Gestational Inulin Supplementation in Low-/High-Fat Sow Diets: Effects on Growth Performance, Lipid Metabolism, and Meat Quality of Offspring Pigs
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Diet and Feeding
2.3. Sample Collection
2.3.1. Collection of Blood Samples from Newborn Piglets
2.3.2. Collection of Tissue Samples from Newborn Piglets and Finishing Pigs
2.4. Feed Intake and Body Weight Measurements at Different Stages of Pigs
2.5. Concentration of Serum Volatile Fatty Acids in Newborn Piglets
2.6. Hepatic Lipid Content and H&E Staining in Newborn Piglets and Finishing Pigs
2.7. Determination of HDAC Activity and Histone Acetylation in the Liver of Newborn Piglets
2.8. Expression of Key Fatty Acid Oxidation-Related Genes in the Liver
2.9. Organ Indices of Piglets at the Birth, Weaning, and Finishing Stages
2.10. Determination of Carcass Traits and Meat Quality in Finishing Pigs
2.11. Statistics
3. Results
3.1. Organ Index of Piglets at Birth and Weaning
3.2. Growth Performance of Piglets from Weaning to Finishing
3.3. Organ Indices and Carcass Traits of Pigs at Finishing Stage
3.4. Meat Quality of Pigs at Finishing Stage
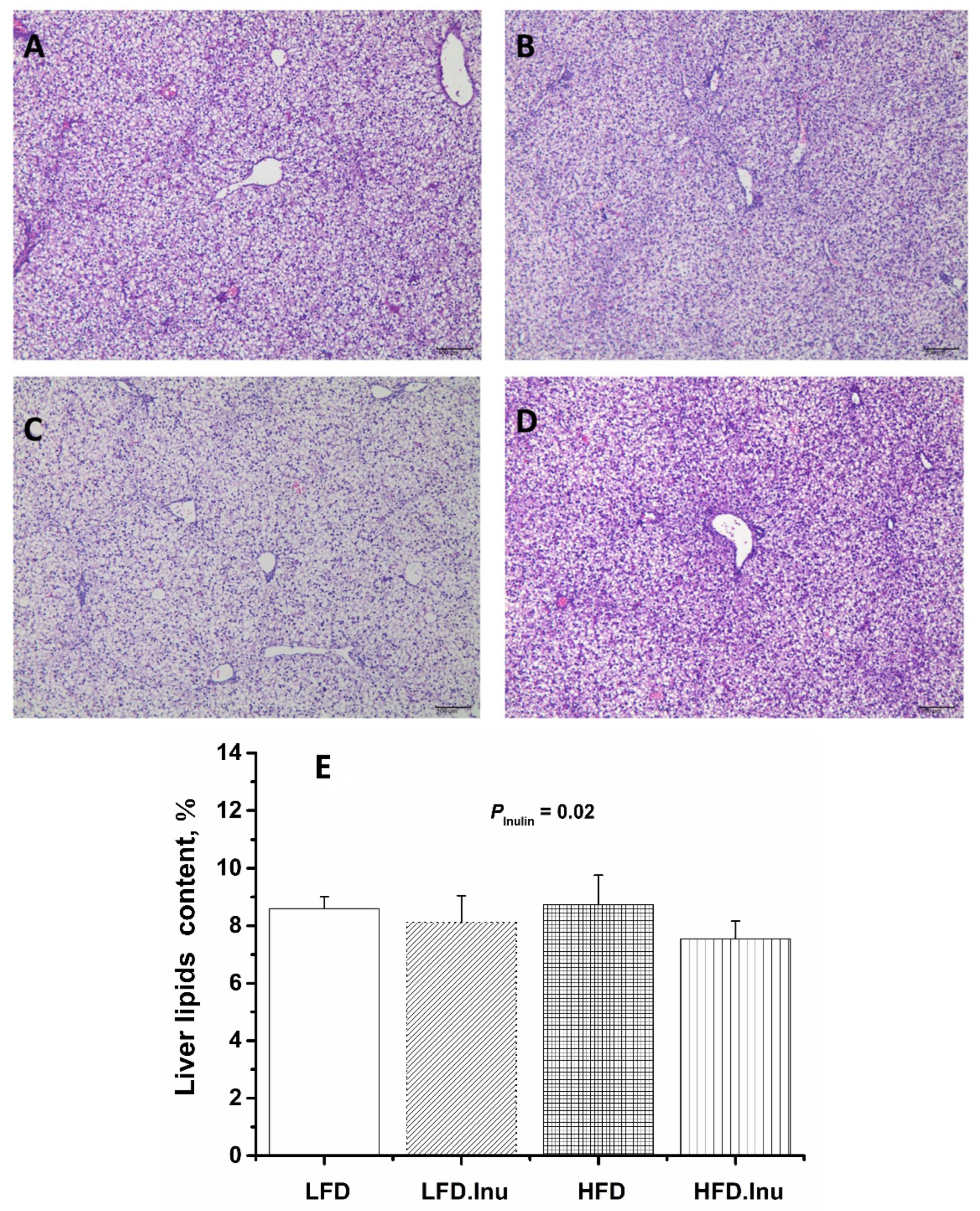
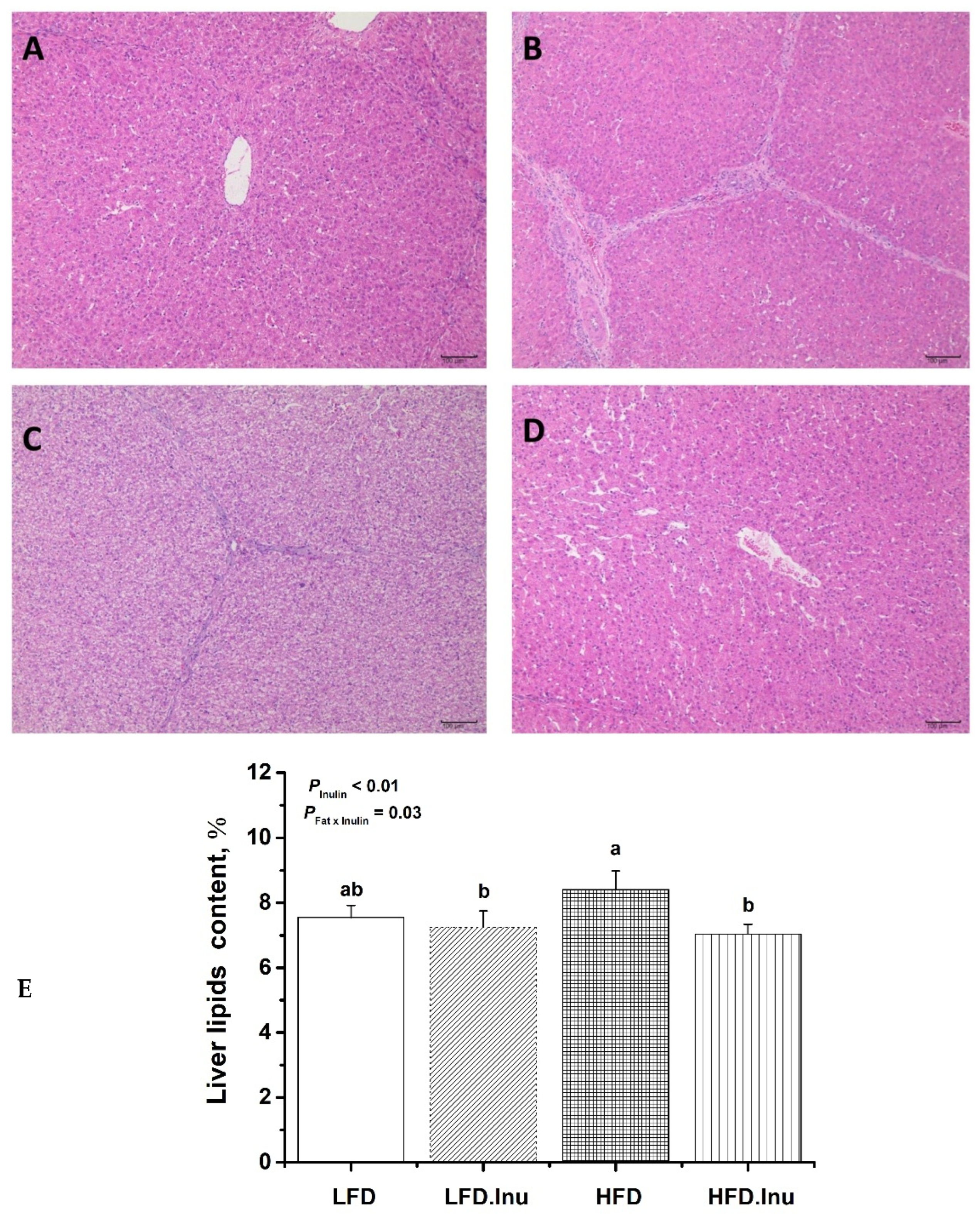
3.5. Hepatic Lipid Deposition in Pigs at Birth and Finishing Stages
3.6. Serum VFA Concentrations in Newborn Piglets
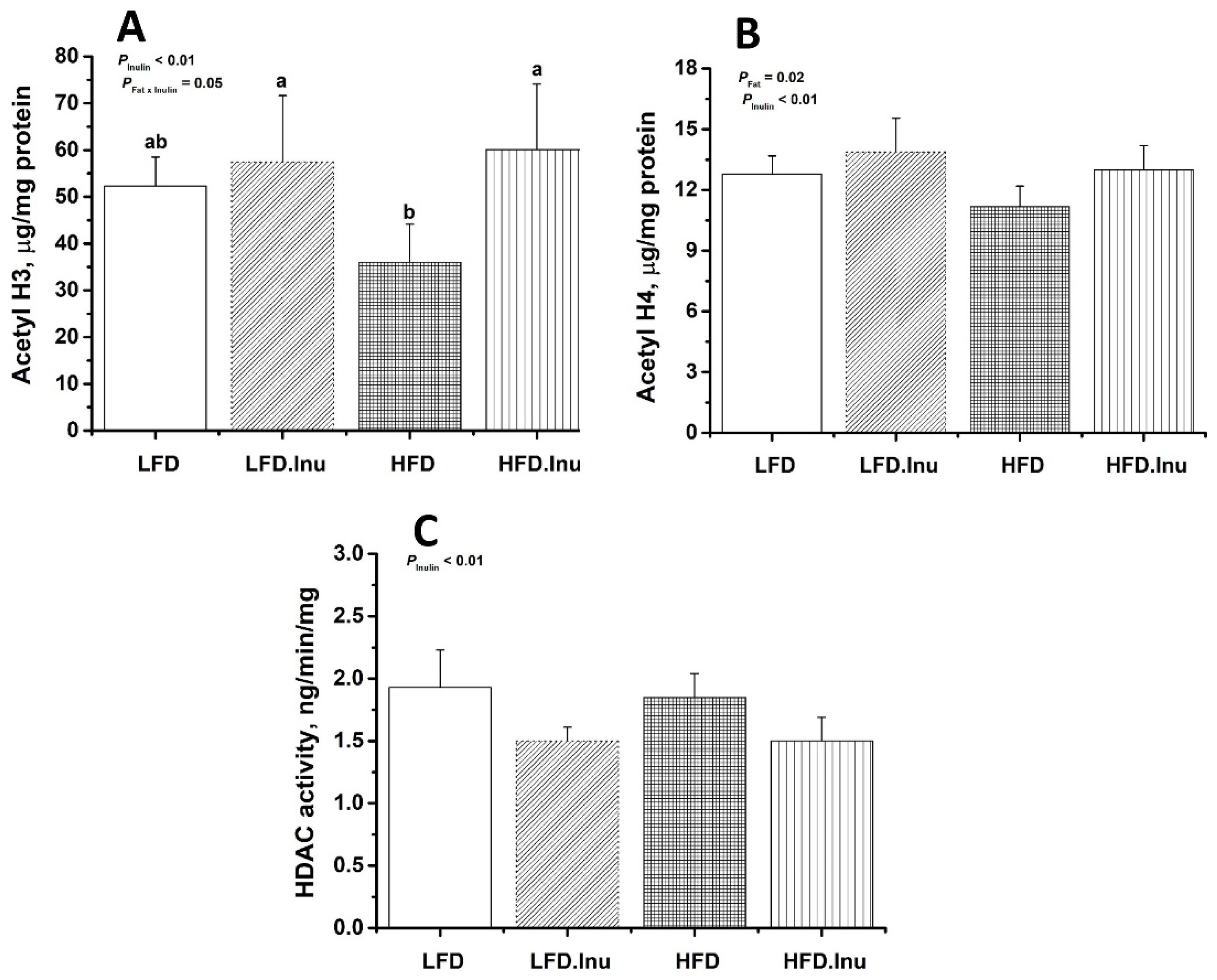
3.7. HDAC Activity and Histone Acetylation in the Liver of Newborn Piglets
3.8. Expression of Key Genes Involved in Hepatic Fatty Acid Oxidation in Pigs at Birth and Finishing Stage
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hallam, M.C.; Reimer, R.A. Impact of diet composition in adult offspring is dependent on maternal diet during pregnancy and lactation in rats. Nutrients 2016, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Bazer, F.W.; Spencer, T.E.; Wu, G.; Cudd, T.A.; Meininger, C.J. Maternal nutrition and fetal development. J. Nutr. 2004, 134, 2169–2172. [Google Scholar] [CrossRef] [PubMed]
- Mazenc, A.; Mervant, L.; Maslo, C.; Lencina, C.; Bézirard, V.; Levêque, M.; Ahn, I.; Alquier-Bacquié, V.; Naud, N.; Héliès-Toussaint, C.; et al. Maternal heme-enriched diet promotes a gut pro-oxidative status associated with microbiota alteration, gut leakiness and glucose intolerance in mice offspring. Redox Biol. 2022, 53, 102333. [Google Scholar] [CrossRef] [PubMed]
- Bianco-Miotto, T.; Craig, J.M.; Gasser, Y.P.; Van Dijk, S.J.; Ozanne, S.E. Epigenetics and DOHaD: From basics to birth and beyond. J. Dev. Orig. Health Dis. 2017, 8, 513–519. [Google Scholar] [CrossRef]
- Li, D.; Li, Y.; Yang, S.; Lu, J.; Jin, X.; Wu, M. Diet-gut microbiota-epigenetics in metabolic diseases: From mechanisms to therapeutics. Biomed. Pharmacother. 2022, 153, 113290. [Google Scholar] [CrossRef]
- Ferrari, A.; Fiorino, E.; Giudici, M.; Gilardi, F.; Galmozzi, A.; Mitro, N.; Cermenati, G.; Godio, C.; Caruso, D.; De Fabiani, E.; et al. Linking epigenetics to lipid metabolism: Focus on histone deacetylases. Mol. Membr. Biol. 2012, 29, 257–266. [Google Scholar] [CrossRef]
- Bekdash, R.A. Epigenetics, Nutrition, and the Brain: Improving Mental Health through Diet. Int. J. Mol. Sci. 2024, 25, 4036. [Google Scholar] [CrossRef]
- Maeshima, K.; Iida, S.; Shimazoe, M.A.; Tamura, S.; Ide, S. Is euchromatin really open in the cell? Trends Cell Biol. 2024, 34, 7–17. [Google Scholar] [CrossRef]
- Consalvi, S.; Saccone, V.; Mozzetta, C. Histone deacetylase inhibitors: A potential epigenetic treatment for Duchenne muscular dystrophy. Epigenomics 2014, 6, 547–560. [Google Scholar] [CrossRef]
- Swaroop, S.; Batabyal, A.; Bhattacharjee, A. HAT/HDAC: The epigenetic regulators of inflammatory gene expression. Int. J. Epigen. 2021, 1, 5. [Google Scholar] [CrossRef]
- López-Rodas, G.; Brosch, G.; Georgieva, E.I.; Sendra, R.; Franco, L.; Loidl, P. Histone deacetylase: A key enzyme for the binding of regulatory proteins to chromatin. FEBS Lett. 1993, 317, 175–180. [Google Scholar] [CrossRef]
- Li, X.; Wang, M.; Liu, S.; Chen, X.; Qiao, Y.; Yang, X.; Yao, J.; Wu, S. Paternal transgenerational nutritional epigenetic effect: A new insight into nutritional manipulation to reduce the use of antibiotics in animal feeding. Anim. Nutr. 2022, 11, 142–151. [Google Scholar] [CrossRef]
- Blagojević, J.; Stanimirović, Z.; Glavinić, U.; Vakanjac, S.; Radukić, Ž.; Mirilović, M.; Maletić, M. Impact of Supplemented Nutrition on Semen Quality, Epigenetic-Related Gene Expression, and Oxidative Status in Boars. Animals 2024, 14, 3297. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wu, J.; Zhuo, Y.; Feng, B.; Fang, Z.; Xu, S.; Li, J.; Zhao, H.; Wu, D.; Hua, L.; et al. Effects of maternal methyl donor intake during pregnancy on ileum methylation and function in an intrauterine growth restriction pig model. J. Anim. Sci. Biotechnol. 2024, 15, 19. [Google Scholar] [CrossRef]
- Saito, T.; Whatmore, P.; Taylor, J.F.; Fernandes, J.M.O.; Adam, A.; Tocher, D.R.; Espe, M.; Skjærven, K.H. Micronutrient supplementation affects transcriptional and epigenetic regulation of lipid metabolism in a dose-dependent manner. Epigenetics 2021, 16, 1217–1234. [Google Scholar] [CrossRef] [PubMed]
- Braz, C.U.; Taylor, T.; Namous, H.; Townsend, J.; Crenshaw, T.; Khatib, H. Paternal diet induces transgenerational epigenetic inheritance of DNA methylation signatures and phenotypes in sheep model. Proc. Nalt. Acad. Sci. Nexus 2022, 1, pgac040. [Google Scholar] [CrossRef] [PubMed]
- Ideraabdullah, F.Y.; Zeisel, S.H. Dietary modulation of the epigenome. Physiol. Rev. 2018, 98, 667–695. [Google Scholar] [CrossRef]
- Cai, D.; Tu, M.; Wan, D. The actions of trace element metabolism and epigenetics on animal health and disease. Front. Vet. Sci. 2022, 9, 1086322. [Google Scholar] [CrossRef]
- Rekha, K.; Venkidasamy, B.; Samynathan, R.; Nagella, P.; Rebezov, M.; Khayrullin, M.; Ponomarev, E.; Bouyahya, A.; Sarkar, T.; Shariati, M. Short-chain fatty acid: An updated review on signaling, metabolism, and therapeutic effects. Crit. Rev. Food Sci. Nutr. 2024, 64, 2461–2489. [Google Scholar] [CrossRef]
- Nshanian, M.; Gruber, J.J.; Geller, B.S.; Chleilat, F.; Lancaster, S.M.; White, S.M.; Alexandrova, L.; Camarillo, J.M.; Kelleher, N.L.; Zhao, Y.; et al. Short-chain fatty acid metabolites propionate and butyrate are unique epigenetic regulatory elements linking diet, metabolism and gene expression. Nat. Metab. 2025, 7, 196–211. [Google Scholar] [CrossRef]
- Kibbie, J.J.; Dillon, S.M.; Thompson, T.A.; Purba, C.M.; McCarter, M.D.; Wilson, C.C. Butyrate directly decreases human gut lamina propria CD4 T cell function through histone deacetylase (HDAC) inhibition and GPR43 signaling. Immunobiology 2021, 226, 152126. [Google Scholar] [CrossRef] [PubMed]
- Davie, J.R. Inhibition of histone deacetylase activity by butyrate. J. Nutr. 2003, 133, 2485S–2493S. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Gao, Z.; Zhang, J.; Ye, X.; Xu, A.; Ye, J.; Jia, W. Sodium butyrate stimulates expression of fibroblast growth factor 21 in liver by inhibition of histone deacetylase 3. Diabetes 2012, 61, 797–806. [Google Scholar] [CrossRef]
- Hong, J.; Jia, Y.; Pan, S.; Jia, F.; Li, H.; Han, Z.; Cai, D.; Zhao, R. Butyrate alleviates high fat diet-induced obesity through activation of adiponectin-mediated pathway and stimulation of mitochondrial function in the skeletal muscle of mice. Oncotarget 2016, 7, 56071. [Google Scholar] [CrossRef]
- Zong, Q.; Qu, H.; Zhao, Y.; Liu, H.; Wu, S.; Wang, S.; Bao, W.; Cai, D. Sodium butyrate alleviates deoxynivalenol-induced hepatic cholesterol metabolic dysfunction via RORγ-mediated histone acetylation modification in weaning piglets. J. Anim. Sci. Biotechnol. 2022, 13, 133. [Google Scholar] [CrossRef] [PubMed]
- Honma, K.; Oshima, K.; Takami, S.; Goda, T. Regulation of hepatic genes related to lipid metabolism and antioxidant enzymes by sodium butyrate supplementation. Metab. Open 2020, 7, 100043. [Google Scholar] [CrossRef]
- Gates, L.A.; Reis, B.S.; Lund, P.J.; Paul, M.R.; Leboeuf, M.; Djomo, A.M.; Nadeem, Z.; Lopes, M.; Vitorino, F.N.; Unlu, G.; et al. Histone butyrylation in the mouse intestine is mediated by the microbiota and associated with regulation of gene expression. Nat. Metab. 2024, 6, 697–707. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, J.; Liu, Z.; Jiang, A.; Li, S.; Wu, D.; Zhang, Y.; Zhu, X.; Zhou, E.; Wei, Z.; et al. Sodium butyrate alleviates lipopolysaccharide-induced inflammatory responses by down-regulation of NF-κB, NLRP3 signaling pathway, and activating histone acetylation in bovine macrophages. Front. Vet. Sci. 2020, 7, 579674. [Google Scholar] [CrossRef]
- Couto, M.R.; Gonçalves, P.; Magro, F.; Martel, F. Microbiota-derived butyrate regulates intestinal inflammation: Focus on inflammatory bowel disease. Pharmacol. Res. 2020, 159, 104947. [Google Scholar] [CrossRef]
- Zong, Q.; Li, K.; Qu, H.; Hu, P.; Xu, C.; Wang, H.; Wu, S.; Wang, S.; Liu, H.; Cai, D.; et al. Sodium butyrate ameliorates Deoxynivalenol-induced oxidative stress and inflammation in the porcine liver via NR4A2-mediated histone acetylation. J. Agric. Food Chem. 2023, 71, 10427–10437. [Google Scholar] [CrossRef]
- Luo, C.H.; Lai, A.; Chang, Y.J. Butyrate inhibits Staphylococcus aureus-aggravated dermal IL-33 expression and skin inflammation through histone deacetylase inhibition. Front. Immunol. 2023, 14, 1114699. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Wei, G.; Wang, Y.; Ma, N.; Chang, G.; Shen, X. Sodium butyrate promotes lipopolysaccharide-induced innate immune responses by enhancing mitogen-activated protein kinase activation and histone acetylation in bovine mammary epithelial cells. J. Dairy Sci. 2020, 103, 11636–11652. [Google Scholar] [CrossRef]
- Yang, J.; Yang, S.; Liao, Y.; Deng, Y.; Jiao, Y. Histone deacetylase inhibitor butyrate inhibits the cellular immunity and increases the serum immunity of pearl oyster Pinctada fucata martensii. Fish Shellfish Immunol. 2023, 133, 108529. [Google Scholar] [CrossRef]
- Yip, W.; Hughes, M.R.; Li, Y.; Cait, A.; Hirst, M.; Mohn, W.W.; McNagny, K.M. Butyrate shapes immune cell fate and function in allergic asthma. Front. Immunol. 2021, 12, 628453. [Google Scholar] [CrossRef] [PubMed]
- Eshleman, E.M.; Rice, T.; Potter, C.; Waddell, A.; Hashimoto-Hill, S.; Woo, V.; Sydney Field, S.; Engleman, L.; Lim, H.W.; Schumacher, M.A.; et al. Microbiota-derived butyrate restricts tuft cell differentiation via histone deacetylase 3 to modulate intestinal type 2 immunity. Immunity 2024, 57, 319–332.e6. [Google Scholar] [CrossRef]
- Lu, H.; Su, S.; Ajuwon, K.M. Butyrate supplementation to gestating sows and piglets induces muscle and adipose tissue oxidative genes and improves growth performance. J. Anim. Sci. 2012, 90, 430–432. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Fang, Z.F.; Che, L.Q.; Xu, S.Y.; Wu, D.; Wu, C.M.; Wu, X.Q. Use of sodium butyrate as an alternative to dietary fiber: Effects on the embryonic development and anti-oxidative capacity of rats. PLoS ONE 2014, 9, e97838. [Google Scholar] [CrossRef]
- Zhou, J.; Gao, S.; Chen, J.; Zhao, R.; Yang, X. Maternal sodium butyrate supplement elevates the lipolysis in adipose tissue and leads to lipid accumulation in offspring liver of weaning-age rats. Lipids Health Dis. 2016, 15, 119. [Google Scholar] [CrossRef]
- Huang, Y.; Gao, S.; Jun, G.; Zhao, R.; Yang, X. Supplementing the maternal diet of rats with butyrate enhances mitochondrial biogenesis in the skeletal muscles of weaned offspring. Br. J. Nutr. 2017, 117, 12–20. [Google Scholar] [CrossRef]
- Huang, Y.J.; Wang, P.M.; Tang, K.S.; Chen, C.J.; Huang, Y.H.; Tiao, M.M. Butyrate ameliorates maternal high-fat diet-induced fetal liver cellular apoptosis. PLoS ONE 2022, 17, e0270657. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, D.; Huang, Y.; Wu, D.; Ji, X.; Zhou, X.; Xia, D.; Yang, X. Maternal butyrate supplementation affects the lipid metabolism and fatty acid composition in the skeletal muscle of offspring piglets. Anim. Nutr. 2021, 7, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Zhang, L.; Zhang, B.; Kong, L.; Pan, X.; Goossens, T.; Song, Z. Dietary sodium butyrate improves female broiler breeder performance and offspring immune function by enhancing maternal intestinal barrier and microbiota. Poult. Sci. 2023, 102, 102658. [Google Scholar] [CrossRef]
- Wu, K.L.H.; Liu, W.C.; Wu, C.W.; Fu, M.H.; Huang, H.M.; Tain, Y.L.; Liang, C.K.; Hung, C.Y.; Chen, I.C.; Hung, P.L.; et al. Butyrate reduction and HDAC4 increase underlie maternal high fructose-induced metabolic dysfunction in hippocampal astrocytes in female rats. J. Nutr. Biochem. 2024, 126, 109571. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhong, X.; He, Y.; Shi, Y. Butyrate, but not propionate, reverses maternal diet-induced neurocognitive deficits in offspring. Pharmacol. Res. 2020, 160, 105082. [Google Scholar] [CrossRef]
- Zhou, P.; Yan, H.; Zhang, Y.; Qi, R.; Zhang, H.; Liu, J. Growth performance, bile acid profile, fecal microbiome and serum metabolomics of growing-finishing pigs fed diets with bile acids supplementation. J. Anim. Sci. 2023, 101, skad393. [Google Scholar] [CrossRef]
- Zhou, P.; Wang, S.; Li, S.; Zhao, Y.; Deng, K.; Chao, D.; Jin, C.; Zhuo, Y.; Che, L.; Li, J.; et al. Effects of prebiotic inulin addition to low-or high-fat diet on maternal metabolic status and neonatal traits of offspring in a pregnant sow model. J. Funct. Foods 2018, 48, 125–133. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; Horwitz, W., Latimer, G.W., Jr., Eds.; AOAC: Gaithersburg, MD, USA, 2007. [Google Scholar]
- Honikel, K.O. Reference methods for the assessment of physical characteristics of meat. Meat Sci. 1998, 49, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Luo, J.; Yu, B.; Zheng, P.; Huang, Z.; Mao, X.; He, J.; Yu, J.; Chen, J.; Chen, D. Dietary resveratrol supplementation improves meat quality of finishing pigs through changing muscle fiber characteristics and antioxidative status. Meat Sci. 2015, 102, 15–21. [Google Scholar] [CrossRef]
- Wang, Y.S.; Zhou, P.; Liu, H.; Li, S.; Zhao, Y.; Deng, K.; Cao, D.D.; Che, L.Q.; Fang, Z.F.; Xu, S.Y.; et al. Effects of inulin supplementation in low-or high-fat diets on reproductive performance of sows and antioxidant defence capacity in sows and offspring. Reprod. Domest. Anim. 2016, 51, 492–500. [Google Scholar] [CrossRef]
- Nguyen, P.; Leray, V.; Diez, M.; Serisier, S.; Bloc’h, J.L.; Siliart, B.; Dumon, H. Liver lipid metabolism. J. Anim. Physiol. Anim. Nutr. 2008, 92, 272–283. [Google Scholar] [CrossRef]
- Thompson, M.D. Developmental programming of NAFLD by parental obesity. Hepatol. Commun. 2020, 4, 1392–1403. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Chen, C.; Kerr, B.J.; Weber, T.E.; Johnston, L.J.; Shurson, G.C. Influence of thermally oxidized vegetable oils and animal fats on growth performance, liver gene expression, and liver and serum cholesterol and triglycerides in young pigs. J. Anim. Sci. 2014, 92, 2960–2970. [Google Scholar] [CrossRef] [PubMed]
- Adeyanju, O.A.; Badejogbin, O.C.; Areola, D.E.; Olaniyi, K.S.; Dibia, C.; Soetan, O.A.; Oniyide, A.A.; Michael, O.S.; Olatunji, L.A.; Soladoye, A.O. Sodium butyrate arrests pancreato-hepatic synchronous uric acid and lipid dysmetabolism in high fat diet fed Wistar rats. Biomed. Pharmacother. 2021, 133, 110994. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, C.; Jiang, Q.; Yin, Y. Butyrate in energy metabolism: There is still more to learn. Trends Endocrinol Metab. 2021, 32, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Che, L.; Liu, L.; Xu, M.; Fan, Z.; Niu, L.; Chen, Y.; Chang, X.; Zhou, P.; Li, M.; Deng, H.; et al. Valine metabolite, 3-hydroxyisobutyrate, promotes lipid metabolism and cell proliferation in porcine mammary gland epithelial cells. Front. Nutr. 2025, 11, 1524738. [Google Scholar] [CrossRef]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef]
- Villena, J.A. New insights into PGC-1 coactivators: Redefining their role in the regulation of mitochondrial function and beyond. FEBS J. 2015, 282, 647–672. [Google Scholar] [CrossRef]
- Amiri, P.; Hosseini, S.A.; Saghafi-Asl, M.; Roshanravan, N.; Tootoonchian, M. Expression of PGC-1α, PPAR-α and UCP1 genes, metabolic and anthropometric factors in response to sodium butyrate supplementation in patients with obesity: A triple-blind, randomized placebo-controlled clinical trial. Eur. J. Clin. Nutr. 2024, 79, 249–257. [Google Scholar] [CrossRef]
- Mattace Raso, G.; Simeoli, R.; Russo, R.; Iacono, A.; Santoro, A.; Paciello, O.; Ferrante, C.F.; Canani, R.B.; Calignano, A.; Meli, R. Effects of sodium butyrate and its synthetic amide derivative on liver inflammation and glucose tolerance in an animal model of steatosis induced by high fat diet. PLoS ONE 2013, 8, e68626. [Google Scholar] [CrossRef]
- Ye, Q.; Zeng, X.; Wang, S.; Zeng, X.; Yang, G.; Ye, C.; Cai, S.; Chen, M.; Li, S.; Qiao, S. Butyrate drives the acetylation of histone H3K9 to activate steroidogenesis through PPARγ and PGC1α pathways in ovarian granulosa cells. FASEB J. 2021, 35, e21316. [Google Scholar] [CrossRef]
- Zang, Y.; Wang, T.; Xie, W.; Wang-Fischer, Y.L.; Getty, L.; Han, J.; Corkey, B.E.; Guo, W. Regulation of acetyl CoA carboxylase and carnitine palmitoyl transferase-1 in rat adipocytes. Obes. Res. 2005, 13, 1530–1539. [Google Scholar] [CrossRef] [PubMed]
- Berni Canani, R.; Di Costanzo, M.; Leone, L. The epigenetic effects of butyrate: Potential therapeutic implications for clinical practice. Clin. Epigenetics 2012, 4, 4. [Google Scholar] [CrossRef] [PubMed]
- Skinner, M.K.; Manikkam, M.; Guerrero-Bosagna, C. Epigenetic transgenerational actions of environmental factors in disease etiology. Trends Endocrinol. Metab. 2010, 21, 214–222. [Google Scholar] [CrossRef]
- Korolenko, A.; Skinner, M.K. Generational stability of epigenetic transgenerational inheritance facilitates adaptation and evolution. Epigenetics 2024, 19, 2380929. [Google Scholar] [CrossRef] [PubMed]


| Maternal Treatment | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| LFD | LFD.Inu | HFD | HFD.Inu | Fat | Inulin | Fat × Inulin | ||
| Piglet BW, kg | ||||||||
| At birth | 1.25 | 1.24 | 1.32 | 1.27 | 0.01 | 0.06 | 0.22 | 0.46 |
| At weaning | 7.56 | 7.33 | 7.93 | 7.28 | 0.08 | 0.36 | 0.02 | 0.22 |
| Organ index of newborn piglets, % | ||||||||
| Heart | 0.72 | 0.68 | 0.63 | 0.70 | 0.02 | 0.41 | 0.76 | 0.27 |
| Liver | 2.67 | 2.84 | 2.59 | 2.73 | 0.06 | 0.46 | 0.23 | 0.92 |
| Spleen | 0.08 | 0.09 | 0.10 | 0.08 | 0.003 | 0.31 | 0.41 | 0.22 |
| Lung | 1.31 | 1.43 | 1.43 | 1.34 | 0.04 | 0.90 | 0.87 | 0.23 |
| Kidney | 0.63 | 0.65 | 0.66 | 0.61 | 0.02 | 0.86 | 0.71 | 0.43 |
| Pancreas | 0.08 | 0.09 | 0.08 | 0.07 | 0.002 | 0.38 | 0.56 | 0.06 |
| Organ index of weaning piglets, % | ||||||||
| Heart | 0.51 | 0.49 | 0.51 | 0.48 | 0.01 | 0.86 | 0.26 | 0.72 |
| Liver | 2.21 | 2.66 | 2.42 | 2.58 | 0.06 | 0.62 | 0.03 | 0.28 |
| Spleen | 0.24 | 0.28 | 0.19 | 0.33 | 0.02 | 0.99 | 0.07 | 0.31 |
| Lung | 1.12 | 1.36 | 1.23 | 1.23 | 0.06 | 0.98 | 0.32 | 0.32 |
| Kidney | 0.48 | 0.51 | 0.47 | 0.53 | 0.02 | 0.79 | 0.15 | 0.65 |
| Pancreas | 0.13 | 0.11 | 0.10 | 0.12 | 0.006 | 0.46 | 0.85 | 0.25 |
| Abdominal fat | 0.30 | 0.31 | 0.37 | 0.27 | 0.01 | 0.54 | 0.09 | 0.05 |
| Maternal Treatment | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| LFD | LFD.Inu | HFD | HFD.Inu | Fat | Inulin | Fat × Inulin | ||
| Average pig weight, kg | ||||||||
| Day 28 | 7.64 | 7.31 | 8.12 | 7.29 | 0.11 | 0.31 | 0.02 | 0.27 |
| Day 70 | 19.55 | 18.91 | 20.18 | 16.89 | 0.35 | 0.33 | 0.01 | 0.07 |
| Day 110 | 47.37 | 47.68 | 46.19 | 41.95 | 0.67 | 0.02 | 0.16 | 0.11 |
| Day 150 | 78.88 | 74.93 | 75.88 | 74.06 | 1.09 | 0.39 | 0.20 | 0.63 |
| Day 180 | 99.76 | 96.76 | 98.24 | 95.91 | 0.93 | 0.53 | 0.17 | 0.86 |
| Average pig weight gain, kg | 92.12 | 89.45 | 90.12 | 88.62 | 0.89 | 0.44 | 0.26 | 0.75 |
| ADFI, g/d | ||||||||
| Day 28 to 70 | 492 | 456 | 480 | 408 | 9 | 0.12 | 0.01 | 0.34 |
| Day 71 to 110 | 1430 | 1184 | 1306 | 1164 | 32 | 0.26 | <0.01 | 0.41 |
| Day 111 to 150 | 2152 | 1952 | 1970 | 1966 | 43 | 0.35 | 0.26 | 0.28 |
| Day 151 to 180 | 2380 | 2470 | 2354 | 2358 | 85 | 0.69 | 0.79 | 0.80 |
| Day 28 to 180 | 1550 | 1440 | 1458 | 1402 | 28 | 0.26 | 0.16 | 0.63 |
| Maternal Treatment | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| LFD | LFD.Inu | HFD | HFD.Inu | Fat | Inulin | Fat × Inulin | ||
| BW, kg | 99.44 | 98.60 | 100.68 | 97.56 | 0.50 | 0.92 | 0.07 | 0.27 |
| Organ index, % | ||||||||
| Heart | 0.32 | 0.34 | 0.29 | 0.32 | 0.006 | 0.12 | 0.12 | 0.70 |
| Liver | 1.42 | 1.58 | 1.49 | 1.53 | 0.02 | 0.79 | 0.01 | 0.10 |
| Spleen | 0.13 | 0.16 | 0.15 | 0.14 | 0.004 | 0.90 | 0.18 | 0.08 |
| Lung | 0.67 b | 1.07 a | 1.04 ab | 0.74 ab | 0.05 | 0.80 | 0.61 | <0.01 |
| Kidney | 0.31 | 0.33 | 0.30 | 0.30 | 0.005 | 0.04 | 0.35 | 0.45 |
| Pancreas | 0.13 | 0.12 | 0.11 | 0.12 | 0.004 | 0.47 | 1.00 | 0.47 |
| Abdominal fat | 0.86 | 0.76 | 0.86 | 0.77 | 0.04 | 0.99 | 0.27 | 0.92 |
| Dressing Percentage, % | 71.24 | 71.70 | 72.87 | 72.43 | 0.39 | 0.15 | 0.99 | 0.57 |
| Average backfat depth, cm | 1.80 | 1.49 | 1.99 | 1.87 | 0.03 | <0.01 | <0.01 | 0.14 |
| LMA 1, cm | 63.29 | 65.73 | 61.76 | 64.07 | 1.07 | 0.31 | 0.14 | 0.97 |
| Maternal Treatment | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| LFD | LFD.Inu | HFD | HFD.Inu | Fat | Inulin | Fat × Inulin | ||
| pH45min | 6.47 | 6.66 | 6.53 | 6.77 | 0.05 | 0.47 | 0.06 | 0.80 |
| pH24h | 5.39 | 5.59 | 5.22 | 5.34 | 0.05 | 0.07 | 0.13 | 0.70 |
| pHdif | 1.09 | 1.07 | 1.31 | 1.43 | 0.07 | 0.04 | 0.70 | 0.60 |
| Meat color24h | ||||||||
| L* | 50.76 | 49.38 | 52.41 | 49.12 | 0.47 | 0.46 | 0.02 | 0.32 |
| a* | 6.14 | 6.39 | 6.19 | 6.37 | 0.10 | 0.94 | 0.29 | 0.87 |
| b* | 6.55 | 6.20 | 6.49 | 6.34 | 0.19 | 0.93 | 0.52 | 0.81 |
| Cooking loss, % | 31.86 | 29.87 | 32.47 | 29.94 | 0.91 | 0.85 | 0.23 | 0.89 |
| Drip loss, % | 2.74 | 2.53 | 2.53 | 2.46 | 0.06 | 0.27 | 0.26 | 0.58 |
| Shear force, N | 4.75 | 4.83 | 4.76 | 4.78 | 0.04 | 0.82 | 0.49 | 0.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, P.; Wu, Y.; Shen, J.; Duan, T.; Che, L.; Zhang, Y.; Zhao, Y.; Yan, H. Gestational Inulin Supplementation in Low-/High-Fat Sow Diets: Effects on Growth Performance, Lipid Metabolism, and Meat Quality of Offspring Pigs. Foods 2025, 14, 1314. https://doi.org/10.3390/foods14081314
Zhou P, Wu Y, Shen J, Duan T, Che L, Zhang Y, Zhao Y, Yan H. Gestational Inulin Supplementation in Low-/High-Fat Sow Diets: Effects on Growth Performance, Lipid Metabolism, and Meat Quality of Offspring Pigs. Foods. 2025; 14(8):1314. https://doi.org/10.3390/foods14081314
Chicago/Turabian StyleZhou, Pan, Yachao Wu, Jianbo Shen, Tao Duan, Long Che, Yong Zhang, Yang Zhao, and Honglin Yan. 2025. "Gestational Inulin Supplementation in Low-/High-Fat Sow Diets: Effects on Growth Performance, Lipid Metabolism, and Meat Quality of Offspring Pigs" Foods 14, no. 8: 1314. https://doi.org/10.3390/foods14081314
APA StyleZhou, P., Wu, Y., Shen, J., Duan, T., Che, L., Zhang, Y., Zhao, Y., & Yan, H. (2025). Gestational Inulin Supplementation in Low-/High-Fat Sow Diets: Effects on Growth Performance, Lipid Metabolism, and Meat Quality of Offspring Pigs. Foods, 14(8), 1314. https://doi.org/10.3390/foods14081314





