Comparison of the Structure, Physicochemical Properties, and Impact on Intestinal Flora of Processed and Unprocessed Polygonum multiflorum Starch
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Starch Isolation
2.3. Chemical Composition
2.4. Water Solubility and Swelling Power
2.5. Morphology and Particle Size
2.6. Molecular Weight
2.7. Crystal Structure
2.8. Fourier Transform Infrared (FT-IR) Spectroscopy
2.9. Thermal Stability Analysis (TGA)
2.10. Determination of Pasting Properties
2.11. In Vitro Digestion of Starch
2.12. Animal Experimental Design
2.13. Gut Microbiota Analysis
2.14. Statistical Analysis
3. Results
3.1. Comparison of the Chemical Composition of Different Starches
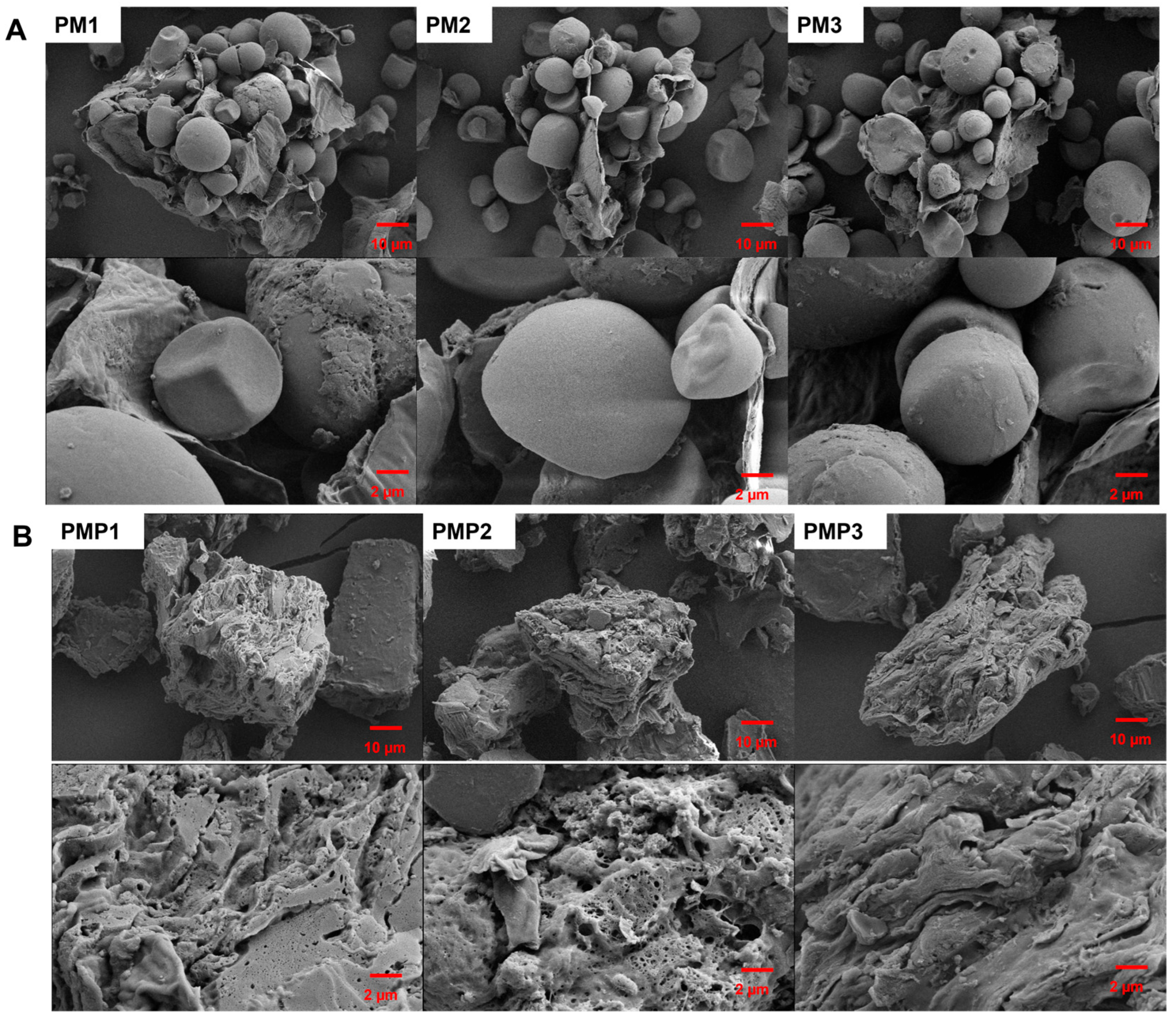
3.2. Morphological Analysis
3.3. Molecular Weight and Pasting Properties
3.4. X-Ray Diffraction
3.5. FT-IR Analysis
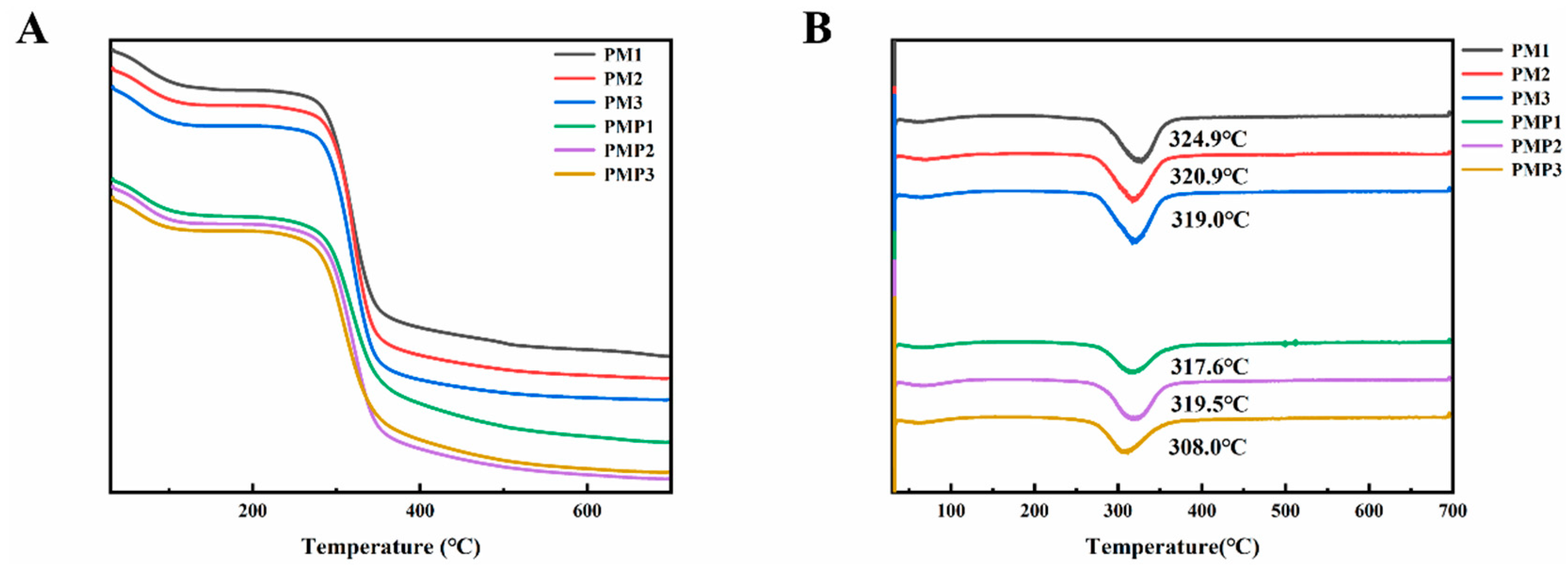
3.6. Thermal Stability Analysis
3.7. In Vitro Digestion Property
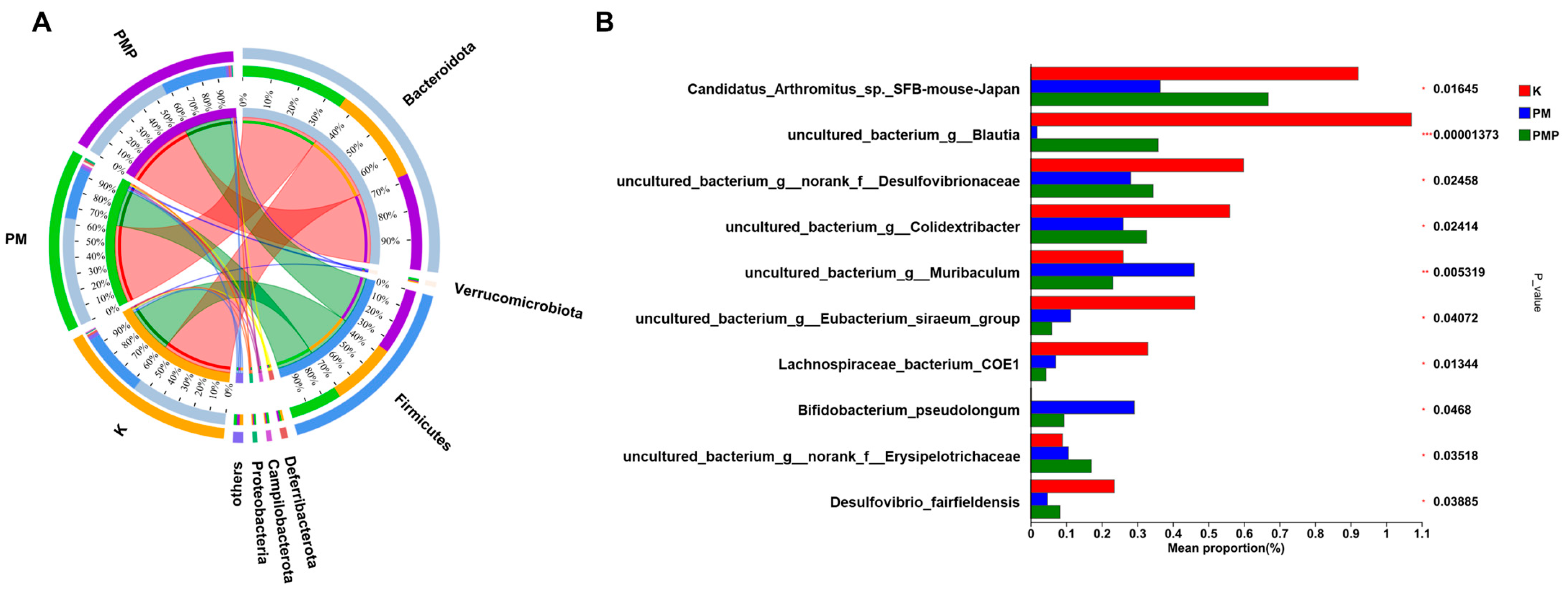
3.8. Microbial Community Distribution
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Cao, P.; Wu, G.; Yao, Z.; Wang, Z.; Li, E.; Yu, S.; Liu, Q.; Gilbert, R.G.; Li, S. Effects of amylose and amylopectin molecular structures on starch electrospinning. Carbohydr. Polym. 2022, 296, 119959. [Google Scholar] [CrossRef] [PubMed]
- Shim, Y.E.; Song, Y.B.; Yoo, S.H.; Lee, B.H. Production of highly branched α-limit dextrins with enhanced slow digestibility by various glycogen-branching enzymes. Carbohydr. Polym. 2023, 15, 120730. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Lin, Y. Effect of Thermal Processing on Flow Properties and Stability of Thickened Fluid Matrices Formulated by Tapioca Starch, Hydroxyl Distarch Phosphate (E-1442), and Xanthan Gum Associating Dysphagia-Friendly Potential. Polymers 2021, 13, 162. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chao, C.; Cai, J.; Niu, B.; Copeland, L.; Wang, S. Starch-lipid and starch-lipid-protein complexes: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1056–1079. [Google Scholar] [CrossRef]
- Jiang, S.; Cen, J.; Zhou, Y.; Wang, Y.; Wu, D.; Wang, Z.; Sun, J.; Shu, X. Physicochemical characterizations of five Dioscorea alata L. starches from China. Int. J. Biol. Macromol. 2023, 237, 124225. [Google Scholar] [CrossRef]
- Ai, Z.; Xie, Y.; Li, X.; Lei, D.; Ambrose, K.; Liu, Y. Revealing color change and drying mechanisms of pulsed vacuum steamed Cistanche deserticola through bioactive components, microstructural and starch gelatinization properties. Food Res. Int. 2022, 162, 112079. [Google Scholar] [CrossRef]
- Xia, Y.; Gao, W.; Jiang, Q.; Li, X.; Huang, L.; Xiao, P. Comparison of the physicochemical and functional properties of Aconitum carmichaeli and Aconiti lateralis Preparata starches. Starch-Starke 2011, 63, 765–770. [Google Scholar] [CrossRef]
- Yang, X.; Dai, J.; Guo, D.; Zhao, S.; Huang, Y.; Chen, X.; Huang, Q. Changes in the properties of Radix Aconiti Lateralis Preparata (Fuzi, processed aconite roots) starch during processing. J. Food Sci. Technol. 2019, 56, 24–29. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, W.; Chen, Z.; He, J.; Feng, L.; Wang, L.; Chen, S. Resistant starch reduces glycolysis by HK2 and suppresses high-fructose corn syrup-induced colon tumorigenesis. J. Gastroenterol. 2024, 59, 905–920. [Google Scholar] [CrossRef]
- Zhang, C.; Qiu, M.; Wang, T.; Luo, L.; Xu, W.; Wu, J.; Zhao, F.; Liu, K.; Zhang, Y.; Wang, X. Preparation, structure characterization, and specific gut microbiota properties related to anti-hyperlipidemic action of type 3 resistant starch from Canna edulis. Food Chem. 2021, 351, 129340. [Google Scholar] [CrossRef]
- Huang, H.; Jiang, Q.; Chen, Y.; Li, X.; Mao, X.; Chen, X.; Huang, L.; Gao, W. Preparation, physico-chemical characterization and biological activities of two modified starches from yam (Dioscorea opposita Thunb.). Food Hydrocoll. 2016, 55, 244–253. [Google Scholar] [CrossRef]
- Mao, X.; Lu, J.; Huang, H.; Gao, X.; Zheng, H.; Chen, Y. Four types of winged yam (Dioscorea alata L.) resistant starches and their effects on ethanol-induced gastric injury in vivo. Food Hydrocoll. 2018, 85, 21–29. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Song, Y.H.; Zhao, R.; Xia, L.; Chen, Y.; Cui, Y.P.; Rao, Z.Y.; Zhou, Y.; Zhuang, W.; et al. Effects of the resistant starch on glucose, insulin, insulin resistance, and lipid parameters in overweight or obese adults: A systematic review and meta-analysis. Nutr. Diabetes 2019, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Vahdat, M.; Hosseini, S.A.; Khalatbari Mohseni, G.; Heshmati, J.; Rahimlou, M. Effects of resistant starch interventions on circulating inflammatory biomarkers: A systematic review and meta-analysis of randomized controlled trials. Nutr. J. 2020, 19, 33. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, X.; Meng, Y.; Wang, Q.; Xu, H.; Chen, L. The Effects of Resistant Starch on Biomarkers of Inflammation and Oxidative Stress: A Systematic Review and Meta-Analysis. Nutr. Cancer 2022, 74, 2337–2350. [Google Scholar] [CrossRef]
- Jia, L.; Dong, X.; Li, X.; Jia, R.; Zhang, H.L. Benefits of resistant starch type 2 for patients with end-stage renal disease under maintenance hemodialysis: A systematic review and meta-analysis. Int. J. Med. Sci. 2021, 18, 811–820. [Google Scholar] [CrossRef]
- Xu, J.; Kong, H.; Li, C.; Ban, X.; Li, Z. Effect of resistant starch supplementation on the diversity and composition of human gut microbiota: A systematic review and meta-analysis. Food Sci. Hum. Wellness 2025, 14, 9250055. [Google Scholar] [CrossRef]
- Chen, W.; Wang, P.; Chen, H.; Xing, Y.; Liu, C.; Pan, G.; Dou, Z.; Han, L. The composition differences between small black beans and big black beans from different habitats and its effects on the processing of Polygonum multiflorum. Phytochem. Anal. 2021, 32, 767–779. [Google Scholar] [CrossRef]
- Yang, J.B.; Ye, F.; Tian, J.Y.; Song, Y.F.; Gao, H.Y.; Liu, Y.; Wang, Q.; Wang, Y.; Ma, S.C.; Cheng, X.L.; et al. Multiflorumisides H—K, stilbene glucosides isolated from Polygonum multiflorum and their in vitro PTP1B inhibitory activities. Fitoterapia 2020, 146, 104703. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, J.; Jin, H.; Gu, D.; Wang, Q.; Liu, Y.; Zan, K.; Fan, J.; Wang, R.; Wei, F.; et al. Comparisons of physicochemical features and hepatoprotective potentials of unprocessed and processed polysaccharides from Polygonum multiflorum Thunb. Int. J. Biol. Macromol. 2023, 235, 123901. [Google Scholar] [CrossRef]
- Ma, X.; Chang, P.R.; Zheng, W.; Yu, J.; Ma, X. Characterization of new starches separated from several traditional Chinese medicines. Carbohydr. Polym. 2010, 82, 148–152. [Google Scholar] [CrossRef]
- Yu, J.L.; Wang, S. Morphological and Crystalline Properties of Starches from New Sources-Traditional Chinese Medicines (TCMs). Starch-Starke 2008, 60, 110–114. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, X.; Zheng, B.; Zhang, Y. Structural and physicochemical properties of lotus seed starch-chlorogenic acid complexes prepared by microwave irradiation. J. Food Sci. Technol. 2020, 58, 4157–4166. [Google Scholar] [CrossRef] [PubMed]
- Gisbert, M.; Aleixandre, A.; Sineiro, J.; Rosell, C.M.; Moreira, R. Interactions between Ascophyllum nodosum Seaweeds Polyphenols and Native and Gelled Corn Starches. Foods 2022, 18, 1165. [Google Scholar] [CrossRef]
- Liang, W.; Zhang, Q.; Duan, H.; Zhou, S.; Zhou, Y.; Li, W.; Yan, W. Understanding CaCl2 induces surface gelatinization to promote cold plasma modified maize starch: Structure-effect relations. Carbohydr. Polym. 2023, 320, 121200. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, D.; Liu, X.; Zheng, J.; Liang, W.; Shen, H.; Ge, X.; Hu, Y.; Li, W. Effect of electron beam irradiation on granular cold-water swelling chestnut starch: Improvement of cold-water solubility, multiscale structure, and rheological properties. Carbohydr. Polym. 2023, 319, 121164. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, Z.; Wang, Z.; Chen, R.; Zhang, S. Molecular interactions between apigenin and starch with different amylose/amylopectin ratios revealed by X-ray diffraction, FT-IR and solid-state NMR. Carbohydr. Polym. 2023, 310, 120737. [Google Scholar] [CrossRef]
- Kumar, S.R.; Tangsrianugul, N.; Sriprablom, J.; Wongsagonsup, R.; Wansuksri, R.; Suphantharika, M. Effect of heat-moisture treatment on the physicochemical properties and digestibility of proso millet flour and starch. Carbohydr. Polym. 2023, 307, 120630. [Google Scholar] [CrossRef]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46, S33–S50. [Google Scholar]
- Lagod, P.P.; Naser, S.A. The Role of Short-Chain Fatty Acids and Altered Microbiota Composition in Autism Spectrum Disorder: A Comprehensive Literature Review. Int. J. Mol. Sci. 2023, 24, 17432. [Google Scholar] [CrossRef]
- Sun, S.; Hong, Y.; Gu, Z.; Cheng, L.; Ban, X.; Li, Z.; Li, C. Different starch varieties influence the complexing state and digestibility of the resulting starch-lipid complexes. Food Hydrocoll. 2023, 141, 108679. [Google Scholar] [CrossRef]
- Liu, Z.; Deng, N.; Luo, S.; Liu, C.; Hu, X. Fermentation of resistant starch from the starch-ferulic acid inclusion complex compared with high-amylose corn starch. Int. J. Biol. Macromol. 2023, 246, 125647. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Fan, J.; Tian, Z.; Ma, L.; Meng, Y.; Yang, Z.; Zeng, X.; Liu, X.; Kang, L.; Nan, X. Effects of treatment methods on the formation of resistant starch in purple sweet potato. Food Chem. 2022, 367, 130580. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yu, P.; Fan, L.; Sun, Y. Effects of ultrasound treatment on the starch properties and oil absorption of potato chips. Ultrason Sonochem. 2021, 70, 105347. [Google Scholar] [CrossRef]
- Vamadevan, V.; Bertoft, E. Observations on the impact of amylopectin and amylose structure on the swelling of starch granules. Food Hydrocoll. 2020, 103, 105663. [Google Scholar] [CrossRef]
- Obadi, M.; Xu, B. Review on the physicochemical properties, modifications, and applications of starches and its common modified forms used in noodle products. Food Hydrocoll. 2021, 112, 106286. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, Y.; Herbuger, K.; Petersen, B.; Cui, Y.; Blennow, A.; Liu, X.; Zhong, Y. High-pressure pasting performance and multilevel structures of short-term microwave-treated high-amylose maize starch. Carbohydr. Polym. 2023, 322, 121366. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Su, P.; Pan, N.; Liu, X.; Zhang, Y.; Zhang, H. Insights into the aggregation structure and physicochemical properties of heat-moisture treated wheat starch and its associated effects on noodle quality. J. Cereal Sci. 2023, 112, 103704. [Google Scholar] [CrossRef]
- Sun, R.; Chao, C.; Yu, J.; Copeland, L.; Wang, S. Type 5 Resistant Starch Can Effectively Alleviate Experimentally Induced Colitis in Mice by Modulating Gut Microbiota. J. Agric. Food Chem. 2025, 73, 2103–2113. [Google Scholar] [CrossRef]
- Huang, M.; Wang, Y.; Ahmad, M.; Ying, R.; Wang, Y.; Tan, C. Fabrication of pickering high internal phase emulsions stabilized by pecan protein/xanthan gum for enhanced stability and bioaccessibility of quercetin. Food Chem. 2021, 357, 129732. [Google Scholar] [CrossRef]
- Krueger, B.; Knutson, C.; Inglett, G.; Walker, C. A differential scanning calorimetry study on the effect of annealing on gelatinization behavior of corn starch. J. Food Sci. 1987, 52, 715–718. [Google Scholar] [CrossRef]
- Lee, Y.-E.; Osman, E.M. Correlation of morphological changes of rice starch granules with rheological properties during heating in excess water. Appl. Biol. Chem. 1991, 34, 379–385. [Google Scholar]
- Ma, H.; Liu, M.; Liang, Y.; Zheng, X.; Sun, L.; Dang, W.; Li, J.; Li, L.; Liu, C. Research progress on properties of pre-gelatinized starch and its application in wheat flour products. Grain Oil Sci. Technol. 2022, 5, 87–97. [Google Scholar] [CrossRef]
- Bajka, B.H.; Clarke, J.M.; Topping, D.L.; Cobiac, L.; Abeywardena, M.Y.; Patten, G.S. Butyrylated starch increases large bowel butyrate levels and lowers colonic smooth muscle contractility in rats. Nutr. Res. 2010, 30, 427–434. [Google Scholar] [CrossRef]
- Lemos, P.; Marcelino, H.; Cardoso, L.; Souza, C.; Druzian, J. Starch chemical modifications applied to drug delivery systems: From fundamentals to FDA-approved raw materials. Int. J. Biol. Macromol. 2021, 184, 218–234. [Google Scholar] [CrossRef]
- Wijegunawardhana, D.; Wijesekara, I.; Liyanage, R.; Truong, T.; Silva, M.; Chandrapala, J. The Impact of Varying Lactose-to-Maltodextrin Ratios on the Physicochemical and Structural Characteristics of Pasteurized and Concentrated Skim and Whole Milk-Tea Blends. Foods 2024, 13, 3016. [Google Scholar] [CrossRef]
- Kong, H.; Yu, L.; Li, C.; Ban, X.; Gu, Z.; Li, Z. Short-Clustered Maltodextrin Activates Ileal Glucose-Sensing and Induces Glucagon-like Peptide 1 Secretion to Ameliorate Glucose Homeostasis in Type 2 Diabetic Mice. J. Agric. Food Chem. 2022, 70, 12604–12619. [Google Scholar] [CrossRef]
- Zhong, Y.; Li, Y.; Qu, J.; Zhang, X.; Seytahmetovna, S.A.; Blennow, A.; Guo, D. Structural features of five types of maize starch granule subgroups sorted by flow cytometry. Food Chem. 2021, 356, 129657. [Google Scholar] [CrossRef]
- Mariappan, G.; Sundaraganesan, N.; Manoharan, S. The spectroscopic properties of anticancer drug Apigenin investigated by using DFT calculations, FT-IR, FT-Raman and NMR analysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 95, 86–99. [Google Scholar] [CrossRef]
- Karonen, M. Insights into Polyphenol-Lipid Interactions: Chemical Methods, Molecular Aspects and Their Effects on Membrane Structures. Plants 2022, 11, 1809. [Google Scholar] [CrossRef]
- Yang, Z.; Hao, H.; Wu, Y.; Liu, Y.; Ouyang, J. Influence of moisture and amylose on the physicochemical properties of rice starch during heat treatment. Int. J. Biol. Macromol. 2021, 168, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Liu, P.; Zhu, J.; Hou, H.; Li, X.; Cui, B. Physicochemical properties of corn starch affected by the separation of granule shells. Int. J. Biol. Macromol. 2020, 164, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Dong, Y.; Gao, W.; Wu, Z.; Yu, B.; Yuan, C.; Cui, B. Effects of water/ionic liquid ratios on the physicochemical properties of high amylose maize starch-lauric acid complex. Food Hydrocoll. 2023, 135, 108134. [Google Scholar] [CrossRef]
- Ali, N.A.; Dash, K.; Routray, W. Physicochemical characterization of modified lotus seed starch obtained through acid and heat moisture treatment. Food Chem. 2020, 319, 126513. [Google Scholar] [CrossRef]
- Dong, F.; Gao, W.; Liu, P.; Kang, X.; Yu, B.; Cui, B. Digestibility, structural and physicochemical properties of microcrystalline butyrylated pea starch with different degree of substitution. Carbohydr. Polym. 2023, 314, 120927. [Google Scholar] [CrossRef]
- Liu, X.; Mao, B.; Gu, J. Blautia—A new functional genus with potential probiotic properties? Gut Microbes 2021, 13, 1875796. [Google Scholar] [CrossRef]



| Sample | Moisture Content (%) | Ash Content (%) | Total Starch Content (mg/g) | Amylose Content (mg/g) | Swelling Power | Solubility (%) |
|---|---|---|---|---|---|---|
| PM1 | 9.32 ± 0.03 | 4.93 ± 0.01 | 197.83 ± 6.98 | 87.70 ± 6.12 | 12.71 ± 1.68 | 2.79 ± 0.11 |
| PM2 | 7.81 ± 0.01 | 4.22 ± 0.00 | 127.97 ± 5.97 | 88.22 ± 5.32 | 15.86 ± 0.84 | 2.43 ± 0.08 |
| PM3 | 8.61 ± 0.01 | 4.68 ± 0.00 | 147.76 ± 2.04 | 82.30 ± 2.16 | 14.49 ± 0.96 | 2.89 ± 0.02 |
| PMP1 | 9.54 ± 0.01 | 2.68 ± 0.02 | 197.27 ± 6.47 | 72.81 ± 2.87 | 6.04 ± 0.05 | 4.74 ± 0.14 |
| PMP2 | 9.79 ± 0.01 | 2.54 ± 0.00 | 140.14 ± 5.62 | 74.99 ± 3.00 | 7.89 ± 0.09 | 4.87 ± 0.17 |
| PMP3 | 8.44 ± 0.01 | 2.97 ± 0.00 | 113.51 ± 1.87 | 79.16 ± 1.40 | 8.62 ± 0.29 | 4.07 ± 0.12 |
| Sample | d (0.25) (μm) | d (0.50) (μm) | d (0.75) (μm) | Median Particle Size | Mean Particle Size | Modal Particle Size |
|---|---|---|---|---|---|---|
| PM1 | 10.95 ± 0.25 | 18.86 ± 0.09 | 32.73 ± 0.32 | 18.86 ± 0.25 | 17.105 ± 0.42 | 17.14 ± 0.12 |
| PM2 | 10.05 ± 0.08 | 15.57 ± 0.12 | 24.08 ± 0.36 | 15.57 ± 0.26 | 13.876 ± 0.40 | 17.14 ± 0.16 |
| PM3 | 10.57 ± 0.09 | 18.48 ± 0.23 | 34.36 ± 0.35 | 18.48 ± 0.34 | 16.960 ± 0.44 | 13.58 ± 0.09 |
| PMP1 | 39.71 ± 0.14 | 56.38 ± 0.26 | 77.45 ± 0.32 | 56.38 ± 0.45 | 54.412 ± 0.25 | 54.92 ± 0.35 |
| PMP2 | 30.11 ± 0.15 | 46.39 ± 0.42 | 67.91 ± 0.33 | 46.39 ± 0.56 | 41.350 ± 0.35 | 54.92 ± 0.32 |
| PMP3 | 29.58 ± 0.12 | 45.84 ± 0.45 | 67.51 ± 0.35 | 45.84 ± 0.34 | 41.374 ± 0.34 | 54.92 ± 0.36 |
| Sample | Mw (kDa) | Mn (kDa) | Mw/Mn | Relative Crystallinity (%) | Ratio at 1047/1022 cm−1 |
|---|---|---|---|---|---|
| PM1 | 24,236.474 | 7645.310 | 3.170 | 27.20 | 0.90 |
| PM2 | 44,421.388 | 12,225.316 | 3.634 | 26.88 | 0.90 |
| PM3 | 25,292.823 | 7300.397 | 3.465 | 27.79 | 0.93 |
| PMP1 | 2158.057 | 1488.433 | 1.450 | 28.57 | 0.95 |
| PMP2 | 976.872 | 551.430 | 1.772 | 32.75 | 0.95 |
| PMP3 | 279.093 | 176.865 | 1.578 | 30.03 | 0.94 |
| Sample | Peak Viscosity (PV) | Trough Viscosity (Pa⋅s) | Breakdown (BD) | Final Viscosity (FV) | Setback (SB) | Peak Time | Pasting Temp |
|---|---|---|---|---|---|---|---|
| PM1 | 634 ± 0.82 | 409 ± 1.63 | 225 ± 2.45 | 586 ± 3.27 | 177 ± 0.47 | 4.00 ± 0.05 | 74.05 ± 0.0 |
| PM2 | 1139 ± 2.05 | 667 ± 4.08 | 472 ± 3.27 | 1050 ± 4.08 | 383 ± 2.45 | 3.80 ± 0.05 | 72.35 ± 0.41 |
| PM3 | 880 ± 4.90 | 521 ± 3.27 | 359 ± 3.27 | 794 ± 4.08 | 273 ± 3.27 | 4.00 ± 0.09 | 74.80 ± 0.57 |
| PMP1 | 16 ± 0.8 | 15 ± 0.82 | 1 ± 0.00 | 19 ± 0.47 | 4 ± 0.47 | 4.07 ± 0.06 | - |
| PMP2 | 47 ± 2.45 | 44 ± 0.47 | 3 ± 0.47 | 78 ± 0.47 | 34 ± 0.47 | 6.73 ± 0.05 | - |
| PMP3 | 19 ± 0.82 | 18 ± 0.47 | 1 ± 0.00 | 29 ± 0.82 | 11 ± 0.47 | 6.07 ± 0.06 | - |
| Sample | RDS (%) | SDS (%) | RS (%) |
|---|---|---|---|
| PM1 | 37.11 ± 0.47 | 4.53 ± 0.11 | 58.35 ± 1.27 |
| PM2 | 36.30 ± 0.33 | 13.10 ± 0.16 | 50.60 ± 1.27 |
| PM3 | 38.29 ± 0.30 | 16.04 ± 0.29 | 45.66 ± 1.33 |
| PMP1 | 13.10 ± 0.17 | 21.75 ± 0.63 | 65.15 ± 0.95 |
| PMP2 | 13.10 ± 0.15 | 26.69 ± 0.99 | 60.21 ± 1.05 |
| PMP3 | 10.60 ± 0.41 | 22.21 ± 1.30 | 67.19 ± 0.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, G.; Wang, Y.; Hu, Y.; Liu, Y.; Li, Q.; Ma, S. Comparison of the Structure, Physicochemical Properties, and Impact on Intestinal Flora of Processed and Unprocessed Polygonum multiflorum Starch. Foods 2025, 14, 1578. https://doi.org/10.3390/foods14091578
Yang G, Wang Y, Hu Y, Liu Y, Li Q, Ma S. Comparison of the Structure, Physicochemical Properties, and Impact on Intestinal Flora of Processed and Unprocessed Polygonum multiflorum Starch. Foods. 2025; 14(9):1578. https://doi.org/10.3390/foods14091578
Chicago/Turabian StyleYang, Guiya, Ying Wang, Yuying Hu, Yue Liu, Quan Li, and Shuangcheng Ma. 2025. "Comparison of the Structure, Physicochemical Properties, and Impact on Intestinal Flora of Processed and Unprocessed Polygonum multiflorum Starch" Foods 14, no. 9: 1578. https://doi.org/10.3390/foods14091578
APA StyleYang, G., Wang, Y., Hu, Y., Liu, Y., Li, Q., & Ma, S. (2025). Comparison of the Structure, Physicochemical Properties, and Impact on Intestinal Flora of Processed and Unprocessed Polygonum multiflorum Starch. Foods, 14(9), 1578. https://doi.org/10.3390/foods14091578








