Novel Active Films with Semolina and Jatoba (Hymenaea courbaril L.): Preparation, Properties, and Sustainability Aspects
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Plant Extracts
2.3. Extraction of Pectic Extract from Yellow Passion Fruit
2.4. Preparation of Inverted Sugar
2.5. Experimental Design and Film Preparation
2.6. Film Characterization
2.6.1. Mechanical Properties and Thickness
2.6.2. Color Analysis
2.6.3. Water Solubility
2.6.4. Water Vapor Permeability (WVP)
2.6.5. Total Phenolic Compound Content and Antioxidant Activity
2.6.6. Thermogravimetric Analysis (TG/DTG)
2.6.7. Differential Scanning Calorimetry (DSC)
2.6.8. X-Ray Diffraction (XRD)
2.6.9. Fourier-Transform Infrared Spectroscopy (FT-IR)
2.6.10. Degradability
2.7. Statistical Analysis
3. Results and Discussion
3.1. Optimization of Film Production Conditions
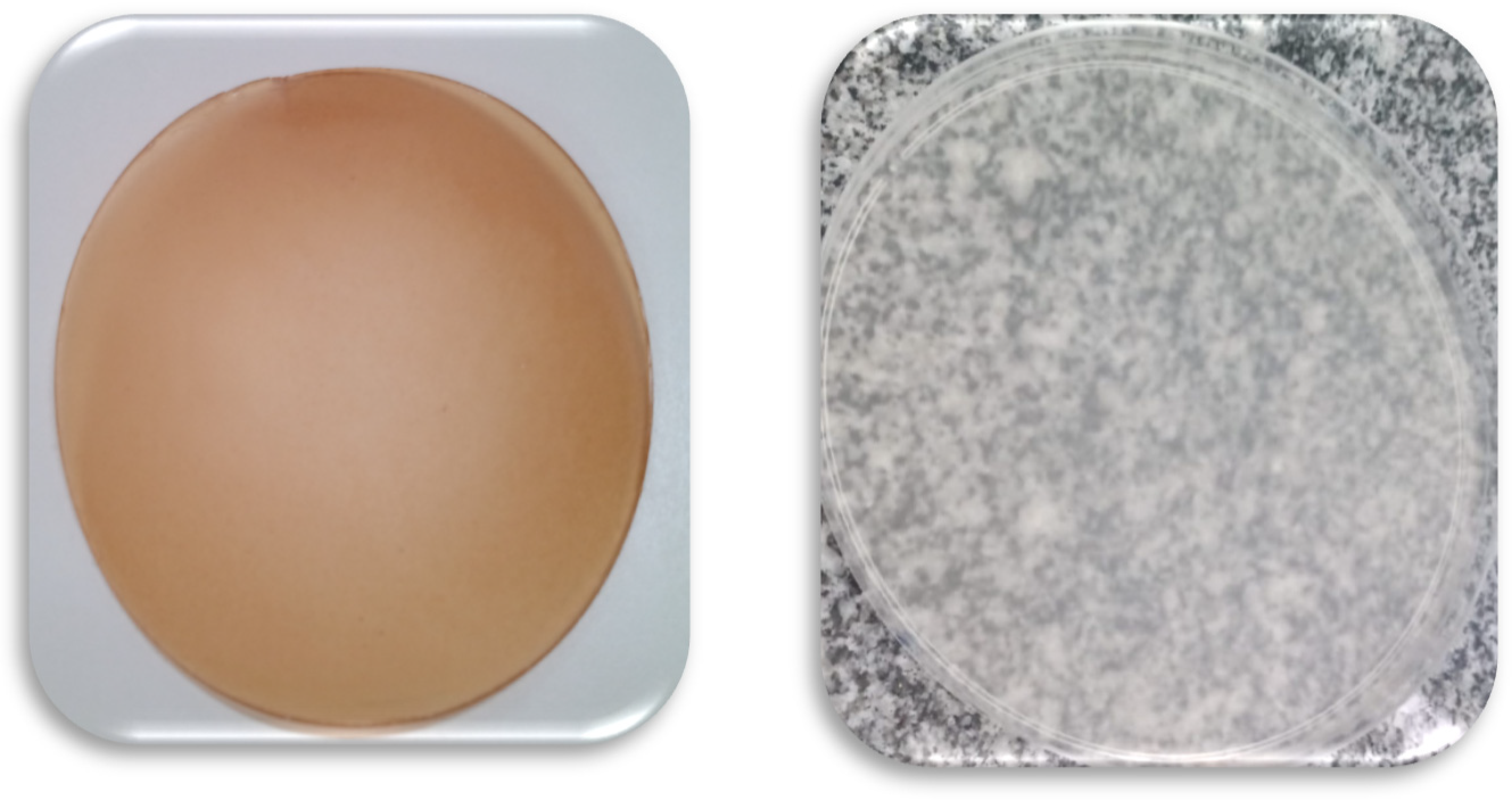
3.2. Characteristics of the Optimized (FO) and Control (FC) Films
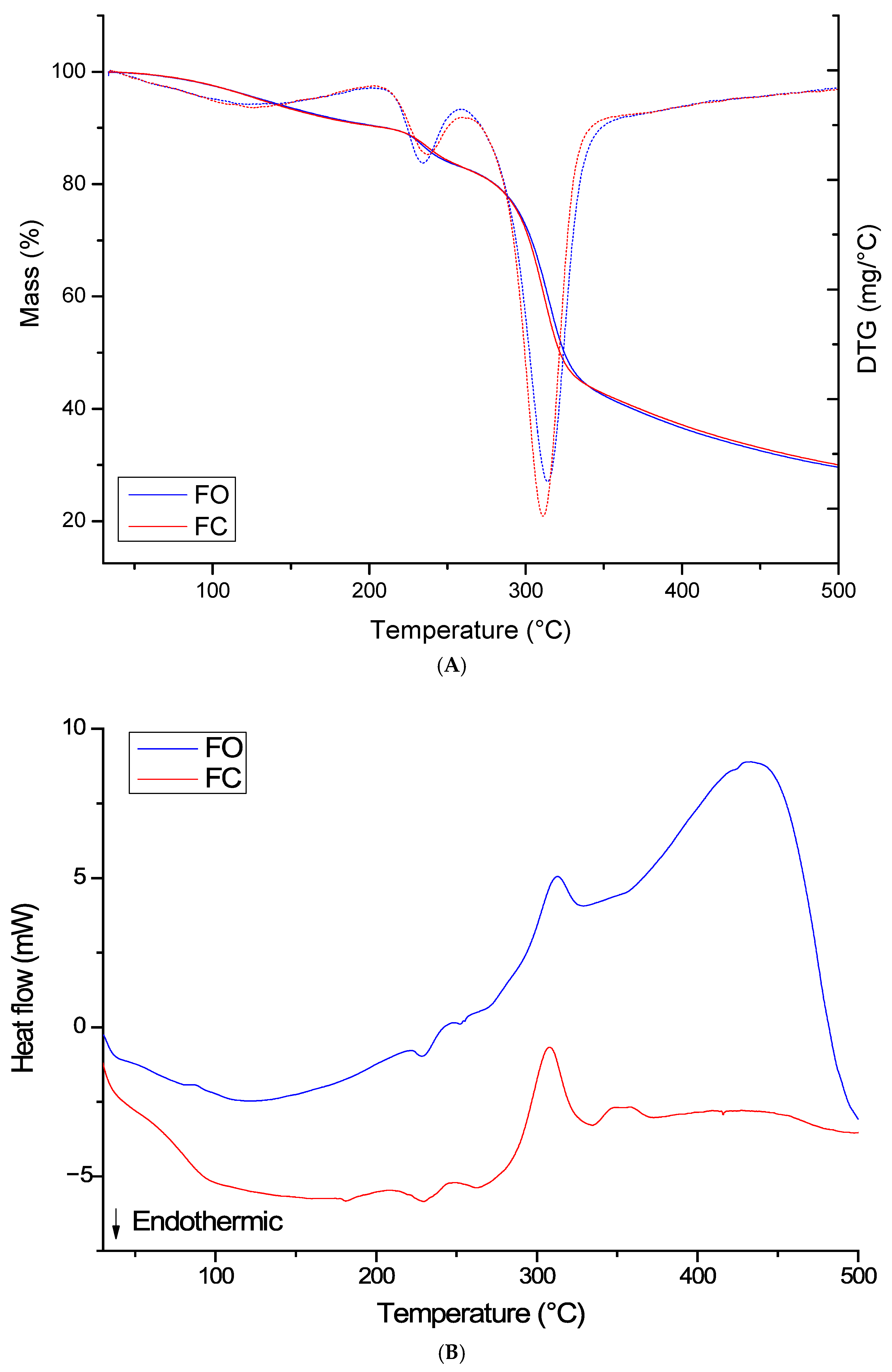
3.3. Thermal Properties
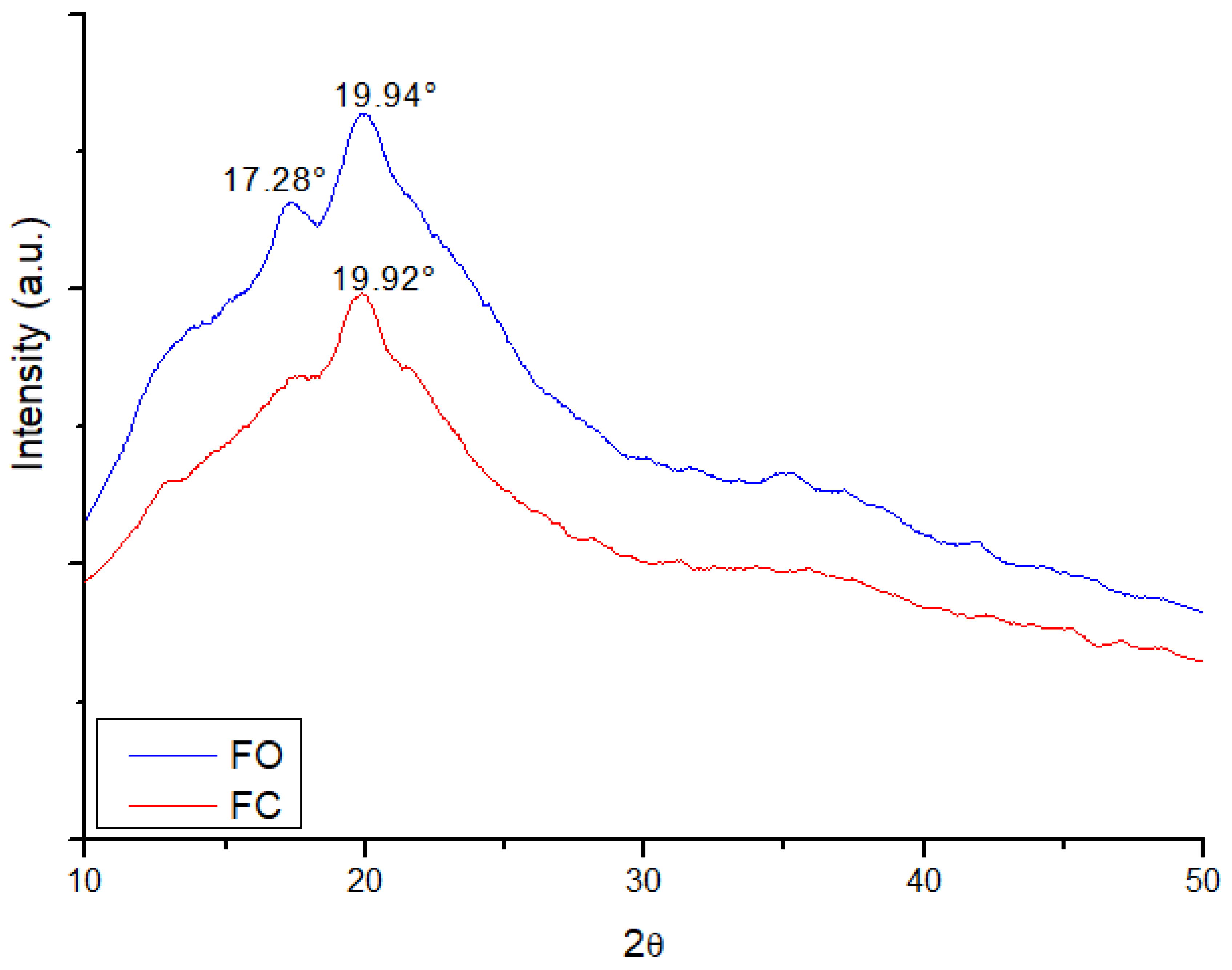
3.4. Structural Characterization by X-Ray Diffraction (XRD)
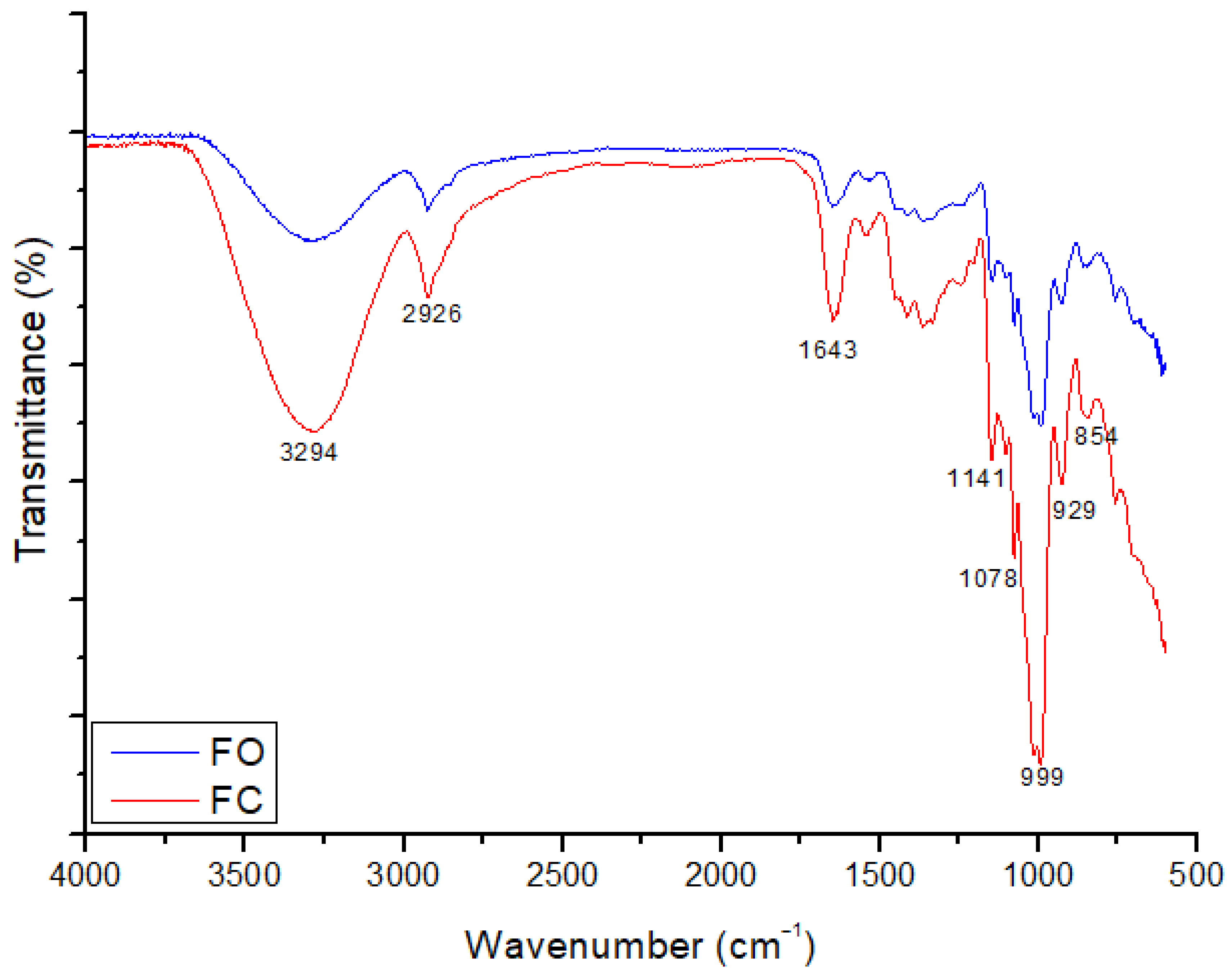
3.5. FTIR Spectrophotometry of the Films
3.6. Soil Degradation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Oliveira Filho, J.G.; Bertolo, M.R.V.; Fernandes, S.S.; Lemes, A.C.; da Cruz Silva, G.; Junior, S.B.; Egea, M.B. Intelligent and active biodegradable biopolymeric films containing carotenoids. Food Chem. 2023, 434, 137454. [Google Scholar] [CrossRef] [PubMed]
- Amin, U.; Khan, M.K.I.; Maan, A.A.; Nazir, A.; Riaz, S.; Khan, M.U.; Sultan, M.; Munekata, P.E.S.; Lorenzo, J.M. Biodegradable active, intelligent, and smart packaging materials for food applications. Food Packag. Shelf Life 2022, 33, 100903. [Google Scholar] [CrossRef]
- Dong, Z.; Du, Z.; Wu, X.; Zhai, K.; Wei, Z.; Rashed, M.M.A. Fabrication and characterization of ZnO nanofilms using extracted pectin of Premna microphylla Turcz leaves and carboxymethyl cellulose. Int. J. Biol. Macromol. 2022, 209, 525–532. [Google Scholar] [CrossRef]
- Adhikary, N.D.; Bains, A.; Sridhar, K.; Kaushik, R.; Chawla, P.; Sharma, M. Recent advances in plant-based ternary polysaccharide complexes for biodegradable packaging. Int. J. Biol. Macromol. 2023, 253, 126725. [Google Scholar] [CrossRef]
- Cheng, J.; Gao, R.; Zhu, Y.; Lin, Q. Applications of biodegradable materials in food packaging: A review. Alex. Eng. J. 2024, 91, 70–83. [Google Scholar] [CrossRef]
- Kola, V.; Carvalho, I.S. Plant extracts as additives in biodegradable films and coatings in active food packaging. Food Biosci. 2023, 54, 102860. [Google Scholar] [CrossRef]
- Nastasi, J.R.; Kontogiorgos, V.; Daygon, V.D.; Fitzgerald, M.A. Pectin-based films and coatings with plant extracts as natural preservatives: A systematic review. Trends Food Sci. Technol. 2022, 120, 193–211. [Google Scholar] [CrossRef]
- Santos, R.T.S.; Biasoto, A.C.T.; Rybka, A.C.P.; Castro, C.D.C.; Aidar, S.D.T.; Borges, G.S.C.; Silva, F.L.H. Physicochemical characterization, bioactive compounds, in vitro antioxidant activity, sensory profile and consumer acceptability of fermented alcoholic beverage obtained from Caatinga passion fruit (Passiflora cincinnata Mast.). LWT 2021, 48, 111714. [Google Scholar] [CrossRef]
- Alves-Silva, G.F.; Romani, V.P.; Martins, V.G. Jatoba (Hymenaea stigonocarpa) pulp films: Properties, antioxidant potential and biodegradability. Food Packag. Shelf Life 2022, 34, 100923. [Google Scholar] [CrossRef]
- Zhang, Y.; Min, T.; Zhao, Y.; Cheng, C.; Yin, H.; Yue, J. The developments and trends of electrospinning active food packaging: A review and bibliometrics analysis. Food Control 2024, 160, 110291. [Google Scholar] [CrossRef]
- Rocha, L.S.; Gonçalves, D.A.; Arakaki, D.G.; Tschinkel, P.F.S.; de Lima, N.V.; de Oliveira, L.C.S.; de Cássia Avellaneda Guimarães, R.; do Nascimento, V.A. Data on elemental composition of the medicinal plant Hymenaea martiana Hayne (Jatoba). Data Brief 2018, 19, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.B.D.; Silva, A.K.C.; Lodete, A.R.; Egea, M.B.; Lima, M.C.P.M.; Silva, F.G. Assessment of chemical and bioactive properties of native fruits from the Brazilian Cerrado. Nutr. Food Sci. 2019, 49, 381–392. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Alias, A.K.; Ariffin, F.; Mahmud, S. Physico-mechanical and microstructural properties of semolina flour films as influenced by different sorbitol/glycerol concentrations. Int. J. Food Prop. 2018, 21, 983–995. [Google Scholar] [CrossRef]
- Alcântara, M.A.; de Lima Brito Polari, I.; de Albuquerque Meireles, B.R.L.; de Lima, A.E.A.; da Silva Junior, J.C.; de Andrade Vieira, É.; dos Santos, N.A.; de Magalhães Cordeiro, A.M.T. Effect of the solvent composition on the profile of phenolic compounds extracted from chia seeds. Food Chem. 2019, 275, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.N.P.; Grisi, C.V.B.; Duarte, C.R.; de Almeida, Y.M.B.; Vinhas, G.M. Active packaging of corn starch with pectin extract and essential oil of Turmeric Longa Linn: Preparation, characterization and application in sliced bread. Int. J. Biol. Macromol. 2023, 226, 1352–1359. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.d.M.; Grisi, C.V.B.; Silva, R.d.C.A.d.; Monção, É.d.C.; de Barros, G.A.; Nascimento, S.d.P.D.; Maciel, J.F.; Cordeiro, A.M.T.d.M.; Queiroz, N.; Souza, A.L. Antimicrobial Packaging from Potato Starch and Pectin with Citric Acid and Bioactive Compounds from Cashew Apple: Preparation, Characterization, and Application in Bread. ACS Omega 2025, 10, 17807–17819. [Google Scholar] [CrossRef]
- ASTM D882–12; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM—American Society for Testing and Materials: West Conshohocken, PA, USA, 2012.
- ASTM E308-17; Standard Practice for Computing the Colors of Objects by Using the CIE System. ASTM—American Society For Testing and Materials: West Conshohocken, PA, USA, 2017.
- Ojagh, S.M.; Rezaei, M.; Razavi, S.H.; Hosseini, S.M.H. Effect of chitosan coatings enriched with cinnamon oil on the quality of refrigerated rainbow trout. Food Chem. 2010, 120, 193–198. [Google Scholar] [CrossRef]
- ASTM E96/E96M-16; Standard Test Methods for Water Vapor Transmission of Materials. ASTM—American Society for Testing and Materials: West Conshohocken, PA, USA, 2016.
- Slinkard, K.; Singleton, V.L. Total Phenol Analysis: Automation and Comparison with Manual Methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Rufino, M.d.S.M.; Alves, R.E.; de Brito, E.S.; de Morais, S.M.; Sampaio, C.d.G.; Pérez-Jiménez, J.; Saura-Calixto, F.D. Metodologia Científica: Determinação da atividade antioxidante total em frutas pelo método de redução do ferro (FRAP) In Comunicado Técnico 125; Embrapa: Brasília, Brazil, 2006; ISSN 1679-6535. [Google Scholar]
- Lima, D.A.S.; Grisi, C.V.B.; Florentino, G.I.B.; Santos, M.M.F.; Madruga, M.S.; da Silva, F.A.P. Preparation and characterization of sustainable active packaging based on myofibrillar proteins and protein hydrolysates from the cutting by-product of Scomberomorus brasiliensis filleting on the band saw machine. Food Chem. 2024, 460, 140490. [Google Scholar] [CrossRef]
- Spagnol, C.; Fragal Elizângela, H.; Witt, M.A.; Follmann, H.D.M.; Silva, R.; Rubira, A.F. Mechanically improved polyvinyl alcohol-composite films using modified cellulose nanowhiskers as nano-reinforcement. Carbohydr. Polym. 2018, 191, 25–34. [Google Scholar] [CrossRef] [PubMed]
- ASTM D5477–11; Standard Practice for Identification of Polymer Layers or Inclusions by Fourier Transform Infrared Microspectroscopy (FT-IR). ASTM—American Society for Testing and Materials: West Conshohocken, PA, USA, 2011.
- Chandra, R.; Rustgi, R. Biodegradation of maleated linear low-density polyethylene and starch blends. Polym. Degrad. Stab. 1997, 56, 185–202. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. DPPH radical scavenging assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant activity/capacity measurement. 1. Classification, physicochemical principles, mechanisms, and electron transfer (ET)-based assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef] [PubMed]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef]
- Scaramussa, S.A.d.L.; Soares, L.d.A.; Santana, L.C.L.d.A. Extracts from Jatoba (Hymenaea courbaril L.) peel and seeds: Antioxidant and antimicrobial activities and synergistic effect of extract combinations. Food Sci. Technol. Int. 2022, 130, 128–136. [Google Scholar] [CrossRef]
- Nascimento, T.A.; Calado, V.; Carvalho, C.W.P. Development and characterization of flexible film based on starch and passion fruit mesocarp flour with nanoparticles. Food Res. Int. 2012, 49, 588–595. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Le, T.Q.; Nguyen, T.T.A.; Nguyen, L.T.M.; Nguyen, D.T.C.; Tran, T.V. Characterizations and antibacterial activities of passion fruit peel pectin/chitosan composite films incorporated Piper betle L. leaf extract for preservation of purple eggplants. Heliyon 2022, 8, e10096. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, Y.; Cai, Y.; Zhou, K.; Gu, X.; Yu, J. How to establish the relationship between wetting parameters and the mechanical properties of a flexible thin film. Tribol. Int. 2024, 192, 109255. [Google Scholar] [CrossRef]
- Anatoly, C.; Pavel, Z.; Tatiana, C.; Alexei, R.; Svetlana, Z. Water Vapor Permeability through Porous Polymeric Membranes with Various Hydrophilicity as Synthetic and Natural Barriers. Polymers 2020, 12, 282. [Google Scholar] [CrossRef]
- McGuiggan, P.M.; Gates, G.A. A Review of the Gas and Vapor Transport Through Single Polymer Films: Implications for Their Use in Book and Paper Conservation. Restaur. Int. J. Preserv. Libr. Arch. Mater. 2024, volume, page. [Google Scholar] [CrossRef]
- Camilo, C.J.; Nonato, C.F.A.; da Silva, J.T.P.; Rodrigues, F.F.G.; Salazara, G.J.T.; da Costa, J.G.M. Interference of Hymenaea courbaril L. (Jatoba) extract on the antibacterial activity of aminoglycosides. Interfaces Revista. 2020, 8, 372–379. [Google Scholar] [CrossRef]
- Athanasopoulou, E.; Bigi, F.; Maurizzi, E.; Karellou, E.I.E.; Pappas, C.S.; Quartieri, A.; Tsironi, T. Synthesis and characterization of polysaccharide- and protein-based edible films and application as packaging materials for fresh fish fillets. Sci. Rep. 2024, 14, 517. [Google Scholar] [CrossRef]
- Mohsin, A.; Waqas Qamar Zaman Guo, M.; Ahmed, W.; Imran Mahmood Khan Niazi, S.; Rehman, A.; Hang, H.; Zhuang, Y. Xanthan-Curdlan nexus for synthesizing edible food packaging films. Int. J. Biol. Macromol. 2020, 162, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Oladzadabbasabadi, N.; Mohammadi Nafchi, A.; Ariffin, F.; Karim, A.A. Plant extracts as packaging aids. In Plant Extracts: Applications in the Food Industry; Academic Press—Elsevier: Amsterdam, The Netherlands, 2022; pp. 225–268. [Google Scholar] [CrossRef]
- Sani, M.A.; Khezerlou, A.; Tavassoli, M.; Abedini, A.H.; McClements, D.J. Development of sustainable UV-screening food packaging materials: A review of recent advances. Trends Food Sci. Technol. 2024, 145, 104366. [Google Scholar] [CrossRef]
- Avramescu, S.M.; Butean, C.; Popa, C.V.; Ortan, A.; Moraru, I.; Temocico, G. Edible and Functionalized Films/Coatings—Performances and Perspectives. Coatings 2020, 10, 687. [Google Scholar] [CrossRef]
- Souza, C.O.; Silva, L.T.; Silva, J.R.; López, J.A.; Veiga-Santos, P.; Druzian, J.I. Mango and Acerola Pulps as Antioxidant Additives in Cassava Starch Bio-based Film. J. Agric. Food Chem. 2011, 59, 2248–2254. [Google Scholar] [CrossRef]
- Dash, K.K.; Ali, N.A.; Das, D.; Mohanta, D. Thorough evaluation of sweet potato starch and lemon-waste pectin based-edible films with nano-titania inclusions for food packaging applications. Int. J. Biol. Macromol. 2019, 139, 449–458. [Google Scholar] [CrossRef]
- Biswas, P.; Jayaseelan, P.; Das, M.; Sikder, A.; Chaudhury, K.; Banerjee, R. Processing of semolina, a wonder resource for resistant starch production: In vitro digestibility and biochemical evaluation. Int. J. Biol. Macromol. 2022, 222, 1918–1924. [Google Scholar] [CrossRef]
- Medina-Jaramillo, C.; Ochoa-Yepes, O.; Bernal, C.; Famá, L. Active and smart biodegradable packaging based on starch and natural extracts. Carbohydr. Polym. 2017, 176, 187–194. [Google Scholar] [CrossRef]
- Yang, N.; Zou, F.; Tao, H.; Guo, L.; Cui, B.; Fang, Y.; Lu, L.; Wu, Z.; Yuan, C.; Zhao, M.; et al. Effects of primary, secondary and tertiary structures on functional properties of thermoplastic starch biopolymer blend films. Int. J. Biol. Macromol. 2023, 236, 124006. [Google Scholar] [CrossRef] [PubMed]
- Lipatova, I.M.; Yusova, A.A.; Makarova, L.I. Functional films based on mechanoactivated starch with prolonged release of preservative. Food Biosci. 2022, 47, 101694. [Google Scholar] [CrossRef]
- Rodrigues, M.Á.V.; Marangon, C.A.; Martins, V.D.C.A.; de Guzzi Plepis, A.M. Chitosan/gelatin films with Jatoba resin: Control of properties by vegetal resin inclusion and degree of acetylation modification. Int. J. Biol. Macromol. 2021, 182, 1737–1745. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Rhim, J.-W. Preparation of pectin/agar-based functional films integrated with zinc sulfide nano petals for active packaging applications. Colloids Surf. B Biointerface 2021, 207, 111999. [Google Scholar] [CrossRef]
- Bodana, V.; Swer, T.L.; Kumar, N.; Singh, A.; Samtiya, M.; Sari, T.P.; Babar, O.A. Development and characterization of pomegranate peel extract-functionalized jackfruit seed starch-based edible films and coatings for prolonging the shelf life of white grapes. Int. J. Biol. Macromol. 2024, 254, 127234. [Google Scholar] [CrossRef]
- Gao, H.X.; He, Z.; Sun, Q.; He, Q.; Zeng, W.C. A functional polysaccharide film forming by pectin, chitosan, and tea polyphenols. Carbohydr. Polym. 2019, 215, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, J.; Ying, Y.; Hu, L.; Hu, Y. pH-sensitive and antibacterial films developed by incorporating anthocyanins extracted from purple potato or roselle into chitosan/polyvinyl alcohol/nano-ZnO matrix: Comparative study. Int. J. Biol. Macromol. 2021, 178, 104–112. [Google Scholar] [CrossRef]
- Chen, N.; Gao, H.X.; He, Q.; Zeng, W.C. Potential application of phenolic compounds with different structural complexity in maize starch-based film. Food Struct. 2023, 36, 100318. [Google Scholar] [CrossRef]
- Nandi, S.; Guha, P. Organic acid-compatibilized potato starch/guar gum blend films. Mater. Chem. Phys. 2021, 268, 124714. [Google Scholar] [CrossRef]
- Patil, S.; Bharimalla, A.K.; Mahapatra, A.; Dhakane-Lad, J.; Arputharaj, A.; Kumar, M.; Raja, A.S.M.; Kambli, N. Effect of polymer blending on mechanical and barrier properties of starch-polyvinyl alcohol based biodegradable composite films. Food Biosci. 2021, 44, 101352. [Google Scholar] [CrossRef]
- Lin, Y.; An, F.; He, H.; Geng, F.; Song, H.; Huang, Q. Structural and rheological characterization of pectin from passion fruit (Passiflora edulis f. flavicarpa) peel extracted by high-speed shearing. Food Hydrocoll. 2021, 114, 106555. [Google Scholar] [CrossRef]
- Wang, F.; Yu, G.; Yang, Q.; Yi, X.; Fu, L.; Li, H. Antibacterial Gelidium amansii polysaccharide-based edible films containing cyclic adenosine monophosphate for bioactive packaging. Int. J. Biol. Macromol. 2022, 212, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Fathiraja, P.; Gopalrajan, S.; Karunanithi, M.; Obaiah, M.C.; Rajasekaran, B.; Vedhi, C. Process optimization and characterization of composite biopolymer films obtained from fish scale gelatin, agar and chitosan using response surface methodology. Polym. Bull. 2022, 80, 10775–10807. [Google Scholar] [CrossRef]
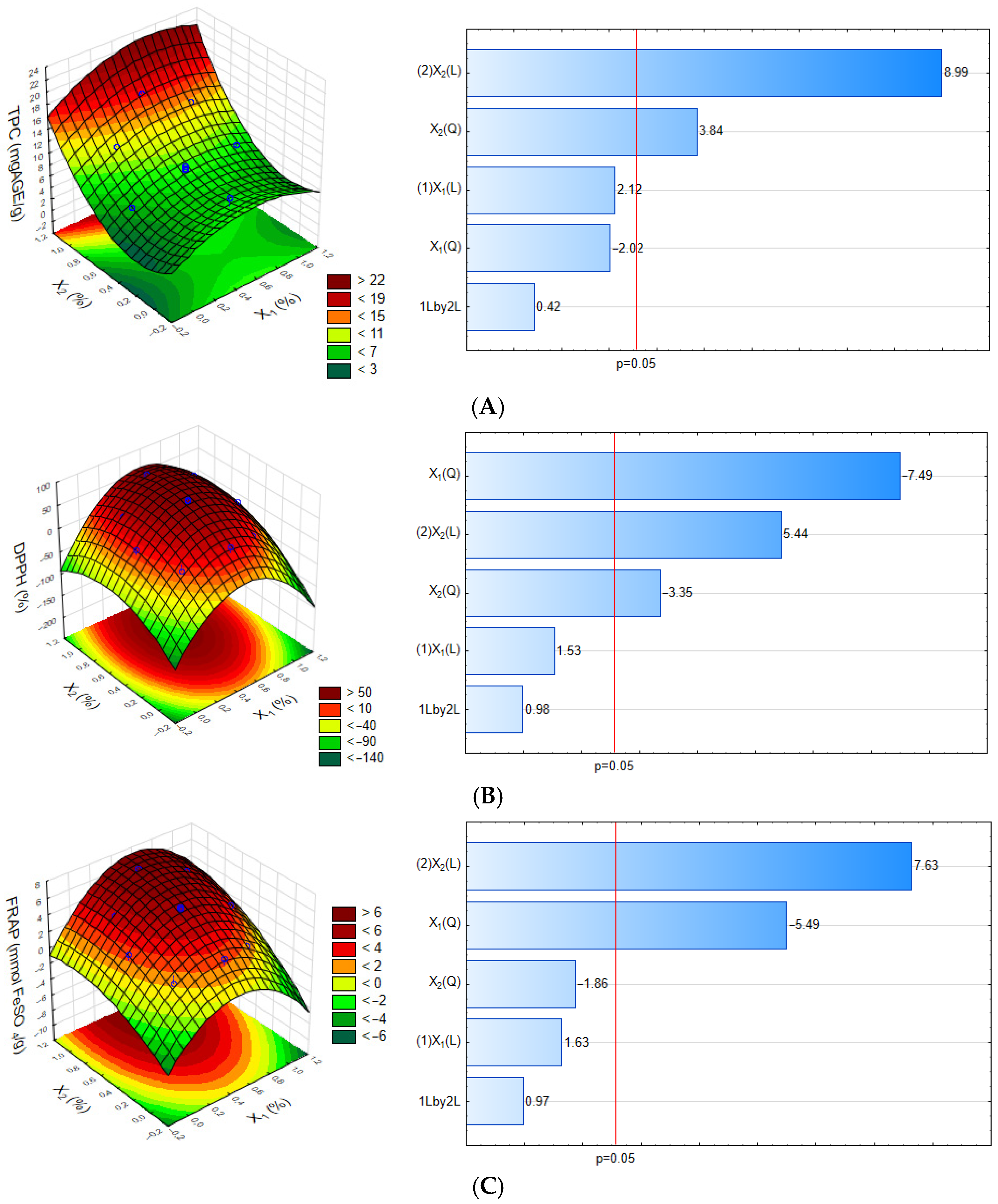
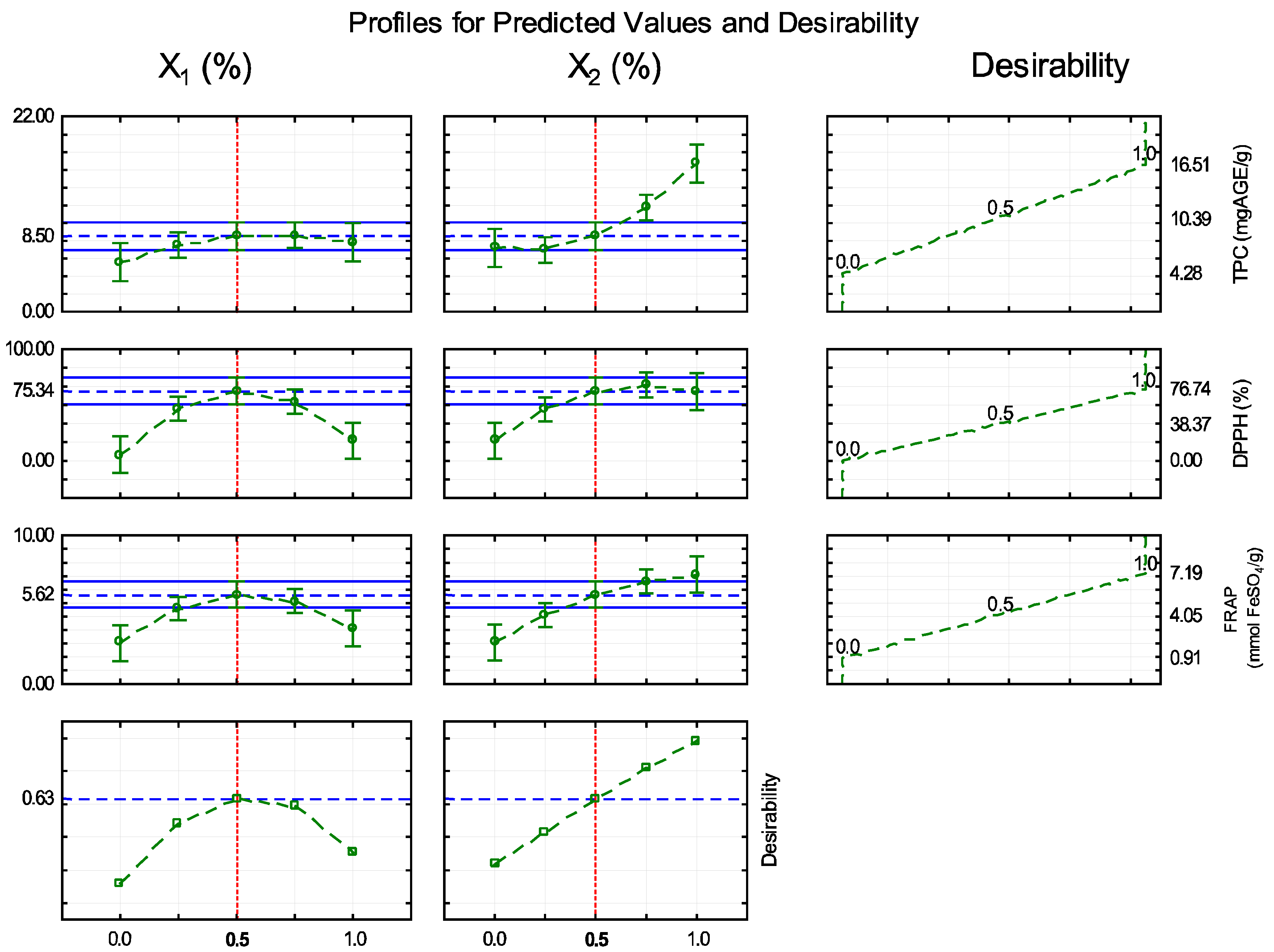
| Independent Variables | Levels | ||||
|---|---|---|---|---|---|
| −α | −1 | 0 | +1 | +α | |
| X1 (%) | 0.00 | 0.15 | 0.50 | 0.85 | 1.00 |
| X2 (%) | 0.00 | 0.15 | 0.50 | 0.85 | 1.00 |
| Independent Variables | Response Variables | ||||
|---|---|---|---|---|---|
| X1 (%) | X2 (%) | TPC (mgGAE/g) | DPPH (%) | FRAP (mmol FeSO4/g) | |
| F1 | 0.15 | 0.15 | 4.28 | 0.00 | 0.91 |
| F2 | 0.15 | 0.85 | 11.68 | 34.05 | 4.28 |
| F3 | 0.85 | 0.15 | 5.28 | 5.02 | 1.43 |
| F4 | 0.85 | 0.85 | 13.57 | 58.12 | 6.08 |
| F5 | 0.00 | 0.50 | 6.08 | 16.25 | 2.75 |
| F6 | 1.00 | 0.50 | 8.53 | 25.48 | 3.26 |
| F7 | 0.50 | 0.00 | 8.55 | 32.57 | 2.83 |
| F8 | 0.50 | 1.00 | 16.51 | 76.74 | 7.19 |
| F9 | 0.50 | 0.50 | 8.80 | 74.89 | 5.73 |
| F10 | 0.50 | 0.50 | 8.25 | 75.28 | 5.52 |
| F11 | 0.50 | 0.50 | 8.45 | 75.87 | 5.63 |
| Parameters | FO | FC |
|---|---|---|
| Thickness (mm) | 0.150 ± 0.02 b | 0.160 ± 0.02 a |
| Elongation at break (%) | 23.82 ± 4.71 a | 8.22 ± 0.53 b |
| TS (MPa) | 5.62 ± 1.77 b | 7.39 ± 1.73 a |
| WVP (10−4 gH2O·mm/m2·h·mmHg) | 1.84 ± 0.10 a | 1.31 ± 0.11 b |
| Water solubility (%) | 65.40 ± 9.90 a | 63.00 ± 1.80 a |
| L* | 49.93 ± 1.30 b | 59.77 ± 0.88 a |
| a* | 4.24 ± 0.72 a | −2.02 ± 0.15 b |
| b* | 20.16 ± 1.36 a | 1.23 ± 0.30 b |
| ΔE | 22.23 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grisi, C.V.B.; Silva, F.C.G.d.; Silva, R.d.C.A.; Silva, R.P.d.; Silva, F.A.P.d.; Cordeiro, A.M.T.d.M. Novel Active Films with Semolina and Jatoba (Hymenaea courbaril L.): Preparation, Properties, and Sustainability Aspects. Foods 2025, 14, 2391. https://doi.org/10.3390/foods14132391
Grisi CVB, Silva FCGd, Silva RdCA, Silva RPd, Silva FAPd, Cordeiro AMTdM. Novel Active Films with Semolina and Jatoba (Hymenaea courbaril L.): Preparation, Properties, and Sustainability Aspects. Foods. 2025; 14(13):2391. https://doi.org/10.3390/foods14132391
Chicago/Turabian StyleGrisi, Cristiani Viegas Brandão, Flávia Cosmo Guedes da Silva, Rita de Cassia Andrade Silva, Rene Pinto da Silva, Fábio Anderson Pereira da Silva, and Angela Maria Tribuzy de Magalhães Cordeiro. 2025. "Novel Active Films with Semolina and Jatoba (Hymenaea courbaril L.): Preparation, Properties, and Sustainability Aspects" Foods 14, no. 13: 2391. https://doi.org/10.3390/foods14132391
APA StyleGrisi, C. V. B., Silva, F. C. G. d., Silva, R. d. C. A., Silva, R. P. d., Silva, F. A. P. d., & Cordeiro, A. M. T. d. M. (2025). Novel Active Films with Semolina and Jatoba (Hymenaea courbaril L.): Preparation, Properties, and Sustainability Aspects. Foods, 14(13), 2391. https://doi.org/10.3390/foods14132391






