Effect of Heat Pasteurization and Sterilization on Milk Safety, Composition, Sensory Properties, and Nutritional Quality
Abstract
1. Introduction
2. Pasteurization and Sterilization Processes and Bacterial Destruction
| Heat Treatments | Temperature–Time Combination Required | Time | Pathogens Destroyed |
|---|---|---|---|
| Thermization | 57–68 °C | 5 s–30 min | Non-spore-forming pathogens and psychrotropic spoilage bacteria |
| Flash pasteurization | 72–80 °C | 15–30 s | Non-spore-forming pathogens and psychrotropic spoilage bacteria |
| Extended shelf life pasteurization (ESLP) | 125–140 °C | 1–10 s | Psychrotropic, mesophilic, and non-spore-forming bacteria |
| HTST | 72–74 °C | 15–20 s | Coxiella burnetii, the most heat-resistant pathogen in raw milk |
| Ultra-high-temperature (UHT) indirect heating | 130–145 °C | 5–20 s | Clostridium Botulinum and target Coxiella burnetii; bacterial endospore |
| Ultra-high-temperature (UHT) direct heating | 142–150 °C | 2–6 s | Heat-resistant spore formers without excessive chemical damage |
| Sterilization | 110–120 °C or 125 °C | 10–20 min 5 min | All non-spore-forming bacteria except heat-resistant spore-forming bacteria |
| Innovative steam injection (ISI) | 160–180 °C | 0.1 s | Heat-resistant spores |
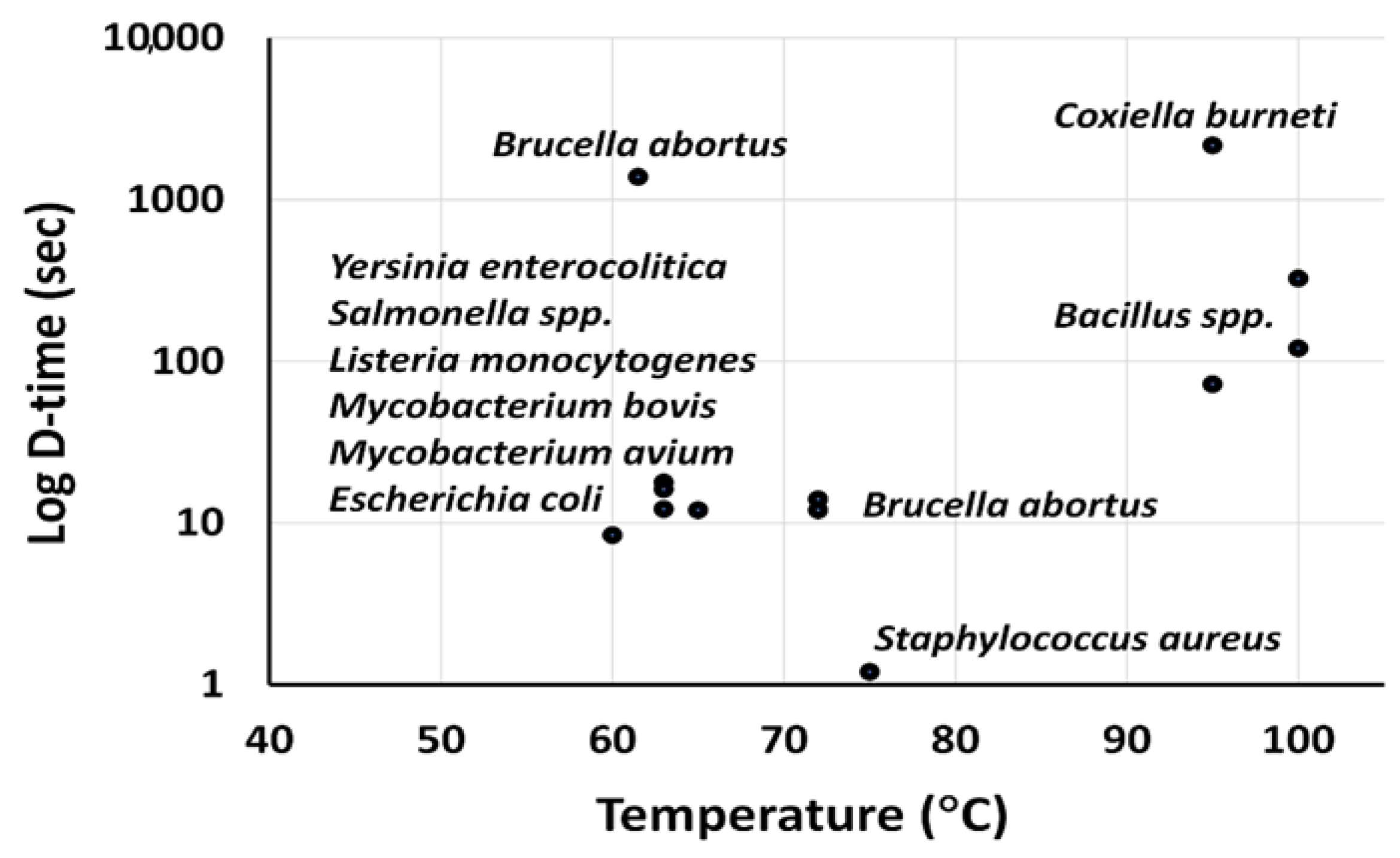
3. Effects of Pasteurization and Sterilization on Milk Safety
| Year | Pathogen | State | Outbreak Information | Reference |
|---|---|---|---|---|
| 2015 | Campylobacter jejuni | Italy | Campylobacter jejuni contamination of raw milk across several Italian regions was estimated to cause between 230,776 and 301,785 cases per year (D–R I model) and up to 5.25 million cases per year under worst-case assumptions (D–R II model) depending on storage conditions. | [43] |
| 2014 | Staphylococcus aureus | Italy | There were no reported outbreaks, but an estimated 485 servings per year contained ≥20 ng enterotoxin A. | [44] |
| 2013 | Campylobacter spp. L. monocytogenes Salmonella spp. | New Zealand | A total of 93 cases per 100,000 servings contained Campylobacter spp., 201 cases contained Shiga toxin-producing E. coli (STEC), and 15 cases contained Salmonella spp. for Listeria monocytogenes. | [45] |
| 2011 | Listeria monocytogenes E. coli O157:H7, Campylobacter, Salmonella | New York | A quantitative risk assessment in New York estimated Listeria monocytogenes infections from raw milk consumption to range from 2.7 × 10−7 to 1.0 × 10−4 cases per person per year. | [46] |
| 2007–2011 | Campylobacter jejuni E. coli O157:H7 | Italy | Between 2007 and 2011, an estimated 6.3–7.2 cases of HUS (Hemolytic Uremic Syndrome) were linked to raw milk consumption in Italy, caused by E. coli O157:H7. Additionally, outbreaks of Campylobacter jejuni were reported in the Veneto and Marche regions during the 2008–2009 period, and two E. coli O157:H7 outbreaks occurred in Emilia Romagna over the same period. | [47] |
| 2009 | S.aureus Staphylococcus enterotoxin A | California | A total of 25.3% of 51,963 raw milk samples tested positive for Staphylococcus aureus, indicating a substantial contamination rate. Additionally, Staphylococcal Enterotoxin A (SEA) exposure levels at these high percentiles could reach 94 ng/serving. | [48] |
| 2008 | Campylobacter spp. | California | Of 16 cases, 4 cases were CC for Campylobacter; 3/4 drank raw milk; and the rest were employees. Two individuals were hospitalized, including one with a form of Guillain–Barré syndrome. | [49] |
| 2007 | C. jejuni | Kansas | Of 25 cases, 7 cases were CC, 18 probably occurred over several months; 16/28 persons who consumed raw milk at a gathering became ill. | [50] |
| 2007 | Salmonella typhimurium | Pennsylvania | There were 29 cases, with an age range of 5 months–76 years; 16/29 were <7 years, 29 cases were CC, there were identical PFGE patterns, and two individuals were hospitalized. | [51] |
| 2007 | C. jejuni | Kansas | There were 68 cases, and 4 cases were CC for C. jejuni; two individuals were hospitalized. | [52] |
| 2006–2007 | Salmonella | There were 85 cases, primarily including Hispanic people. A total of 85 cases were CC, with identical PFGE patterns; 36 individuals were hospitalized. | [53] | |
| 2006 | E. coli O157:H7 | California | There were 6 cases, with 5 CC with identical PFGE patterns. There was one non-CC case, HUS; three individuals were hospitalized. | [54] |
| 2005 | E. coli O157:H7 | Washington | Of 18 cases, 8 cases were CC; 7/8 had identical PFGE patterns. Five people were hospitalized, and four had HUS. | [53] |
| 2002–2003 | S. typhimurium | Multi-State in the USA | There were 62 cases, and 62 were CC, with identical PFGE patterns and an epidemiologic link to an implicated dairy outbreak strain isolated from milk, cream, and butter samples. | [55] |
| 2002 | C. jejuni | Utah | Of 13 cases, 5/6 cases were CC; six individuals sought medical attention and none were hospitalized. | [56] |
| 2001 | Salmonella | - | A total of 26 cases were CC for MDR-SN; 23 individuals were treated with antibiotics, and 8 were hospitalized. | [57] |
| 2001 | C. jejuni | Wisconsin | Of 75 cases, 28 cases were CC; the PFGE patterns of 21 tested individuals were identical. | [58] |
4. Effect of Pasteurization and Sterilization on Milk Constituents
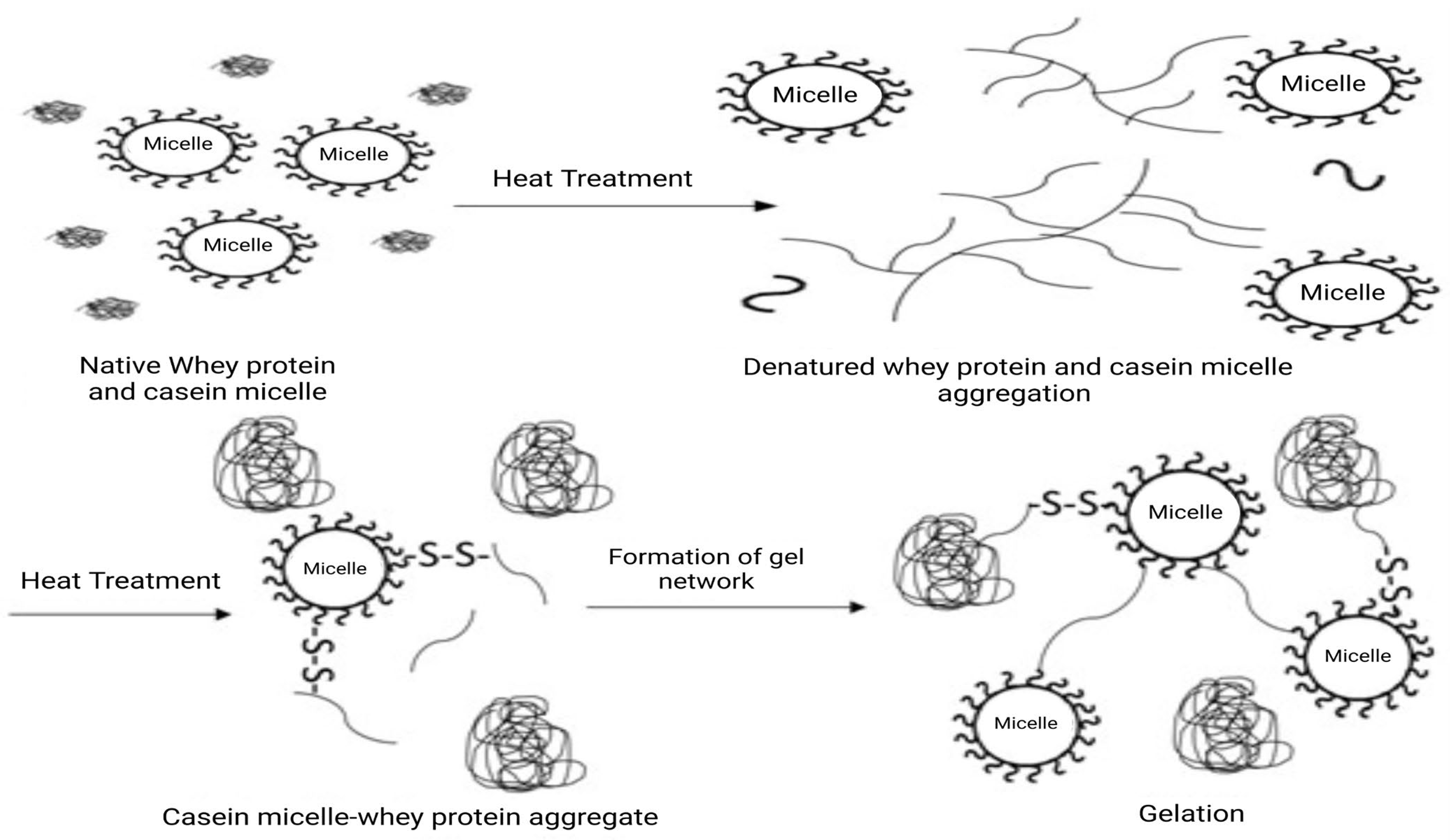
4.1. Effects on Milk Protein Structure and Functionality
4.2. Effect on Antimicrobial Systems
5. The Effects of Pasteurization and Sterilization on the Physical Properties and Sensory Quality of Milk
6. The Effects of Pasteurization and Sterilization on the Nutritional Quality of Milk
6.1. Effects on Vitamins and Minerals
6.2. Effect on Milk Digestibility and Gut Health
6.3. Effects on Lactose Intolerance, Allergy, and Immunity
6.4. Effect on Fatty Acids and Milk Fat Globule Membrane (MFGM)
7. Alternative Processing Methods
8. Economic Impact of Milk Processing Methods
9. Future Prospective
10. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garcia, R.; Adrian, J. Nicolas Appert: Inventor and Manufacturer. Food Rev. Int. 2009, 25, 115–125. [Google Scholar] [CrossRef]
- Westhoff, D.C. Heating Milk for Microbial Destruction: A Historical Outline and Update. J. Food Prot. 1978, 41, 122–130. [Google Scholar] [CrossRef]
- Koplik, H. The History of the First Milk Depot or Gouttes De Lait with Consultations in America. J. Am. Med. Assoc. 1914, LXIII, 1574–1575. [Google Scholar] [CrossRef]
- Smith-Howard, K. Pure and Modern Milk: An Environmental History Since 1900; Oxford University Press: Oxford, UK, 2017. [Google Scholar]
- Andreoletti, O.; Lau Baggesen, D.; Bolton, D.; Butaye, P.; Cook, P.; Davies, R.; Fernández Escámez, P.S.; Griffin, J.; Hald, T.; Havelaar, A.; et al. Scientific Opinion on the Public Health Risks Related to the Consumption of Raw Drinking Milk. EFSA J. 2015, 13, 3940. [Google Scholar] [CrossRef]
- The Dangers of Raw Milk: Unpasteurized Milk Can Pose a Serious Health Risk—FDA. Available online: https://www.fda.gov/food/buy-store-serve-safe-food/dangers-raw-milk-unpasteurized-milk-can-pose-serious-health-risk (accessed on 2 April 2025).
- Raw Milk—Food Safety—CDC. Available online: https://www.cdc.gov/food-safety/foods/raw-milk.html (accessed on 2 April 2025).
- Haug, A.; Høstmark, A.T.; Harstad, O.M. Bovine Milk in Human Nutrition—A Review. Lipids Health Dis. 2007, 6, 25. [Google Scholar] [CrossRef]
- Lisboa, H.M.; Pasquali, M.B.; dos Anjos, A.I.; Sarinho, A.M.; de Melo, E.D.; Andrade, R.; Batista, L.; Lima, J.; Diniz, Y.; Barros, A. Innovative and Sustainable Food Preservation Techniques: Enhancing Food Quality, Safety, and Environmental Sustainability. Sustainability 2024, 16, 8223. [Google Scholar] [CrossRef]
- Zhao, Y.M.; de Alba, M.; Sun, D.W.; Tiwari, B. Principles and Recent Applications of Novel Non-Thermal Processing Technologies for the Fish Industry—A Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Khaliq, A.; Mishra, A.K.; Rabbani, A. Comparative Antimicrobial Evaluation of Synthetic Antibiotics and Essential Oils Against Human Pathogenic Bacteria and Fungi. Innov. Agric. 2024, 7, 1–7. [Google Scholar] [CrossRef]
- Knipschildt, M.E.; Andersen, G.G. Drying of Milk and Milk Products. Robinson Mod. Dairy Technol. 1994, 1, 159–254. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations/World Health Organization (FAO/WHO). Code of Hygienic Practice for Milk and Milk Products. Joint FAO/WHO Food Standards Programme-Codex Committee on Food Hygiene, 26th Session, 29 March–2 April 2004, Washington, DC, USA. 2004. Available online: http://www.fao.org/fileadmin/user_upload/livestockgov/documents/CXP_057e.pdf (accessed on 2 April 2025).
- Barbano, D.M.; Ma, Y.; Santos, M.V. Influence of Raw Milk Quality on Fluid Milk Shelf Life. J. Dairy Sci. 2006, 89, E15–E19. [Google Scholar] [CrossRef]
- Ma, Y.; Ryan, C.; Barbano, D.M.; Galton, D.M.; Rudan, M.A.; Boor, K.J. Effects of Somatic Cell Count on Quality and Shelf-Life of Pasteurized Fluid Milk. J. Dairy. Sci. 2000, 83, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Enright, J.; Sadler, W.; Thomas, R. Thermal Inactivation of Coxiella Burnetii and Its Relation to Pasteurization of Milk; US Government Printing: Washington, WA, USA, 1957.
- Tamime, A.Y. Milk Processing and Quality Management; Wiley-Blackwell: Hoboken, NJ, USA, 2009; pp. 1–324. [Google Scholar] [CrossRef]
- Hall, C.W.; Trout, G.M. Milk Pasteurization; AVI Publishing: Delhi, India, 1968. [Google Scholar]
- Varnam, A.; Sutherland, J. Milk and Milk Products: Technology, Chemistry and Microbiology; Aspen Publishers: Boston, MA, USA, 2001. [Google Scholar]
- Sharma, S.K.; Sehgal, N.; Kumar, A. Dry-Reagent Strips for Testing Milk Pasteurization. LWT Food Sci. Technol. 2003, 36, 567–571. [Google Scholar] [CrossRef]
- Ludikhuyze, L.; Claeys, W.; Hendrickx, M. Combined Pressure—Temperature Inactivation of Alkaline Phosphatase in Bovine Milk: A Kinetic Study. J. Food Sci. 2000, 65, 155–160. [Google Scholar] [CrossRef]
- Peng, Z.; Li, Y.; Yan, L.; Yang, S.; Yang, D. Correlation Analysis of Microbial Contamination and Alkaline Phosphatase Activity in Raw Milk and Dairy Products. Int. J. Env. Res. Public. Health 2023, 20, 1825. [Google Scholar] [CrossRef] [PubMed]
- Deeth, H.; Smithers, G. Heat Treatment of Milk–Overview. Inernational Dairy Federation, IDF Factsheet. pp. 1–4. Available online: https://www.fil-idf.org/wp-content/uploads/2018/02/Factsheet-001_Heat-treatment-1-1.pdf (accessed on 17 February 2025).
- Martin, N.H.; Boor, K.J.; Wiedmann, M. Symposium Review: Effect of Post-Pasteurization Contamination on Fluid Milk Quality. J. Dairy Sci. 2018, 101, 861–870. [Google Scholar] [CrossRef]
- Kirwan, M. Handbook of Paper and Paperboard Packaging Technology; Wiley-Blackwell: Hoboken, NJ, USA, 2012. [Google Scholar]
- Twede, D.; Selke, S.E.M.; Kamdem, D.-P.; Pira, S. Cartons, Crates and Corrugated Board: Handbook of Paper and Wood Packaging Technology; Destech Pubns Inc.: Lancaster, PA, USA, 2014. [Google Scholar]
- LeJeune, J.T.; Rajala-Schultz, P.J. Unpasteurized Milk: A Continued Public Health Threat. Clin. Infect. Dis. 2009, 48, 93–100. [Google Scholar] [CrossRef]
- Van Brandt, L.; Van der Plancken, I.; De Block, J.; Vlaemynck, G.; Van Coillie, E.; Herman, L.; Hendrickx, M. Adequacy of Current Pasteurization Standards to Inactivate Mycobacterium Paratuberculosis in Milk and Phosphate Buffer. Int. Dairy J. 2011, 21, 295–304. [Google Scholar] [CrossRef]
- Sarkar, S. Microbiological Considerations: Pasteurized Milk. Int. J. Dairy Sci. 2015, 10, 206–218. [Google Scholar] [CrossRef]
- Adly, E.; Hegazy, A.A.; Kamal, M.; Abu-Hussien, S.H. Midguts of Culex Pipiens L. (Diptera: Culicidae) as a Potential Source of Raw Milk Contamination with Pathogens. Sci. Rep. 2022, 12, 13183. [Google Scholar] [CrossRef]
- Ullah, S.; Khan, S.U.H.; Khan, M.J.; Khattak, B.; Fozia, F.; Ahmad, I.; Wadaan, M.A.; Khan, M.F.; Baabbad, A.; Goyal, S.M. Multiple-Drug Resistant Shiga Toxin-Producing E. Coli in Raw Milk of Dairy Bovine. Trop. Med. Infect. Dis. 2024, 9, 64. [Google Scholar] [CrossRef]
- Gume, B.; Berhanu, L.; Kassa, T.; Bediru, H.; Fikre, A.G.; Dadi, L.S.; Mereta, S.T. Bacterial Hazard Identification and Exposure Assessment of Raw Milk Consumption in Jimma Zone, South West Ethiopia. BMC Microbiol. 2023, 23, 166. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, M.W. The Microbiological Safety of Raw Milk. Improv. Saf. Qual. Milk. 2010, 1, 27–63. [Google Scholar] [CrossRef]
- Fusco, V.; Chieffi, D.; Fanelli, F.; Logrieco, A.F.; Cho, G.S.; Kabisch, J.; Böhnlein, C.; Franz, C.M.A.P. Microbial Quality and Safety of Milk and Milk Products in the 21st Century. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2013–2049. [Google Scholar] [CrossRef]
- Murphy, M.; Buckley, J.F.; Whyte, P.; O’Mahony, M.; Anderson, W.; Wall, P.G.; Fanning, S. Surveillance of Dairy Production Holdings Supplying Raw Milk to the Farmhouse Cheese Sector for Escherichia Coli O157, O26 and O111. Zoonoses Public. Health 2007, 54, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, H.J.; Mathisen, T.; Løvseth, A.; Omoe, K.; Qvale, K.S.; Loncarevic, S. An Outbreak of Staphylococcal Food Poisoning Caused by Enterotoxin H in Mashed Potato Made with Raw Milk. FEMS Microbiol. Lett. 2005, 252, 267–272. [Google Scholar] [CrossRef]
- Langer, A.J.; Ayers, T.; Grass, J.; Lynch, M.; Angulo, F.J.; Mahon, B.E. Nonpasteurized Dairy Products, Disease Outbreaks, and State Laws—United States, 1993–2006. Emerg. Infect. Dis. 2012, 18, 385. [Google Scholar] [CrossRef]
- Lucey, J.A. Raw Milk Consumption: Risks and Benefits. Nutr. Today 2015, 50, 189. [Google Scholar] [CrossRef] [PubMed]
- Mungai, E.A.; Behravesh, C.B.; Gould, L.H. Increased Outbreaks Associated with Nonpasteurized Milk, United States, 2007–2012. Emerg. Infect. Dis. 2015, 21, 119. [Google Scholar] [CrossRef]
- Robinson, T.J.; Scheftel, J.M.; Smith, K.E. Raw Milk Consumption among Patients with Non–Outbreak-Related Enteric Infections, Minnesota, USA, 2001–2010. Emerg. Infect. Dis. 2014, 20, 38. [Google Scholar] [CrossRef]
- D’Amico, D.J.; Groves, E.; Donnelly, C.W. Low Incidence of Foodborne Pathogens of Concern in Raw Milk Utilized for Farmstead Cheese Production. J. Food Prot. 2008, 71, 1580–1589. [Google Scholar] [CrossRef]
- Jamali, H.; Paydar, M.; Radmehr, B.; Ismail, S.; Dadrasnia, A. Prevalence and Antimicrobial Resistance of Staphylococcus Aureus Isolated from Raw Milk and Dairy Products. Food Control 2015, 54, 383–388. [Google Scholar] [CrossRef]
- Giacometti, F.; Bonilauri, P.; Amatiste, S.; Arrigoni, N.; Bianchi, M.; Losio, M.N.; Bilei, S.; Cascone, G.; Comin, D.; Daminelli, P.; et al. Human Campylobacteriosis Related to the Consumption of Raw Milk Sold by Vending Machines in Italy: Quantitative Risk Assessment Based on Official Controls over Four Years. Prev. Vet. Med. 2015, 121, 151–158. [Google Scholar] [CrossRef]
- Crotta, M.; Rizzi, R.; Varisco, G.; Daminelli, P.; Cunico, E.C.; Luini, M.; Graber, H.U.; Paterlini, F.; Guitian, J. Multiple-Strain Approach and Probabilistic Modeling of Consumer Habits in Quantitative Microbial Risk Assessment: A Quantitative Assessment of Exposure to Staphylococcal Enterotoxin A in Raw Milk. J. Food Prot. 2016, 79, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Soboleva, T.; French, N. Assessment of the Microbiological Risks Associated with the Consumption of Raw Milk; Ministry for Primary Industries: Wellington, New Zealand, 2014.
- Latorre, A.A.; Pradhan, A.K.; Van Kessel, J.A.S.; Karns, J.S.; Boor, K.J.; Rice, D.H.; Mangione, K.J.; Grohn, Y.T.; Schukken, Y.H. Quantitative Risk Assessment of Listeriosis Due to Consumption of Raw Milk. J. Food Prot. 2011, 74, 1268–1281. [Google Scholar] [CrossRef] [PubMed]
- Giacometti, F.; Serraino, A.; Bonilauri, P.; Ostanello, F.; Daminelli, P.; Finazzi, G.; Losio, M.N.; Marchetti, G.; Liuzzo, G.; Zanoni, R.G.; et al. Quantitative Risk Assessment of Verocytotoxin-Producing Escherichia Coli O157 and Campylobacter Jejuni Related to Consumption of Raw Milk in a Province in Northern Italy. J. Food Prot. 2012, 75, 2031–2038. [Google Scholar] [CrossRef] [PubMed]
- Heidinger, J.C.; Winter, C.K.; Cullor, J.S. Quantitative Microbial Risk Assessment for Staphylococcus Aureus and Staphylococcus Enterotoxin A in Raw Milk. J. Food Prot. 2009, 72, 1641–1653. [Google Scholar] [CrossRef]
- California Department of Public Health, Food and Drug Branch, Emergency Response Unit. Environmental Investigation of a Campylobacter Jejuni Outbreak in 2012 Associated with Claravale Farms Raw Whole Milk Final Report. Available online: https://realrawmilkfacts.com/PDFs/2012-claravale-farms-raw-milk-outbreak-final-report.pdf (accessed on 5 March 2025).
- Kansas Department of Health and Environment. Outbreak of Campylobacter Jejuni Infections Associated with Consumption of Cheese Made from Raw Milk-Western Kansas; Kansas Department of Health and Environment: Topeka, KS, USA, 2007.
- Salmonella Typhimurium Infection Associated with Raw Milk and Cheese Consumption—Pennsylvania. 2007. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5644a3.htm (accessed on 24 February 2025).
- Department of Kansas Government Information. Campylobacteriosis Outbreak Associated with Unpasteurized Milk, Reno County and Butler County, August–December 2007; [Final Report]—Health and Environment; Department of Kansas Government Information: Topeka, KS, USA. Available online: https://kgi.contentdm.oclc.org/digital/collection/p16884coll4/id/1349/ (accessed on 24 February 2025).
- Oliver, S.P.; Boor, K.J.; Murphy, S.C.; Murinda, S.E. Food Safety Hazards Associated with Consumption of Raw Milk. Foodborne Pathog. Dis. 2009, 6, 793–806. [Google Scholar] [CrossRef]
- Escherichia Coli 0157:H7 Infections in Children Associated with Raw Milk and Raw Colostrum from Cows—California. 2006. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5723a2.htm (accessed on 24 February 2025).
- Multistate Outbreak of Salmonella Serotype Typhimurium Infections Associated with Drinking Unpasteurized Milk—Illinois, Indiana, Ohio, and Tennessee, 2002–2003. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5226a3.htm (accessed on 24 February 2025).
- Davis, K.R.; Dunn, A.C.; Burnett, C.; McCullough, L.; Dimond, M.; Wagner, J.; Smith, L.; Carter, A.; Willardson, S.; Nakashima, A.K. Campylobacter Jjejuni Infections Associated with Raw Milk Consumption—Utah, 2014. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 301–305. [Google Scholar] [CrossRef]
- McCarthy, K.S.; Lopetcharat, K.; Drake, M.A. Milk Fat Threshold Determination and the Effect of Milk Fat Content on Consumer Preference for Fluid Milk. J. Dairy Sci. 2017, 100, 1702–1711. [Google Scholar] [CrossRef]
- Outbreak of Campylobacter Jejuni Infections Associated with Drinking Unpasteurized Milk Procured through a Cow-Leasing Program—Wisconsin. 2001. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5125a2.htm (accessed on 24 February 2025).
- Sakkas, L.; Moutafi, A.; Moschopoulou, E.; Moatsou, G. Assessment of Heat Treatment of Various Types of Milk. Food Chem. 2014, 159, 293–301. [Google Scholar] [CrossRef]
- Guinee, T.P. Effect of High-Temperature Treatment of Milk and Whey Protein Denaturation on the Properties of Rennet—Curd Cheese: A Review. Int. Dairy J. 2021, 121, 105095. [Google Scholar] [CrossRef]
- Zhang, Y.; Yi, S.; Lu, J.; Pang, X.; Xu, X.; Lv, J.; Zhang, S. Effect of Different Heat Treatments on the Maillard Reaction Products, Volatile Compounds and Glycation Level of Milk. Int. Dairy J. 2021, 123, 105182. [Google Scholar] [CrossRef]
- Esteghlal, S.; Gahruie, H.H.; Niakousari, M.; Barba, F.J.; Bekhit, A.E.D.; Mallikarjunan, K.; Roohinejad, S. Bridging the Knowledge Gap for the Impact of Non-Thermal Processing on Proteins and Amino Acids. Foods 2019, 8, 262. [Google Scholar] [CrossRef] [PubMed]
- Montilla, A.; Calvo, M.M. Goat’s Milk Stability during Heat Treatment: Effect of PH and Phosphates. J. Agric. Food Chem. 1997, 45, 931–934. [Google Scholar] [CrossRef]
- Al haj, O.A.; Al Kanhal, H.A. Compositional, Technological and Nutritional Aspects of Dromedary Camel Milk. Int. Dairy J. 2010, 20, 811–821. [Google Scholar] [CrossRef]
- Wijayanti, H.B.; Bansal, N.; Deeth, H.C. Stability of Whey Proteins during Thermal Processing: A Review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1235–1251. [Google Scholar] [CrossRef]
- Patel, P.B.; Thakkar, V.R.; Patel, J.S. Cellular Effect of Curcumin and Citral Combination on Breast Cancer Cells: Induction of Apoptosis and Cell Cycle Arrest. J. Breast Cancer 2015, 18, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, S.; Barac, M.; Macej, O.; Vucic, T.; Lacnjevac, C. SDS-PAGE Analysis of Soluble Proteins in Reconstituted Milk Exposed to Different Heat Treatments. Sensors 2007, 7, 371–383. [Google Scholar] [CrossRef]
- Chen, G.Q.; Qu, Y.; Gras, S.L.; Kentish, S.E. Separation Technologies for Whey Protein Fractionation. Food Eng. Rev. 2023, 15, 438–465. [Google Scholar] [CrossRef]
- Donato, L.; Guyomarc’h, F. Formation and Properties of the Whey Protein/κ-Casein Complexes in Heated Skim Milk—A Review. Dairy Sci. Technol. 2009, 89, 3–29. [Google Scholar] [CrossRef]
- Tolkach, A.; Kulozik, U.; Tolkach, A.; Kulozik, U. Reaction Kinetic Pathway of Reversible and Irreversible Thermal Denaturation of β-Lactoglobulin. Lait 2007, 87, 301–315. [Google Scholar] [CrossRef]
- Brew, K. Milk Proteins | α-Lactalbumin. Encycl. Dairy Sci. Second. Ed. 2011, 780–786. [Google Scholar] [CrossRef]
- McSweeney, P.L.H.; Fox, P.F. Advanced Dairy Chemistry: Volume 1A: Proteins: Basic Aspects, 4th ed.; Springer Science & Business Media: Berlin, Germany, 2013; pp. 1–548. [Google Scholar] [CrossRef]
- Dalabasmaz, S.; Pischetsrieder, M. Design of a Prediction Model for the Differentiation of Pasteurized Milk from Heated ESL Milk by Peptide Profiling. Proteomics 2019, 19, 1800292. [Google Scholar] [CrossRef] [PubMed]
- Ismail, B.; Nielsen, S.S. Invited Review: Plasmin Protease in Milk: Current Knowledge and Relevance to Dairy Industry. J. Dairy Sci. 2010, 93, 4999–5009. [Google Scholar] [CrossRef]
- Rauh, V.M.; Johansen, L.B.; Ipsen, R.; Paulsson, M.; Larsen, L.B.; Hammershøj, M. Plasmin Activity in UHT Milk: Relationship between Proteolysis, Age Gelation, and Bitterness. J. Agric. Food Chem. 2014, 62, 6852–6860. [Google Scholar] [CrossRef] [PubMed]
- Ryskaliyeva, A.; Henry, C.; Miranda, G.; Faye, B.; Konuspayeva, G.; Martin, P. Combining Different Proteomic Approaches to Resolve Complexity of the Milk Protein Fraction of Dromedary, Bactrian Camels and Hybrids, from Different Regions of Kazakhstan. PLoS ONE 2018, 13, e0197026. [Google Scholar] [CrossRef]
- Scollard, P.G.; Beresford, T.P.; Needs, E.C.; Murphy, P.M.; Kelly, A.L. Plasmin Activity, β-Lactoglobulin Denaturation and Proteolysis in High Pressure Treated Milk. Int. Dairy J. 2000, 10, 835–841. [Google Scholar] [CrossRef]
- Khaliq, A.; Mishra, A.K.; Niroula, A.; Baba, W.N.; Shaukat, M.N.; Rabbani, A. An Updated Comprehensive Review of Camel Milk: Composition, Therapeutic Properties, and Industrial Applications. Food Biosci. 2024, 62, 105531. [Google Scholar] [CrossRef]
- Shimamura, T.; Kurogi, Y.; Katsuno, S.; Kashiwagi, T.; Ukeda, H. Demonstration of the Presence of Aminoreductone Formed during the Maillard Reaction in Milk. Food Chem. 2011, 129, 1088–1092. [Google Scholar] [CrossRef]
- Erbersdobler, H.F.; Somoza, V. Forty Years of Furosine—Forty Years of Using Maillard Reaction Products as Indicators of the Nutritional Quality of Foods. Mol. Nutr. Food Res. 2007, 51, 423–430. [Google Scholar] [CrossRef]
- Jaeger, H.; Janositz, A.; Knorr, D. The Maillard Reaction and Its Control during Food Processing. The Potential of Emerging Technologies. Pathol. Biol. 2010, 58, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Swanepoel, N.; Robinson, P.H.; Erasmus, L.J. Amino Acid Needs of Lactating Dairy Cows: Impact of Feeding Lysine in a Ruminally Protected Form on Productivity of Lactating Dairy Cows. Anim. Feed. Sci. Technol. 2010, 157, 79–94. [Google Scholar] [CrossRef]
- Felfoul, I.; Jardin, J.; Gaucheron, F.; Attia, H.; Ayadi, M.A. Proteomic Profiling of Camel and Cow Milk Proteins under Heat Treatment. Food Chem. 2017, 216, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Hillier, R.M.; Lyster, R.L.J. Whey Protein Denaturation in Heated Milk and Cheese Whey. J. Dairy Res. 1979, 46, 95–102. [Google Scholar] [CrossRef]
- Atuonwu, J.C.; Ray, J.; Stapley, A.G.F. A Kinetic Model for Whey Protein Denaturation at Different Moisture Contents and Temperatures. Int. Dairy J. 2017, 75, 41–50. [Google Scholar] [CrossRef]
- Dannenberg, F.; Kessler, H.-G. Reaction Kinetics of the Denaturation of Whey Proteins in Milk. J. Food Sci. 1988, 53, 258–263. [Google Scholar] [CrossRef]
- Dannenberg, F.; Kessler, H.G. Effect of Denaturation of β-Lactoglobulin on Texture Properties of Set-Style Nonfat Yoghurt. 1. Syneresis. Milchwissenschaft 1988, 43, 632–635. [Google Scholar]
- Bulca, S.; Dumpler, J.; Kulozik, U. Kinetic Description of Heat-Induced Cross-Linking Reactions of Whey Protein-Free Casein Solutions. Int. J. Dairy Technol. 2016, 69, 489–496. [Google Scholar] [CrossRef]
- Pitt, W.; Harden, T.; Hull, R.R. Investigation of the Antimicrobial Activity of Raw Milk against Several Foodborne Pathogens. Milchwissenschaft 2000, 55, 249–252. [Google Scholar]
- YuQian, L.; Yousef, A.E. Characteristics of Listeria Monocytogenes Important to Food Processors. In Listeria, Listeriosis, and Food Safety; CRC Press: Boca Raton, FL, USA, 1999; pp. 131–224. [Google Scholar]
- Gay, M.; Amgar, A. Factors Moderating Listeria Monocytogenes Growth in Raw Milk and in Soft Cheese Made from Raw Milk. Lait 2005, 85, 153–170. [Google Scholar] [CrossRef]
- Dewettinck, K.; Rombaut, R.; Thienpont, N.; Le, T.T.; Messens, K.; Van Camp, J. Nutritional and Technological Aspects of Milk Fat Globule Membrane Material. Int. Dairy J. 2008, 18, 436–457. [Google Scholar] [CrossRef]
- German, J.B.; Dillard, C.J. Composition, Structure and Absorption of Milk Lipids: A Source of Energy, Fat-Soluble Nutrients and Bioactive Molecules. Crit. Rev. Food Sci. Nutr. 2007, 46, 57–92. [Google Scholar] [CrossRef] [PubMed]
- Spreer, E.; Mixa, A. Milk and Dairy Product Technology; Routledge: New York, NY, USA, 2017; pp. 1–483. [Google Scholar] [CrossRef]
- Ceni, G.; Fernandes Silva, M.; Valério, C.; Cansian, R.L.; Oliveira, J.V.; Dalla Rosa, C.; Mazutti, M.A. Continuous Inactivation of Alkaline Phosphatase and Escherichia Coli in Milk Using Compressed Carbon Dioxide as Inactivating Agent. J. CO2 Util. 2016, 13, 24–28. [Google Scholar] [CrossRef]
- Wheeler, T.T.; Hodgkinson, A.J.; Prosser, C.G.; Davis, S.R. Immune Components of Colostrum and Milk—A Historical Perspective. J. Mammary Gland. Biol. Neoplasia 2007, 12, 237–247. [Google Scholar] [CrossRef]
- Needs, E. High Pressure Processing of Dairy Products; Springer: Boston, MA, USA, 2001; pp. 269–296. [Google Scholar] [CrossRef]
- Rodrigues, L.R. Milk Minor Constituents, Enzymes, Hormones, Growth Factors, and Organic Acids. In Milk and Dairy Products in Human Nutrition: Production, Composition and Health; Wiley-Blackwell: New York, NY, USA, 2013; pp. 220–245. [Google Scholar] [CrossRef]
- Bogahawaththa, D.; Chandrapala, J.; Vasiljevic, T. Thermal Denaturation of Bovine Immunoglobulin G and Its Association with Other Whey Proteins. Food Hydrocoll. 2017, 72, 350–357. [Google Scholar] [CrossRef]
- Martínez, B.; Bravo, D.; Rodríguez, A. Consequences of the Development of Nisin-Resistant Listeria Monocytogenes in Fermented Dairy Products. J. Food Prot. 2005, 68, 2383–2388. [Google Scholar] [CrossRef]
- Li, Y.; Weng, P.; Wu, Z.; Liu, Y. Extending the Shelf Life of Raw Milk and Pasteurized Milk with Plantaricin FB-2. Foods 2023, 12, 608. [Google Scholar] [CrossRef]
- González-Chávez, S.A.; Arévalo-Gallegos, S.; Rascón-Cruz, Q. Lactoferrin: Structure, Function and Applications. Int. J. Antimicrob. Agents 2009, 33, 301.e1–301.e8. [Google Scholar] [CrossRef]
- Claeys, W.L.; Ludikhuyze, L.R.; Van Loey, A.M.; Hendrickx, M.E. Inactivation Kinetics of Alkaline Phosphatase and Lactoperoxidase, and Denaturation Kinetics of β-Lactoglobulin in Raw Milk under Isothermal and Dynamic Temperature Conditions. J. Dairy Res. 2001, 68, 95–107. [Google Scholar] [CrossRef]
- Campbell, R.E.; Drake, M.A. Invited Review: The Effect of Native and Nonnative Enzymes on the Flavor of Dried Dairy Ingredients. J. Dairy Sci. 2013, 96, 4773–4783. [Google Scholar] [CrossRef]
- Fox, P.F.; Kelly, A.L. Indigenous Enzymes in Milk: Overview and Historical Aspects—Part 2. Int. Dairy J. 2006, 16, 517–532. [Google Scholar] [CrossRef]
- Prado, B.M.; Sombers, S.E.; Ismail, B.; Hayes, K.D. Effect of Heat Treatment on the Activity of Inhibitors of Plasmin and Plasminogen Activators in Milk. Int. Dairy J. 2006, 16, 593–599. [Google Scholar] [CrossRef]
- Stevens, C.R.; Millar, T.M.; Clinch, J.G.; Kanczler, J.M.; Bodamyali, T.; Blake, D.R. Antibacterial Properties of Xanthine Oxidase in Human Milk. Lancet 2000, 356, 829–830. [Google Scholar] [CrossRef]
- Alvarez, V.B. Sensory Evaluation of Milk and Milk Products. In Dairy Processing and Quality Assurance; Wiley-Blackwell: New York, NY, USA, 2015; pp. 467–487. [Google Scholar] [CrossRef]
- Kokkinidou, S.; Peterson, D.G. Control of Maillard-Type off-Flavor Development in Ultrahigh-Temperature-Processed Bovine Milk by Phenolic Chemistry. J. Agric. Food Chem. 2014, 62, 8023–8033. [Google Scholar] [CrossRef]
- Schamberger, G.P.; Labuza, T.P. Effect of Green Tea Flavonoids on Maillard Browning in UHT Milk. LWT Food Sci. Technol. 2007, 40, 1410–1417. [Google Scholar] [CrossRef]
- Nozière, P.; Graulet, B.; Lucas, A.; Martin, B.; Grolier, P.; Doreau, M. Carotenoids for Ruminants: From Forages to Dairy Products. Anim. Feed. Sci. Technol. 2006, 131, 418–450. [Google Scholar] [CrossRef]
- Li, Y.; Joyner, H.S.; Carter, B.G.; Drake, M.A. Effects of Fat Content, Pasteurization Method, Homogenization Pressure, and Storage Time on the Mechanical and Sensory Properties of Bovine Milk. J. Dairy Sci. 2018, 101, 2941–2955. [Google Scholar] [CrossRef]
- Gathercole, J.; Reis, M.G.; Agnew, M.; Reis, M.M.; Humphrey, R.; Harris, P.; Clerens, S.; Haigh, B.; Dyer, J.M. Molecular Modification Associated with the Heat Treatment of Bovine Milk. Int. Dairy J. 2017, 73, 74–83. [Google Scholar] [CrossRef]
- Datta, N.; Deeth, H.C. Age Gelation of UHT Milk—A Review. Food Bioprod. Process. 2001, 79, 197–210. [Google Scholar] [CrossRef]
- Chojnicka-Paszun, A.; de Jongh, H.H.J.; de Kruif, C.G. Sensory Perception and Lubrication Properties of Milk: Influence of Fat Content. Int. Dairy J. 2012, 26, 15–22. [Google Scholar] [CrossRef]
- Nguyen, P.T.M.; Bhandari, B.; Prakash, S. Tribological Method to Measure Lubricating Properties of Dairy Products. J. Food Eng. 2016, 168, 27–34. [Google Scholar] [CrossRef]
- Clark, S.; Costello, M.; Drake, M.A.; Bodyfelt, F. The Sensory Evaluation of Dairy Products. In The Sensory Evaluation of Dairy Products; Van Nostrand Reinhold: New York, NY, USA, 2009; pp. 1–573. [Google Scholar] [CrossRef]
- Reineccius, G. Flavor Chemistry and Technology; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar] [CrossRef]
- Grebenteuch, S.; Kanzler, C.; Klaußnitzer, S.; Kroh, L.W.; Rohn, S. The Formation of Methyl Ketones during Lipid Oxidation at Elevated Temperatures. Molecules 2021, 26, 1104. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.P.; Barbano, D.M.; Drake, M.A. The Influence of Ultra-Pasteurization by Indirect Heating versus Direct Steam Injection on Skim and 2% Fat Milks. J. Dairy Sci. 2017, 100, 1688–1701. [Google Scholar] [CrossRef]
- Zhao, J.; Zhou, B.; Wang, P.; Ren, F.; Mao, X. Physicochemical Properties of Fluid Milk with Different Heat Treatments and HS-GC-IMS Identification of Volatile Organic Compounds. Int. Dairy J. 2023, 142, 105654. [Google Scholar] [CrossRef]
- Feng, D.; Wang, J.; Ji, X.; Min, W.; Yan, W. HS-GC-IMS Detection of Volatile Organic Compounds in Yak Milk Powder Processed by Different Drying Methods. LWT 2021, 141, 110855. [Google Scholar] [CrossRef]
- Liem, D.G.; Bolhuis, D.P.; Hu, X.; Keast, R.S.J. Short Communication: Influence of Labeling on Australian and Chinese Consumers’ Liking of Milk with Short (Pasteurized) and Long (UHT) Shelf Life. J. Dairy Sci. 2016, 99, 1747–1754. [Google Scholar] [CrossRef]
- Meng, F.; Han, Z.; Zhang, Z.; Ding, H.; Lu, X.; Lu, C.; Ma, L.; Kang, Z.; Wang, B.; Li, Y. Effect of Steam Infusion and Steam Injection Ultra-High Temperature Treatment on Active Proteins and Flavor Compounds in Milk. J. Food Sci. Technol. 2023, 41, 70–80. [Google Scholar] [CrossRef]
- Gennari, A.; Monteiro, B.; Lehn, D.; Fernanda, C.; De Souza, V. Effects of Pasteurization and Ultra-High Temperature Processes on Proximate Composition and Fatty Acid Profile in Bovine Milk. Am. J. Food Technol. 2015, 10, 265–272. [Google Scholar] [CrossRef]
- Council Regulation (EC) No. 2597/97 Laying Down Additional Rules on the Common Organization of the Market in Milk and Milk Products for Drinking Milk. FAOLEX. Available online: https://www.fao.org/faolex/results/details/en/c/LEX-FAOC018629/ (accessed on 5 April 2025).
- Macdonald, L.E.; Brett, J.; Kelton, D.; Majowicz, S.E.; Snedeker, K.; Sargeant, J.M. A Systematic Review and Meta-Analysis of the Effects of Pasteurization on Milk Vitamins, and Evidence for Raw Milk Consumption and Other Health-Related Outcomes. J. Food Prot. 2011, 74, 1814–1832. [Google Scholar] [CrossRef]
- Raynal-Ljutovac, K.; Lagriffoul, G.; Paccard, P.; Guillet, I.; Chilliard, Y. Composition of Goat and Sheep Milk Products: An Update. Small Rumin. Res. 2008, 79, 57–72. [Google Scholar] [CrossRef]
- Lyocks; Ayo, S.W.J.; Tanimu, J.; Olajide. Mineral Elements Content in Raw Milk of Grazing Cattle in Kaduna Metropolis and Environs. Niger. J. Agric. Food Environ. 2013, 9, 22–27. [Google Scholar]
- Butler, M.I.; Bastiaanssen, T.F.S.; Long-Smith, C.; Berding, K.; Morkl, S.; Cusack, A.M.; Strain, C.; Busca, K.; Porteous-Allen, P.; Claesson, M.J.; et al. Recipe for a Healthy Gut: Intake of Unpasteurised Milk Is Associated with Increased Lactobacillus Abundance in the Human Gut Microbiome. Nutrients 2020, 12, 1468. [Google Scholar] [CrossRef] [PubMed]
- Heckman, J.R. Securing Fresh Food from Fertile Soil, Challenges to the Organic and Raw Milk Movements. Renew. Agric. Food Syst. 2019, 34, 472–485. [Google Scholar] [CrossRef]
- Schaafsma, G. Lactose and Lactose Derivatives as Bioactive Ingredients in Human Nutrition. Int. Dairy J. 2008, 18, 458–465. [Google Scholar] [CrossRef]
- Law, D.; Conklin, J.; Pimentel, M. Erratum: Lactose Intolerance and the Role of the Lactose Breath Test. Am. J. Gastroenterol. 2010, 105, 2308. [Google Scholar] [CrossRef]
- Claeys, W.L.; Cardoen, S.; Daube, G.; De Block, J.; Dewettinck, K.; Dierick, K.; De Zutter, L.; Huyghebaert, A.; Imberechts, H.; Thiange, P.; et al. Raw or Heated Cow Milk Consumption: Review of Risks and Benefits. Food Control 2013, 31, 251–262. [Google Scholar] [CrossRef]
- Mummah, S.; Oelrich, B.; Hope, J.; Vu, Q.; Gardner, C.D. Effect of Raw Milk on Lactose Intolerance: A Randomized Controlled Pilot Study. Ann. Fam. Med. 2014, 12, 134–141. [Google Scholar] [CrossRef]
- Brooks, C.; Pearce, N.; Douwes, J. The Hygiene Hypothesis in Allergy and Asthma: An Update. Curr. Opin. Allergy Clin. Immunol. 2013, 13, 70–77. [Google Scholar] [CrossRef]
- Brick, T.; Schober, Y.; Böcking, C.; Pekkanen, J.; Genuneit, J.; Loss, G.; Dalphin, J.C.; Riedler, J.; Lauener, R.; Nockher, W.A.; et al. ω-3 Fatty Acids Contribute to the Asthma-Protective Effect of Unprocessed Cow’s Milk. J. Allergy Clin. Immunol. 2016, 137, 1699–1706.e13. [Google Scholar] [CrossRef]
- Chrischilles, E.; Ahrens, R.; Kuehl, A.; Kelly, K.; Thorne, P.; Burmeister, L.; Merchant, J. Asthma Prevalence and Morbidity among Rural Iowa Schoolchildren. J. Allergy Clin. Immunol. 2004, 113, 66–71. [Google Scholar] [CrossRef]
- Ege, M.J.; Frei, R.; Bieli, C.; Schram-Bijkerk, D.; Waser, M.; Benz, M.R.; Weiss, G.; Nyberg, F.; van Hage, M.; Pershagen, G.; et al. Not All Farming Environments Protect against the Development of Asthma and Wheeze in Children. J. Allergy Clin. Immunol. 2007, 119, 1140–1147. [Google Scholar] [CrossRef] [PubMed]
- Perkin, M.R.; Strachan, D.P. Which Aspects of the Farming Lifestyle Explain the Inverse Association with Childhood Allergy? J. Allergy Clin. Immunol. 2006, 117, 1374–1381. [Google Scholar] [CrossRef]
- Riedler, J.; Braun-Fahrländer, C.; Eder, W.; Schreuer, M.; Waser, M.; Maisch, S.; Carr, D.; Schierl, R.; Nowak, D.; Von Mutius, E. Exposure to Farming in Early Life and Development of Asthma and Allergy: A Cross-Sectional Survey. Lancet 2001, 358, 1129–1133. [Google Scholar] [CrossRef]
- Tse, K.; Horner, A.A. Allergen Tolerance versus the Allergic March: The Hygiene Hypothesis Revisited. Curr. Allergy Asthma Rep. 2008, 8, 475–483. [Google Scholar] [CrossRef]
- Von Mutius, E. The Environmental Predictors of Allergic Disease. J. Allergy Clin. Immunol. 2000, 105, 9–19. [Google Scholar] [CrossRef]
- Von Mutius, E.; Vercelli, D. Farm Living: Effects on Childhood Asthma and Allergy. Nat. Rev. Immunol. 2010, 10, 861–868. [Google Scholar] [CrossRef]
- Waser, M.; Michels, K.B.; Bieli, C.; Flöistrup, H.; Pershagen, G.; Von Mutius, E.; Ege, M.; Riedler, J.; Schram-Bijkerk, D.; Brunekreef, B.; et al. Inverse Association of Farm Milk Consumption with Asthma and Allergy in Rural and Suburban Populations across Europe. Clin. Exp. Allergy 2007, 37, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Wickens, K.; Lane, J.M.; Fitzharris, P.; Siebers, R.; Riley, G.; Douwes, J.; Smith, T.; Crane, J. Farm Residence and Exposures and the Risk of Allergic Diseases in New Zealand Children. Allergy 2002, 57, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Rosenlund, H. Protective Factors in Childhood Allergy Related to Diet and Lifestyle; Karolinska Institutet: Stockholm, Sweden, 2010. [Google Scholar]
- Loss, G.; Depner, M.; Ulfman, L.H.; Van Neerven, R.J.J.; Hose, A.J.; Genuneit, J.; Karvonen, A.M.; Hyvärinen, A.; Kaulek, V.; Roduit, C.; et al. Consumption of Unprocessed Cow’s Milk Protects Infants from Common Respiratory Infections. J. Allergy Clin. Immunol. 2015, 135, 56–62.e2. [Google Scholar] [CrossRef]
- Strachan, D.P. Hay Fever, Hygiene, and Household Size. BMJ 1989, 299, 1259–1260. [Google Scholar] [CrossRef]
- Morin, S.; Bernard, H.; Przybylski-Nicaise, L.; Corthier, G.; Rabot, S.; Wal, J.M.; Hazebrouck, S. Allergenic and Immunogenic Potential of Cow’s Milk β-Lactoglobulin and Caseins Evidenced Without Adjuvant in Germ-Free Mice. Mol. Nutr. Food Res. 2011, 55, 1700–1707. [Google Scholar] [CrossRef] [PubMed]
- Barnes, M.; Cullinan, P.; Athanasaki, P.; MacNeill, S.; Hole, A.M.; Harris, J.; Kalogeraki, S.; Chatzinikolaou, M.; Drakonakis, N.; Bibaki-Liakou, V.; et al. Crete: Does Farming Explain Urban and Rural Differences in Atopy? Clin. Exp. Allergy 2001, 31, 1822–1828. [Google Scholar] [CrossRef]
- Remes, S.T.; Iivanainen, K.; Koskela, H.; Pekkanen, J. Which Factors Explain the Lower Prevalence of Atopy Amongst Farmers’ Children? Clin. Exp. Allergy 2003, 33, 427–434. [Google Scholar] [CrossRef]
- Radon, K.; Windstetter, D.; Eckart, J.; Dressel, H.; Leitritz, L.; Reichert, J.; Schmid, M.; Praml, G.; Schosser, M.; Von Mutius, E.; et al. Farming Exposure in Childhood, Exposure to Markers of Infections and the Development of Atopy in Rural Subjects. Clin. Exp. Allergy 2004, 34, 1178–1183. [Google Scholar] [CrossRef] [PubMed]
- Bieli, C.; Eder, W.; Frei, R.; Braun-Fahrländer, C.; Klimecki, W.; Waser, M.; Riedler, J.; von Mutius, E.; Scheynius, A.; Pershagen, G.; et al. A Polymorphism in CD14 Modifies the Effect of Farm Milk Consumption on Allergic Diseases and CD14 Gene Expression. J. Allergy Clin. Immunol. 2007, 120, 1308–1315. [Google Scholar] [CrossRef]
- Pfefferle, P.I.; Büchele, G.; Blümer, N.; Roponen, M.; Ege, M.J.; Krauss-Etschmann, S.; Genuneit, J.; Hyvärinen, A.; Hirvonen, M.R.; Lauener, R.; et al. Cord Blood Cytokines Are Modulated by Maternal Farming Activities and Consumption of Farm Dairy Products during Pregnancy: The PASTURE Study. J. Allergy Clin. Immunol. 2010, 125, 108–115.e3. [Google Scholar] [CrossRef] [PubMed]
- Khaliq, A.; Rabbani, A. Exploring Commercially Available Camel Milk Dairy Products: Medicinal, Nutritional and Antimicrobial Properties. Res. Innov. Food Sci. Technol. 2025, 14, 37–48. [Google Scholar] [CrossRef]
- Mainer, G.; Sánchez, L.; Ena, J.M.; Calvo, M. Kinetic and Thermodynamic Parameters for Heat Denaturation of Bovine Milk IgG, IgA and IgM. J. Food Sci. 1997, 62, 1034–1038. [Google Scholar] [CrossRef]
- Kulczycki, A. Bovine IgG Can Aggregate at Conditions Simulating Pasteurization and Binds to Some Human Fcγ Receptors. Mol. Immunol. 1987, 24, 259–266. [Google Scholar] [CrossRef]
- Donnet-Hughes, A.; Duc, N.; Serrant, P.; Vidal, K.; Schiffrin, E.J. Bioactive Molecules in Milk and Their Role in Health and Disease: The Role of Transforming Growth Factor-β. Immunol. Cell Biol. 2000, 78, 74–79. [Google Scholar] [CrossRef]
- Van Neerven, R.J.J.; Knol, E.F.; Heck, J.M.L.; Savelkoul, H.F.J. Which Factors in Raw Cow’s Milk Contribute to Protection Against Allergies? J. Allergy Clin. Immunol. 2012, 130, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Blaser, M.J.; Duncan, D.J.; Osterholm, M.T.; Istre, G.R.; Wang, W.L. Serologic Study of Two Clusters of Infection Due to Campylobacter Jejuni. J. Infect. Dis. 1983, 147, 820–823. [Google Scholar] [CrossRef]
- Capuano, E.; Ferrigno, A.; Acampa, I.; Ait-Ameur, L.; Fogliano, V. Characterization of the Maillard Reaction in Bread Crisps. Eur. Food Res. Technol. 2008, 228, 311–319. [Google Scholar] [CrossRef]
- Masatcioglu, M.T.; Gokmen, V.; Ng, P.K.W.; Koksel, H. Effects of Formulation, Extrusion Cooking Conditions, and CO2 Injection on the Formation of Acrylamide in Corn Extrudates. J. Sci. Food Agric. 2014, 94, 2562–2568. [Google Scholar] [CrossRef]
- Melnik, B.C.; Schmitz, G. Exosomes of Pasteurized Milk: Potential Pathogens of Western Diseases. J. Transl. Med. 2019, 17, 3. [Google Scholar] [CrossRef] [PubMed]
- Khaliq, A.; Mishra, A.K.; Shaukat, M.N.; Rabbani, A. Plant-Based Dairy Substitutes: Current Scenario and Future Prospects. Innov. Agric. 2023, 6, e32880. [Google Scholar] [CrossRef]
- Badawy, S.; Liu, Y.; Guo, M.; Liu, Z.; Xie, C.; Marawan, M.A.; Ares, I.; Lopez-Torres, B.; Martínez, M.; Maximiliano, J.E.; et al. Conjugated Linoleic Acid (CLA) as a Functional Food: Is It Beneficial or Not? Food Res. Int. 2023, 172, 113158. [Google Scholar] [CrossRef]
- Ali, M.A.; Kamal, M.M.; Rahman, M.H.; Siddiqui, M.N.; Haque, M.A.; Saha, K.K.; Rahman, M.A. Functional Dairy Products as a Source of Bioactive Peptides and Probiotics: Current Trends and Future Prospectives. J. Food Sci. Technol. 2021, 59, 1263–1279. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Khan, S.; Bahmid, N.A.; Nagdalian, A.A.; Jafari, S.M.; Castro-Muñoz, R. Impact of High-Pressure Processing on the Bioactive Compounds of Milk—A Comprehensive Review. J. Food Sci. Technol. 2024, 61, 1632–1651. [Google Scholar] [CrossRef]
- Smithers, G.; Versteeg, C.; Augustin, M.; Kelly, A.; Kothari, K.; Simpson, A.; Mulvihill, D.; Kelly, P. Symposium: Dairy Foods: Emerging Non-Thermal Food Processing Technologies-Their Potential in Dairy Systems. Available online: https://www.jtmtg.org/JAM/2008/abstracts/0553.PDF (accessed on 5 March 2025).
- Pitino, M.A.; Unger, S.; Doyen, A.; Pouliot, Y.; Aufreiter, S.; Stone, D.; Kiss, A.; O’Connor, D.L. High Hydrostatic Pressure Processing Better Preserves the Nutrient and Bioactive Compound Composition of Human Donor Milk. J. Nutr. 2019, 149, 497–504. [Google Scholar] [CrossRef]
- Chawla, R.; Patil, G.R.; Singh, A.K. High Hydrostatic Pressure Technology in Dairy Processing: A Review. J. Food Sci. Technol. 2011, 48, 260. [Google Scholar] [CrossRef] [PubMed]
- Rademacher, B.; Hinrichs, J. Effects of High Pressure Treatment on Indigenous Enzymes in Bovine Milk: Reaction Kinetics, Inactivation and Potential Application. Int. Dairy J. 2006, 16, 655–661. [Google Scholar] [CrossRef]
- Pegu, K.; Arya, S.S. Non-Thermal Processing of Milk: Principles, Mechanisms and Effect on Milk Components. J. Agric. Food Res. 2023, 14, 100730. [Google Scholar] [CrossRef]
- Farkas, D.F.; Hoover, D.G. High Pressure Processing. J. Food Sci. 2000, 65, 47–64. [Google Scholar] [CrossRef]
- Hite, B.H. The Effect of Pressure in the Preservation of Milk: A Preliminary Report. In West Virginia Agricultural and Forestry Experiment Station Bulletins; Generic: Flemington, Australia, 1899. [Google Scholar] [CrossRef]
- Sehrawat, R.; Kaur, B.P.; Nema, P.K.; Tewari, S.; Kumar, L. Microbial Inactivation by High Pressure Processing: Principle, Mechanism and Factors Responsible. Food Sci. Biotechnol. 2021, 30, 19–35. [Google Scholar] [CrossRef]
- Ribeiro, L.R.; Magalhães, I.S.; Tribst, A.A.L.; de Castro Leite Júnior, B.R. Effects of High-Pressure Processing on Enzyme Activity in Milk and Dairy Products: Science and Applications. In Foundations and Frontiers in Enzymology; Academic Press: Cambridge, MA, USA, 2023; pp. 169–193. [Google Scholar] [CrossRef]
- Borda, D.; Van Loey, A.; Smout, C.; Hendrickx, M. Mathematical Models for Combined High Pressure and Thermal Plasmin Inactivation Kinetics in Two Model Systems. J. Dairy Sci. 2004, 87, 4042–4049. [Google Scholar] [CrossRef]
- Huppertz, T.; Fox, P.F.; Kelly, A.L. Plasmin Activity and Proteolysis in High Pressure-Treated Bovine Milk. Lait 2004, 84, 297–304. [Google Scholar] [CrossRef]
- Yu, T.; Zhang, X.; Feng, R.; Wang, C.; Wang, X.; Wang, Y. Comparison of the Effects of High Hydrostatic Pressure and Pasteurization on Quality of Milk during Storage. Foods 2022, 11, 2837. [Google Scholar] [CrossRef]
- Ravichandran, C.; Jayachandran, L.E.; Kothakota, A.; Pandiselvam, R.; Balasubramaniam, V.M. Influence of High Pressure Pasteurization on Nutritional, Functional and Rheological Characteristics of Fruit and Vegetable Juices and Purees-an Updated Review. Food Control 2023, 146, 109516. [Google Scholar] [CrossRef]
- Akdeniz, V.; Akalın, A.S. Recent Advances in Dual Effect of Power Ultrasound to Microorganisms in Dairy Industry: Activation or Inactivation. Crit. Rev. Food Sci. Nutr. 2022, 62, 889–904. [Google Scholar] [CrossRef]
- Bermudez-Aguirre, D.; Niemira, B.A. Pasteurization of Foods with Ultrasound: The Present and the Future. Appl. Sci. 2022, 12, 10416. [Google Scholar] [CrossRef]
- Islam, M.N.; Zhang, M.; Adhikari, B. The Inactivation of Enzymes by Ultrasound—A Review of Potential Mechanisms. Food Rev. Int. 2014, 30, 1–21. [Google Scholar] [CrossRef]
- Villamiel, M.; De Jong, P. Influence of High-Intensity Ultrasound and Heat Treatment in Continuous Flow on Fat, Proteins, and Native Enzymes of Milk. J. Agric. Food Chem. 2000, 48, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Chiozzi, V.; Agriopoulou, S.; Varzakas, T. Advances, Applications, and Comparison of Thermal (Pasteurization, Sterilization, and Aseptic Packaging) Against Non-Thermal (Ultrasounds, UV Radiation, Ozonation, High Hydrostatic Pressure) Technologies in Food Processing. Appl. Sci. 2022, 12, 2202. [Google Scholar] [CrossRef]
- Xu, S.; Qiao, Y.; Liu, X.; Church, C.C.; Wan, M. Fundamentals of Cavitation. In Cavitation in Biomedicine; Springer: Boston, MA, USA, 2015; pp. 1–46. [Google Scholar] [CrossRef]
- Chauhan, O.P.; Sayanfar, S.; Toepfl, S. Effect of Pulsed Electric Field on Texture and Drying Time of Apple Slices. J. Food Sci. Technol. 2018, 55, 2251–2258. [Google Scholar] [CrossRef]
- Wouters, P.C.; Alvarez, I.; Raso, J. Critical Factors Determining Inactivation Kinetics by Pulsed Electric Field Food Processing. Trends Food Sci. Technol. 2001, 12, 112–121. [Google Scholar] [CrossRef]
- Roobab, U.; Abida, A.; Chacha, J.S.; Athar, A.; Madni, G.M.; Ranjha, M.M.A.N.; Rusu, A.V.; Zeng, X.A.; Aadil, R.M.; Trif, M. Applications of Innovative Non-Thermal Pulsed Electric Field Technology in Developing Safer and Healthier Fruit Juices. Molecules 2022, 27, 4031. [Google Scholar] [CrossRef]
- Michalac, S.; Alvarez, V.; Ji, T.; Zhang, Q.H. Inactivation of Selected Microorganisms and Properties of Pulsed Electric Field Processed Milk. J. Food Process Preserv. 2003, 27, 137–151. [Google Scholar] [CrossRef]
- Sampedro, F.; Rodrigo, M.; Martínez, A.; Rodrigo, D.; Barbosa-Cánovas, G.V. Quality and Safety Aspects of PEF Application in Milk and Milk Products. Crit. Rev. Food Sci. Nutr. 2007, 45, 25–47. [Google Scholar] [CrossRef]
- Sepulveda, D.R.; Góngora-Nieto, M.M.; Guerrero, J.A.; Barbosa-Cánovas, G.V. Production of Extended-Shelf Life Milk by Processing Pasteurized Milk with Pulsed Electric Fields. J. Food Eng. 2005, 67, 81–86. [Google Scholar] [CrossRef]
- Šalaševičius, A.; Uždavinytė, D.; Visockis, M.; Ruzgys, P.; Šatkauskas, S. Comparative Analysis of Pulsed Electric Fields (PEF) and Traditional Pasteurization Techniques: Comparative Effects on Nutritional Attributes and Bacterial Viability in Milk and Whey Products. Appl. Sci. 2023, 13, 12127. [Google Scholar] [CrossRef]
- Su, W.; Wang, Q.; Li, J.; Qiu, Z.; Qiu, Y. Effects of Pulsed Electric Field Technology on the Nutritional Value and Biological Function of Plant Food. Front. Sustain. Food Syst. 2024, 8, 1385533. [Google Scholar] [CrossRef]
- Dhillon, P.K.; Singla, P. Advances in Non-Thermal Membrane Processing for Nutrient Recovery in the Dairy Industry: A Comprehensive Review. In Non-Thermal Processing of Functional Foods; CRC Press: Boca Raton, FL, USA, 2024; pp. 223–245. [Google Scholar] [CrossRef]
- Rabbani, A.; Srikumar, S.; Niroula, A.; Khan, M.K.I.; Nazir, A. Stability and Shelf-Life Modeling of Lemongrass Essential Oil-in-Water Nanoemulsions. Eur. J. Lipid Sci. Technol. 2025, 127, e202400096. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, L.; Pang, X.; Lu, J.; Kong, F.; Lv, J. Use of Microfiltration to Improve Quality and Shelf Life of Ultra-High Temperature Milk. J. Food Process Preserv. 2016, 40, 707–714. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Li, Z.; Xu, S.; Zhang, J.; Hettinga, K.; Zhou, P. Effects of Microfiltration Combined with Ultrasonication on Shelf Life and Bioactive Protein of Skim Milk. Ultrason. Sonochem 2021, 77, 105668. [Google Scholar] [CrossRef] [PubMed]
- Khatkar, S.K.; Dudi, K.; Lonkar, S.A.; Sidhu, K.S.; Khatkar, A.B.; Chandla, N.K.; Panghal, A. An Overview of Membrane Technology in Dairy & Food Industry. In Novel Technologies in Food Science; Wiley-Scrivener: Austin, TX, USA, 2022; pp. 65–108. [Google Scholar] [CrossRef]
- Żbik, K.; Onopiuk, A.; Górska-Horczyczak, E.; Wierzbicka, A. Trends and Opportunities in the Dairy Industry: A2 Milk and Processing Methods. Appl. Sci. 2024, 14, 6513. [Google Scholar] [CrossRef]
- Becker, K.M.; Parsons, R.L.; Kolodinsky, J.; Matiru, G.N. A Cost and Returns Evaluation of Alternative Dairy Products to Determine Capital Investment and Operational Feasibility of a Small-Scale Dairy Processing Facility. J. Dairy Sci. 2007, 90, 2506–2516. [Google Scholar] [CrossRef]
- Lau, S.; Wiedmann, M.; Adalja, A. Economic and Environmental Analysis of Processing Plant Interventions to Reduce Fluid Milk Waste. J. Dairy Sci. 2023, 106, 4773–4784. [Google Scholar] [CrossRef]
- Merlino, V.M.; Massaglia, S.; Borra, D.; Mimosi, A.; Cornale, P. Which Factors Drive Consumer Decisions During Milk Purchase? New Individuals’ Profiles Considering Fresh Pasteurized and Uht Treated Milk. Foods 2022, 11, 77. [Google Scholar] [CrossRef]
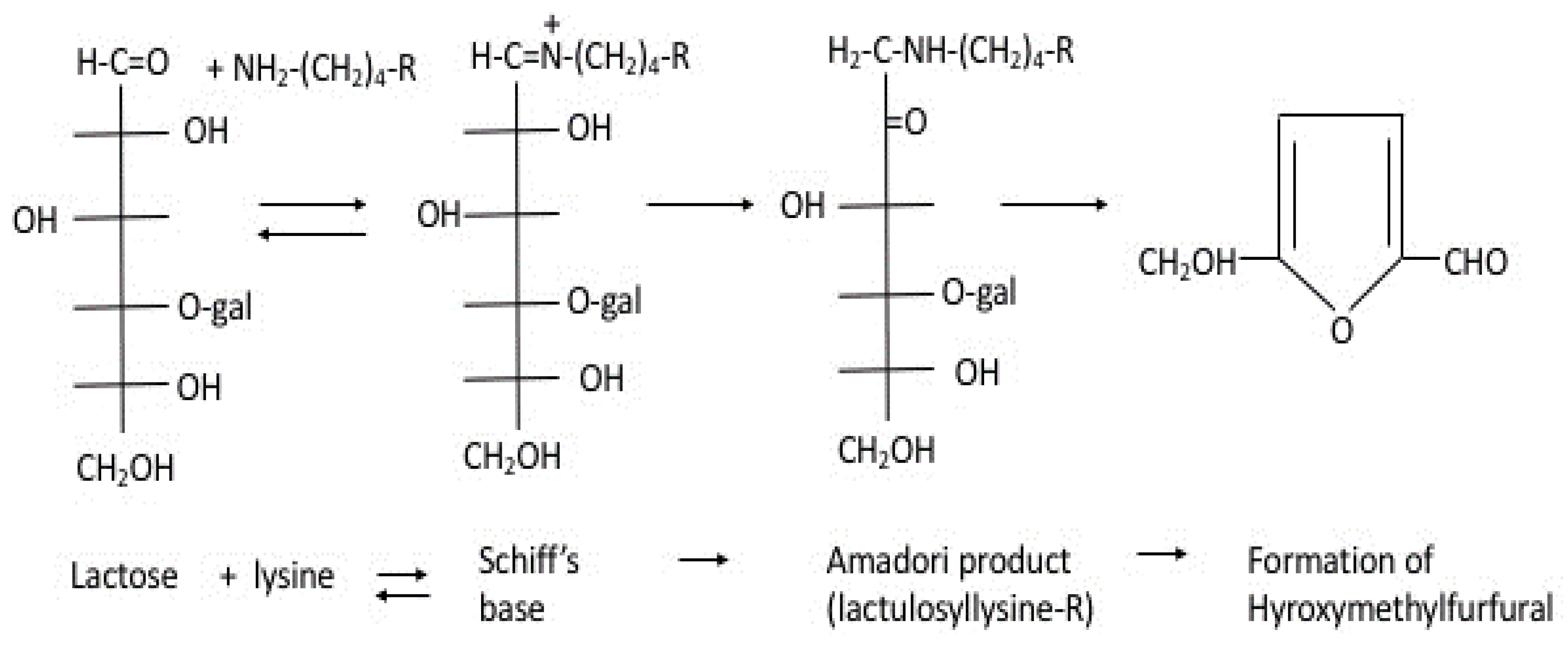
| Protein | -SH Groups | S-S Bonds | Reference |
|---|---|---|---|
| α-LA | None | 4 (Cys6-Cys120, Cys28-Cys111, Cys61-Cys77, and Cys73-Cys91) | [71] |
| β-LG | 1 (Cys121) | 2 (Cys66-Cys160 and Cys106-Cys119) | [72] |
| BSA | 1 (Cys34) | 17 | [65] |
| Precursor | Peptide Sequence (Position) | Peptide (m/z) | Released by |
|---|---|---|---|
| αS1-Casein | IPNPIGSENSEKTTMPLW (182–199) | 2014.0 | Heat |
| SDIPNPIGSENSEKTTMPLW (180–199) | 2216.1 | Cathepsin G | |
| RPKHPIKHQGLPQEVLNENLLRFF (1–24) | 2910.6 | Cathepsin B, Cathepsin D | |
| β-Casein | EMPFPKYPVEPFTESQSL (108–125) | 2126.0 | Plasmin, Cathepsin D |
| HKEMPFPKYPVEPFTESQSL (106–125) | 2391.2 | Plasmin, Cathepsin D |
| Component | Temperature Range ( °C) | ln k0 | Ea (kJmol−l) | n | Reference |
|---|---|---|---|---|---|
| Bovine serum albumin | 82–150 | 13.18 | 49 | 1 | [84] |
| Immunoglobulin | 60–76 | 90.38 | 275 | 1 | [85] |
| 76–82 | 54.21 | 170 | 1 | ||
| α-Lactalbumin | 70–85 | 84.92 | 269 | 1 | [86] |
| 85–150 | 16.95 | 69 | 1 | ||
| β-Lactoglobulin | 70–90 | 89.43 | 280 | 1.5 | [86,87] |
| 90–150 | 12.66 | 48 | 1.5 | ||
| 75–85 | 120.64 | 374 | 1.8 | ||
| Lysine (AA) | 130–160 | 8.77 | 109 | 2 | [88] |
| Component | Role in Milk | Effect of Pasteurization | References |
|---|---|---|---|
| Alkaline Phosphatase (EC 3.1.3.1) | Potent anti-inflammatory enzyme | Since this enzyme is destroyed by heat, it is used as sensitive indicator for adequate pasteurization of milk | [98] |
| Bovine immunoglobulin | Immunogenic proteins | 59–76% of activity is retained after HTST treatment | [99] |
| Bacteriocins | Antimicrobial peptides produced by certain milk bacteria with narrow spectrum of antimicrobial activity mainly against Gram-positive bacteria | No effect | [100,101] |
| Lactoferrin | Broad-spectrum antibacterial agent that binds to iron and reduces free iron supply for proliferation of bacteria, fungi, and protozoa | No effect | [102] |
| Lactoperoxidase(LPO, E.C. 1.11.1.7) | Acts together with hydrogen peroxide and thiocyanate ions as antibacterial agents | 70–90% of enzyme activity is retained after HTST treatment; activity is gradually lost during refrigeration of pasteurized milk | [103,104] |
| Lysozyme | Breaks cell walls primarily affecting Gram-positive bacteria | >75% of enzyme activity is retained after heating (80 °C, 15 s) | [105] |
| Plasmin (EC 3.4.21.7) | Milk protease causes alterations in protein structure and function | Survives pasteurization but may be destroyed at high temperature | [106] |
| Xanthine oxidase | Claimed to have antimicrobial properties by supplying hydrogen peroxidetolactoperoxidase | No effect | [96,107] |
| County Where Study Was Conducted | Exposure | Results | Reference |
|---|---|---|---|
| Crete (Greece) | Unpasteurized milk products | Adj. OR (and 95% CI) of atopy and unpasteurized farm milk consumption with and without simultaneous farm animal contact: 0.32 (0.13–0.78) and 0.58 (0.34–0.98), respectively | [151] |
| Austria, Germany, Switzerland | Milk directly produced or purchased on a farm | Consumption of farm milk during first year of life significantly inversely associated with asthma, hayfever, and atopy independent of other farm exposures | [141] |
| New Zealand | Unpasteurized milk, yogurt at least weekly before age of two years | Adj. OR and (95% CI) for early yogurt consumption and hay fever 0.30 (0.1–0.7); any unpasteurized milk and atopic eczema: 0.2 (0.1–0.8); no significant association between unpasteurized milk consumption and asthma or atopy | [146] |
| Finland | Farm milk in infancy | Farm milk consumption not associated with atopy; no other allergic health outcomes reported | [152] |
| Northern Germany | Raw, unboiled farm milk | Raw milk consumption and atopy adj. OR (and 95% CI): 0.65 (0.36–1.18); in those visiting animal houses before age of 7 years, raw milk consumption and atopy: 0.35 (0.17–0.74) | [153] |
| England | Unpasteurized milk | Current unpasteurized milk consumption associated with less eczema adj. Or and (95% CI) of 0.59 (0.40–0.87) and atopy of 0.42 (0.10–0.53), and higher production of whole blood stimulated IFN-γ; effect independent of farming status; no effect on asthma | [140] |
| Sweden, Austria, the Netherlands, Germany, Switzerland | Milk directly produced or purchased on a farm | Association between farm milk and asthma varied between genotypes of CD14/-1721; similar patterns for symptoms of hay fever and pollen sensitization; CD14/-1721 also modified association between farm milk and CD14 gene expression | [154] |
| Sweden, the Netherlands, Austria, Germany, Switzerland | Milk directly produced or purchased on a farm | Adj. OR and (95%CI) of farm milk consumption ever in life and asthma: 0.47 (0.61–0.88), rhinoconjunctivitis: 0.56 (0.43–0.73), sensitization to pollen: 0.67 (0.47–0.96), and food mix: 0.42 (0.19–0.92); association observed in all subgroups independent of farm-related exposures | [145] |
| Finland, France Austria, Germany, Switzerland | Skimmed and unskimmed farm milk, farm-produced butter, and yogurt during pregnancy | Maternal consumption of farm-produced butter during pregnancy associated with increased IFN-γ and TNF-α production in cord blood, and farm-produced yogurt inversely related to these cytokines | [155] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabbani, A.; Ayyash, M.; D’Costa, C.D.C.; Chen, G.; Xu, Y.; Kamal-Eldin, A. Effect of Heat Pasteurization and Sterilization on Milk Safety, Composition, Sensory Properties, and Nutritional Quality. Foods 2025, 14, 1342. https://doi.org/10.3390/foods14081342
Rabbani A, Ayyash M, D’Costa CDC, Chen G, Xu Y, Kamal-Eldin A. Effect of Heat Pasteurization and Sterilization on Milk Safety, Composition, Sensory Properties, and Nutritional Quality. Foods. 2025; 14(8):1342. https://doi.org/10.3390/foods14081342
Chicago/Turabian StyleRabbani, Ahmad, Mutamed Ayyash, Crystal D. C. D’Costa, Gang Chen, Yajun Xu, and Afaf Kamal-Eldin. 2025. "Effect of Heat Pasteurization and Sterilization on Milk Safety, Composition, Sensory Properties, and Nutritional Quality" Foods 14, no. 8: 1342. https://doi.org/10.3390/foods14081342
APA StyleRabbani, A., Ayyash, M., D’Costa, C. D. C., Chen, G., Xu, Y., & Kamal-Eldin, A. (2025). Effect of Heat Pasteurization and Sterilization on Milk Safety, Composition, Sensory Properties, and Nutritional Quality. Foods, 14(8), 1342. https://doi.org/10.3390/foods14081342










