Evaluation of Glycerol Concentration in the Production of Lemon Oil Incorporated Pectin-Based Films Using Principal Component Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
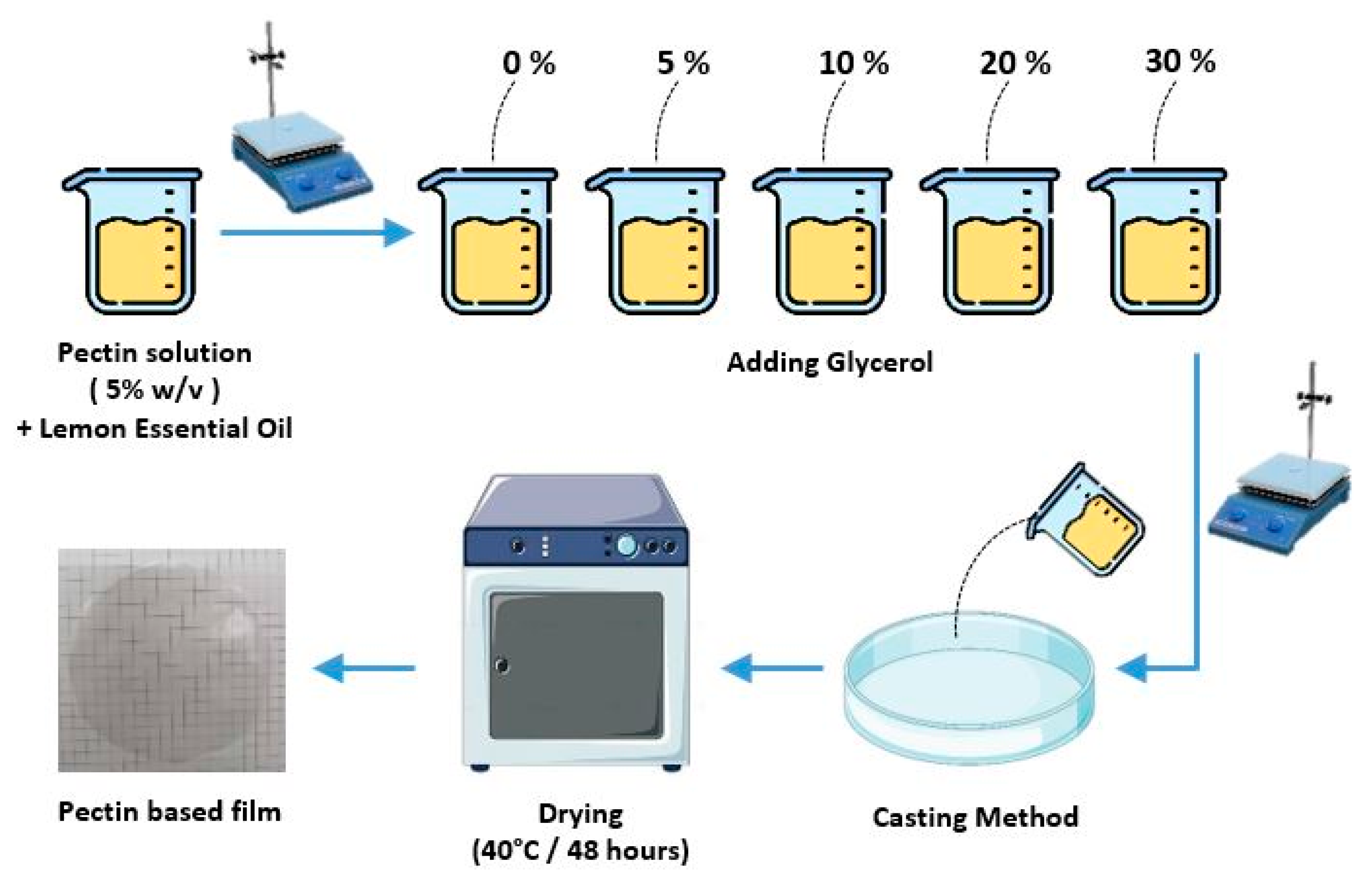
2.2. Preparation of Encapsulated Lemon Essential Oil (LEO)
2.3. Preparation of Pectin Films with Varying Glycerol Concentrations
2.4. Film Thickness
2.5. Moisture Content
2.6. Solubility
2.7. Swelling Ratio
2.8. Water Vapor Permeability
2.9. Contact Angle Measurements
2.10. Film Transparency
2.11. Film Transmittance
2.12. Fourier Transform Infrared Spectroscopy (FT-IR)
2.13. Mechanical Properties
2.14. Burst Strength
2.15. Film Morphology
2.16. Statistical Analyses
3. Results
3.1. Film Thickness
3.2. Moisture Content
3.3. Solubility
3.4. Swelling Ratio
3.5. Water Vapor Permeability
3.6. Contact Angle Measurements
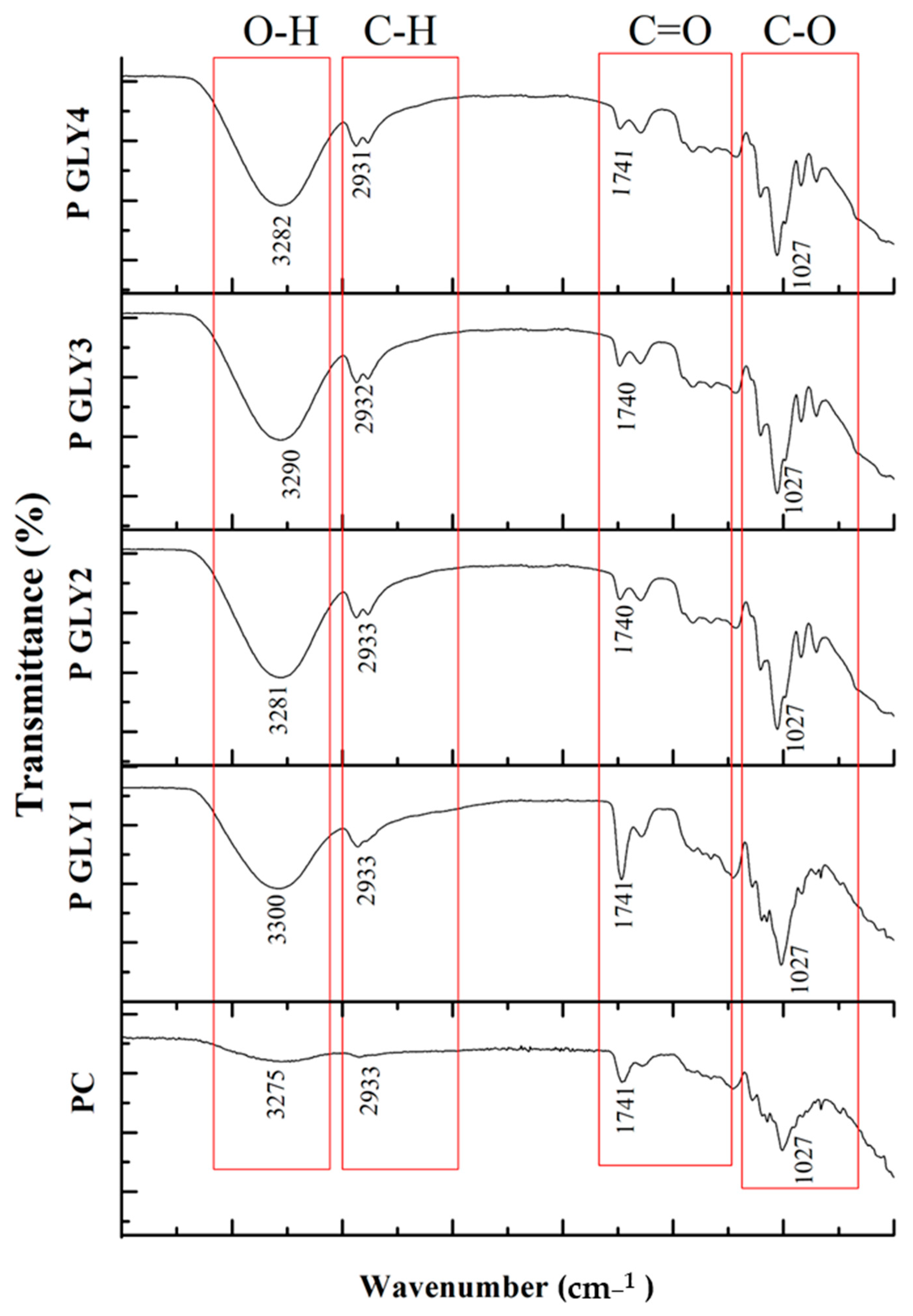
3.7. Fourier Transform Infrared Spectroscopy (FT-IR)
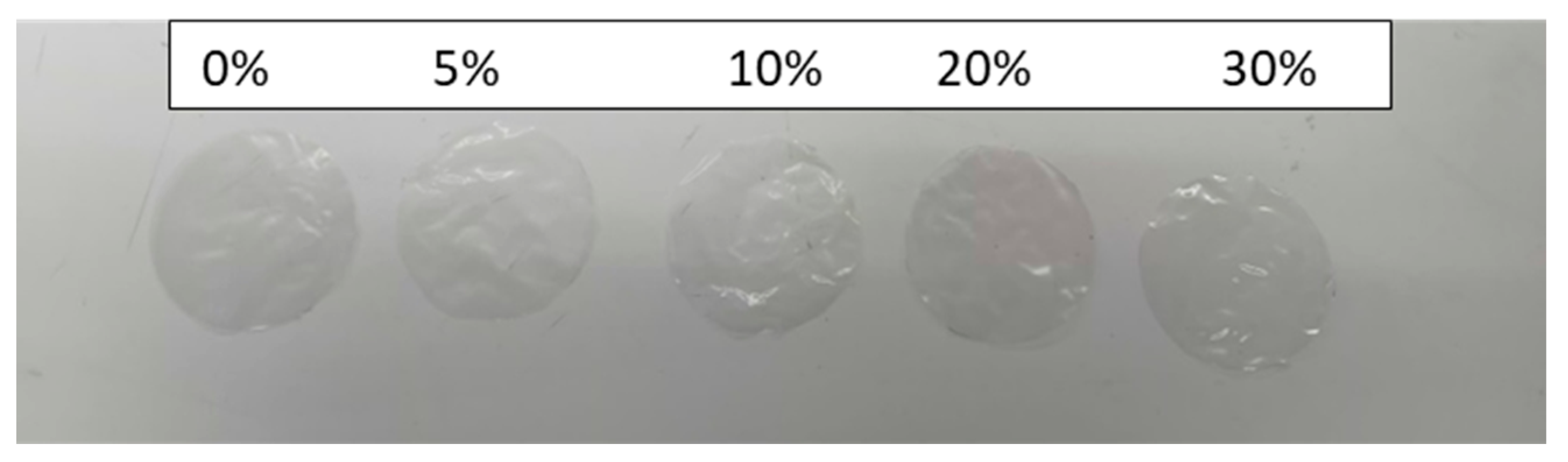
3.8. Optical Properties
3.9. Mechanical Properties
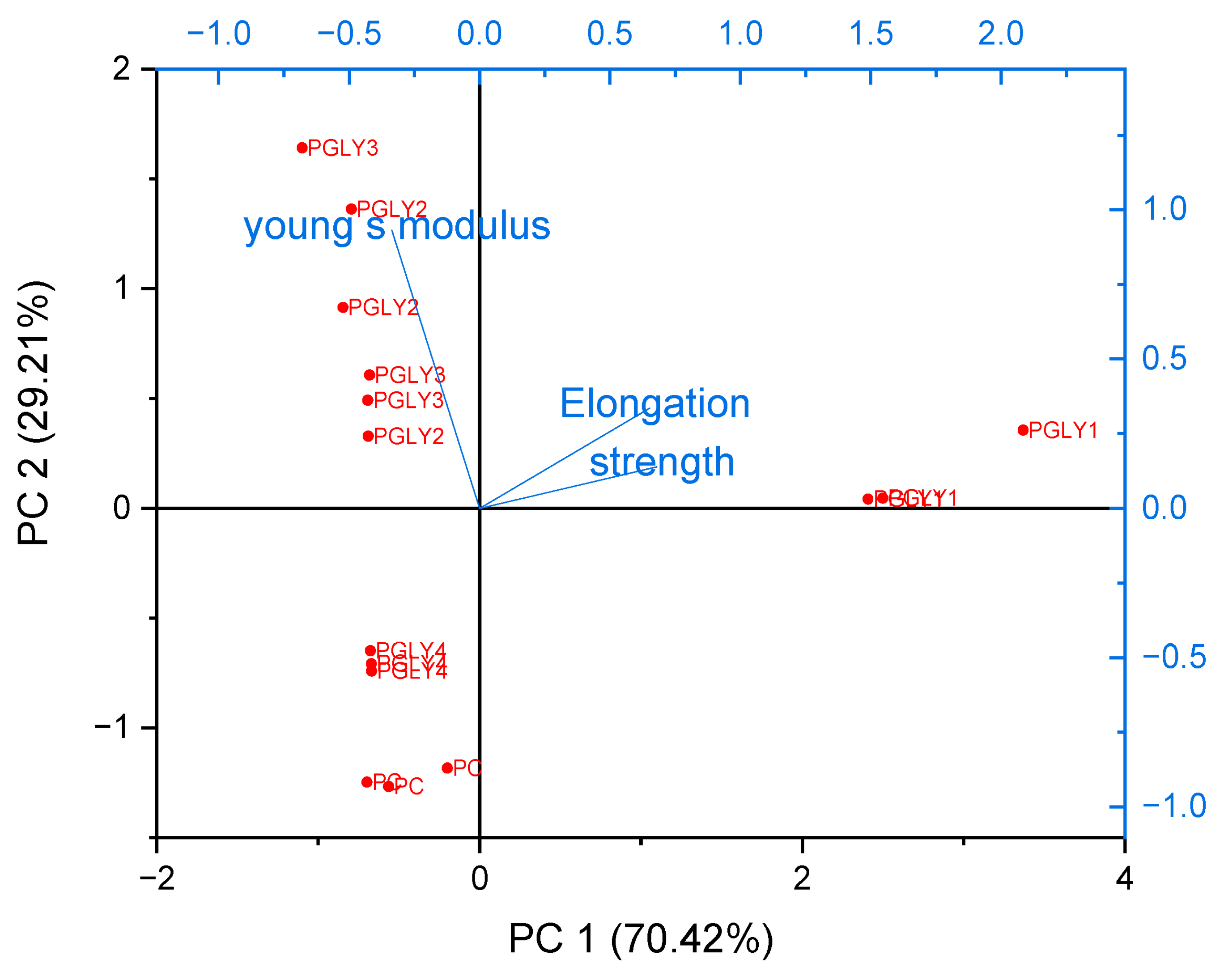
3.10. Principal Component Analysis for the Mechanical Properties
3.11. Burst Strength
3.12. Film Morphology
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- King, C.D.; Stephens, C.G.; Lynch, J.P.; Jordan, S.N. Farmers’ attitudes towards agricultural plastics—Management and disposal, awareness and perceptions of the environmental impacts. Sci. Total Environ. 2023, 864, 160955. [Google Scholar] [CrossRef] [PubMed]
- Panou, A.; Karabagias, I.K. Biodegradable packaging materials for foods preservation: Sources, advantages, limitations, and future perspectives. Coatings 2023, 13, 1176. [Google Scholar] [CrossRef]
- Khandeparkar, A.S.; Paul, R.; Sridhar, A.; Lakshmaiah, V.V.; Nagella, P. Eco-friendly innovations in food packaging: A sustainable revolution. Sustain. Chem. Pharm. 2024, 39, 101579. [Google Scholar] [CrossRef]
- Singaram, A.J.V.; Guruchandran, S.; Ganesan, N.D. Review on functionalized pectin films for active food packaging. Packag. Technol. Sci. 2024, 37, 237–262. [Google Scholar] [CrossRef]
- Barbut, S.; Harper, A. Influence of relative humidity on dried ca++-alginate films and composites made with soy and pectin. Ital. J. Food Sci. 2020, 32, 195–208. [Google Scholar]
- Mayakrishnan, V.; Madhavan, A.A. 11 Bio-based materials in advanced packaging applications. Sustain. Bio-Based Compos. Biomed. Eng. Appl. 2024, 20, 205. [Google Scholar]
- Ma, Y.; Luo, J.; Xu, Y. Co-preparation of pectin and cellulose from apple pomace by a sequential process. J. Food Sci. Technol. 2019, 56, 4091–4100. [Google Scholar] [CrossRef]
- Lim, X.Y.; Li, J.; Yin, H.M.; He, M.; Li, L.; Zhang, T. Stabilization of Essential Oil: Polysaccharide-Based Drug Delivery System with Plant-like Structure Based on Biomimetic Concept. Polymers 2023, 15, 3338. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, S.; Hardie, W.J.; Li, X.; Xiao, M.; Huang, T.; Xiong, T.; Xie, M. Microcapsules of a cinnamon, peppermint, and lemon essential oil mix by spray drying: Preparation, characterization and antibacterial functions. Food Hydrocoll. 2023, 145, 109103. [Google Scholar] [CrossRef]
- Akachat, B.; Himed, L.; Salah, M.; D’Elia, M.; Rastrelli, L.; Barkat, M. Development of Pectin-Based Films with Encapsulated Lemon Essential Oil for Active Food Packaging: Improved Antioxidant Activity and Biodegradation. Foods 2025, 14, 353. [Google Scholar] [CrossRef]
- Qiang, T.; Ren, W.; Chen, L. Biodegradable, high mechanical strength, and eco-friendly pectin-based plastic film. Food Hydrocoll. 2024, 149, 109539. [Google Scholar] [CrossRef]
- Eça, K.S.; Machado, M.T.; Hubinger, M.D.; Menegalli, F.C. Development of active films from pectin and fruit extracts: Light protection, antioxidant capacity, and compounds stability. J. Food Sci. 2015, 80, C2389–C2396. [Google Scholar] [CrossRef]
- Paudel, S.; Regmi, S.; Janaswamy, S. Effect of glycerol and sorbitol on cellulose-based biodegradable films. Food Packag. Shelf Life 2023, 37, 101090. [Google Scholar] [CrossRef]
- Dick, M.; Costa, T.M.H.; Gomaa, A.; Subirade, M.; de Oliveira Rios, A.; Flôres, S.H. Edible film production from chia seed mucilage: Effect of glycerol concentration on its physicochemical and mechanical properties. Carbohydr. Polym. 2015, 130, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Vityazev, F.V.; Khramova, D.S.; Saveliev, N.Y.; Ipatova, E.A.; Burkov, A.A.; Beloserov, V.S.; Belyi, V.A.; Kononov, L.O.; Martinson, E.A.; Litvinets, S.G.; et al. Pectin–glycerol gel beads: Preparation, characterization and swelling behaviour. Carbohydr. Polym. 2020, 238, 116166. [Google Scholar] [CrossRef]
- Liu, F.; Chiou, B.S.; Avena-Bustillos, R.J.; Zhang, Y.; Li, Y.; McHugh, T.H.; Zhong, F. Study of combined effects of glycerol and transglutaminase on properties of gelatin films. Food Hydrocoll. 2017, 65, 1–9. [Google Scholar] [CrossRef]
- Cai, C.; Ma, R.; Duan, M.; Deng, Y.; Liu, T.; Lu, D. Effect of starch film containing thyme essential oil microcapsules on physicochemical activity of mango. Lwt 2020, 131, 109700. [Google Scholar] [CrossRef]
- Bishnoi, A.; Chandla, N.K.; Talwar, G.; Khatkar, S.K.; Sharma, A. Mechanical Strength, Solubility, and Functional Studies of Developed Composite Biopolymeric Film. J. Food Process. Preserv. 2023, 2023, 5108490. [Google Scholar] [CrossRef]
- ASTM D570-22; ASTM License Agreement. ASTM: West Conshohocken, PA, USA, 2018.
- Ilyas, R.A.; Sapuan, S.M.; Ishak, M.R.; Zainudin, E.S. Sugar palm nanocrystalline cellulose reinforced sugar palm starch composite: Degradation and water-barrier properties. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Selangor, Malaysia, 21–23 November 2017; IOP Publishing: Bristol, UK, 2018; Volume 368, p. 012006. [Google Scholar]
- Remedio, L.N.; dos Santos, J.W.S.; Maciel, V.B.V.; Yoshida, C.M.P.; de Carvalho, R.A. Characterization of active chitosan films as a vehicle of potassium sorbate or nisin antimicrobial agents. Food Hydrocoll. 2019, 87, 830–838. [Google Scholar] [CrossRef]
- ASTM International. ASTM D882-12, Standard Test Method for Tensile Properties of Thin Plastic Sheeting; ASTM International: West Conshohocken, PA, USA, 2012. [Google Scholar]
- Terzioğlu, P.; Güney, F.; Parın, F.N.; Şen, İ.; Tuna, S. Biowaste orange peel incorporated chitosan/polyvinyl alcohol composite films for food packaging applications. Food Packag. Shelf Life 2021, 30, 100742. [Google Scholar] [CrossRef]
- Pirinç, F.T.; Dağdelen, A.F.; Sarıcaoğlu, F.T. Optical and mechanical properties of bi-layer biodegradable films from poly lactic acid and bovine gelatin. Eur. Food Sci. Eng. 2020, 1, 13–17. [Google Scholar]
- Geleta, T.T.; Habtegebreil, S.A.; Tolesa, G.N. Physical, mechanical, and optical properties of enset starch from bulla films influenced by different glycerol concentrations and temperatures. J. Food Process. Preserv. 2020, 44, e14586. [Google Scholar] [CrossRef]
- Zakaria, N.H.; Muhammad, N.; Abdullah, M.M.A.B. Effect of glycerol content on mechanical, microstructure and physical properties of thermoplastic potato starch film. AIP Conf. Proc. 2018, 2030, 020230. [Google Scholar] [CrossRef]
- Ertürk, N.; Ay, S.B. Effect of Ethylene Glycol and Glycerol Concentrations on Properties of Rye-Based Films. Avrupa Bilim Ve Teknol. Derg. 2022, 34, 705–710. [Google Scholar] [CrossRef]
- Malmir, S.; Montero, B.; Rico, M.; Barral, L.; Bouza, R. Morphology, thermal and barrier properties of biodegradable films of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) containing cellulose nanocrystals. Compos. Part A Appl. Sci. Manuf. 2017, 93, 41–48. [Google Scholar] [CrossRef]
- Hernando, H.; Julianti, E.; Nuryawan, A.; Amaturrahim, S.A.; Piliang, A.F.R.; Yanhar, M.R.; Goei, R.; Soykeabkaew, N.; Saputra, A.M.A.; Gea, S. Impact of glycerol on oil palm trunk starch bioplastics enhanced with citric-acid epoxidized palm oil oligomers. Case Stud. Chem. Environ. Eng. 2024, 10, 100839. [Google Scholar] [CrossRef]
- Syafiq, R.M.O.; Sapuan, S.M.; Zuhri, M.Y.M.; Othman, S.H.; Ilyas, R.A. Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films. Nanotechnol. Rev. 2022, 11, 423–437. [Google Scholar] [CrossRef]
- Shafqat, A.; Al-Zaqri, N.; Tahir, A.; Alsalme, A. Synthesis and characterization of starch based bioplatics using varying plant-based ingredients, plasticizers and natural fillers. Saudi J. Biol. Sci. 2021, 28, 1739–1749. [Google Scholar] [CrossRef]
- Abedini, A.A.; Pircheraghi, G.; Kaviani, A. The role of calcium crosslinking and glycerol plasticizing on the physical and mechanical properties of superabsorbent: Alginate/Quince Seed Gum films. J. Polym. Res. 2023, 30, 20. [Google Scholar] [CrossRef]
- da Silva, M.A.; Bierhalz, A.C.K.; Kieckbusch, T.G. Alginate and pectin composite films crosslinked with Ca2+ ions: Effect of the plasticizer concentration. Carbohydr. Polym. 2009, 77, 736–742. [Google Scholar] [CrossRef]
- Santhosh, R.; Sarkar, P. Jackfruit seed starch/tamarind kernel xyloglucan/zinc oxide nanoparticles-based composite films: Preparation, characterization, and application on tomato (Solanum lycopersicum) fruits. Food Hydrocoll. 2022, 133, 107917. [Google Scholar] [CrossRef]
- Sothornvit, R.; Krochta, J.M. Plasticizers in edible films and coatings. In Innovations in Food Packaging; Academic Press: Cambridge, MA, USA, 2005; pp. 403–433. [Google Scholar]
- Zhang, Y.; Deng, L.; Zhong, H.; Pan, J.; Li, Y.; Zhang, H. Superior water stability and antimicrobial activity of electrospun gluten nanofibrous films incorporated with glycerol monolaurate. Food Hydrocoll. 2020, 109, 106116. [Google Scholar] [CrossRef]
- Agustin, S.; Cahyanto, M.N.; Wahyuni, E.T. Effect of glycerol plasticizer on the structure and characteristics of bacterial cellulose-based biocomposite films. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Yogyakarta, Indonesia, 12–13 December 2023; IOP Publishing: Bristol, UK, 2024; Volume 1377, p. 012046. [Google Scholar]
- Tarique, J.; Sapuan, S.M.; Khalina, A. Effect of glycerol plasticizer loading on the physical, mechanical, thermal, and barrier properties of arrowroot (Maranta arundinacea) starch biopolymers. Sci. Rep. 2021, 11, 13900. [Google Scholar] [CrossRef] [PubMed]
- Ngo, T.M.P.; Nguyen, T.H.; Dang, T.M.Q.; Tran, T.X.; Rachtanapun, P. Characteristics and antimicrobial properties of active edible films based on pectin and nanochitosan. Int. J. Mol. Sci. 2020, 21, 2224. [Google Scholar] [CrossRef]
- Sreekumar, P.A.; Leblanc, N.; Saiter, J.M. Effect of glycerol on the properties of 100% biodegradable thermoplastic based on wheat flour. J. Polym. Environ. 2013, 21, 388–394. [Google Scholar] [CrossRef]
- Fu, Z.Q.; Guo, S.X.; Sun, Y.; Wu, H.J.; Huang, Z.G.; Wu, M. Effect of glycerol content on the properties of potato flour films. Starch-Stärke 2021, 73, 2000203. [Google Scholar] [CrossRef]
- Rai, S.K.; Chaturvedi, K.; Yadav, S.K. Evaluation of structural integrity and functionality of commercial pectin based edible films incorporated with corn flour, beetroot, orange peel, muesli and rice flour. Food Hydrocoll. 2019, 91, 127–135. [Google Scholar]
- Cao, L.; Liu, W.; Wang, L. Developing a green and edible film from Cassia gum: The effects of glycerol and sorbitol. J. Clean. Prod. 2018, 175, 276–282. [Google Scholar] [CrossRef]
- Wang, X.; Ullah, N.; Sun, X.; Guo, Y.; Chen, L.; Li, Z.; Feng, X. Development and characterization of bacterial cellulose reinforced biocomposite films based on protein from buckwheat distiller’s dried grains. Int. J. Biol. Macromol. 2017, 96, 353–360. [Google Scholar] [CrossRef]
- Jeevahan, J.; Chandrasekaran, M. Nanoedible films for food packaging: A review. J. Mater. Sci. 2019, 54, 12290–12318. [Google Scholar] [CrossRef]
- Phumkacha, A.; Leejarkpai, T.; Kirdponpattara, S. Effects of Plasticizers on Physical and Mechanical Properties of Tamarind Kernel Powder Film. In Materials Science Forum; Trans Tech Publications Ltd.: Wollerau, Switzerland, 2023; Volume 1098, pp. 65–69. [Google Scholar]
- Matta, E.; Bertola, N. Development and characterization of high methoxyl pectin film by using isomalt as plasticizer. J. Food Process. Preserv. 2020, 44, e14568. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Hasan, Z.; Omran, A.A.B.; Elfaghi, A.M.; Khattak, M.A.; Ilyas, R.A.; Sapuan, S.M. Effect of various plasticizers in different concentrations on physical, thermal, mechanical, and structural properties of wheat starch-based films. Polymers 2022, 15, 63. [Google Scholar] [CrossRef] [PubMed]
- Katili, S.; Harsunu, B.T.; Irawan, S. Effect of plasticizer concentration of glycerol and chitosan compositions in the solvent on the physical properties of chitosan edible film. J. Teknol. 2013, 6, 29–38. [Google Scholar]
- Liu, H.; Adhikari, R.; Guo, Q.; Adhikari, B. Preparation and characterization of glycerol plasticized (high-amylose) starch–chitosan films. J. Food Eng. 2013, 116, 588–597. [Google Scholar] [CrossRef]
- Shanbhag, C.; Shenoy, R.; Shetty, P.; Srinivasulu, M.; Nayak, R. Formulation and characterization of starch-based novel biodegradable edible films for food packaging. J. Food Sci. Technol. 2023, 60, 2858–2867. [Google Scholar] [CrossRef]
- Mishra, S.P.; Sarkar, U.; Taraphder, S.; Datta, S.; Swain, D.; Saikhom, R.; Panda, S.; Laishram, M. Multivariate statistical data analysis-principal component analysis (PCA). Int. J. Livest. Res. 2017, 7, 60–78. [Google Scholar]
- Bharti, B.M.; Bhuvana, T.; Chandraprakash, C. Burst and physicochemical characteristics of glycerol-added chitosan films for food packaging. ACS Food Sci. Technol. 2023, 3, 772–780. [Google Scholar] [CrossRef]
- Cazón, P.; Vázquez, M.; Velazquez, G. Composite films with UV-barrier properties of bacterial cellulose with glycerol and poly (vinyl alcohol): Puncture properties, solubility, and swelling degree. Biomacromolecules 2019, 20, 3115–3125. [Google Scholar] [CrossRef]

| Sample Code | Pectin (% w/v) | Essential Oil (% w/v) | Glycerol (% wt of Polymer) | Citric Acid (% w/w) |
|---|---|---|---|---|
| PC | 5 | 1 | 0 | 10 |
| PGLY1 | 5 | 1 | 5 | 10 |
| PGLY2 | 5 | 1 | 10 | 10 |
| PGLY3 | 5 | 1 | 20 | 10 |
| PGLY4 | 5 | 1 | 30 | 10 |
| Film | Thickness (mm) | Moisture Content (%) | Solubility (%) | Swelling Ratio (%) | WVP (g m−1 s−1 Pa−1) |
|---|---|---|---|---|---|
| PC | 0.066 ± 0.002 | 18.00 ± 0.06 | 13.83 ± 0.28 | 17.81 ± 0.50 | 3.64 × 10−1⁰ ± 0.08 |
| PGLY1 | 0.086 ± 0.001 | 21.00 ± 0.09 | 19.79 ± 0.42 | 31.81 ± 0.61 | 3.26 × 10−11 ± 0.01 |
| PGLY2 | 0.283 ± 0.005 | 21.59 ± 0.10 | 25.74 ± 0.54 | 48.96 ± 0.96 | 2.65 × 10−11 ± 0.01 |
| PGLY3 | 0.325 ± 0.000 | 22.00 ± 0.11 | 26.48 ± 0.59 | 56.35 ± 1.12 | 2.04 × 10−11 ± 0.01 |
| PGLY4 | 0.453 ± 0.001 | 25.00 ± 0.14 | 32.00 ± 0.67 | 76.67 ± 1.24 | 1.13 × 10−1⁰ ± 0.05 |
| Films | Contact Angle ° | Image |
|---|---|---|
| PC | PC 69.19 ± 0.67 | 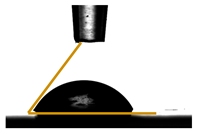 |
| PGLY1 | 56.98 ± 0.69 | 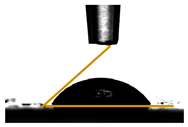 |
| PGLY2 | 28.98 ± 0.30 | 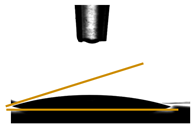 |
| PGLY3 | 22.69 ± 0.70 | 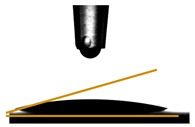 |
| PGLY4 | 18.54 ± 0.74 | 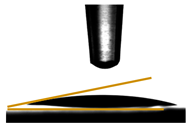 |
| Film Sample | Transmission (%) | Transparency UV Range (280 nm) | Transparency Visible Range (600 nm) |
|---|---|---|---|
| PC | 62.50 ± 0.25 | 72.55 ± 0.50 | 1.624 ± 0.02 |
| PGLY1 | 61.25 ± 0.30 | 78.30 ± 0.40 | 1.246 ± 0.03 |
| PGLY2 | 31.03 ± 0.20 | 59.52 ± 0.60 | 0.814 ± 0.01 |
| PGLY3 | 28.71 ± 0.15 | 45.06 ± 0.50 | 0.795 ± 0.02 |
| PGLY4 | 28.64 ± 0.12 | 45.05 ± 0.45 | 0.753 ± 0.01 |
| Film Samples | Elongation (%) | Strength (MPa) | Young’s Modulus (MPa) |
|---|---|---|---|
| PC | 1.25 ± 0.93 a | 2.10 ± 1.56 a | 1.09 ± 0.40 a |
| PGLY1 | 20.62 ± 2.19 d | 20.43 ± 3.89 b | 2.83 ± 0.48 a |
| PGLY2 | 6.16 ± 1.13 bc | 2.66 ± 0.51 a | 22.33 ± 5.13 b |
| PGLY3 | 5.28 ± 1.11 c | 2.40 ± 0.65 a | 21.75 ± 6.93 b |
| PGLY4 | 2.90 ± 1.00 a | 0.87 ± 0.45 a | 6.45 ± 0.45 a |
| Film Sample | Burst Strength (g) | Distance at Burst (mm) |
|---|---|---|
| PC | 771.70 ± 2.31 | 0.930 ± 0.03 |
| PGLY1 | 4272.27 ± 128.17 | 3.610 ± 0.11 |
| PGLY2 | 673.25 ± 20.18 | 3.560 ± 0.11 |
| PGLY3 | 559.40 ± 16.78 | 3.568 ± 0.11 |
| PGLY4 | 219.20 ± 6.56 | 1.570 ± 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akachat, B.; Himed, L.; Torche, A.; Khelef, Y.; Barkat, M.; Salah, M.; D’Elia, M.; Rastrelli, L.; Terzioğlu, P. Evaluation of Glycerol Concentration in the Production of Lemon Oil Incorporated Pectin-Based Films Using Principal Component Analysis. Foods 2025, 14, 1576. https://doi.org/10.3390/foods14091576
Akachat B, Himed L, Torche A, Khelef Y, Barkat M, Salah M, D’Elia M, Rastrelli L, Terzioğlu P. Evaluation of Glycerol Concentration in the Production of Lemon Oil Incorporated Pectin-Based Films Using Principal Component Analysis. Foods. 2025; 14(9):1576. https://doi.org/10.3390/foods14091576
Chicago/Turabian StyleAkachat, Belkis, Louiza Himed, Assala Torche, Yahia Khelef, Malika Barkat, Merniz Salah, Maria D’Elia, Luca Rastrelli, and Pınar Terzioğlu. 2025. "Evaluation of Glycerol Concentration in the Production of Lemon Oil Incorporated Pectin-Based Films Using Principal Component Analysis" Foods 14, no. 9: 1576. https://doi.org/10.3390/foods14091576
APA StyleAkachat, B., Himed, L., Torche, A., Khelef, Y., Barkat, M., Salah, M., D’Elia, M., Rastrelli, L., & Terzioğlu, P. (2025). Evaluation of Glycerol Concentration in the Production of Lemon Oil Incorporated Pectin-Based Films Using Principal Component Analysis. Foods, 14(9), 1576. https://doi.org/10.3390/foods14091576








