Kinetics of Quality Degradation and Water Removal During Air Drying of Osmodehydrated Oyster Mushrooms Impregnated with Rosa damascena Distillation By-Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Osmotic Dehydration Pretreatment
2.3. Air Drying Process
2.4. Determination of Quality Characteristics During Air Drying Process
2.4.1. Water Activity
2.4.2. Color
2.4.3. Textural Properties
2.5. Mathematical Modeling of Drying Kinetics and Properties
2.5.1. Mass Transfer Kinetics
| Model | Model Equation | References |
|---|---|---|
| Lewis | (14) | [51,53] |
| Page | (15) | [33,54] |
| Modified Page | (16) | [55,56] |
| Henderson and Pabis | (17) | [34,57] |
| Weibull | (18) | [58] |
| Midilli | (19) | [35,59] |
2.5.2. Color and Texture Kinetic Modeling
2.6. Data Analysis
3. Results and Discussion
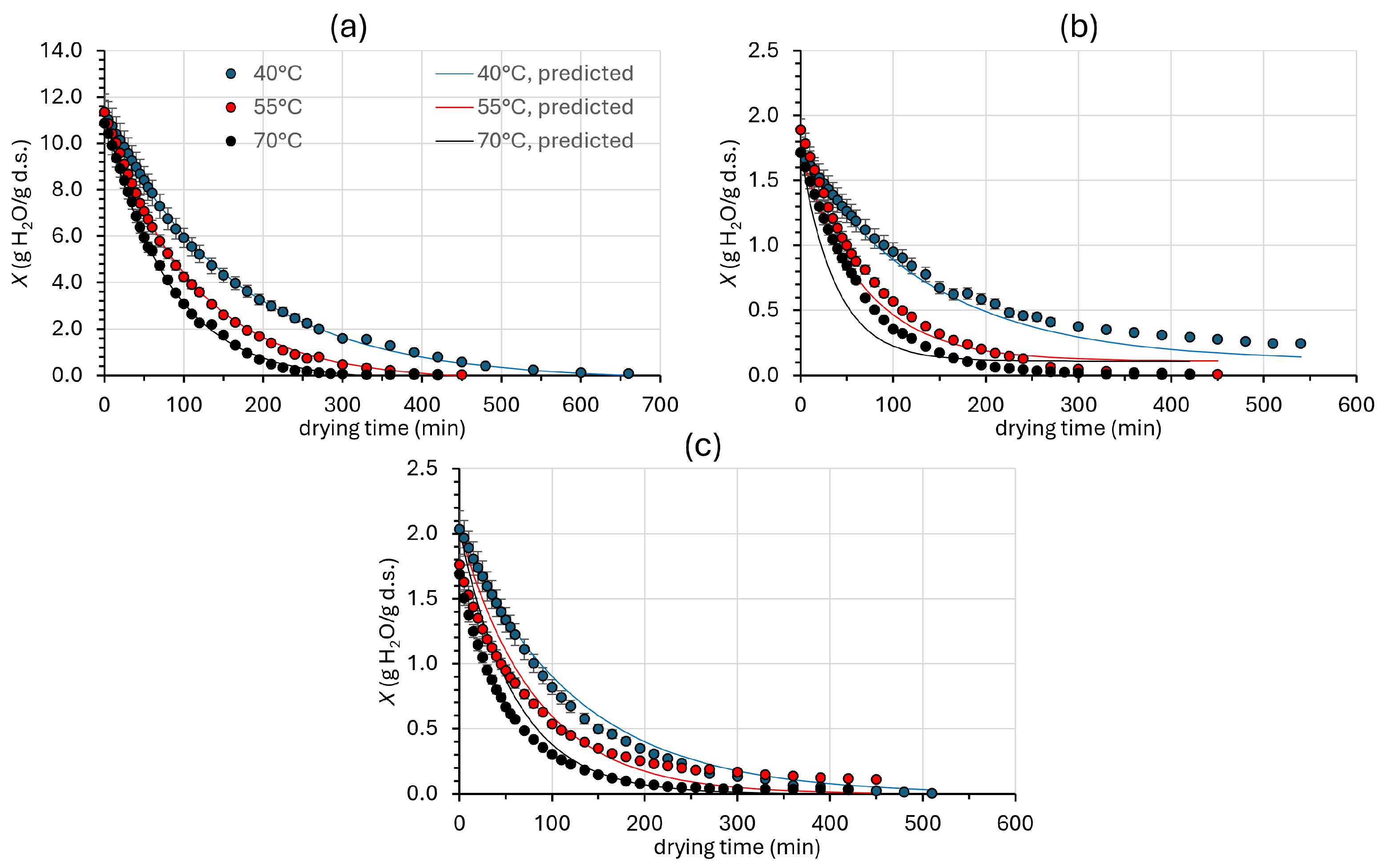
3.1. Effect of Drying Temperature on Drying Kinetics
3.1.1. Water Content Reduction
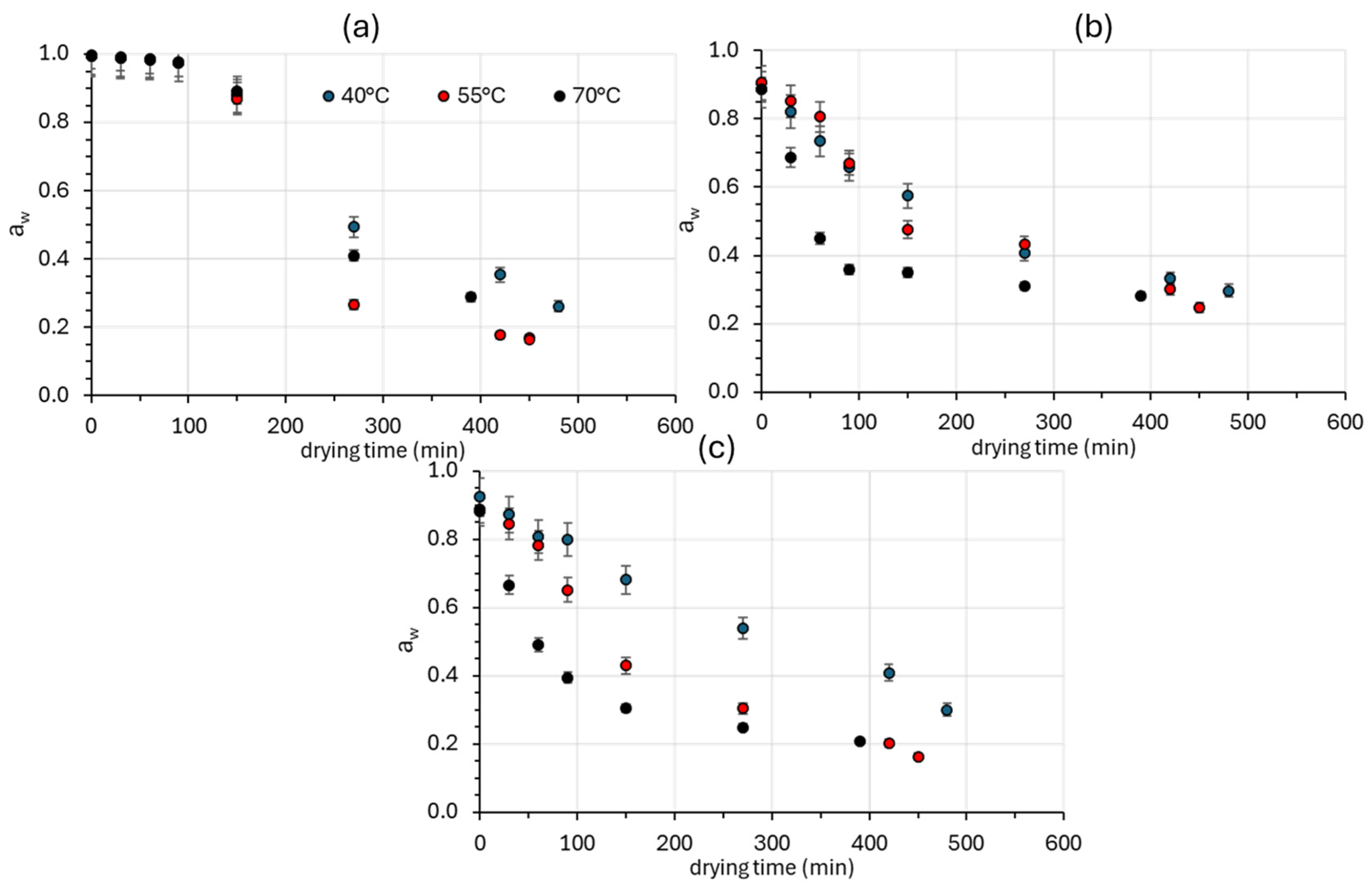
3.1.2. Water Activity Reduction
3.2. Effect of Drying Temperature on Physicochemical Characteristics
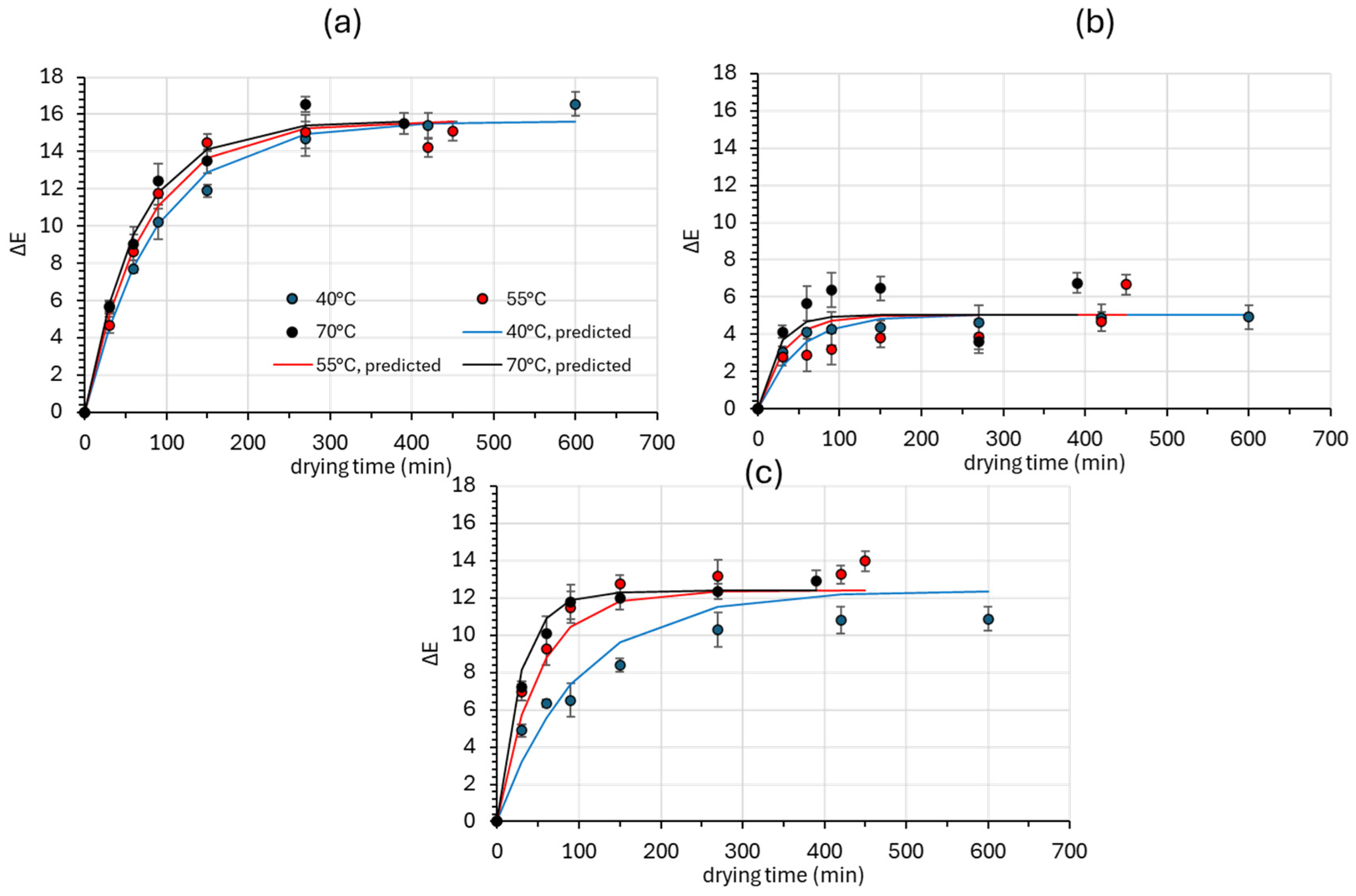
3.2.1. Color Parameters
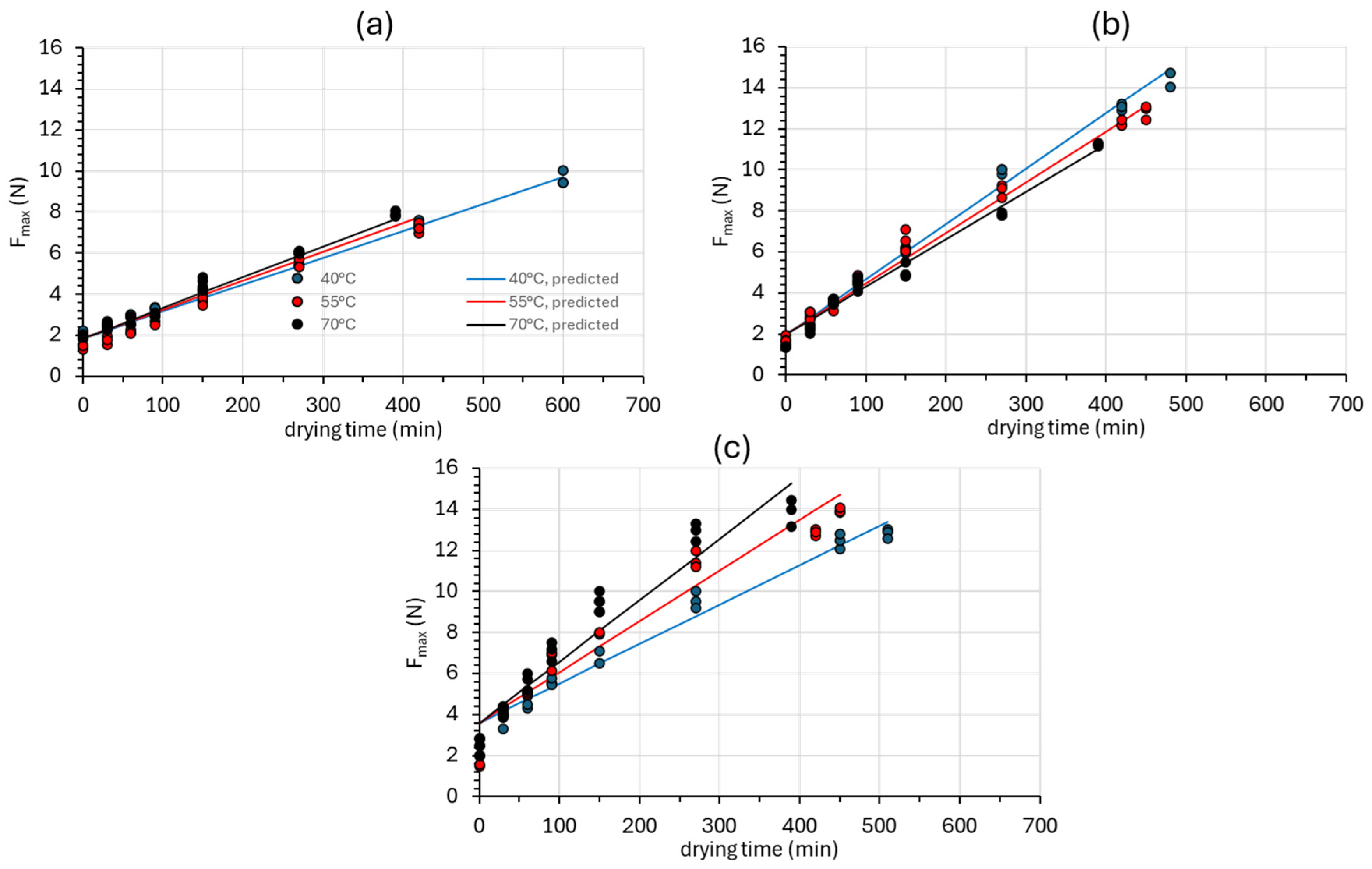
3.2.2. Texture
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mutukwa, I.B.; Hall III, C.A.; Cihacek, L.; Lee, C.W. Evaluation of drying method and pretreatment effects on the nutritional and antioxidant properties of oyster mushroom (Pleurotus ostreatus). J. Food Process. Preserv. 2019, 43, e13910. [Google Scholar] [CrossRef]
- Omari, A.; Behroozi-Khazaei, N.; Sharifian, F. Drying kinetic and artificial neural network modeling of mushroom drying process in microwave-hot air dryer. J. Food Process. Eng. 2018, 41, e12849. [Google Scholar] [CrossRef]
- Rubina, T.; Aboltins, A. Drying characteristics of mushrooms. In Proceedings of the Engineering for Rural Development, Jelgava, Latvia, 26–28 May 2021; Volume 20, pp. 557–563. [Google Scholar] [CrossRef]
- Qin, L.; Gao, J.X.; Xue, J.; Chen, D.; Lin, S.Y.; Dong, X.P.; Zhu, B.W. Changes in aroma profile of shiitake mushroom (Lentinus edodes) during different stages of hot air drying. Foods 2020, 9, 444. [Google Scholar] [CrossRef] [PubMed]
- Salehi, F.; Kashaninejad, M.; Jafarianlari, A. Drying kinetics and characteristics of combined infrared-vacuum drying of button mushroom slices. Heat Mass Transf. 2017, 53, 1751–1759. [Google Scholar] [CrossRef]
- Doymaz, I. Drying kinetics and rehydration characteristics of convective hot-air dried white button mushroom slices. J. Chem. 2014, 2014, 453175. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Mujumdar, A.S. Development of flavor during drying and applications of edible mushrooms: A review. Dry. Technol. 2021, 39, 1685–1703. [Google Scholar] [CrossRef]
- Das, I.; Arora, A. Alternate microwave and convective hot air application for rapid mushroom drying. J. Food Eng. 2018, 223, 208–219. [Google Scholar] [CrossRef]
- Wang, Q.; Li, S.; Han, X.; Ni, Y.; Zhao, D.; Hao, J. Quality evaluation and drying kinetics of shitake mushrooms dried by hot air, infrared and intermittent microwave–assisted drying methods. LWT 2019, 107, 236–242. [Google Scholar] [CrossRef]
- Mirzaei-Baktash, H.; Hamdami, N.; Torabi, P.; Fallah-Joshaqani, S.; Dalvi-Isfahan, M. Impact of different pretreatments on drying kinetics and quality of button mushroom slices dried by hot-air or electrohydrodynamic drying. LWT 2022, 155, 112894. [Google Scholar] [CrossRef]
- Moutia, I.; Lakatos, E.; Kovács, A.J. Impact of Dehydration Techniques on the Nutritional and Microbial Profiles of Dried Mushrooms. Foods 2024, 13, 3245. [Google Scholar] [CrossRef]
- Das, M.; Mayookha, V.P.; Geetha, V.; Chetana, R.; Suresh Kumar, G. Influence of different drying techniques on quality parameters of mushroom and its utilization in development of ready to cook food formulation. J. Food Sci. Technol. 2023, 60, 1342–1354. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, Y.; Li, M.; Peng, C.; Liu, Q.; Guan, X.; Liang, Z.; Wang, Q. Research progress of mushroom drying pretreatment technology. J. Food Process. Eng. 2024, 47, e14648. [Google Scholar] [CrossRef]
- Kaur, K.; Kumar, S.; Alam, M.S. Air drying kinetics and quality characteristics of oyster mushroom (Pleurotus ostreatus) influenced by osmotic dehydration. Agric. Eng. Int. CIGR J. 2014, 16, 214–222. [Google Scholar]
- Duan, H.; Yu, Q.; Ni, Y.; Li, J.; Yu, L.; Fan, L. Calcium combined with vacuum treatment improves postharvest storage quality of Agaricus bisporus by regulating polyamine metabolism. Postharvest Biol. Technol. 2024, 210, 112735. [Google Scholar] [CrossRef]
- Abrahão, F.R.; Corrêa, J.L.G. Osmotic dehydration: More than water loss and solid gain. Crit. Rev. Food Sci. Nutr. 2023, 63, 2970–2989. [Google Scholar] [CrossRef]
- Asghari, A.; Zongo, P.A.; Osse, E.F.; Aghajanzadeh, S.; Raghavan, V.; Khalloufi, S. Review of osmotic dehydration: Promising technologies for enhancing products’ attributes, opportunities, and challenges for the food industries. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13346. [Google Scholar] [CrossRef]
- Kowalski, S.; Mierzwa, D. Influence of preliminary osmotic dehydration on drying kinetics and final quality of carrot (Daucus carota L.). Chem. Process Eng. 2011, 32, 185–194. [Google Scholar] [CrossRef][Green Version]
- Oliveira, S.M.; Brandao, T.R.; Silva, C.L. Influence of drying processes and pretreatments on nutritional and bioactive characteristics of dried vegetables: A review. Food Eng. Rev. 2016, 8, 134–163. [Google Scholar] [CrossRef]
- Corrêa, J.L.G.; Rasia, M.C.; Mulet, A.; Cárcel, J.A. Influence of ultrasound application on both the osmotic pretreatment and subsequent convective drying of pineapple (Ananas comosus). Innov. Food Sci. Emerg. Technol. 2017, 41, 284–291. [Google Scholar] [CrossRef]
- Mandala, I.G.; Anagnostaras, E.F.; Oikonomou, C.K. Influence of osmotic dehydration conditions on apple air-drying kinetics and their quality characteristics. J. Food Eng. 2005, 69, 307–316. [Google Scholar] [CrossRef]
- Pavkov, I.; Radojčin, M.; Stamenković, Z.; Kešelj, K.; Tylewicz, U.; Sipos, P.; Ponjičan, O.; Sedlar, A. Effects of osmotic dehydration on the hot air drying of apricot halves: Drying kinetics, mass transfer, and shrinkage. Processes 2021, 9, 202. [Google Scholar] [CrossRef]
- Ghanem Romdhane, N.; Djendoubi, N.; Bonazzi, C.; Kechaou, N.; Boudhrioua Mihoubi, N. Effect of combined air-drying-osmotic dehydration on kinetics of techno-functional properties, color and total phenol contents of lemon (Citrus limon. v. lunari) peels. Int. J. Food Eng. 2016, 12, 515–525. [Google Scholar] [CrossRef]
- Şahin, U.; Öztürk, H.K. Effects of pulsed vacuum osmotic dehydration (PVOD) on drying kinetics of figs (Ficus carica L.). Innov. Food Sci. Emerg. Technol. 2016, 36, 104–111. [Google Scholar] [CrossRef]
- Dermesonlouoglou, E.; Chalkia, A.; Dimopoulos, G.; Taoukis, P. Combined effect of pulsed electric field and osmotic dehydration pre-treatments on mass transfer and quality of air dried goji berry. Innov. Food Sci. Emerg. Technol. 2018, 49, 106–115. [Google Scholar] [CrossRef]
- Guiamba, I.; Ahrné, L.; Khan, M.A.; Svanberg, U. Retention of β-carotene and vitamin C in dried mango osmotically pretreated with osmotic solutions containing calcium or ascorbic acid. Food Bioprod. Process. 2016, 98, 320–326. [Google Scholar] [CrossRef]
- Tolera, K.D.; Abera, S. Nutritional quality of Oyster Mushroom (Pleurotus ostreatus) as affected by osmotic pretreatments and drying methods. Food Sci. Nutr. 2017, 5, 989–996. [Google Scholar] [CrossRef]
- Torringa, E.; Esveld, E.; Scheewe, I.; van den Berg, R.; Bartels, P. Osmotic dehydration as a pre-treatment before combined microwave-hot-air drying of mushrooms. J. Food Eng. 2001, 49, 185–191. [Google Scholar] [CrossRef]
- Katsoufi, S.; Lazou, A.E.; Giannakourou, M.C.; Krokida, M.K. Air drying kinetics and quality characteristics of osmodehydrated-candied pumpkins using alternative sweeteners. Dry. Technol. 2021, 39, 2194–2205. [Google Scholar] [CrossRef]
- Biswas, R.; Hossain, M.A.; Zzaman, W. Thin layer modeling of drying kinetics, rehydration kinetics and color changes of osmotic pre-treated pineapple (Ananas comosus) slices during drying: Development of a mechanistic model for mass transfer. Innov. Food Sci. Emerg. Technol. 2022, 80, 103094. [Google Scholar] [CrossRef]
- Ertekin, C.; Firat, M.Z. A comprehensive review of thin-layer drying models used in agricultural products. Crit. Rev. Food Sci. Nutr. 2017, 57, 701–717. [Google Scholar] [CrossRef]
- Inyang, U.E.; Oboh, I.O.; Etuk, B.R. Kinetic models for drying techniques—Food materials. J. Adv. Chem. Eng. 2018, 8, 27–48. [Google Scholar] [CrossRef]
- Onwude, D.I.; Hashim, N.; Janius, R.B.; Nawi, N.M.; Abdan, K. Modeling the thin-layer drying of fruits and vegetables: A review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 599–618. [Google Scholar] [CrossRef] [PubMed]
- Artnaseaw, A.; Theerakulpisut, S.; Benjapiyaporn, C. Drying characteristics of Shiitake mushroom and Jinda chili during vacuum heat pump drying. Food Bioprod. Process. 2010, 88, 105–114. [Google Scholar] [CrossRef]
- Ohaco, E.H.; Valiente, L.; Ichiyama, B.; de Michelis, A. Drying kinetics of frozen oyster mushrooms (Pleurotus ostreatus). Micol. Apl. Int. 2016, 28, 17–28. [Google Scholar]
- Pei, F.; Xiao, K.; Chen, L.; Yang, W.; Zhao, L.; Fang, Y.; Ma, N.; Mariga, A.Μ.; Hu, Q. Mass transfer characteristics during ultrasound-assisted osmotic dehydration of button mushroom (Agaricus bisporus). J. Food Sci. Technol. 2019, 56, 2213–2223. [Google Scholar] [CrossRef]
- Giannakourou, M.C.; Tsironi, T.; Thanou, I.; Tsagri, A.M.; Katsavou, E.; Lougovois, V.; Kyrana, V.; Kasapidis, G.; Sinanoglou, V.J. Shelf life extension and improvement of the nutritional value of fish fillets through osmotic treatment based on the sustainable use of rosa damascene distillation by-products. Foods 2019, 8, 421. [Google Scholar] [CrossRef]
- Konteles, S.J.; Stavropoulou, N.A.; Thanou, I.V.; Mouka, E.; Kousiaris, V.; Stoforos, G.N.; Gogou, E.; Giannakourou, M.C. Enriching Cured Meat Products with Bioactive Compounds Recovered from Rosa damascene and Rosmarinus officinalis L. Distillation By-Products: The Pursuit of Natural Antimicrobials to Reduce the Use of Nitrites. Appl. Sci. 2023, 13, 13085. [Google Scholar] [CrossRef]
- Tsiaka, T.; Stavropoulou, N.A.; Giannakourou, M.C.; Strati, I.F.; Sinanoglou, V.J. Optimization of Ultrasound-Assisted Extraction and Characterization of the Phenolic Compounds in Rose Distillation Side Streams Using Spectrophotometric Assays and High-Throughput Analytical Techniques. Molecules 2023, 28, 7403. [Google Scholar] [CrossRef]
- Stavropoulou, N.A.; Giannakourou, M.C. Combined effect of bioactive compound enrichment using Rosa damascene distillation side streams and an optimized osmotic treatment on the stability of frozen oyster mushrooms. Appl. Sci. 2023, 13, 9734. [Google Scholar] [CrossRef]
- Official Methods of Analysis of AOAC International, 16th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1998; Volume II, Chapter 37; p. 4.
- Andreou, V.; Strati, I.F.; Fotakis, C.; Liouni, M.; Zoumpoulakis, P.; Sinanoglou, V.J. Herbal distillates: A new era of grape marc distillates with enriched antioxidant profile. Food Chem. 2018, 253, 171–178. [Google Scholar] [CrossRef]
- Ghellam, M.; Zannou, O.; Pashazadeh, H.; Galanakis, C.M.; Aldawoud, T.M.; Ibrahim, S.A.; Koca, I. Optimization of osmotic dehydration of autumn olive berries using response surface methodology. Foods 2021, 10, 1075. [Google Scholar] [CrossRef] [PubMed]
- Macedo, L.L.; Corrêa, J.L.G.; Araújo, C.D.S.; Oliveira, D.D.S.; Teixeira, L.J.Q. Use of coconut sugar as an alternative agent in osmotic dehydration of strawberries. J. Food Sci. 2023, 88, 3786–3806. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Miao, J.; Huang, L.; Li, B. Microwave freeze-drying of button mushroom (Agaricus bisporus) based on non-volatile taste components by controlling microwave power density. Int. J. Food Sci. Technol. 2022, 57, 379–389. [Google Scholar] [CrossRef]
- Pathak, S.S.; Sonawane, A.; Srinivas, A.; Pradhan, R.C. Application of image analysis for detecting the browning of unripe banana slices. ACS Food Sci. Technol. 2021, 1, 1507–1513. [Google Scholar] [CrossRef]
- Takeungwongtrakul, S.; Sai-Ut, S.; Waraput, T.; Supapvanich, S. Thermal processes improving antibrowning potential of mixed Aloe vera and pineapple core extract solution on browning inhibition of fresh-cut apples. Int. J. Food Sci. Technol. 2022, 57, 6881–6889. [Google Scholar] [CrossRef]
- Stavropoulou, N.A.; Pavlidis, V.A.; Giannakourou, M.C. Optimization of osmotic dehydration of white mushrooms by Response Surface Methodology for shelf-life extension and quality improvement of frozen end-products. Foods 2022, 11, 2354. [Google Scholar] [CrossRef]
- Castillo-Gironés, S.; Masztalerz, K.; Lech, K.; Issa-Issa, H.; Figiel, A.; Carbonell-Barrachina, A.A. Impact of osmotic dehydration and different drying methods on the texture and sensory characteristic of sweet corn kernels. J. Food Process. Preserv. 2021, 45, e15383. [Google Scholar] [CrossRef]
- Nishinari, K.; Kohyama, K.; Kumagai, H.; Funami, T.; Bourne, M.C. Parameters of texture profile analysis. Food Sci. Technol. Res. 2013, 19, 519–521. [Google Scholar] [CrossRef]
- Henderson, S.M. Progress in developing the thin layer drying equation. Trans. ASAE 1974, 17, 1167–1168. [Google Scholar] [CrossRef]
- Taheri-Garavand, A.; Rafiee, S.; Keyhani, A. Mathematical modeling of thin layer drying kinetics of tomato influence of air dryer conditions. Int. Trans. J. Eng. Manag. Appl. Sci. Technol. 2011, 2, 147–160. [Google Scholar]
- Lewis, W.K. The rate of drying of solid materials. J. Ind. Eng. Chem. 1921, 13, 427–432. [Google Scholar] [CrossRef]
- Zhang, Q.; Litchfleld, J.B. An optimization of intermittent corn drying in a laboratory scale thin layer dryer. Dry. Technol. 1991, 9, 383–395. [Google Scholar] [CrossRef]
- Togrul, H. Suitable drying model for infrared drying of carrot. J. Food Eng. 2006, 77, 610–619. [Google Scholar] [CrossRef]
- Yaldiz, O.; Ertekin, C.; Uzun, H.I. Mathematical modeling of thin layer solar drying of sultana grapes. Energy 2001, 26, 457–465. [Google Scholar] [CrossRef]
- Aghbashlo, M.; Kianmehr, M.H.; Khani, S.; Ghasemi, M. Mathematical modelling of thin layer drying of carrot. Int. Agrophys. 2009, 23, 313–317. [Google Scholar]
- Corzo, O.; Bracho, N.; Pereira, A.; Vasquez, A. Weibull distribution for modeling air drying of coroba slices. LWT-Food Sci. Technol. 2008, 41, 2023–2028. [Google Scholar] [CrossRef]
- Midilli, A.; Kucuk, H.; Yapar, Z. A new model for single layer drying. Dry. Technol. 2002, 20, 1503–1513. [Google Scholar] [CrossRef]
- Engin, D. Effect of drying temperature on color and desorption characteristics of oyster mushroom. Food Sci. Technol. 2019, 40, 187–193. [Google Scholar] [CrossRef]
- Tran, T.N.T.; Khoo, K.S.; Chew, K.W.; Phan, T.Q.; Nguyen, H.S.; Nguyen-Sy, T.; Chen, W.H.; Show, P.L. Modelling drying kinetic of oyster mushroom dehydration–The optimization of drying conditions for dehydratation of Pleurotus species. Mater. Sci. Energy Technol. 2020, 3, 840–845. [Google Scholar] [CrossRef]
- Simal, S.; Deya, E.; Frau, M.; Rossello, C. Simple modelling of air drying curves of fresh and osmotically pre-dehydrated apple cubes. J. Food Eng. 1997, 33, 139–150. [Google Scholar] [CrossRef]
- Marçal, S.; Sousa, A.S.; Taofiq, O.; Antunes, F.; Morais, A.M.; Freitas, A.C.; Barros, L.; Ferreira, I.C.F.R.; Pintado, M. Impact of postharvest preservation methods on nutritional value and bioactive properties of mushrooms. Trends Food Sci. Technol. 2021, 110, 418–431. [Google Scholar] [CrossRef]
- Maleki, M.; Shahidi, F.; Varidi, M.J.; Azarpazhooh, E. Hot air drying kinetics of novel functional carrot snack: Impregnated using polyphenolic rich osmotic solution with ultrasound pretreatment. J. Food Process. Eng. 2020, 43, e13331. [Google Scholar] [CrossRef]
- Kadir, N.; Yeasmen, N.; Bhuiyan, M.H.R.; Khan, M.J.; Iqbal, A. Osmotic and convective hot air drying of sweet gourd. Food Sci. Biotechnol. 2022, 33, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Madamba, P.S.; Driscoll, R.H.; Buckle, K.A. The thin-layer drying characteristics of garlic slices. J. Food Eng. 1996, 29, 75–97. [Google Scholar] [CrossRef]
- Akhondi, E.; Kazemi, A.; Maghsoodi, V. Determination of a suitable thin layer drying curve model for saffron (Crocus sativus L.) stigmas in an infrared dryer. Sci. Iran. 2011, 18, 1397–1401. [Google Scholar] [CrossRef]
- Ayadi, M.; Mabrouk, S.B.; Zouari, I.; Bellagi, A. Kinetic study of the convective drying of spearmint. J. Saudi Soc. Agric. Sci. 2014, 13, 1–7. [Google Scholar] [CrossRef]
- Bi, Y.X.; Zielinska, S.; Ni, J.B.; Li, X.X.; Xue, X.F.; Tian, W.L.; Peng, W.J.; Fang, X.M. Effects of hot-air drying temperature on drying characteristics and color deterioration of rape bee pollen. Food Chem. X 2022, 16, 100464. [Google Scholar] [CrossRef]
- Liang, Y.; Luo, K.; Wang, B.; Huang, B.; Fei, P.; Zhang, G. Inhibition of polyphenol oxidase for preventing browning in edible mushrooms: A review. J. Food Sci. 2024, 89, 6796–6817. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, J.; Wang, X.; Lin, Q.; Liu, W.; Xie, X.; Wang, Z.; Guan, W. Effects of UV-C irradiation on the physiological and antioxidant responses of button mushrooms (Agaricus bisporus) during storage. Int. J. Food Sci. Technol. 2016, 51, 1502–1508. [Google Scholar] [CrossRef]
- Shankar, M.A.; Sehrawat, R.; Pareek, S.; Nema, P.K. Physico-chemical properties and drying kinetic evaluation of hot air and vacuum dried pre-treated oyster mushroom under innovative multi-mode developed dryer. Int. J. Hortic. Sci. Technol. 2022, 9, 363–374. [Google Scholar] [CrossRef]
- Guo, L.; Lan, N.; Li, H.; Xiang, P.; Kan, H. Effect of hot air drying temperature on the quality and antioxidant activity of Boletus edulis Bull.: Fr. J. Food Process. Preserv. 2021, 45, e15540. [Google Scholar] [CrossRef]
- Nudar, J.T.; Roy, M.; Ahmed, S. Combined Osmotic Pretreatment and Hot Air Dying: Evaluation of Drying Kinetics and Quality Parameters of Adajamir (Citrus assamensis). Heliyon 2023, 9, e19545. [Google Scholar] [CrossRef] [PubMed]
- Wanderley, R.D.O.S.; de Figueirêdo, R.M.F.; de Melo Queiroz, A.J.; dos Santos, F.S.; de França Silva, A.P.; Paiva, Y.F.; Moura, H.V.; Silva, E.T.d.V.; Carvalho, A.J.d.B.A.; Lima, M.d.S.; et al. Effect of drying temperature on antioxidant activity, phenolic compound profile and hygroscopic behavior of pomegranate peel and seed flours. LWT 2023, 189, 115514. [Google Scholar] [CrossRef]
- Miranda, M.; Vega-Gálvez, A.; López, J.; Parada, G.; Sanders, M.; Aranda, M.; Uribe, E.; Di Scala, K. Impact of air-drying temperature on nutritional properties, total phenolic content and antioxidant capacity of quinoa seeds (Chenopodium quinoa Willd.). Ind. Crops Prod. 2010, 32, 258–263. [Google Scholar] [CrossRef]
- Chen, J.P.; Wang, Y.; Zhang, X.Y.; Sun, P.; Wu, Z.F.; Shang, Y.F.; Yang, S.-H.; Ma, Y.-L.; Wei, Z.J. Effect of air drying temperature on the phenolics and antioxidant activity of Xuan-Mugua fruit. Food Sci. Technol. 2022, 42, e45322. [Google Scholar] [CrossRef]
- Pandiselvam, R.; Tak, Y.; Olum, E.; Sujayasree, O.; Tekgül, Y.; Çalışkan Koç, G.; Kaur, M.; Nayi, P.; Kothakota, A.; Kumar, M. Advanced osmotic dehydration techniques combined with emerging drying methods for sustainable food production: Impact on bioactive components, texture, color, and sensory properties of food. J. Texture Stud. 2022, 53, 737–762. [Google Scholar] [CrossRef]
- Mari, A.; Parisouli, D.N.; Krokida, M. Exploring osmotic dehydration for food preservation: Methods, modelling, and modern applications. Foods 2024, 13, 2783. [Google Scholar] [CrossRef]
- Haneef, N.; Hanif, N.; Hanif, T.; Raghavan, V.; Garièpy, Y.; Wang, J. Food fortification potential of osmotic dehydration and the impact of osmo-combined techniques on bioactive component saturation in fruits and vegetables. Braz. J. Food Technol. 2024, 27, e2023028. [Google Scholar] [CrossRef]


| Parameter | Sample Category | ||
|---|---|---|---|
| Control | OD | ODR | |
| Lewis model (Equation (14)) | |||
| k1,MR | 1.2807 ± 0.0286 | 1.7827 ± 0.0613 | 1.6728 ± 0.0907 |
| k0,MR | 0.00938 ± 0.00006 | 0.01042 ± 0.00015 | 0.01284 ± 0.00027 |
| Page model (Equation (15)) | |||
| k1,MR | 1.3578 ± 0.0975 | 1.6866 ± 0.0632 | 1.6309 ± 0.0169 |
| k0,MR | 0.0068 ± 0.0002 | 0.0139 ± 0.0012 | 0.0144 ± 0.0019 |
| n | 1.0688 ± 0.0169 | 0.9359 ± 0.0184 | 0.9719 ± 0.0304 |
| Modified Page model (Equation (16)) | |||
| k1,MR | 1.2703 ± 0.0181 | 1.8021 ± 0.0001 | 1.6781 ± 0.0921 |
| k0,MR | 0.00940 ± 0.00004 | 0.0104 ± 0.0012 | 0.0128 ± 0.0003 |
| n | 1.0688 ± 0.0069 | 0.9358 ± 0.0164 | 0.9719 ± 0.0204 |
| Henderson and Pabis model (Equation (17)) | |||
| k1,MR | 1.2733 ± 0.0217 | 1.7946 ± 0.0611 | 1.6734 ± 0.0898 |
| k0,MR | 0.00970 ± 0.00006 | 0.0102 ± 0.0002 | 0.0131 ± 0.0004 |
| n | 1.0256 ± 0.0035 | 0.9925 ± 0.0093 | 1.0119 ± 0.0147 |
| Weibull model (Equation (18)) | |||
| k1,MR | −1.2703 ± 0.0181 | −1.8021 ± 0.0599 | −1.6781 ± 0.0921 |
| k0,MR | 106.21 ± 0.45 | 96.36 ± 1.37 | 78.04 ± 1.69 |
| n | 1.0688 ± 0.0068 | 0.9359 ± 0.0184 | 0.9719 ± 0.0304 |
| Midilli model (Equation (19)) | |||
| k1,MR | 1.3367 ± 0.0226 | 1.3367 ± 0.0226 | 1.5918 ± 0.1034 |
| k0,MR | 0.0076 ± 0.0004 | 0.0122 ± 0.0019 | 0.0164 ± 0.0036 |
| n | 1.0469 ± 0.0118 | 0.9729 ± 0.0965 | 0.9585 ± 0.0477 |
| b | −1.20 × 10−5 ± 0.05 × 10−5 | 6.90 × 10−5 ± 0.24 × 10−5 | 6.10 × 10−5 ± 0.32 × 10−5 |
| a | 1.0083 ± 0.0044 | 1.0017 ± 0.0137 | 1.0342 ± 0.0214 |
| Model | CONTROL | OD | ODR | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| χ2 | SSE | RMSE | R2 | χ2 | SSE | RMSE | R2 | χ2 | SSE | RMSE | R2 | |
| Lewis | 0.000232 | 0.075 | 0.01517 | 0.999 | 0.001029 | 0.328 | 0.031985 | 0.990 | 0.002072 | 0.655 | 0.04538 | 0.990 |
| Page | 0.000099 | 0.032 | 0.009922 | 0.999 | 0.00091 | 0.289 | 0.030018 | 0.991 | 0.002059 | 0.649 | 0.045161 | 0.981 |
| Modified Page | 0.000098 | 0.031 | 0.009920 | 0.999 | 0.00091 | 0.290 | 0.030043 | 0.991 | 0.002059 | 0.649 | 0.045161 | 0.980 |
| Henderson and Pabis | 0.000139 | 0.045 | 0.011719 | 0.999 | 0.000991 | 0.315 | 0.031337 | 0.990 | 0.002063 | 0.650 | 0.045206 | 0.981 |
| Weibull | 0.000099 | 0.032 | 0.009922 | 0.999 | 0.00091 | 0.289 | 0.030018 | 0.991 | 0.002059 | 0.649 | 0.045161 | 0.980 |
| Midilli | 0.000100 | 0.032 | 0.00925 | 0.999 | 0.001007 | 0.318 | 0.031489 | 0.991 | 0.002033 | 0.636 | 0.044734 | 0.981 |
| Type of Samples | Parameters of Quality Degradation Indices | ||
|---|---|---|---|
| Browing Index | |||
| k1,BI | k0,BI | R2 | |
| Control | 2.78015 ± 0.83306 | 0.02601 ± 0.00707 | 0.985 |
| OD | 3.2572 ± 0.9832 | 0.03236 ± 0.00986 | 0.984 |
| ODR | 1.60568 ± 0.33306 | 0.00867 ± 0.00096 | 0.973 |
| ΔE | |||
| k1,ΔE | k0,ΔE | R2 | |
| Control | 0.54885 ± 0.03533 | 0.01391 ± 0.00153 | 0.997 |
| OD | 1.3306 ± 0.5465 | 0.03182 ± 0.00676 | 0.952 |
| ODR | 2.27573 ± 0.78228 | 0.02069 ± 0.00185 | 0.991 |
| Hardness (Fmax, N) | |||
| k1,F | k0,F | R2 | |
| Control | 0.2247 ± 0.056 | 0.014101 ± 0.000616 | 0.994 |
| OD | 0.2652 ± 0.0884 | 0.02751 ± 0.00073 | 0.996 |
| ODR | 0.7856 ± 0.0862 | 0.02472 ± 0.00159 | 0.987 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stavropoulou, N.A.; Lazou, A.E.; Giannakourou, M.C. Kinetics of Quality Degradation and Water Removal During Air Drying of Osmodehydrated Oyster Mushrooms Impregnated with Rosa damascena Distillation By-Products. Foods 2025, 14, 1543. https://doi.org/10.3390/foods14091543
Stavropoulou NA, Lazou AE, Giannakourou MC. Kinetics of Quality Degradation and Water Removal During Air Drying of Osmodehydrated Oyster Mushrooms Impregnated with Rosa damascena Distillation By-Products. Foods. 2025; 14(9):1543. https://doi.org/10.3390/foods14091543
Chicago/Turabian StyleStavropoulou, Natalia A., Andriana E. Lazou, and Maria C. Giannakourou. 2025. "Kinetics of Quality Degradation and Water Removal During Air Drying of Osmodehydrated Oyster Mushrooms Impregnated with Rosa damascena Distillation By-Products" Foods 14, no. 9: 1543. https://doi.org/10.3390/foods14091543
APA StyleStavropoulou, N. A., Lazou, A. E., & Giannakourou, M. C. (2025). Kinetics of Quality Degradation and Water Removal During Air Drying of Osmodehydrated Oyster Mushrooms Impregnated with Rosa damascena Distillation By-Products. Foods, 14(9), 1543. https://doi.org/10.3390/foods14091543







