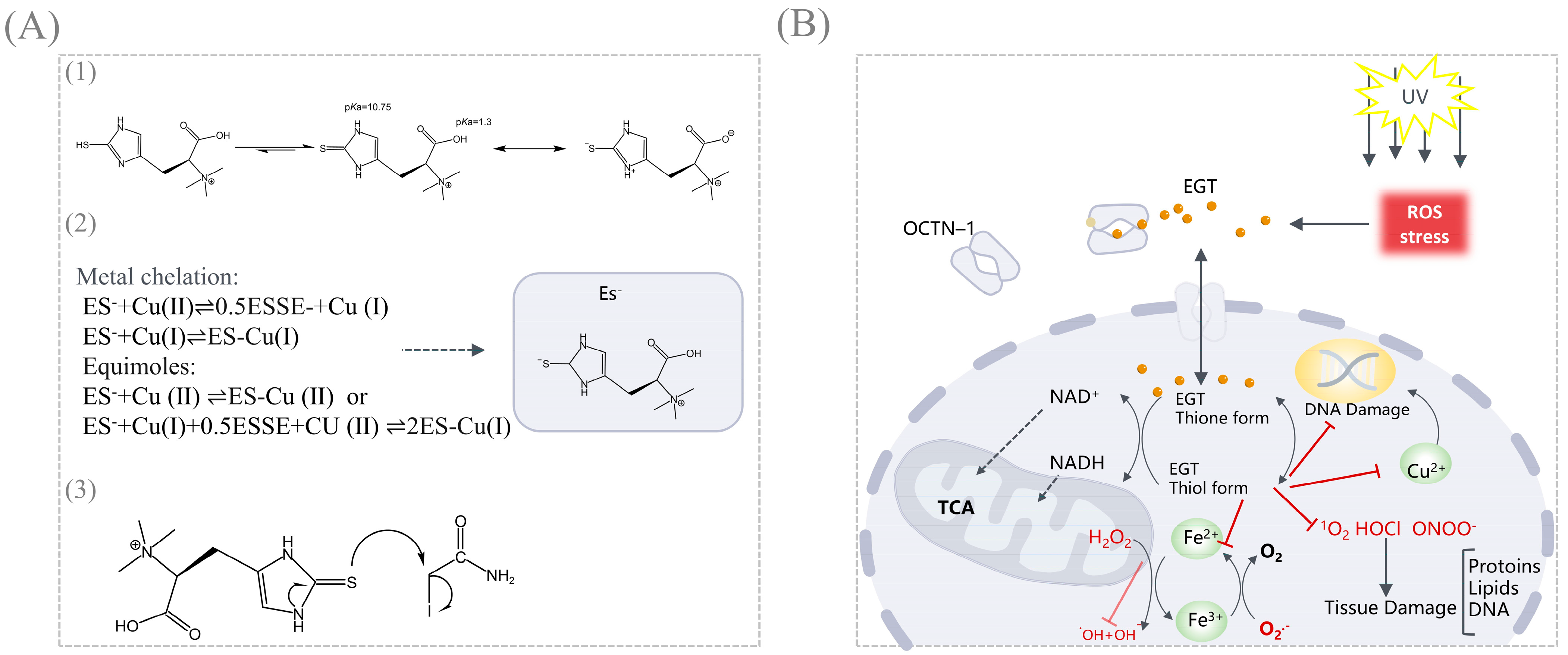
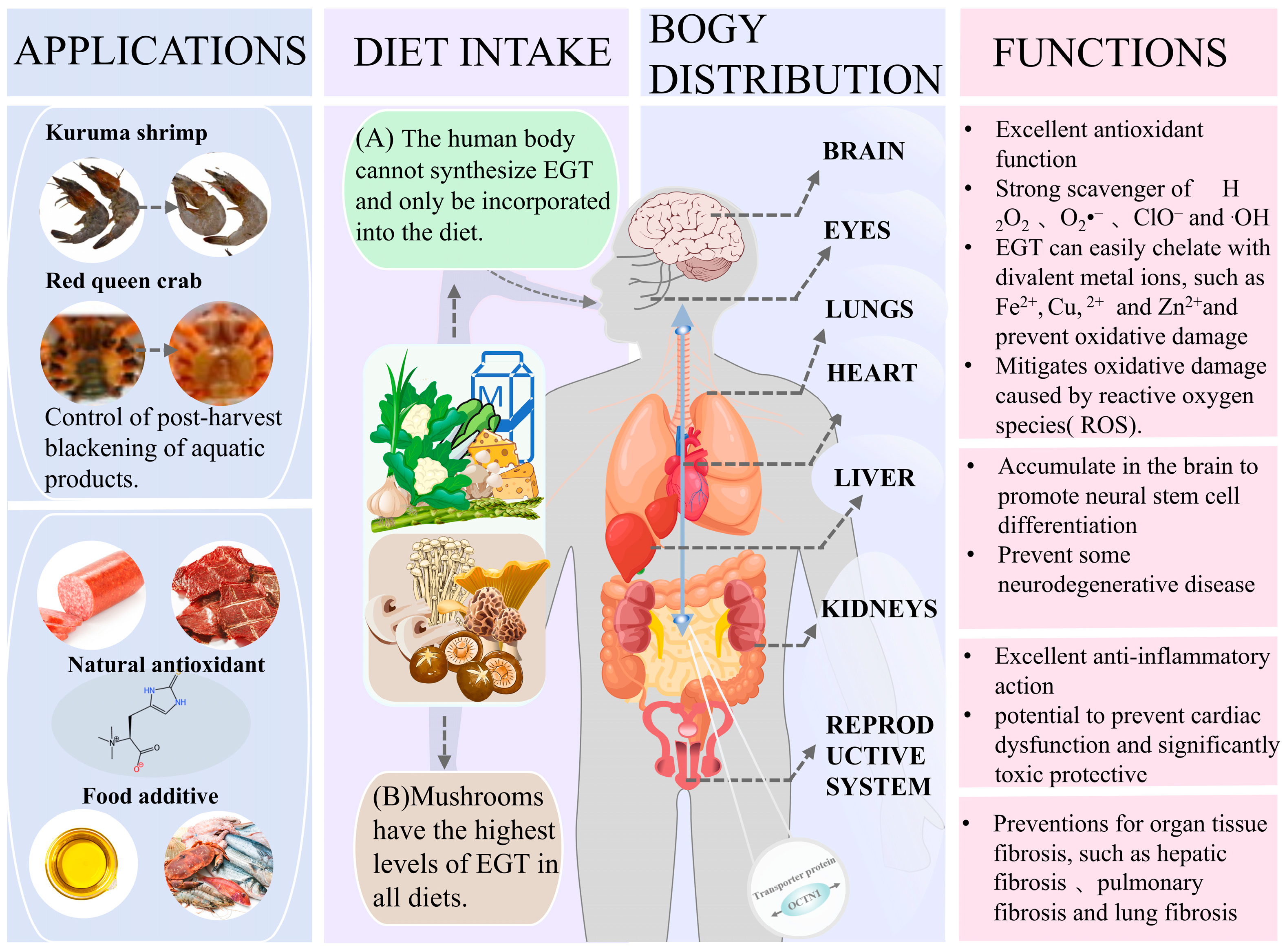
4. Functions and Applications of EGT
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Turck, D.; Bresson, J.L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; Naska, A. Statement on the safety of synthetic l-ergothioneine as a novel food—Supplementary dietary exposure and safety assessment for infants and young children, pregnant and breastfeeding women. EFSA J. Eur. Food Saf. Auth. 2017, 15, e05060. [Google Scholar] [CrossRef]
- Petermann, I.; Triggs, C.M.; Huebner, C.; Han, D.Y.; Gearry, R.B.; Barclay, M.L.; Demmers, P.S.; McCulloch, A.; Ferguson, L.R. Mushroom intolerance: A novel diet–gene interaction in Crohn’s disease. Br. J. Nutr. 2009, 102, 506–508. [Google Scholar] [CrossRef] [PubMed]
- Hartman, P.E. Ergothioneine as antioxidant. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1990; Volume 186, pp. 310–318. [Google Scholar] [CrossRef]
- Halliwell, B.; Cheah, I.K.; Tang, R.M.Y. Ergothioneine—A diet-derived antioxidant with therapeutic potential. FEBS Lett. 2018, 592, 3357–3366. [Google Scholar] [CrossRef]
- Tanret, C. The new base drawn from rye ergot, ergothioneine. CR Hebd. Acad. Sci 1909, 25, 222–224. [Google Scholar] [CrossRef]
- Bello, M.H.; Barrera-Perez, V.; Morin, D.; Epstein, L. The Neurospora crassa mutant Nc Δ Egt-1 identifies an ergothioneine biosynthetic gene and demonstrates that ergothioneine enhances conidial survival and protects against peroxide toxicity during conidial germination. Fungal Genet. Biol. 2012, 49, 160–172. [Google Scholar] [CrossRef]
- Seebeck, F.P. In vitro reconstitution of Mycobacterial ergothioneine biosynthesis. J. Am. Chem. Soc. 2010, 132, 6632–6633. [Google Scholar] [CrossRef] [PubMed]
- Alamgir, K.M.; Masuda, S.; Fujitani, Y.; Fukuda, F.; Tani, A. Production of ergothioneine by Methylobacterium species. Front. Microbiol. 2015, 6, 1185. [Google Scholar] [CrossRef]
- Dubost, N.J.; Ou, B.; Beelman, R.B. Quantification of polyphenols and ergothioneine in cultivated mushrooms and correlation to total antioxidant capacity. Food Chem. 2007, 105, 727–735. [Google Scholar] [CrossRef]
- Dubost, N.J.; Beelman, R.B.; Peterson, D.; Royse, D.J. Identification and Quantification of Ergothioneine in Cultivated Mushrooms by Liquid Chromatography-Mass Spectroscopy. Int. J. Med. Mushrooms 2006, 8, 215–222. [Google Scholar] [CrossRef]
- Takusagawa, S.; Satoh, Y.; Ohtsu, I.; Dairi, T. Ergothioneine production with Aspergillus oryzae. Biosci. Biotechnol. Biochem. 2018, 83, 181–184. [Google Scholar] [CrossRef]
- Chen, B.-X.; Xue, L.-N.; Wei, T.; Ye, Z.-W.; Li, X.-H.; Guo, L.-Q.; Lin, J.-F. Enhancement of ergothioneine production by discovering and regulating its metabolic pathway in Cordyceps militaris. Microb. Cell Factories 2022, 21, 169. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Li, J.; Du, G.; Kang, Z. Construction and optimization of ergothioneine-producing Escherichia coli. Chin. J. Biotechnol. 2022, 38, 796–806. [Google Scholar] [CrossRef]
- van der Hoek, S.A.; Rusnák, M.; Jacobsen, I.H.; Martínez, J.L.; Kell, D.B.; Borodina, I. Engineering ergothioneine production in Yarrowia lipolytica. FEBS Lett. 2021, 596, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.X.; Chen, J.W.; Ke, J.; Hu, F.Y.; Wen, J.C.; Dong, Y.G.; Wang, F.Q.; Xiong, L.B. Co-augmentation of a transport gene mfsT1 in Mycolicibacterium neoaurum with genome engineering to enhance ergothioneine production. J. Basic Microbiol. 2024, 64, e2300705. [Google Scholar] [CrossRef] [PubMed]
- Markova, N.G.; Karaman-Jurukovska, N.; Dong, K.K.; Damaghi, N.; Smiles, K.A.; Yarosh, D.B. Skin cells and tissue are capable of using l -ergothioneine as an integral component of their antioxidant defense system. Free Radic. Biol. Med. 2009, 46, 1168–1176. [Google Scholar] [CrossRef]
- Tang, R.M.Y.; Cheah, I.K.-M.; Yew, T.S.K.; Halliwell, B. Distribution and accumulation of dietary ergothioneine and its metabolites in mouse tissues. Sci. Rep. 2018, 8, 1601. [Google Scholar] [CrossRef] [PubMed]
- Encarnacion, A.B.; Fagutao, F.; Hirono, I.; Ushio, H.; Ohshima, T. Effects of ergothioneine from mushrooms (Flammulina velutipes) on melanosis and lipid oxidation of kuruma shrimp (Marsupenaeus japonicus). J. Agric. Food Chem. 2010, 58, 2577–2585. [Google Scholar] [CrossRef]
- Encarnacion, A.B.; Fagutao, F.; Hirayama, J.; Terayama, M.; Hirono, I.; Ohshima, T. Edible mushroom (Flammulina velutipes) extract inhibits melanosis in Kuruma shrimp (Marsupenaeus japonicus). J. Food Sci. 2011, 76, C52–C58. [Google Scholar] [CrossRef]
- Liu, Y.; Duan, X.; Zhang, M.; Li, C.; Zhang, Z.; Hu, B.; Liu, A.; Li, Q.; Chen, H.; Tang, Z. Extraction, structure characterization, carboxymethylation and antioxidant activity of acidic polysaccharides from Craterellus cornucopioides. Ind. Crops Prod. 2021, 159, 113079. [Google Scholar] [CrossRef]
- Cheah, I.K.; Feng, L.; Tang, R.M.; Lim, K.H.; Halliwell, B. Ergothioneine levels in an elderly population decrease with age and incidence of cognitive decline; a risk factor for neurodegeneration? Biochem. Biophys. Res. Commun. 2016, 478, 162–167. [Google Scholar] [CrossRef]
- Smith, E.; Ottosson, F.; Hellstrand, S.; Ericson, U.; Orho-Melander, M.; Fernandez, C.; Melander, O. Ergothioneine is associated with reduced mortality and decreased risk of cardiovascular disease. Heart (Br. Card. Soc.) 2020, 106, 691–697. [Google Scholar] [CrossRef]
- Servillo, L.; D’Onofrio, N.; Balestrieri, M.L. Ergothioneine antioxidant function: From chemistry to cardiovascular therapeutic potential. J. Cardiovasc. Pharmacol. 2017, 69, 183–191. [Google Scholar] [CrossRef]
- Ames, B.N. Prolonging healthy aging: Longevity vitamins and proteins. Proc. Natl. Acad. Sci. USA 2018, 115, 10836–10844. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, K.L.; Scharf, D.H.; Litomska, A.; Hertweck, C. Enzymatic Carbon-Sulfur Bond Formation in Natural Product Biosynthesis. Chem. Rev. 2017, 117, 5521–5577. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, L.; Owens, R.A.; Dolan, S.K.; O'Keeffe, G.; Schrettl, M.; Kavanagh, K.; Jones, G.W.; Doyle, S. The Aspergillus fumigatus protein GliK protects against oxidative stress and is essential for gliotoxin biosynthesis. Eukaryot. Cell 2012, 11, 1226–1238. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, Z.H.; Cui, M.X. Effect of flavonoid extracts from flowers of Hibiscus Manihot in aging mice. Anhui Agr. Sci. Bull. 2020, 26, 25–28. [Google Scholar] [CrossRef]
- Faponle, A.S.; Seebeck, F.P.; de Visser, S.P. Sulfoxide Synthase versus Cysteine Dioxygenase Reactivity in a Nonheme Iron Enzyme. J. Am. Chem. Soc. 2017, 139, 9259–9270. [Google Scholar] [CrossRef]
- Stampfli, A.R.; Goncharenko, K.V.; Meury, M.; Dubey, B.N.; Schirmer, T.; Seebeck, F.P. An alternative active site architecture for O2 activation in the ergothioneine biosynthetic EgtB from Chloracidobacterium thermophilum. J. Am. Chem. Soc. 2019, 141, 5275–5285. [Google Scholar] [CrossRef]
- Kamide, T.; Takusagawa, S.; Tanaka, N.; Ogasawara, Y.; Kawano, Y.; Ohtsu, I.; Satoh, Y.; Dairi, T. High Production of Ergothioneine in Escherichia coli using the Sulfoxide Synthase from Methylobacterium strains. J. Agric. Food Chem. 2020, 68, 6390–6394. [Google Scholar] [CrossRef]
- Hu, W.; Song, H.; Sae Her, A.; Bak, D.W.; Naowarojna, N.; Elliott, S.J.; Qin, L.; Chen, X.; Liu, P. Bioinformatic and biochemical characterizations of C–S bond formation and cleavage enzymes in the fungus Neurospora crassa ergothioneine biosynthetic pathway. Org. Lett. 2014, 16, 5382–5385. [Google Scholar] [CrossRef]
- Cheng, R.; Wu, L.; Lai, R.; Peng, C.; Naowarojna, N.; Hu, W.; Li, X.; Whelan, S.A.; Lee, N.; Lopez, J.; et al. Single-Step Replacement of an Unreactive C–H Bond by a C–S Bond Using Polysulfide as the Direct Sulfur Source in the Anaerobic Ergothioneine Biosynthesis. ACS Catal. 2020, 10, 8981–8994. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Lai, R.; Peng, C.; Lopez, J.; Li, Z.; Naowarojna, N.; Li, K.; Wong, C.; Lee, N.; Whelan, S.A. Implications for an Imidazole-2-yl Carbene Intermediate in the Rhodanase-Catalyzed C–S Bond Formation Reaction of Anaerobic Ergothioneine Biosynthesis. ACS Catal. 2021, 11, 3319–3334. [Google Scholar] [CrossRef] [PubMed]
- Meyer, D.; Neumann, P.; Ficner, R.; Tittmann, K. Observation of a stable carbene at the active site of a thiamin enzyme. Nat. Chem. Biol. 2013, 9, 488–490. [Google Scholar] [CrossRef] [PubMed]
- Stampfli, A.R.; Blankenfeldt, W.; Seebeck, F.P. Structural basis of ergothioneine biosynthesis. Curr. Opin. Struct. Biol. 2020, 65, 1–8. [Google Scholar] [CrossRef]
- Osawa, R.; Kamide, T.; Satoh, Y.; Kawano, Y.; Ohtsu, I.; Dairi, T. Heterologous and High Production of Ergothioneine in Escherichia coli. J. Agric. Food Chem. 2018, 66, 1191–1196. [Google Scholar] [CrossRef]
- van der Hoek, S.A.; Darbani, B.; Zugaj, K.E.; Prabhala, B.K.; Biron, M.B.; Randelovic, M.; Medina, J.B.; Kell, D.B.; Borodina, I. Engineering the Yeast Saccharomyces cerevisiae for the Production of L-(+)-Ergothioneine. Front. Bioeng. Biotechnol. 2019, 7, 262. [Google Scholar] [CrossRef]
- Chen, Z.; He, Y.; Wu, X.; Wang, L.; Dong, Z.; Chen, X. Toward more efficient ergothioneine production using the fungal ergothioneine biosynthetic pathway. Microb. Cell Factories 2022, 21, 76. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Nagasaka, R.; Ohshima, T. Effects of extraction solvents, cooking procedures and storage conditions on the contents of ergothioneine and phenolic compounds and antioxidative capacity of the cultivated mushroom Flammulina velutipes. Int. J. Food Sci. Technol. 2012, 47, 1193–1205. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Srivastav, P.P.; Mishra, H.N. Optimization of process variables for supercritical fluid extraction of ergothioneine and polyphenols from Pleurotus ostreatus and correlation to free-radical scavenging activity. J. Supercrit. Fluids 2014, 95, 51–59. [Google Scholar] [CrossRef]
- Cremades, O.; Diaz-Herrero, M.M.; Carbonero-Aguilar, P.; Gutierrez-Gil, J.F.; Fontiveros, E.; Bautista, J. White button mushroom ergothioneine aqueous extracts obtained by the application of enzymes and membrane technology. Food Biosci. 2015, 10, 42–47. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Ho, K.-J.; Hsieh, Y.-J.; Wang, L.-T.; Mau, J.-L. Contents of lovastatin, γ-aminobutyric acid and ergothioneine in mushroom fruiting bodies and mycelia. LWT 2012, 47, 274–278. [Google Scholar] [CrossRef]
- Kalaras, M.D.; Richie, J.P.; Calcagnotto, A.; Beelman, R.B. Mushrooms: A rich source of the antioxidants ergothioneine and glutathione. Food Chem. 2017, 233, 429–433. [Google Scholar] [CrossRef]
- Pratti, P.G.; Junior, J.d.S.P.; Petito, N.d.L.; da Silva, B.D.; Conte-Junior, C.A.; Branco, V.N.C.; Araújo, K.G.d.L.; Domingues, J.R. Effect of hot air-drying and pasteurization on ergothioneine content in edible mushrooms. J. Food Compos. Anal. 2024, 126, 105865. [Google Scholar] [CrossRef]
- Zhang, C.; Xie, Y.; Liu, D.; Liu, R.; Han, J. Effects of Drying Process and High Hydrostatic Pressure on Extraction of Antioxidant Ergothioneine from Pleurotus citrinopileatus Singer. Foods 2024, 13, 878. [Google Scholar] [CrossRef] [PubMed]
- Cheah, I.K.; Halliwell, B. Ergothioneine; antioxidant potential, physiological function and role in disease. BBA—Mol. Basis Dis. 2012, 1822, 784–793. [Google Scholar] [CrossRef]
- Xiong, C.; Xia, Y.; Zheng, P.; Wang, C. Increasing oxidative stress tolerance and subculturing stability of Cordyceps militaris by overexpression of a glutathione peroxidase gene. Appl. Microbiol. Biotechnol. 2013, 97, 2009–2015. [Google Scholar] [CrossRef] [PubMed]
- Xiong, K.; Guo, H.; Xue, S.; Liu, M.; Dai, Y.; Lin, X.; Zhang, S. Production optimization of food functional factor ergothioneine in wild-type red yeast Rhodotorula mucilaginosa DL-X01. J. Sci. Food Agric. 2024, 104, 4050–4057. [Google Scholar] [CrossRef]
- Van Laer, K.; Hamilton, C.J.; Messens, J. Low-molecular-weight thiols in thiol–disulfide exchange. Antioxid. Redox Signal. 2013, 18, 1642–1653. [Google Scholar] [CrossRef]
- Misra, H.P. Generation of superoxide free radical during the autoxidation of thiols. J. Biol. Chem. 1974, 249, 2151–2155. [Google Scholar] [CrossRef]
- Kawano, H.; Higuchi, F.; Mayumi, T.; Hama, T. Studies on ergothioneine. VII. Some effects of ergothioneine on glycolytic metabolism in red blood cells from rats. Chem. Pharm. Bull. 1982, 30, 2611–2613. [Google Scholar] [CrossRef]
- Misiti, F.; Castagnola, M.; Zuppi, C.; Giardina, B.; Messana, I. Role of ergothioneine on S-nitrosoglutathione catabolism. Biochem. J. 2001, 356, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Jocelyn, P.C. Biochemistry of the SH Group: The Occurrence, Chemical Properties, Metabolism and Biological Function of Thiols and Disulphides; Academic Press: London, UK, 1972. [Google Scholar]
- Kotha, R.R.; Tareq, F.S.; Yildiz, E.; Luthria, D.L. Oxidative stress and antioxidants—A critical review on in vitro antioxidant assays. Antioxidants 2022, 11, 2388. [Google Scholar] [CrossRef] [PubMed]
- Vergel-Alfonso, A.A.; Arias-Avelenda, R.; Casariego-Año, A.; Giménez, M.J.; Ruíz-Cruz, S.; López-Corona, B.E.; Del-Toro-Sánchez, C.L.; Gonzalez-Bravo, A.L.; Plascencia-Jatomea, M.; Menchaca-Armenta, M.; et al. Development and Characterization of Pectin and Beeswax-Based Coatings Enhanced with Anthocyanins and Its Antioxidant and Antifungal Properties. Processes 2025, 13, 542. [Google Scholar] [CrossRef]
- Del-Toro-Sánchez, C.L.; Rodríguez-Félix, F.; Cinco-Moroyoqui, F.J.; Juárez, J.; Ruiz-Cruz, S.; Wong-Corral, F.J.; Borboa-Flores, J.; Castro-Enríquez, D.D.; Barreras-Urbina, C.G.; Tapia-Hernández, J.A. Recovery of phytochemical from three safflower (Carthamus tinctorius L.) by-products: Antioxidant properties, protective effect of human erythrocytes and profile by UPLC-DAD-MS. J. Food Process. Preserv. 2021, 45, e15765. [Google Scholar] [CrossRef]
- Motohashi, N.; Mori, I.; Sugiura, Y. Complexing of copper ion by ergothioneine. Chem. Pharm. Bull. 1976, 24, 2364–2368. [Google Scholar] [CrossRef] [PubMed]
- Aruoma, O.; Spencer, J.; Mahmood, N. Protection against oxidative damage and cell death by the natural antioxidant ergothioneine. Food Chem. Toxicol. 1999, 37, 1043–1053. [Google Scholar] [CrossRef]
- Halliwell, B. The wanderings of a free radical. Free Radic. Biol. Med. 2009, 46, 531–542. [Google Scholar] [CrossRef]
- Behroozi, P. Free Radicals in Biology and Medicine. In Biology and Medicine; Oxford University: Oxford, UK, 2015; Volume 13, p. 1. [Google Scholar]
- Akanmu, D.; Cecchini, R.; Aruoma, O.I.; Halliwell, B. The antioxidant action of ergothioneine. Arch. Biochem. Biophys. 1991, 288, 10–16. [Google Scholar] [CrossRef]
- Whiteman, M.; Halliwell, B. Thiols and disulphides can aggravate peroxynitrite-dependent inactivation of α1-antiproteinase. FEBS Lett. 1997, 414, S0014–S5793. [Google Scholar] [CrossRef]
- Hanlon, D.P. Interaction of ergothioneine with metal ions and metalloenzymes. J. Med. Chem. 1971, 14, 1084–1087. [Google Scholar] [CrossRef]
- Deiana, M.; Rosa, A.; Casu, V.; Piga, R.; Dessı̀, M.A.; Aruoma, O.I. L-ergothioneine modulates oxidative damage in the kidney and liver of rats in vivo: Studies upon the profile of polyunsaturated fatty acids. Clin. Nutr. 2004, 23, 183–193. [Google Scholar] [CrossRef]
- Brandalise, F.; Roda, E.; Ratto, D.; Goppa, L.; Gargano, M.L.; Cirlincione, F.; Priori, E.C.; Venuti, M.T.; Pastorelli, E.; Savino, E.; et al. Hericium erinaceus in Neurodegenerative Diseases: From Bench to Bedside and Beyond, How Far from the Shoreline? J. Fungi 2023, 9, 551. [Google Scholar] [CrossRef]
- Behof, W.J.; Whitmore, C.A.; Haynes, J.R.; Rosenberg, A.J.; Tantawy, M.N.; Peterson, T.E.; Harrison, F.E.; Beelman, R.B.; Pham, W. A novel antioxidant ergothioneine PET radioligand for in vivo imaging applications. Sci. Rep. 2021, 11, 18450. [Google Scholar] [CrossRef]
- Cheah, I.K.; Halliwell, B. Could Ergothioneine Aid in the Treatment of Coronavirus Patients? Antioxidants 2020, 9, 595. [Google Scholar] [CrossRef] [PubMed]
- Haard, N.F.; Simpson, B.K. Seafood Enzymes: Utilization and Influence on Postharvest Seafood Quality; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar] [CrossRef]
- Encarnacion, A.B.; Fagutao, F.; Shozen, K.-I.; Hirono, I.; Ohshima, T. Biochemical intervention of ergothioneine-rich edible mushroom (Flammulina velutipes) extract inhibits melanosis in crab (Chionoecetes japonicus). Food Chem. 2011, 127, 1594–1599. [Google Scholar] [CrossRef]
- Encarnacion, A.B.; Fagutao, F.; Jintasataporn, O.; Worawattanamateekul, W.; Hirono, I.; Ohshima, T. Application of ergothioneine-rich extract from an edible mushroom Flammulina velutipes for melanosis prevention in shrimp, Penaeus monodon and Litopenaeus vannamei. Food Res. Int. 2012, 45, 232–237. [Google Scholar] [CrossRef]
- Tao, Y.; Xiao, S.; Cai, J.; Wang, J.; Li, L. Effects of Ergothioneine-enriched mushroom extract on oxidative stability, volatile compounds and sensory quality of emulsified sausage. Anim. Biosci. 2021, 34, 1695–1704. [Google Scholar] [CrossRef]
- Pahila, J.; Kaneda, H.; Nagasaka, R.; Koyama, T.; Ohshima, T. Effects of ergothioneine-rich mushroom extracts on lipid oxidation and discoloration in salmon muscle stored at low temperatures. Food Chem. 2017, 233, 273–281. [Google Scholar] [CrossRef]
| Microorganism Species | Experimental Methods | EGT Yield | Reference |
|---|---|---|---|
| Mycobacterium pubescent | Using Escherichia coli as an alternative host to express the egtBCDE gene from M. pubescent for EGT production | 17 ± 1– 24 ± 4 mg/L | [34] |
| Methylobacterium aquaticum | Genetic modification of strain 22A, deletion of the histidine metabolizing enzyme gene hutH, introduction of the genome egtBD, and optimization of cultivation conditions | 7 mg/g dw | [6] |
| Saccharomyces cerevisiae | Investigating the effect of amino acid supplementation of the medium and altering the nitrogen metabolism of S. cerevisiae with knock-out of TOR1 or YIH1 | 598 ± 18 mg/L | [35] |
| Neurospora crassa | Using Aspergillus oryzae as an alternative host for EGT production | 124.5 ± 5.0– 231.0 ± 1.1 mg/kg dw | [9] |
| Using Crabtree-negative, oleaginous yeast Yarrowia lipolytica as an alternative host for EGT production | 1.63 ± 0.04 g/L | [12] | |
| Using Escherichia coli as an alternative host for EGT production, and enhancing the supply of the three precursors, amino acids of EGT | 95.58 ± 3.2– 548.75 mg/L | [11] | |
| Trichoderma reesei | Co-expression of EGT biosynthesis genes of T.reesei (tregt1 and tregt2) in E.coli, then fed-batch fermentation | 0.89–4.34 g/L | [36] |
| Agaricus blazei Murill | Boiling, steaming, and microwaving extract treatments | 0.66–2.0 g/kg dw | [37] |
| Pleurotus ostreatus | Supercritical fluid extraction with CO2 to extract EGT | 0.90–1.35 mg/g dw | [38] |
| Agaricus bisporus | Using enzyme and 5 kDa/UF membrane technology, combined with a concentration step | 0.323 ± 0.012 −118.63 ± 4.11 g/kg dw | [39] |
| Cordyceps militaris | Excavating the EGT synthetases CmE1B and CmEgt2 of C.militaris into the genome and using a strong promoter to modify the EGT synthesis pathway further. | 0.511 ± 0.040– 2.485 ± 0.051 g/kg dw | [10] |
| Mechanism (Category) | Assay | Technology | Results | Reference |
|---|---|---|---|---|
| Thiobarbituic acid (TBA) method | In this assay, TBA and trichloroacetic acid are mixed with the sample solution, placed in the hot water bath for 10 min, centrifuged in the solution, and the supernatant absorbance activity is measured at 550–560 nm | EGT inhibited the peroxidation of arachidonic acid by mixed systems of myoglobin or hemoglobin and H2O2 | [59] | |
| ROS/RNS Scavenging activity/lipid oxidation | Hydroxyl radical scavenging activity (deoxyri-bose assay) | Competitive inhibition of deoxyribose degradation by EGT is used to measure its scavenging capacity, and solubilization studies with pulsed radiation are used to calculate rate constants for the reaction of OH with scavengers | Used to scavenge hydroxyl radicals (OH), proven EGT has strong scavenging power | [59] |
| Peroxynitrite radical scavenging activity | The addition of ONOO- to α1AP at pH 7.4 causes the loss of its elastase-inhibitory capacity, EGT Competitive inhibition of ONOO−, and its scavenging capacity measured by enzyme activity | Used to scavenge hydroxyl radicals, peroxynitrite (ONOO−), proven EGT has strong scavenging power | [60] | |
| ROS/RNS Scavenging activity /lipid oxidation | Total oxyradical scavenging capacity (TOSC) | This assay is based on the reaction of artificially generated oxyradicals with α-keto-γ-methiolbutyric acid (KMBA), which is completely oxidized to ethylene. EGT competitively inhibits ethylene production, and the antioxidant capacity of EGT was determined by measuring the amount of total ethylene | EGT was the most active ROS scavenger agent as compared to GSH, trolox, and uric acid. The scavenging capacity, anti-ROO· (5.51 ± 0.1 units), anti-hydroxyl radicals (0.34 ± 0.09 units), anti-peroxynitrite (5.2 ± 1 units), anti-peroxyl radicals (5.53 ± 1.27 units) | [54] |
| Determination of single-line oxygen scavenging capacity | This assay determines the EGT scavenging capacity by measuring the time of decay of single heavy-state molecular oxygen phosphorescence at 1270 nm in D2O | EGT is the most active singlet oxygen quencher of the compounds studied in the present experiments, and at the normal physiological pH K0bs = 2.3 × 107 | [60] | |
| Metal chelation | Metal-complexes of ergothioneine (Fe2+, Cu2+, Zn2+) | The interaction between EGT and divalent metal ions has been investigated by means of potentiometric titration, visible and near-infrared spectra | Copper has the highest complex formation constant | [61] |
| In vivo | Determination of total lipid content | This assay uses the iron complex of the chelating agent nitrilotriacetic acid (Fe-NTA), based on the profile of polyunsaturated fatty acids at 264 nm for the assessment of antioxidant actions in vivo | Supplementation with EGT not only protects the organs against lipid peroxidation but also conserves the consumption of endogenous glutathione and a-tocopherol | [62] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Liu, Z.; Wang, Z.; Lei, Z.; Jia, Y.; Chen, W.; Shi, R.; Wang, C. A Review of Novel Antioxidant Ergothioneine: Biosynthesis Pathways, Production, Function and Food Applications. Foods 2025, 14, 1588. https://doi.org/10.3390/foods14091588
Zhang H, Liu Z, Wang Z, Lei Z, Jia Y, Chen W, Shi R, Wang C. A Review of Novel Antioxidant Ergothioneine: Biosynthesis Pathways, Production, Function and Food Applications. Foods. 2025; 14(9):1588. https://doi.org/10.3390/foods14091588
Chicago/Turabian StyleZhang, Haijing, Zheng Liu, Zhong Wang, Ziteng Lei, Yan Jia, Wei Chen, Ruoyu Shi, and Chengtao Wang. 2025. "A Review of Novel Antioxidant Ergothioneine: Biosynthesis Pathways, Production, Function and Food Applications" Foods 14, no. 9: 1588. https://doi.org/10.3390/foods14091588
APA StyleZhang, H., Liu, Z., Wang, Z., Lei, Z., Jia, Y., Chen, W., Shi, R., & Wang, C. (2025). A Review of Novel Antioxidant Ergothioneine: Biosynthesis Pathways, Production, Function and Food Applications. Foods, 14(9), 1588. https://doi.org/10.3390/foods14091588








