Ratiometric Fluorescent Probes Based on Isosteviol with Identification of Maleic Acid in Starchy Foods
Abstract
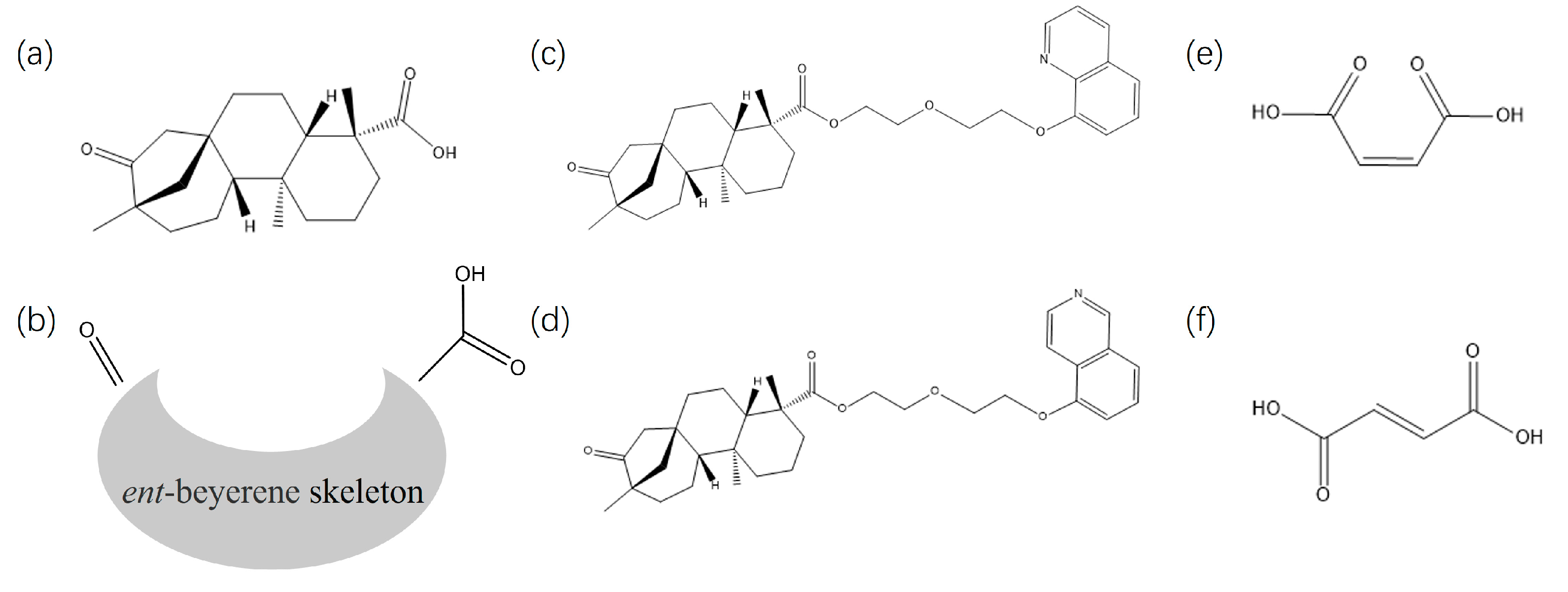
1. Introduction
2. Materials and Methods
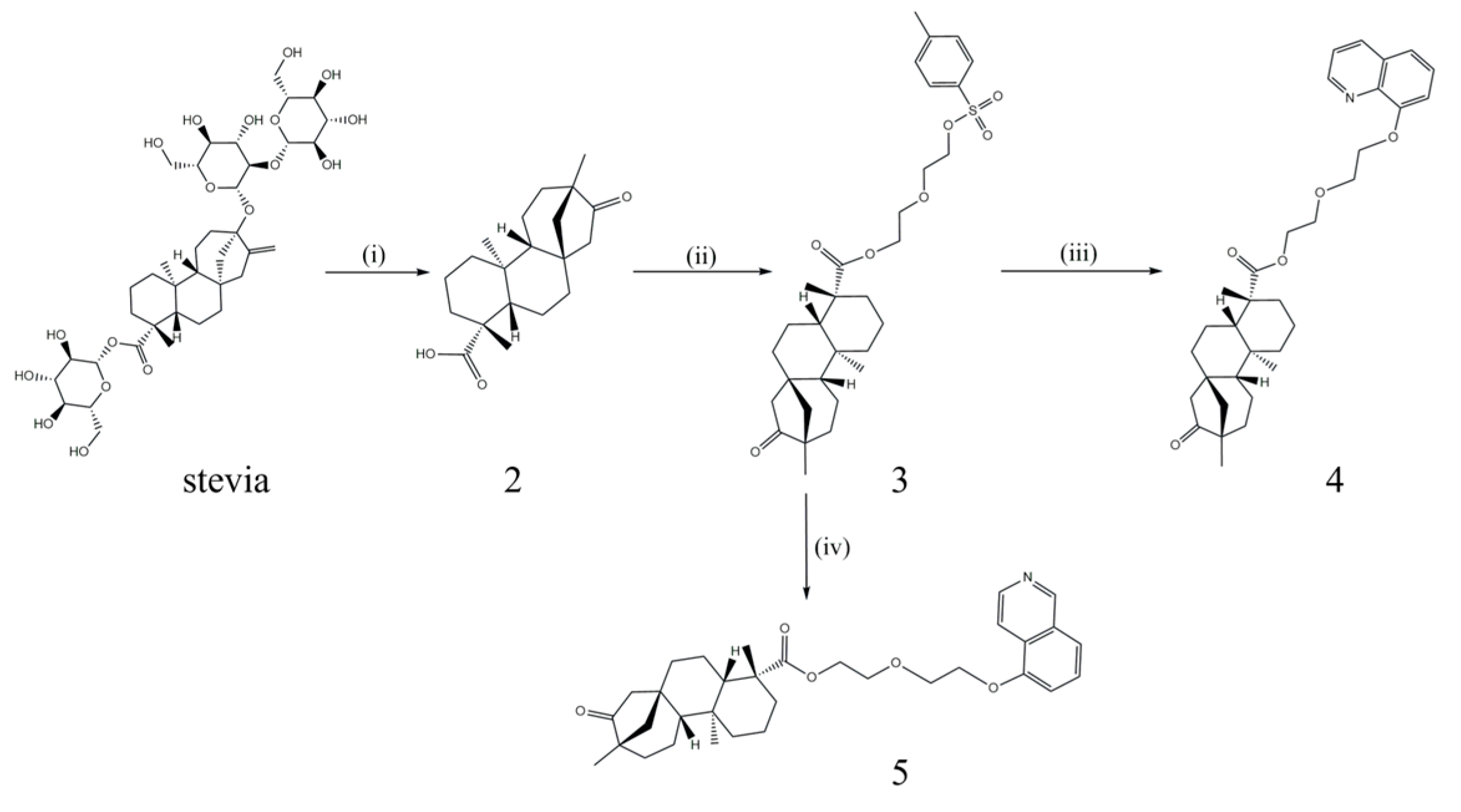
2.1. Synthesis of Probes 4 and 5
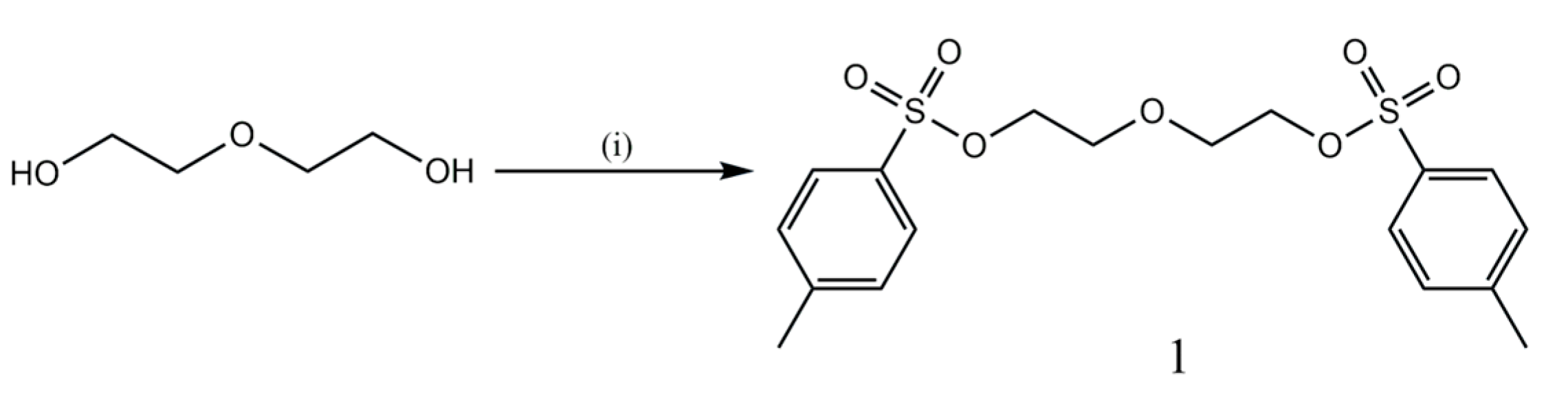
2.1.1. Synthesis of Oxybis (Ethane-2,1-diyl) bis (4-Methylbenzenesulfonate)(1)
2.1.2. Synthesis of Isosteviol(2)
2.1.3. Synthesis of 2-(2-(Tosyloxy)ethoxy)ethyl (4R,4aS,6aR,9S,11aR,11bS)-4,9,11b-trimethyl-8-oxotetradecahydro-6a,9-methanocyclohepta[a]naphthalene-4-carboxylate(3)
2.1.4. Synthesis of 2-(2-(Quinolin-8-yloxy)ethoxy)ethyl (4R,4aS,6aR,9S,11aR,11bS)-4,9,11b-trimethyl-8-oxotetradecahydro-6a,9-methanocyclohepta[a]naphthalene-4-carboxylate(4)
2.1.5. Synthesis of 2-(2-(Isoquinolin-5-yloxy)ethoxy)ethyl (4R,4aS,6aR,9S,11aR,11bS)-4,9,11b-trimethyl-8-oxotetradecahydro-6a,9-methanocyclohepta[a]naphthalene-4-carboxylate(5)
2.2. Fluorescence Spectral Study
2.3. Detection Limit
2.4. DFT Calculation
2.5. Detection of Maleic Acid in Food Additives
3. Results and Discussion
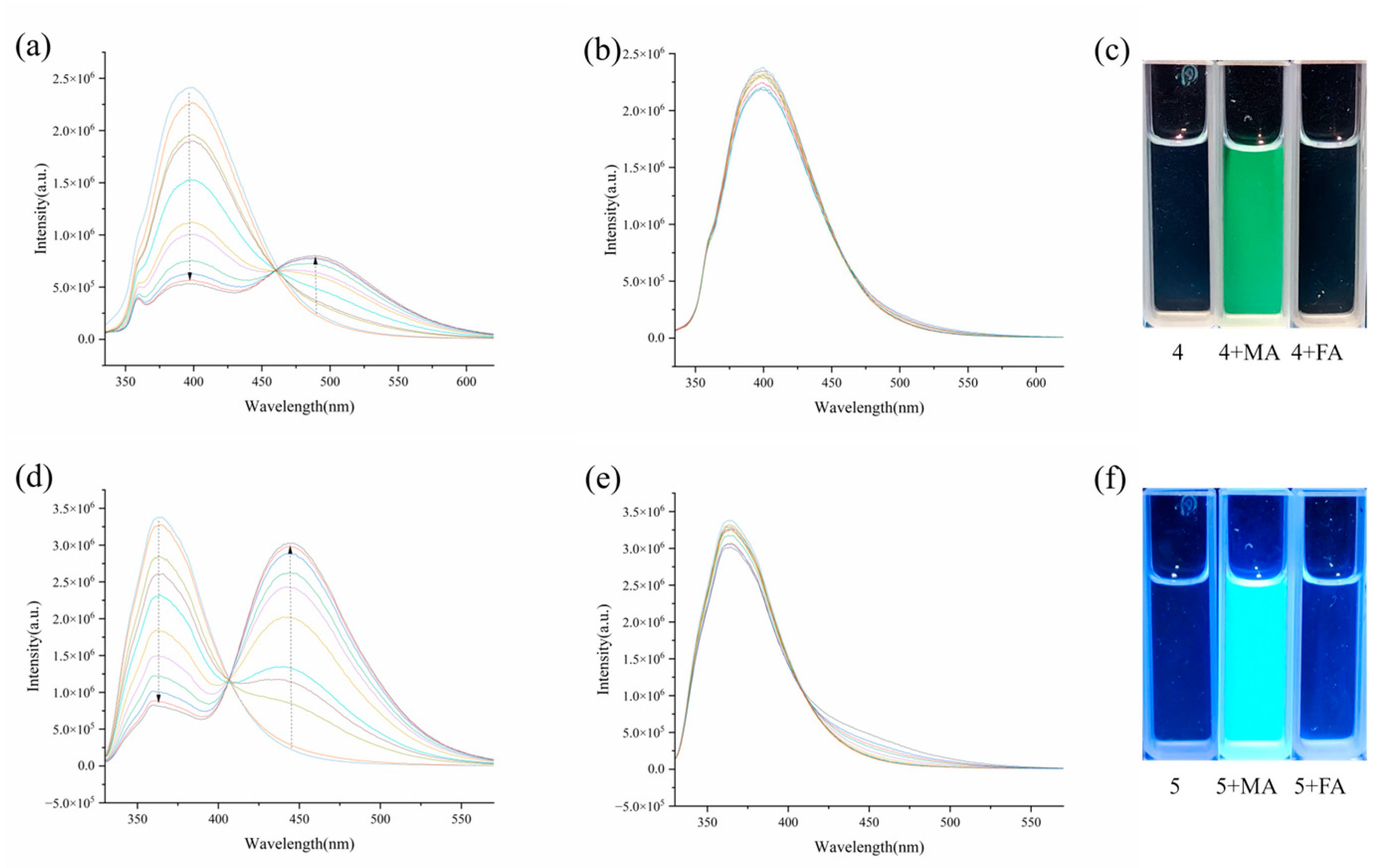
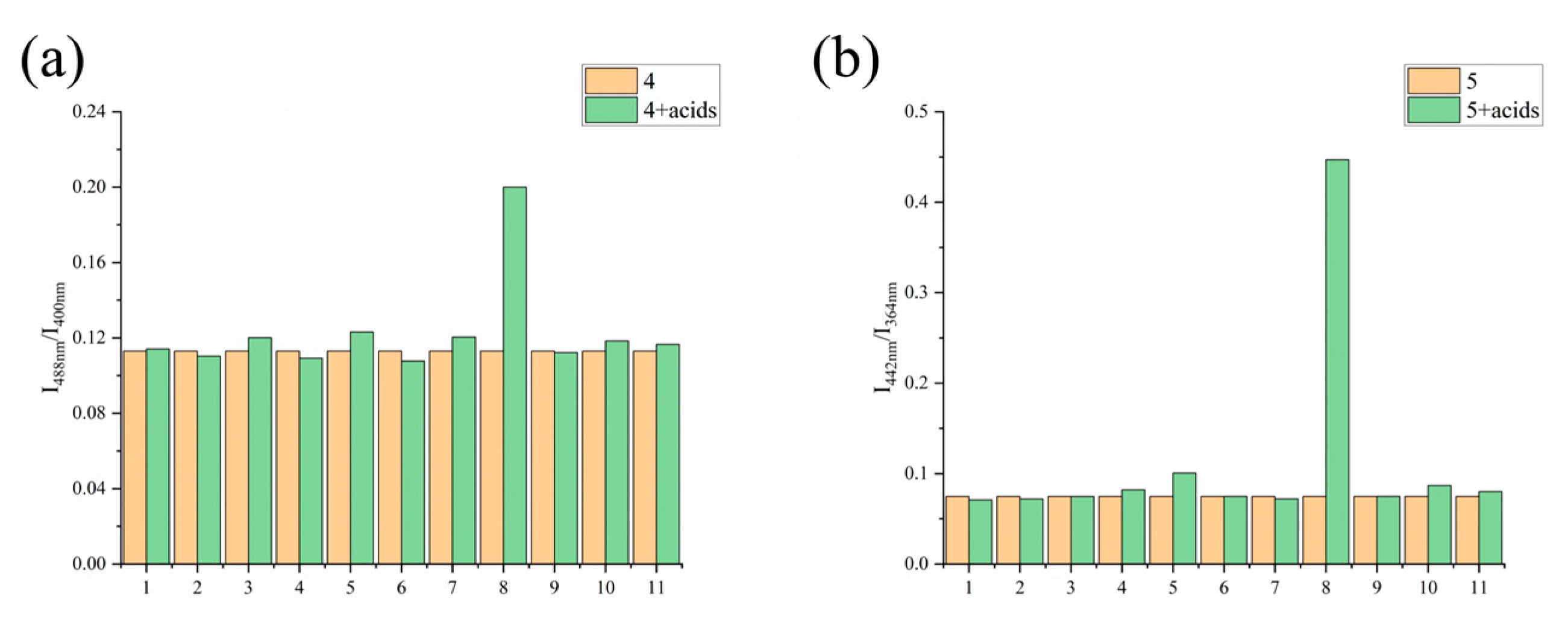
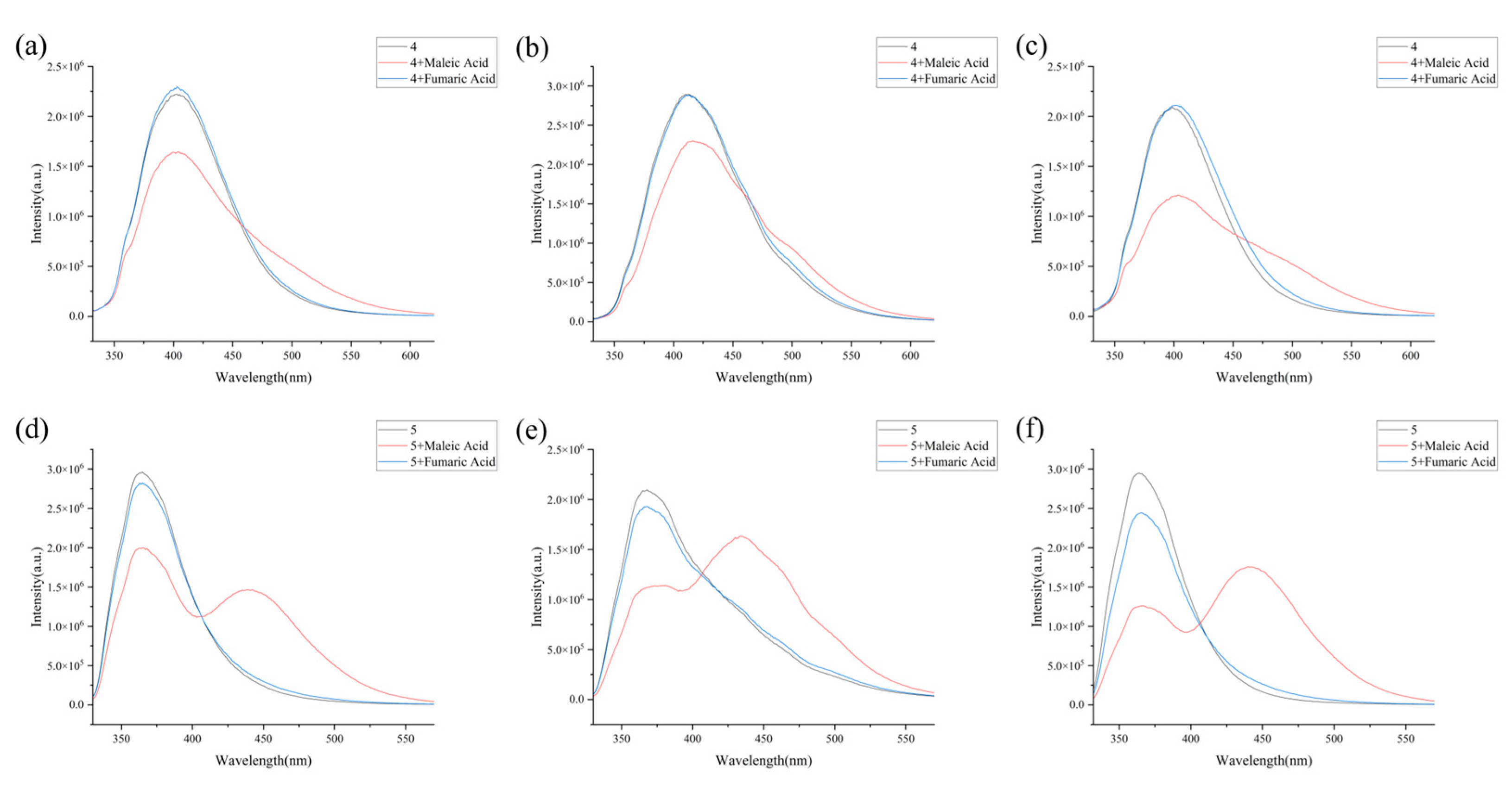
3.1. Fluorescence Sensing Study
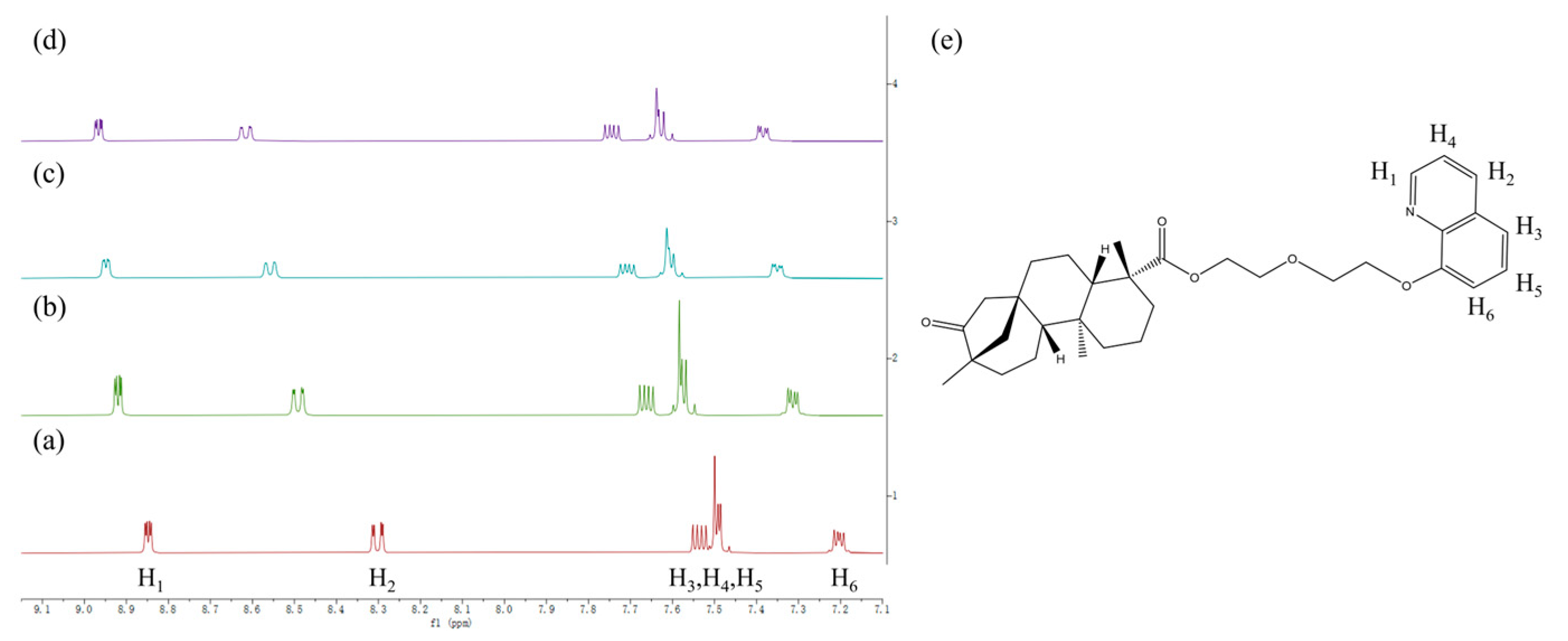
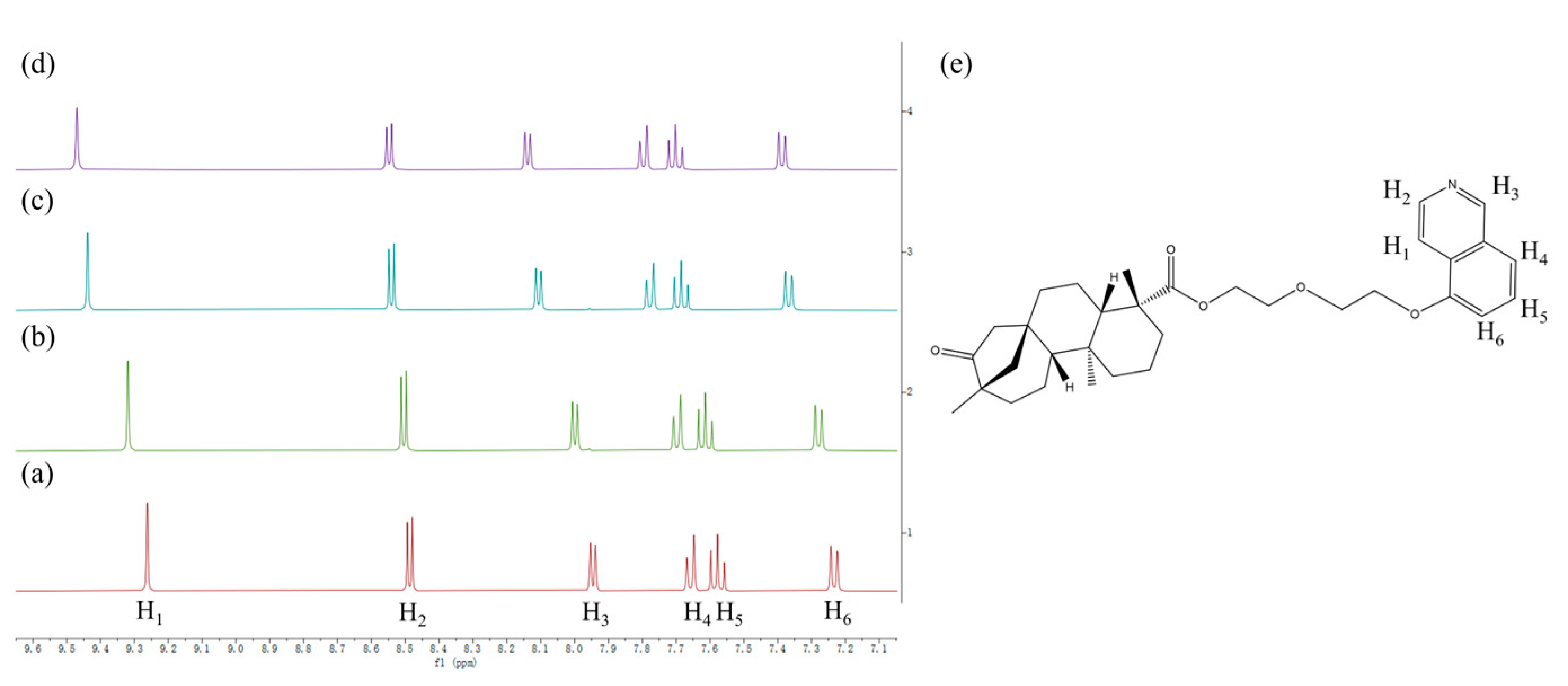
3.2. 1H NMR Study
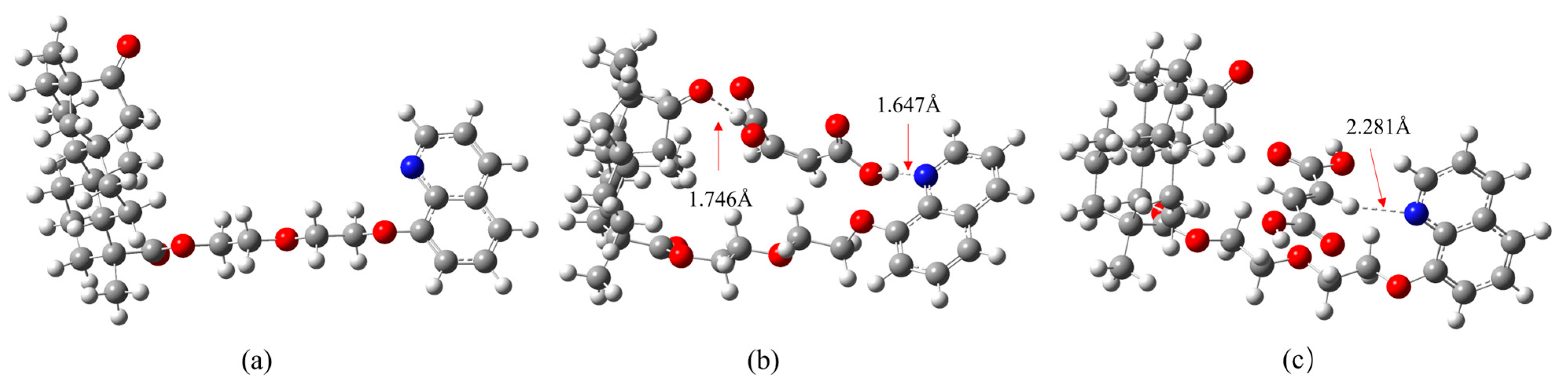
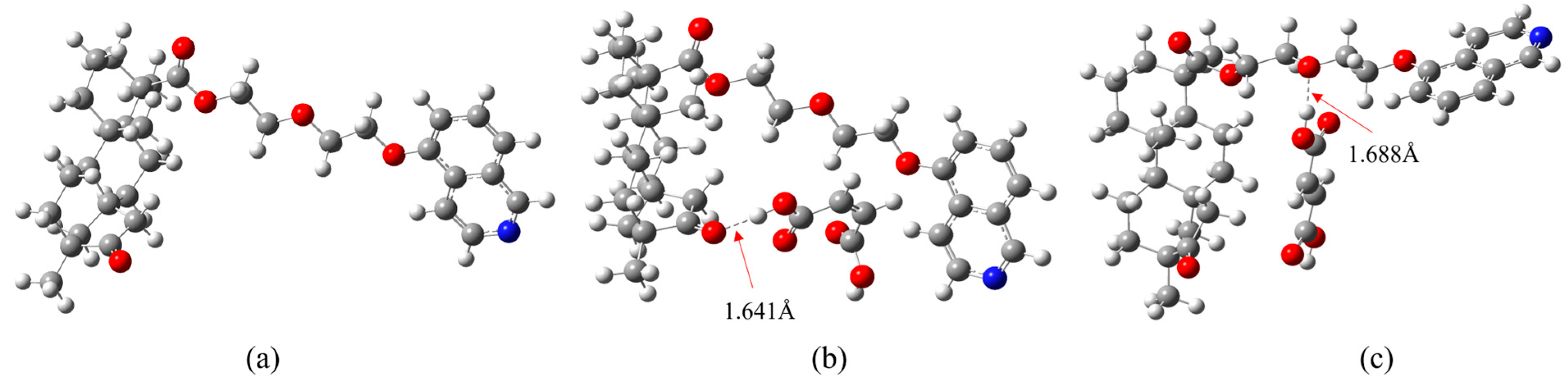
3.3. Density Functional Theory (DFT) Studies
3.4. Identification of Maleic Acid in Starchy Food
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, Y.; Tao, C.-L.; Yu, M.; Xiong, Y.; Ouyang, Y.-N.; Liu, X.-G.; Zhao, Z. Tetraphenylethene-Based Luminescent Metal–Organic Framework for Effective Differentiation of cis/trans Isomers. ACS Appl. Mater. Interfaces 2020, 12, 35266–35272. [Google Scholar] [CrossRef]
- Lo, R.; Roy, S.; Banerjee, T.; Ganguly, B.; Das, A. A new receptor with a FRET based fluorescence response for selective recognition of fumaric and maleic acids in aqueous medium. Chem. Commun. 2013, 49, 9818–9820. [Google Scholar] [CrossRef]
- Mishra, S.; Dash, P.P.; Mohanty, P.; Panda, M.K.; Mohanty, M.; Nanda, P.K.; Behera, S.K.; Sahoo, S.K.; Bhaskaran, R.; Jali, B.R. A Schiff-Base Molecular Probe for Selective Fluorescence Sensing of Maleic Acid with Recognition of Maleic Acid in Food Additives and Cell Imaging. J. Fluoresc. 2024, 35, 1–13. [Google Scholar] [CrossRef]
- Xaviera, A.; Saleem, A.; Akhtar, M.F.; Alshammari, A.; Albekairi, N.A. Fumaric acid per se and in combination with methotrexate arrests inflammation via moderating inflammatory and oxidative stress biomarkers in arthritic rats. Immunopharmacol. Immunotoxicol. 2024, 46, 793–804. [Google Scholar] [CrossRef]
- Altmeyer, P.J.; Matthes, U.; Pawlak, F.; Hoffmann, K.; Frosch, P.J.; Ruppert, P.; Wassilew, S.W.; Horn, T.; Kreysel, H.W.; Lutz, G.; et al. Antipsoriatic effect of fumaric-acid derivatives—Results of a multicenter double-blind-study in 100 patients. J. Am. Acad. Dermatol. 1994, 30, 977–981. [Google Scholar] [CrossRef]
- Sebastian, J.; Osorio-Gonzalez, C.; Rouissi, T.; Hegde, K.; Brar, S.K. Bioderived fumaric acid for sustainable production of key active pharmaceutical ingredients: Dimethyl fumarate and Monomethyl fumarate. Process Biochem. 2022, 120, 35–40. [Google Scholar] [CrossRef]
- Eiam-ong, S.; Spohn, M.; Kurtzman, N.A.; Sabatini, S. Insights into the biochemical mechanism of maleic acid-induced Fanconi syndrome. Kidney Int. 1995, 48, 1542–1548. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-C.; Wang, C.-C.; Tung, C.-W. An in silico toxicogenomics approach for inferring potential diseases associated with maleic acid. Chem.-Biol. Interact. 2014, 223, 38–44. [Google Scholar] [CrossRef]
- Wang, C.-C.; Lin, Y.-C.; Cheng, Y.-H.; Tung, C.-W. Profiling transcriptomes of human SH-SY5Y neuroblastoma cells exposed to maleic acid. PeerJ 2017, 5, e3175. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Dixit, P.; Trivedi, V.; Gandhi, A.; Senger, A.; Guttikar, S.; Singhal, P.; Shrivastav, P.S. Chromatographic separation of (E)- and (Z)-isomers of entacapone and their simultaneous quantitation in human plasma by LC–ESI-MS/MS. J. Chromatogr. B 2009, 877, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, X.; Wang, B.; Ding, Z.-J.; Li, C. Carbon-carbon double bond in pillar[5]arene cavity: Selective binding of cis/trans-olefin isomers. Chin. Chem. Lett. 2020, 31, 3230–3232. [Google Scholar] [CrossRef]
- Li, J.; Gu, X.; Yuan, X.; Qiu, Q.; Sun, J.; Wang, H. Cis/trans Fluorescent Recognition by Naphthalimide Dyes ⊂ CB [7] Assembly. J. Fluoresc. 2016, 26, 1219–1224. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Zhao, Q.W.; Fan, R.; Wang, H.L.; Zhu, J.H.; Wang, X.D. An efficiently ratiometric fluorescent probe based on bis-dihydroxyboron fluorescein complexes for detection of pyrethroid residues in fruit juices. Microchem. J. 2022, 172, 106954. [Google Scholar] [CrossRef]
- Dash, P.P.; Mohanty, P.; Behura, R.; Behera, S.; Naik, S.; Mishra, M.; Sahoo, H.; Barick, A.K.; Mohapatra, P.; Sahoo, S.K.; et al. Rapid Colorimetric and Fluorometric Discrimination of Maleic Acid vs. Fumaric Acid and Detection of Maleic Acid in Food Additives. J. Fluoresc. 2023, 34, 1015–1024. [Google Scholar] [CrossRef]
- Kim, E.-J.; Haldar, U.; Lee, H. Tuning the ability to discriminate between geometric isomers maleic acid and fumaric acid of water-soluble polymeric probes with a donor-π-acceptor skeleton. Polymer 2020, 186, 122040. [Google Scholar] [CrossRef]
- Goswami, S.; Das, N.K.; Sen, D.; Hazra, G.; Goh, J.H.; Sing, Y.C.; Fun, H.-K. Recognition of acids involved in Krebs cycle by 9-anthrylmethyl-di(6-acetylamino-2-picolyl)amine: A case of selective fluorescence enhancement for maleic acid. New J. Chem. 2011, 35, 2811–2819. [Google Scholar] [CrossRef]
- Ghosh, K.; Sen, T.; Patra, A.; Mancini, J.S.; Cook, J.M.; Parish, C.A. (rac)-1,1′-Binaphthyl-Based Simple Receptors Designed for Fluorometric Discrimination of Maleic and Fumaric Acids. J. Phys. Chem. B 2011, 115, 8597–8608. [Google Scholar] [CrossRef]
- Samanta, S.; Kar, C.; Das, G. Colorimetric and Fluorometric Discrimination of Geometrical Isomers (Maleic Acid vs Fumaric Acid) with Real-Time Detection of Maleic Acid in Solution and Food Additives. Anal. Chem. 2015, 87, 9002–9008. [Google Scholar] [CrossRef]
- Huang, X.; Song, J.; Yung, B.C.; Huang, X.; Xiong, Y.; Chen, X. Ratiometric optical nanoprobes enable accurate molecular detection and imaging. Chem. Soc. Rev. 2018, 47, 2873–2920. [Google Scholar] [CrossRef]
- Park, S.-H.; Kwon, N.; Lee, J.-H.; Yoon, J.; Shin, I. Synthetic ratiometric fluorescent probes for detection of ions. Chem. Soc. Rev. 2020, 49, 143–179. [Google Scholar] [CrossRef]
- Shangguan, H.; Liu, Q.; Wang, Y.; Teng, Z.; Tian, R.; Wu, T.; Yang, L.; Jiang, L.; Liu, X.; Wei, L. Bioimaging of a chromenoquinoline-based ratiometric fluorescent probe for detecting ClO−. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 303, 123256. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, H.; Xu, F.; Gao, X.; Li, J.; Xu, S.; Zhang, D.; Wu, X.; Xu, J.; Hua, H.; et al. Diterpenoid lead stevioside and its hydrolysis products steviol and isosteviol: Biological activity and structural modification. Eur. J. Med. Chem. 2018, 156, 885–906. [Google Scholar] [CrossRef] [PubMed]
- Lohoelter, C.; Weckbecker, M.; Waldvogel, S.R. (-)-Isosteviol as a Versatile Ex-Chiral-Pool Building Block for Organic Chemistry. Eur. J. Org. Chem. 2013, 2013, 5539–5554. [Google Scholar] [CrossRef]
- Lohoelter, C.; Brutschy, M.; Lubczyk, D.; Waldvogel, S.R. Novel supramolecular affinity materials based on (−)-isosteviol as molecular templates. Beilstein J. Org. Chem. 2013, 9, 2821–2833. [Google Scholar] [CrossRef]
- An, Y.-J.; Zhang, Y.-X.; Wu, Y.; Liu, Z.-M.; Pi, C.; Tao, J.-C. Simple amphiphilic isosteviol–proline conjugates as chiral catalysts for the direct asymmetric aldol reaction in the presence of water. Tetrahedron Asymmetry 2010, 21, 688–694. [Google Scholar] [CrossRef]
- Ma, Z.-w.; Liu, Y.-x.; Li, P.-l.; Ren, H.; Zhu, Y.; Tao, J.-c. A highly efficient large-scale asymmetric Michael addition of isobutyraldehyde to maleimides promoted by a novel multifunctional thiourea. Tetrahedron Asymmetry 2011, 22, 1740–1748. [Google Scholar] [CrossRef]
- Lü, Q.; Tao, J.; Wang, J.; Wang, C.; Chen, X.; Ma, Z. Chiral Squaramide Catalyzed Enantioselective Michael Addition of Cyclic 1,3-Diketones to β,γ-Unsaturated α-Keto Esters. Chin. J. Org. Chem. 2022, 42, 1520. [Google Scholar] [CrossRef]
- Bonger, K.M.; van den Berg, R.J.B.H.N.; Heitman, L.H.; Ijzerman, A.P.; Oosterom, J.; Timmers, C.M.; Overkleeft, H.S.; van der Marel, G.A. Synthesis and evaluation of homo-bivalent GnRHR ligands. Bioorg. Med. Chem. 2007, 15, 4841–4856. [Google Scholar] [CrossRef]
- Chen, J.; Sun, M.; Cai, J.; Cao, M.; Zhou, W.; Ji, M. The Synthesis and Crystal Structure of (4α,8β,13β,16β)-13-Methyl-16,18-diol-17-Norkaurane: A Simultaneous Reduction Product of Isosteviol. J. Chem. Crystallogr. 2010, 41, 519–522. [Google Scholar] [CrossRef]
- Ali, S.; Ahmad, T.; Tahir, M.Y.; Usman, M.; Chhattal, M.; Hussain, I.; Khan, S.; Hassan, A.M.; Assiri, M.A.; Rosaiah, P.; et al. The emergence of density functional theory for supercapacitors: Recent progress and advances. J. Energy Storage 2023, 73, 109100. [Google Scholar] [CrossRef]
- Kapustin, R.; Grinvald, I.; Agrba, A.; Agrba, M.; Vorotyntsev, A.; Vorotyntsev, V.; Vorotyntsev, I.; Petukhov, A.; Baberkina, E.; Aleksandrova, D. Molecular Interactions and Structural Transformations in Water Complexes with Azoles in Solid Films: IR and DFT Study. ChemistrySelect 2024, 9, e202403714. [Google Scholar] [CrossRef]
- Klamt, A.; Moya, C.; Palomar, J. A Comprehensive Comparison of the IEFPCM and SS(V)PE Continuum Solvation Methods with the COSMO Approach. J. Chem. Theory Comput. 2015, 11, 4220–4225. [Google Scholar] [CrossRef] [PubMed]
- Chuwkwu, U.G.; Louis, H.; Edet, H.O.; Unimuke, T.O.; Olagoke, P.O.; Adeyinka, A.S. Toward Site-Specific Interactions of nH2 (n = 1–4) with Ga12As12 Nanostructured for Hydrogen Storage Applications. Energy Fuels 2023, 37, 1353–1369. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. B.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, X.; Zheng, C.; Zhang, F. Ratiometric Fluorescent Probes Based on Isosteviol with Identification of Maleic Acid in Starchy Foods. Foods 2025, 14, 1541. https://doi.org/10.3390/foods14091541
Qian X, Zheng C, Zhang F. Ratiometric Fluorescent Probes Based on Isosteviol with Identification of Maleic Acid in Starchy Foods. Foods. 2025; 14(9):1541. https://doi.org/10.3390/foods14091541
Chicago/Turabian StyleQian, Xinye, Chunling Zheng, and Fang Zhang. 2025. "Ratiometric Fluorescent Probes Based on Isosteviol with Identification of Maleic Acid in Starchy Foods" Foods 14, no. 9: 1541. https://doi.org/10.3390/foods14091541
APA StyleQian, X., Zheng, C., & Zhang, F. (2025). Ratiometric Fluorescent Probes Based on Isosteviol with Identification of Maleic Acid in Starchy Foods. Foods, 14(9), 1541. https://doi.org/10.3390/foods14091541





