Pre-Treatment Effects on Chemico-Physical Characteristics of Argan Press Cake Used for Bread Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Raw Materials
2.3. APC Treatment
2.4. Proximate Analysis
2.5. Mineral Content
2.6. Phytic Acid Content
2.7. Saponin Content
2.8. Fluorescence Spectroscopy
2.9. Infrared Spectroscopy Measurements
2.10. Breadmaking
2.11. Technological Parameters
2.12. Mixograph
2.13. Loaf Volume
2.14. Image Analysis for Crumb Characteristics
2.15. Statistical Analysis
3. Results and Discussion
3.1. APC Characteristics
3.1.1. Nutritional Content
3.1.2. Mineral Content
3.1.3. Color Characteristics
3.1.4. FTIR and Fluorescence Analysis
3.2. Characterization of Flour Blends
3.3. Characterization of Bread
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Filière de L’argane—Fellah Trade. Available online: https://www.fellah-trade.com/fr/filiere-vegetale/chiffres-cles-arganier (accessed on 21 March 2024).
- El Monfalouti, H.; Guillaume, D.; Denhez, C.; Charrouf, Z. Therapeutic Potential of Argan Oil: A Review. J. Pharm. Pharmacol. 2010, 62, 1669–1675. [Google Scholar] [CrossRef]
- Moutik, S.; Benali, A.; Bendaou, M.; Maadoudi, E.H.; Kabbour, M.R.; El Housni, A.; Es-Safi, N.E. The Effect of Using Diet Supplementation Based on Argane (Argania spinosa) on Fattening Performance, Carcass Characteristics and Fatty Acid Composition of Lambs. Heliyon 2021, 7, e05942. [Google Scholar] [CrossRef]
- Zeghlouli, J.; Guendouz, A.; Duchez, D.; El Modafar, C.; Michaud, P.; Delattre, C. Valorization of Co-Products Generated by Argan Oil Extraction Process: Application to Biodiesel Production. Biofuels 2022, 13, 771–777. [Google Scholar] [CrossRef]
- Demnati, D.; Sánchez, S.; Pacheco, R.; Zahar, M.; Martínez, L. Comparative Study of Argan and Olive Fruits and Oils. Actes Prem. Congrès Int. L’Arganier 2011, 7, 435–441. [Google Scholar]
- El Monfalouti, H.; Charrouf, Z.; Belviso, S.; Ghirardello, D.; Scursatone, B.; Guillaume, D.; Denhez, C.; Zeppa, G. Analysis and Antioxidant Capacity of the Phenolic Compounds from Argan Fruit (Argania spinosa (L.) Skeels). Eur. J. Lipid Sci. Technol. 2012, 114, 446–452. [Google Scholar] [CrossRef]
- Rahib, Y.; Sarh, B.; Chaoufi, J.; Bonnamy, S.; Elorf, A. Physicochemical and Thermal Analysis of Argan Fruit Residues (AFRs) as a New Local Biomass for Bioenergy Production. J. Therm. Anal. Calorim. 2020, 145, 2405–2416. [Google Scholar] [CrossRef]
- Zouhair, F.Z.; Benali, A.; Kabbour, M.R.; EL Kabous, K.; El Maadoudi, E.h.; Bouksaim, M.; Essamri, A. Typical Characterization of Argane Pulp of Various Moroccan Areas: A New Biomass for the Second Generation Bioethanol Production. J. Saudi Soc. Agric. Sci. 2020, 19, 192–198. [Google Scholar] [CrossRef]
- Hilali, M.; Bey, M.; Oubarka, S.; Lebkiri, A. Effects of Argan Cake (Argania spinosa (L.) Saptaceae) Substitution on the Growth Performance, Nutritional Value, and Economic Efficacy of Broiler Chickens. J. Glob. Innov. Agric. Sci. 2022, 10, 55–60. [Google Scholar] [CrossRef]
- Sapna, I.; Jayadeep, A. Cellulolytic and Xylanolytic Enzyme Combinations in the Hydrolysis of Red Rice Bran: A Disparity in the Release of Nutraceuticals and Its Correlation with Bioactivities. LWT 2022, 154, 112856. [Google Scholar] [CrossRef]
- Pereira, P.; Palma, C.; Ferreira-Pêgo, C.; Amaral, O.; Amaral, A.; Rijo, P.; Gregório, J.; Palma, L.; Nicolai, M. Grape Pomace: A Potential Ingredient for the Human Diet. Foods 2020, 9, 1772. [Google Scholar] [CrossRef]
- Foti, P.; Romeo, F.V.; Russo, N.; Pino, A.; Vaccalluzzo, A.; Caggia, C.; Randazzo, C.L. Olive Mill Wastewater as Renewable Raw Materials to Generate High Added-Value Ingredients for Agro-Food Industries. Appl. Sci. 2021, 11, 7511. [Google Scholar] [CrossRef]
- Wedamulla, N.E.; Fan, M.; Choi, Y.-J.; Kim, E.-K. Citrus Peel as a Renewable Bioresource: Transforming Waste to Food Additives. J. Funct. Foods 2022, 95, 105163. [Google Scholar] [CrossRef]
- Rivero Meza, S.L.; Cañizares, L.; Dannenberg, B.; Peres, B.B.; Rodrigues, L.A.; Mardade, C.; de Leon, M.A.; Gaioso, C.A.; Egea, I.; de Oliveira, M. Sustainable Rice Bran Protein: Composition, Extraction, Quality Properties and Applications. Trends Food Sci. Technol. 2024, 145, 104355. [Google Scholar] [CrossRef]
- Kotecka-Majchrzak, K.; Sumara, A.; Fornal, E.; Montowska, M. Oilseed Proteins—Properties and Application as a Food Ingredient. Trends Food Sci. Technol. 2020, 106, 160–170. [Google Scholar] [CrossRef]
- El Abbassi, A.; Khalid, N.; Zbakh, H.; Ahmad, A. Physicochemical Characteristics, Nutritional Properties, and Health Benefits of Argan Oil: A Review. Crit. Rev. Food Sci. Nutr. 2014, 54, 1401–1414. [Google Scholar] [CrossRef]
- Taarji, N.; Rabelo Da Silva, C.A.; Khalid, N.; Gadhi, C.; Hafidi, A.; Kobayashi, I.; Neves, M.A.; Isoda, H.; Nakajima, M. Formulation and Stabilization of Oil-in-Water Nanoemulsions Using a Saponins-Rich Extract from Argan Oil Press-Cake. Food Chem. 2018, 246, 457–463. [Google Scholar] [CrossRef]
- Ali, H.; Houghton, P.J.; Soumyanath, A. α-Amylase Inhibitory Activity of Some Malaysian Plants Used to Treat Diabetes; with Particular Reference to Phyllanthus Amarus. J. Ethnopharmacol. 2006, 107, 449–455. [Google Scholar] [CrossRef]
- Arsov, A.; Tsigoriyna, L.; Batovska, D.; Armenova, N.; Mu, W.; Zhang, W.; Petrov, K.; Petrova, P. Bacterial Degradation of Antinutrients in Foods: The Genomic Insight. Foods 2024, 13, 2408. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Phosanam, A.; Stockmann, R. Perspectives on Saponins: Food Functionality and Applications. Int. J. Mol. Sci. 2023, 24, 13538. [Google Scholar] [CrossRef]
- AACC Approved Methods of Analysis, 10th ed.; Cereals & Grains Association: St. Paul, MN, USA, 2000.
- Thavarajah, P.; Thavarajah, D.; Vandenberg, A. Low Phytic Acid Lentils (Lens culinaris L.): A Potential Solution for Increased Micronutrient Bioavailability. J. Agric. Food Chem. 2009, 57, 9044–9049. [Google Scholar] [CrossRef]
- Edeoga, H.O.; Okwu, D.E.; Mbaebie, B.O. Phytochemical Constituents of Some Nigerian Medicinal Plants. Afr. J. Biotechnol. 2005, 4, 685–688. [Google Scholar] [CrossRef]
- Bosmali, I.; Kotsiou, K.; Matsakidou, A.; Irakli, M.; Madesis, P.; Biliaderis, C.G. Fortification of Wheat Bread with an Alternative Source of Bean Proteins Using Raw and Roasted Phaseolus Coccineus Flours: Impact on Physicochemical, Nutritional and Quality Attributes. Food Hydrocoll. 2025, 158, 110527. [Google Scholar] [CrossRef]
- ISO 5529:2007; Wheat—Determination of the Sedimentation Index—Zeleny Test. International Organization for Standardization: Geneva, Switzerland, 2007.
- Durmus, Y.; Anil, M.; Simsek, S. Discrimination of Glutopeak Test and Mixograph Parameters for Evaluation of Wheat Flour Supplemented with Hazelnut Skin, Cross-Linked Starch, and Oxidized Starch. Foods 2023, 12, 328. [Google Scholar] [CrossRef]
- Hussain, M.; Saeed, F.; Niaz, B.; Afzaal, M.; Ikram, A.; Hussain, S.; Mohamed, A.A.; Alamri, M.S.; Anjum, F.M. Biochemical and Nutritional Profile of Maize Bran-Enriched Flour in Relation to Its End-Use Quality. Food Sci. Nutr. 2021, 9, 3336–3345. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, M.; Singh, G. Effect of Different Pretreatments on the Quality of Mushrooms during Solar Drying. J. Food Sci. Technol. 2013, 50, 165–170. [Google Scholar] [CrossRef]
- Lazo, J.; Tapia, J.; Guerra, F.P. Cadmium and Copper Stress Responses in Soapbark Tree (Quillaja saponaria): Effects on Growth, Metal Accumulation, Saponin Concentration, and Gene Expression. Plants 2025, 14, 709. [Google Scholar] [CrossRef] [PubMed]
- Shinta, Y.C.; Zaman, B.; Sumiyati, S. Citric Acid and EDTA as Chelating Agents in Phytoremediation of Heavy Metal in Polluted Soil: A Review. IOP Conf. Ser. Earth Environ. Sci. 2021, 896, 012023. [Google Scholar] [CrossRef]
- Urbano, G.; López-Jurado, M.; Aranda, P.; Vidal-Valverde, C.; Tenorio, E.; Porres, J. The Role of Phytic Acid in Legumes: Antinutrient or Beneficial Function? J. Physiol. Biochem. 2000, 56, 283–294. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Ali, A.; Ain, H.B.U.; Kausar, S.; Khalil, A.A.; Aadil, R.M.; Zeng, X.-A. Bioaccessibility Mechanisms, Fortification Strategies, Processing Impact on Bioavailability, and Therapeutic Potentials of Minerals in Cereals. Future Foods 2024, 10, 100425. [Google Scholar] [CrossRef]
- Mahgoub, S.E.O.; Elhag, S.A. Effect of Milling, Soaking, Malting, Heat-Treatment and Fermentation on Phytate Level of Four Sudanese Sorghum Cultivars. Food Chem. 1998, 61, 77–80. [Google Scholar] [CrossRef]
- Khan, N.; Zaman, R.; Elahi, M. Effect of Heat Treatments on the Phytic Acid Content of Maize Products. J. Sci. Food Agric. 1991, 54, 153–156. [Google Scholar] [CrossRef]
- Joseph, M.; Guo, Q.; Lindshield, B.; Adedeji, A.A.; Alavi, S. Characterization of Extruded Sorghum-Soy Blends to Develop Pre-Cooked and Nutritionally Dense Fortified Blended Foods. Foods 2025, 14, 779. [Google Scholar] [CrossRef] [PubMed]
- Beal, L.; Mehta, T. Zinc and Phytate Distribution in Peas. Influence of Heat Treatment, Germination, pH, Substrate, and Phosphorus on Pea Phytate and Phytase. J. Food Sci. 1985, 50, 96–100. [Google Scholar] [CrossRef]
- Lakram, N.; Zouhair, F.Z.; Ennahli, Y.; Moutik, S.; Mohamed, B.; Naciri, M.; El Maadoudi, E.; Housni, A.; Kabbour, M. The Impact of Optimizing the Detoxification of Argane (Argania spinosa) Press Cake on Nutritional Quality and Saponin Levels. Iran. J. Appl. Anim. Sci. 2019, 9, 235–246. [Google Scholar]
- Sharma, S.; Goyal, R.; Barwal, S. Domestic Processing Effects on Physicochemical, Nutritional and Anti-Nutritional Attributes in Soybean (Glycine max L. Merill). Int. Food Res. J. 2013, 20. [Google Scholar]
- Osman, M.A. Effect of Different Processing Methods, on Nutrient Composition, Antinutrional Factors, and in Vitro Protein Digestibility of Dolichos Lablab Bean [Lablab purpuresus (L.) Sweet]. Pak. J. Nutr. 2007, 6, 299–303. [Google Scholar] [CrossRef]
- Wanjekeche, E.; Wakasa, V.; Mureithi, J.G. Effect of Germination, Alkaline and Acid Soaking and Boiling on the Nutritional Value of Mature and Immature Mucuna (Mucuna pruriens) Beans. Trop. Subtrop. Agroecosystems 2003, 1, 183–192. [Google Scholar]
- Kuyu, C.G.; Tola, Y.B.; Mohammed, A.; Ramaswamy, H.S. Determination of Citric Acid Pretreatment Effect on Nutrient Content, Bioactive Components, and Total Antioxidant Capacity of Dried Sweet Potato Flour. Food Sci. Nutr. 2018, 6, 1724–1733. [Google Scholar] [CrossRef]
- ALAJAJI, S.A.; EL-ADAWY, T.A. Nutritional Composition of Chickpea (Cicer arietinum L.) as Affected by Microwave Cooking and Other Traditional Cooking Methods. Nutr. Compos. Chickpea Cicer Arietinum Affect. Microw. Cook. Tradit. Cook. Methods 2006, 19, 806–812. [Google Scholar] [CrossRef]
- Tonfack Djikeng, F.; Selle, E.; Morfor, A.T.; Tiencheu, B.; Hako Touko, B.A.; Teboukeu Boungo, G.; Ndomou Houketchang, S.; Karuna, M.S.L.; Linder, M.; Ngoufack, F.Z.; et al. Effect of Boiling and Roasting on Lipid Quality, Proximate Composition, and Mineral Content of Walnut Seeds (Tetracarpidium conophorum) Produced and Commercialized in Kumba, South-West Region Cameroon. Food Sci. Nutr. 2018, 6, 417–423. [Google Scholar] [CrossRef]
- Platel, K.; Srinivasan, K. Bioavailability of Micronutrients from Plant Foods: An Update. Crit. Rev. Food Sci. Nutr. 2016, 56, 1608–1619. [Google Scholar] [CrossRef]
- Guo, F.; Danielski, R.; Santhiravel, S.; Shahidi, F. Unlocking the Nutraceutical Potential of Legumes and Their By-Products: Paving the Way for the Circular Economy in the Agri-Food Industry. Antioxidants 2024, 13, 636. [Google Scholar] [CrossRef]
- Porres, J.m.; Etcheverry, P.; Miller, D.d.; Lei, X.g. Phytase and Citric Acid Supplementation in Whole-Wheat Bread Improves Phytate-Phosphorus Release and Iron Dialyzability. J. Food Sci. 2001, 66, 614–619. [Google Scholar] [CrossRef]
- Zhang, K.-P.; Chen, G.-F.; Zhao, L.; Liu, B.; Xu, X.-B.; Tian, J.-C. Molecular Genetic Analysis of Flour Color Using a Doubled Haploid Population in Bread Wheat (Triticum aestivum L.). Euphytica 2009, 165, 471–484. [Google Scholar] [CrossRef]
- Ngoma, K.; Mashau, M.E.; Silungwe, H. Physicochemical and Functional Properties of Chemically Pretreated Ndou Sweet Potato Flour. Int. J. Food Sci. 2019, 2019, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ameny, M.A.; Wilson, P.W. Relationship between Hunter Color Values and β-Carotene Contents in White-Fleshed African Sweetpotatoes (Ipomoea batatas Lam). J. Sci. Food Agric. 1997, 73, 301–306. [Google Scholar] [CrossRef]
- Akyıldız, A.; Öcal, N.D. Effects of Dehydration Temperatures on Colour and Polyphenoloxidase Activity of Amasya and Golden Delicious Apple Cultivars. J. Sci. Food Agric. 2006, 86, 2363–2368. [Google Scholar] [CrossRef]
- Singh, K.; Tripathi, S.; Chandra, R. Maillard Reaction Product and Its Complexation with Environmental Pollutants: A Comprehensive Review of Their Synthesis and Impact. Bioresour. Technol. Rep. 2021, 15, 100779. [Google Scholar] [CrossRef]
- Wani, A.A.; Sogi, D.S.; Singh, P.; Khatkar, B.S. Influence of Watermelon Seed Protein Concentrates on Dough Handling, Textural and Sensory Properties of Cookies. J. Food Sci. Technol. 2015, 52, 2139–2147. [Google Scholar] [CrossRef]
- Ortolan, F.; Urbano, K.; Netto, F.M.; Steel, C.J. Chemical and Structural Characteristics of Proteins of Non-Vital and Vital Wheat Glutens. Food Hydrocoll. 2022, 125, 107383. [Google Scholar] [CrossRef]
- Bock, J.E.; Connelly, R.K.; Damodaran, S. Impact of Bran Addition on Water Properties and Gluten Secondary Structure in Wheat Flour Doughs Studied by Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy. Cereal Chem. 2013, 90, 377–386. [Google Scholar] [CrossRef]
- Sivam, A.S.; Sun-Waterhouse, D.; Perera, C.O.; Waterhouse, G.I.N. Application of FT-IR and Raman Spectroscopy for the Study of Biopolymers in Breads Fortified with Fibre and Polyphenols. Food Res. Int. 2013, 50, 574–585. [Google Scholar] [CrossRef]
- Nawrocka, A.; Krekora, M.; Niewiadomski, Z.; Miś, A. FTIR Studies of Gluten Matrix Dehydration after Fibre Polysaccharide Addition. Food Chem. 2018, 252, 198–206. [Google Scholar] [CrossRef]
- He, X.; Wang, B.; Zhao, B.; Meng, Y.; Chen, J.; Yang, F. Effect of Hydrothermal Treatment on the Structure and Functional Properties of Quinoa Protein Isolate. Foods 2022, 11, 2954. [Google Scholar] [CrossRef] [PubMed]
- Meziani, S.; Jasniewski, J.; Ribotta, P.; Arab-Tehrany, E.; Muller, J.-M.; Ghoul, M.; Desobry, S. Influence of Yeast and Frozen Storage on Rheological, Structural and Microbial Quality of Frozen Sweet Dough. J. Food Eng. 2012, 109, 538–544. [Google Scholar] [CrossRef]
- Liu, G.; Li, J.; Shi, K.; Wang, S.; Chen, J.; Liu, Y.; Huang, Q. Composition, Secondary Structure, and Self-Assembly of Oat Protein Isolate. J. Agric. Food Chem. 2009, 57, 4552–4558. [Google Scholar] [CrossRef]
- Peng, Q.; Khan, N.A.; Wang, Z.; Yu, P. Moist and Dry Heating-Induced Changes in Protein Molecular Structure, Protein Subfractions, and Nutrient Profiles in Camelina Seeds. J. Dairy Sci. 2014, 97, 446–457. [Google Scholar] [CrossRef]
- Wang, M.; Li, Y.; Ma, C.; Zhang, Z.; Guo, L.; Huang, M.; Sun, J. Stability of Native/Thermally Denatured Myofibrillar Protein Particles: Improvement with Decreasing pH. Food Hydrocoll. 2023, 140, 108628. [Google Scholar] [CrossRef]
- Uranga, J.; Puertas, A.I.; Etxabide, A.; Dueñas, M.T.; Guerrero, P.; de la Caba, K. Citric Acid-Incorporated Fish Gelatin/Chitosan Composite Films. Food Hydrocoll. 2019, 86, 95–103. [Google Scholar] [CrossRef]
- Ramirez, D.O.S.; Carletto, R.A.; Tonetti, C.; Giachet, F.T.; Varesano, A.; Vineis, C. Wool Keratin Film Plasticized by Citric Acid for Food Packaging. Food Packag. Shelf Life 2017, 12, 100–106. [Google Scholar] [CrossRef]
- Locquet, N.; Aït-Kaddour, A.; Cordella, C.B.Y. 3D Fluorescence Spectroscopy and Its Applications. In Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 1–39. ISBN 978-0-470-02731-8. [Google Scholar]
- Gorokhov, V.V.; Knox, P.P.; Korvatovsky, B.N.; Lukashev, E.P.; Goryachev, S.N.; Paschenko, V.Z.; Rubin, A.B. Comparison of Spectral and Temporal Fluorescence Parameters of Aqueous Tryptophan Solutions Frozen in the Light and in the Dark. Chem. Phys. 2023, 571, 111919. [Google Scholar] [CrossRef]
- Najib, M.; Botosoa, E.P.; Hallab, W.; Hallab, K.; Hallab, Z.; Hamze, M.; Delaplace, G.; Karoui, R.; Chihib, N.-E. Utilization of Front-Face Fluorescence Spectroscopy for Monitoring Lipid Oxidation during Lebanese Qishta Aging. LWT 2020, 130, 109693. [Google Scholar] [CrossRef]
- Miriani, M.; Keerati-u-rai, M.; Corredig, M.; Iametti, S.; Bonomi, F. Denaturation of Soy Proteins in Solution and at the Oil–Water Interface: A Fluorescence Study. Food Hydrocoll. 2011, 25, 620–626. [Google Scholar] [CrossRef]
- Rahimi Yazdi, S.; Corredig, M. Heating of Milk Alters the Binding of Curcumin to Casein Micelles. A Fluorescence Spectroscopy Study. Food Chem. 2012, 132, 1143–1149. [Google Scholar] [CrossRef]
- Ji, W.; Yang, F.; Yang, M. Effect of Change in pH, Heat and Ultrasound Pre-Treatments on Binding Interactions between Quercetin and Whey Protein Concentrate. Food Chem. 2022, 384, 132508. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, M.; Rezaei, M.; Jafarpour, A.; Undeland, I. Dynamic Rheological, Microstructural and Physicochemical Properties of Blend Fish Protein Recovered from Kilka (Clupeonella cultriventris) and Silver Carp (Hypophthalmichthys molitrix) by the pH-Shift Process or Washing-Based Technology. Food Chem. 2017, 229, 695–709. [Google Scholar] [CrossRef]
- Pezeshk, S.; Rezaei, M.; Hosseini, H.; Abdollahi, M. Impact of pH-Shift Processing Combined with Ultrasonication on Structural and Functional Properties of Proteins Isolated from Rainbow Trout by-Products. Food Hydrocoll. 2021, 118, 106768. [Google Scholar] [CrossRef]
- Chang, C.; Wang, T.; Hu, Q.; Luo, Y. Caseinate-Zein-Polysaccharide Complex Nanoparticles as Potential Oral Delivery Vehicles for Curcumin: Effect of Polysaccharide Type and Chemical Cross-Linking. Food Hydrocoll. 2017, 72, 254–262. [Google Scholar] [CrossRef]
- Wang, S.; Yang, J.; Shao, G.; Liu, J.; Wang, J.; Yang, L.; Li, J.; Liu, H.; Zhu, D.; Li, Y.; et al. pH-Induced Conformational Changes and Interfacial Dilatational Rheology of Soy Protein Isolated/Soy Hull Polysaccharide Complex and Its Effects on Emulsion Stabilization. Food Hydrocoll. 2020, 109, 106075. [Google Scholar] [CrossRef]
- Ikpeme, C.; Eneji, C.; Igile, G. Nutritional and Organoleptic Properties of Wheat (Triticum aestivum) and Beniseed (Sesame indicum) Composite Flour Baked Foods. J. Food Res. 2012, 1, 84. [Google Scholar] [CrossRef]
- Chinma, C.E.; Gbadamosi, K.B.; Ogunsina, B.S.; Oloyede, O.O.; Salami, S.O. Effect of Addition of Germinated Moringa Seed Flour on the Quality Attributes of Wheat-Based Cake. J. Food Process. Preserv. 2014, 38, 1737–1742. [Google Scholar] [CrossRef]
- Ogunronbi, O.; Jooste, P.J.; Abu, J.O.; Van Der Merwe, B. Chemical Composition, Storage Stability and Effect of Cold-Pressed Flaxseed Oil Cake Inclusion on Bread Quality. J. Food Process. Preserv. 2011, 35, 64–79. [Google Scholar] [CrossRef]
- Sharma, S.; Prabhasankar, P. Effect of Whole Hempseed Flour Incorporation on the Rheological, Microstructural and Nutritional Characteristics of Chapati—Indian Flatbread. LWT 2021, 137, 110491. [Google Scholar] [CrossRef]
- Shongwe, S.G.; Kidane, S.W.; Shelembe, J.S.; Nkambule, T.P. Dough Rheology and Physicochemical and Sensory Properties of Wheat–Peanut Composite Flour Bread. Legume Sci. 2022, 4. [Google Scholar] [CrossRef]
- Gupta, S.; Shimray, C.A.; Venkateswara Rao, G. Influence of Organic Acids on Rheological and Bread-Making Characteristics of Fortified Wheat Flour. Int. J. Food Sci. Nutr. 2012, 63, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Bora, P.; Ragaee, S.; Abdel-Aal, E.-S.M. Effect of Incorporation of Goji Berry By-Product on Biochemical, Physical and Sensory Properties of Selected Bakery Products. LWT 2019, 112, 108225. [Google Scholar] [CrossRef]
- Hussain, A.; Kausar, T.; Aslam, J.; Quddoos, M.Y.; Ali, A.; Kauser, S.; Zerlasht, M.; Rafique, A.; Noreen, S.; Iftikhar, K.; et al. Physical and Rheological Studies of Biscuits Developed with Different Replacement Levels of Pumpkin (Cucurbita maxima) Peel, Flesh, and Seed Powders. J. Food Qual. 2023, 2023, e4362094. [Google Scholar] [CrossRef]
- Shilbi, A.Z.N.A.; Murtini, E.S. Optimization of Ginger (Zingiber officinale) and Cinnamon (Cinnamomum verum) on Total Phenolics, Antioxidant Activity and Loaf Volume of Bread. Adv. Food Sci. Sustain. Agric. Agroindustrial Eng. 2022, 5, 102–110. [Google Scholar] [CrossRef]
- Bhatt, S.M.; Gupta, R.K. Bread (Composite flour) Formulation and Study of Its Nutritive, Phytochemical and Functional Properties. J. Pharmacogn. Phytochem. 2015, 4, 254–268. [Google Scholar]
- Ragaee, S.; Abdel-Aal, E.-S.M. Pasting Properties of Starch and Protein in Selected Cereals and Quality of Their Food Products. Food Chem. 2006, 95, 9–18. [Google Scholar] [CrossRef]
- Ikegwu, O.J.; Okechukwu, P.E.; Ekumankana, E.O. Physico-Chemical and Pasting Characteristics of Flour and Starch from Achi Brachystegia Eurycoma Seed. J. Food Technol. 2010, 8, 58–66. [Google Scholar] [CrossRef]
- Julianti, E.; Rusmarilin, H.; Ridwansyah; Yusraini, E. Functional and Rheological Properties of Composite Flour from Sweet Potato, Maize, Soybean and Xanthan Gum. J. Saudi Soc. Agric. Sci. 2017, 16, 171–177. [Google Scholar] [CrossRef]
- Martins, Z.E.; Pinho, O.; Ferreira, I.M.P.L.V.O. Fortification of Wheat Bread with Agroindustry By-Products: Statistical Methods for Sensory Preference Evaluation and Correlation with Color and Crumb Structure. J. Food Sci. 2017, 82, 2183–2191. [Google Scholar] [CrossRef] [PubMed]
- Fenn, D.; Lukow, O.M.; Humphreys, G.; Fields, P.G.; Boye, J.I. Wheat-Legume Composite Flour Quality. Int. J. Food Prop. 2010, 13, 381–393. [Google Scholar] [CrossRef]
- Jafari, M.; Koocheki, A.; Milani, E. Effect of Extrusion Cooking of Sorghum Flour on Rheology, Morphology and Heating Rate of Sorghum–Wheat Composite Dough. J. Cereal Sci. 2017, 77, 49–57. [Google Scholar] [CrossRef]
- Sun, X.; Ma, L.; Zhong, X.; Liang, J. Potential of Raw and Fermented Maize Gluten Feed in Bread Making: Assess of Dough Rheological Properties and Bread Quality. LWT 2022, 162, 113482. [Google Scholar] [CrossRef]
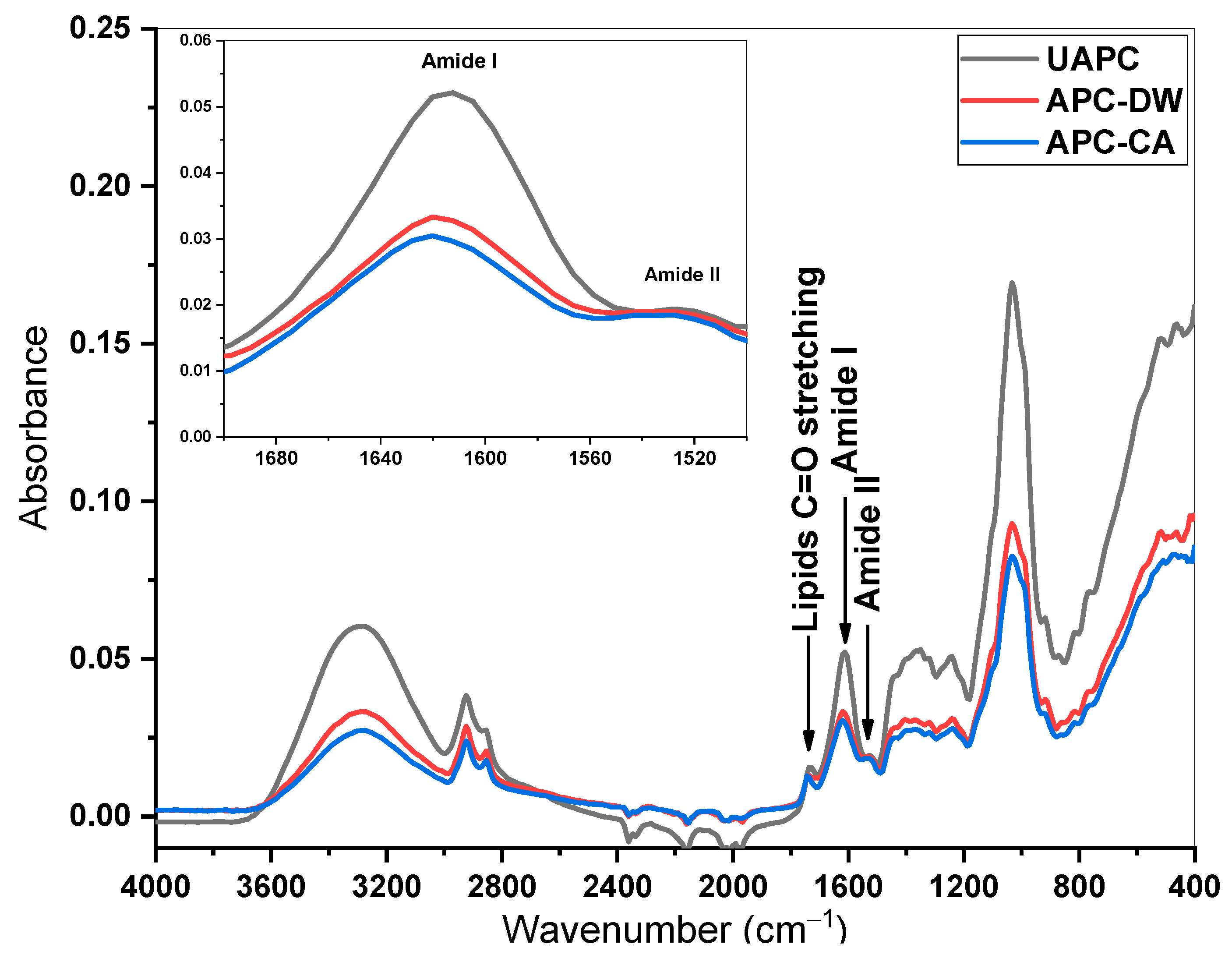
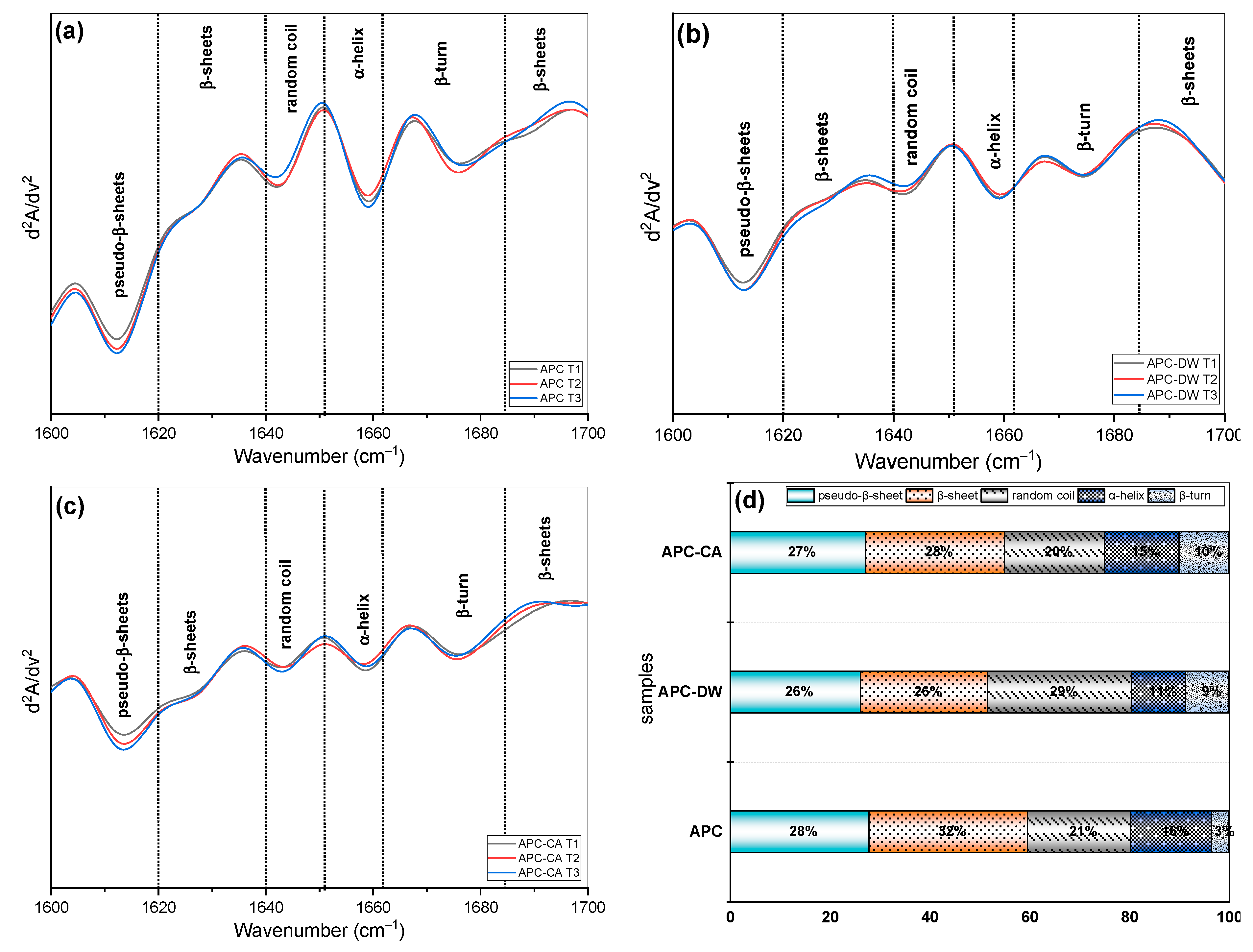
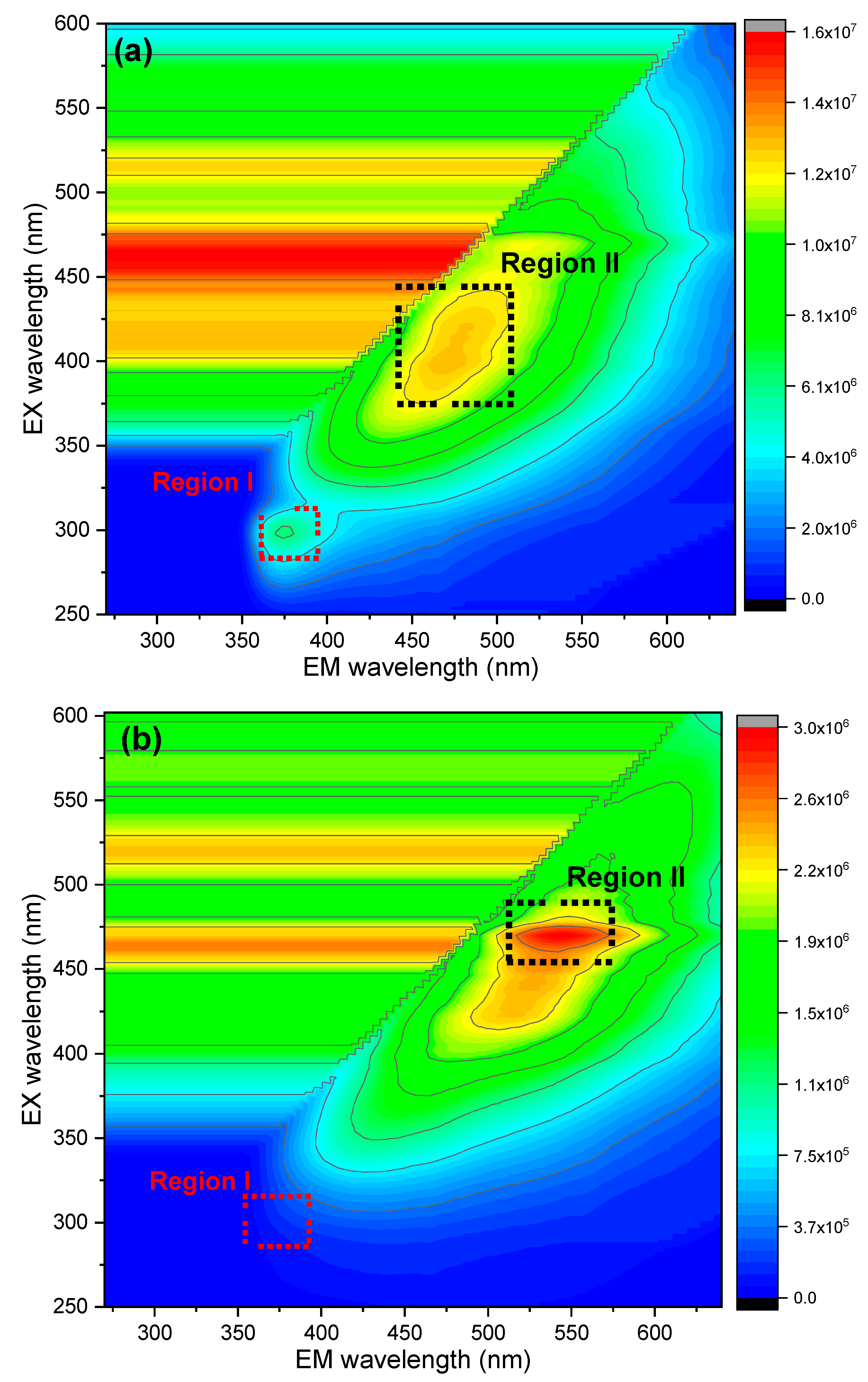


| Traits | UAPC | APC-CA | APC-DW |
|---|---|---|---|
| Saponin (mg/g) | 4.71 ± 0.10 a | 0.80 ± 0.12 b | 1.20± 0.10 c |
| Lipid (%) | 20.30 ± 0.20 a | 15.30 ± 0.10 b | 13.20 ± 0.20 c |
| Ash (%) | 5.09 ± 0.02 a | 3.18 ± 0.05 b | 4.66 ± 0.03 c |
| Fiber (%) | 17.83 ± 0.20 a | 14.13 ± 0.10 b | 15.82 ± 0.20 c |
| Protein (%) | 47.70 ± 0.10 a | 47.40 ± 0.10 a | 46.88 ± 0.06 b |
| Moisture (%) | 9.00 ± 0.02 a | 7.33 ± 0.03 b | 8.01 ± 0.25 c |
| Ca (mg/100 g) | 690.5 ± 0.13 a | 553.4 ± 0.10 b | 464.7± 0.11 c |
| Fe (mg/100 g) | 8.7 ± 0.04 a | 11.6 ± 0.02 b | 13.2 ± 0.01 c |
| Zn (mg/100 g) | 6.3 ± 0.00 a | 3.1 ± 0.01 b | 9.3 ± 0.00 c |
| Pythic acid (g/100 g) | 1.63 ± 0.10 a | 1.05 ± 0.01 b | 1.03 ± 0.01 b |
| Phytic Acid/Iron | 15.98 ± 0.67 a | 7.73 ± 0.18 b | 6.58 ± 0.6 b |
| Phytic Acid/Zinc | 25.74 ± 0.06 a | 33.71 ± 0.87 b | 10.95 ± 0.06 c |
| L* | 80.21 ± 0.02 a | 78.18 ± 0.05 b | 79.53 ± 0.10 c |
| a* | 1.70 ± 0.01 a | 1.64 ± 0.01 b | 1.58 ± 0.01 c |
| b* | 12.21 ± 0.02 a | 12.28 ± 0.03 a | 12.24 ± 0.50 a |
| Traits | WWF | 5%APC-CA | 10%APC-CA | 15%APC-CA | 20%APC-CA | 5%APC-DW | 10%APC-DW | 15%APC-DW | 20%APC-DW |
|---|---|---|---|---|---|---|---|---|---|
| Lipid (%) | 1.71 ± 0.03 a | 2.69 ± 0.01 b | 3.37 ± 0.04 c | 3.92 ± 0.03 d | 4.89 ± 0.04 e | 2.17 ± 0.06 ab | 3.31 ± 0.03 c | 4.02 ± 0.06 d | 4.36 ± 0.05 d |
| Ash (%) | 1.54 ± 0.03 a | 1.55 ± 0.03 ab | 1.63 ± 0.02 ab | 1.72 ± 0.01 ac | 1.77 ± 0.01 c | 1.67 ± 0.01 b | 1.90 ± 0.01 d | 1.94 ± 0.01 de | 2.04 ± 0.02 e |
| Fiber (%) | 2.60 ± 0.10 a | 3.27± 0.05 b | 4.73 ± 0.03 c | 5.22 ± 0.05 de | 5.35 ± 0.06 ef | 3.38 ± 0.02 b | 4.90 ± 0.01 cd | 5.36 ± 0.04 ef | 5.69 ± 0.03 f |
| Protein (%) | 13.56 ± 0.03 a | 14.51 ± 0.02 b | 15.15 ± 0.01 c | 15.85 ± 0.01 de | 16.62 ± 0.01 ef | 14.17 ± 0.01 f | 15.13 ± 0.03 c | 15.62 ± 0.03 d | 16.33 ± 0.03 f |
| Moisture (%) | 11.40 ± 0.10 a | 11.25 ± 0.02 a | 10.92 ± 0.02 ab | 10.87 ± 0.01 ab | 10.64 ± 0.01 bc | 10.91 ± 0.01 ab | 10.55 ± 0.01 bc | 10.33 ± 0.01 cd | 10.11 ± 0.01 d |
| Ca (mg/100 g) | 15.15 ± 0.01 a | 15.24 ± 0.04 a | 16.48 ± 0.03 b | 17.28 ± 0.03 bd | 17.75 ± 0.09 bd | 15.92 ± 0.03 a | 17.28 ± 0.03 c | 18.71 ± 0.06 d | 19.88 ± 0.03 d |
| Fe (mg/100 g) | 3.92 ± 0.01 a | 4.51 ± 0.02 b | 5.42 ± 0.03 cd | 5.60 ± 0.02 cd | 5.65 ± 0.03 d | 5.01 ± 0.02 bc | 5.63 ± 0.01 d | 5.80 ± 0.01 d | 5.92 ± 0.01 d |
| Zn (mg/100 g) | 2.40 ± 0.02 a | 2.41 ± 0.01 a | 2.60 ± 0.03 a | 2.63 ± 0.02 a | 2.81 ± 0.03 ab | 3.42 ± 0.01 abc | 3.74 ± 0.02 abc | 3.93 ± 0.01 c | 4.12 ± 0.02 c |
| Pythic acid (g/100 g) | 0.62 ± 0.01 a | 0.73 ± 0.01 bc | 0.81 ± 0.02 cd | 0.88 ± 0.01 de | 0.95 ± 0.02 e | 0.69 ± 0.01 ab | 0.74 ± 0.02 bc | 0.83 ± 0.02 d | 0.92 ± 0.02 ef |
| Phytic acid/iron | 13.76 ± 0.20 ab | 13.69 ± 0.19 abc | 12.65 ± 0.31 bcd | 13.67 ± 0.16 ab | 14.39 ± 0.30 a | 11.86 ± 0.24 cd | 11.40 ± 0.31 d | 12.32 ± 0.31 bcd | 13.39 ± 0.29 ae |
| Phytic acid/zinc | 27.83 ± 0.42 a | 29.98 ± 0.51 a | 33.26 ± 0.71 b | 33.35 ± 0.38 b | 33.14 ± 0.70 b | 20.13 ± 0.30 d | 19.48 ± 0.52 d | 21.81 ± 0.52 cd | 22.88 ± 0.50 c |
| L* (Flour) | 77.14 ± 0.08 a | 85.31 ± 0.09 d | 85.27 ± 0.02 cd | 85.20 ± 0.57 cd | 84.29 ± 0.02 bc | 83.68 ± 0.08 b | 85.26 ± 0.11 cd | 84.60 ± 0.06 bcd | 84.17 ± 0.05 b |
| a* (Flour) | 1.79 ± 0.05 a | 0.69 ± 0.01 bd | 0.56 ± 0.01 c | 0.57 ± 0.02 c | 0.68 ± 0.01 bd | 0.69 ± 0.00 bd | 0.62 ± 0.01 bc | 0.73 ± 0.02 d | 0.76 ± 0.01 d |
| b* (Flour) | 12.56 ± 0.03 a | 12.78 ± 0.05 g | 12.10 ± 0.02 bd | 12.10 ± 0.01 bd | 11.97 ± 0.02 bc | 11.83 ± 0.03 c | 12.41 ± 0.07 aef | 12.65 ± 0.04 g | 12.46 ± 0.02 af |
| WWF | 5%APC-CA | 10%APC-CA | 15%APC-CA | 20%APC-CA | 5%APC-DW | 10%APC-DW | 15%APC-DW | 20%APC-DW | |
|---|---|---|---|---|---|---|---|---|---|
| Gluten straight (mL) | 56.46 ± 0.1 a | 51.55 ± 0.2 b | 44.19 ± 0.1 c | 42.71 ± 0.5 cd | 39.28 ± 0.2 e | 47.62 ± 0.5 f | 41.73 ± 0.1 cde | 40.75 ± 0.5 de | 39.28 ± 0.0 c |
| Mixing time (min) | 4.72 ± 0.2 a | 3.98 ± 0.1 b | 2.96 ± 0.2 c | 3.96 ± 0.1 d | 4.40 ± 0.0 e | 4.12 ± 0.2 f | 3.08 ± 0.1 c | 3.06 ± 0.1 c | 3.16 ± 0.2 g |
| Peak height (%) | 43.8 ± 0.0 a | 47.6 ± 0.1 b | 50.0 ± 0.0 c | 47.6 ± 0.0 b | 50.7 ± 0.1 bc | 45.32 ± 0.0 e | 43.04 ± 0.0 a | 43.00 ± 0.0 a | 43.0 ± 0.0 a |
| Water absorption (%) | 63.4 ± 0.0 a | 64.4 ± 0.1 b | 65.0 ± 0.0 c | 66.0 ± 0.1 d | 66.9 ± 0.1 e | 64.5 ± 0.2 b | 65.4 ± 0.0 f | 66.4 ± 0.0 g | 67.6 ± 0.2 h |
| WWF | 5%APC-CA | 10%APC-CA | 5%APC-DW | 10%APC-DW | 15%APC-DW | 20%APCDW | |
|---|---|---|---|---|---|---|---|
| Cells numbers | 66.6 ± 0.03 a | 35.20 ± 0.0 b | 75.25 ± 0.0 c | 54.33 ± 0.02 d | 84.75 ± 0.03 e | 84.25 ± 0.03 e | 107.5 ± 0.03 f |
| Total area | 288.09 ± 0.02 a | 265.31 ± 0.01 a | 113.98 ± 0.01 b | 272.88 ± 0.02 a | 305.03 ± 0.02 c | 236.52 ± 0.0 d | 247.55 ± 0.02 e |
| Average size (mm2) | 4.4 ± 0.02 a | 7.83 ± 0.01 b | 1.53 ± 0.01 c | 5.01 ± 0.02 d | 3.59 ± 0.02 e | 2.81 ± 0.0 f | 2.32 ± 0.02 g |
| Porosity (%) | 32.01 ± 0.02 a | 29.47 ± 0.01 ab | 12.66 ± 0.01 c | 30.32 ± 0.02 a | 33.89 ± 0.02 a | 26.28 ± 0.0 b | 27.50 ± 0.02 b |
| L* (Bread) | 64.03 ± 0.02 a | 62.86 ± 0.01 b | 60.44 ± 0.02 c | 58.87 ± 0.02 d | 58.20 ± 0.02 d | 57.27 ± 0.03 d | 56.90 ± 0.03 d |
| a* (Bread) | 4.21 ± 0.02 a | 4.35 ± 0.01 a | 4.61 ± 0.02 b | 5.35 ± 0.03 c | 5.53 ± 0.03 d | 5.69 ± 0.02 e | 5.71 ± 0.02 e |
| b* (Bread) | 25.37 ± 0.03 a | 24.25 ± 0.03 b | 23.72 ± 0.01 c | 24.98 ± 0.01 d | 24.27 ± 0.02 b | 22.63 ± 0.02 e | 21.44 ± 0.02 f |
| Loaf volume (cm3) | 1150.00 ± 2.00 a | 1150.00 ± 1.00 a | 750.00 ± 2.00 b | 850.00 ± 1.00 c | 700.00 ± 1.00 d | 670.00 ± 1.00 e | 650.00 ± 2.00 f |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Kaourat, A.; Choukri, H.; Kartah, B.E.; Snoussi, A.; Zeppa, G.; Benali, A.; Taghouti, M.; El Monfalouti, H. Pre-Treatment Effects on Chemico-Physical Characteristics of Argan Press Cake Used for Bread Production. Foods 2025, 14, 1315. https://doi.org/10.3390/foods14081315
El Kaourat A, Choukri H, Kartah BE, Snoussi A, Zeppa G, Benali A, Taghouti M, El Monfalouti H. Pre-Treatment Effects on Chemico-Physical Characteristics of Argan Press Cake Used for Bread Production. Foods. 2025; 14(8):1315. https://doi.org/10.3390/foods14081315
Chicago/Turabian StyleEl Kaourat, Asma, Hasnae Choukri, Badr Eddine Kartah, Ahmed Snoussi, Giuseppe Zeppa, Aouatif Benali, Mouna Taghouti, and Hanae El Monfalouti. 2025. "Pre-Treatment Effects on Chemico-Physical Characteristics of Argan Press Cake Used for Bread Production" Foods 14, no. 8: 1315. https://doi.org/10.3390/foods14081315
APA StyleEl Kaourat, A., Choukri, H., Kartah, B. E., Snoussi, A., Zeppa, G., Benali, A., Taghouti, M., & El Monfalouti, H. (2025). Pre-Treatment Effects on Chemico-Physical Characteristics of Argan Press Cake Used for Bread Production. Foods, 14(8), 1315. https://doi.org/10.3390/foods14081315







