Development and Application of a Rapid Detection Technique for 2-Amino-1-methyl-6-phenyl-imidazo [4,5-b] Pyridine in Meat Products Based on Hydrogel-Molecular-Imprinting Electrochemical Sensing Technology
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Equipment
2.2. Method
2.2.1. Preparation of Molecularly Imprinted Hydrogels
2.2.2. Preparation of the Molecularly Imprinted-Hydrogel Electrochemical Sensor
2.2.3. Electrochemical Testing Conditions
2.2.4. Sample Processing
3. Results and Discussion
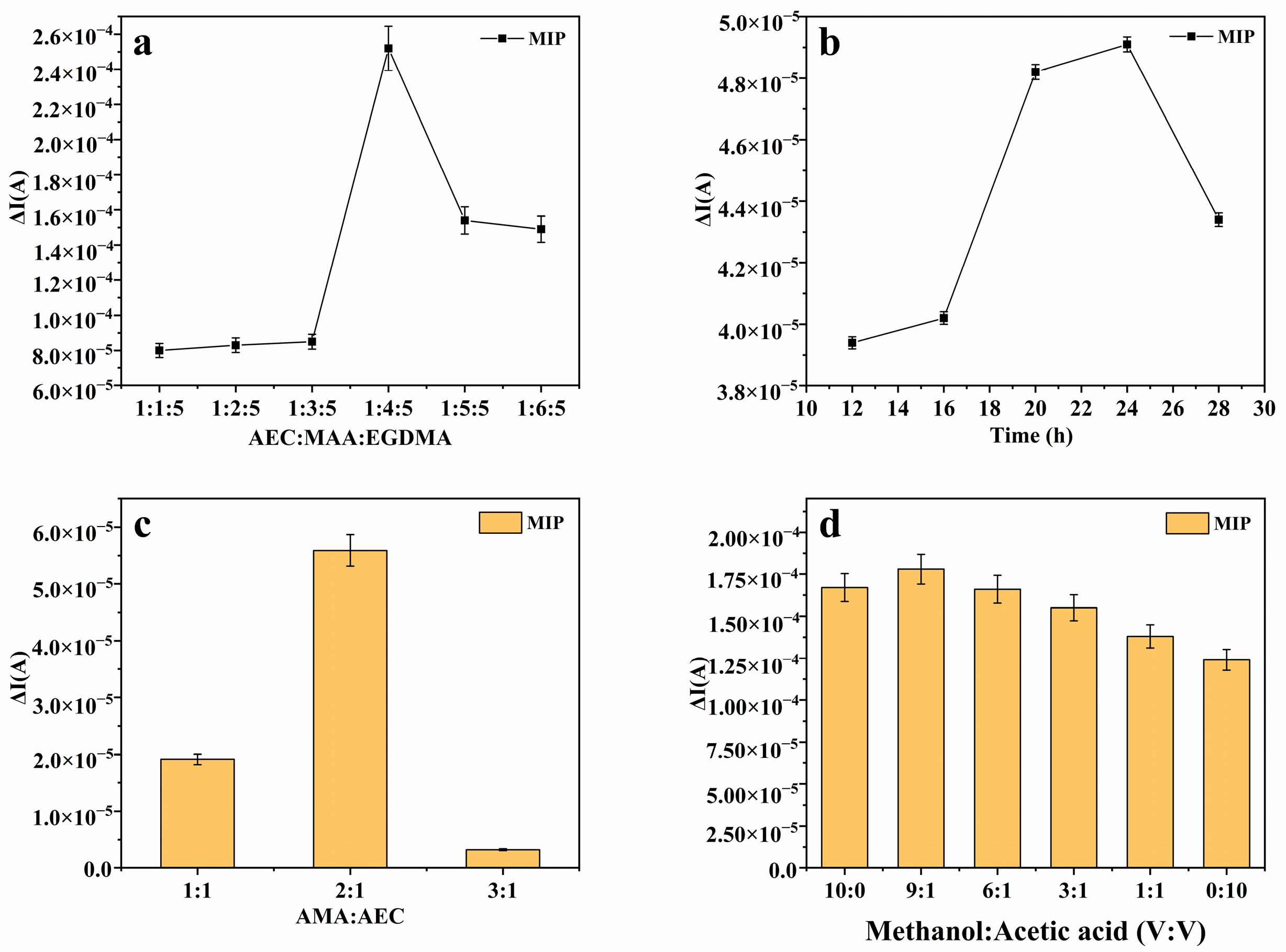
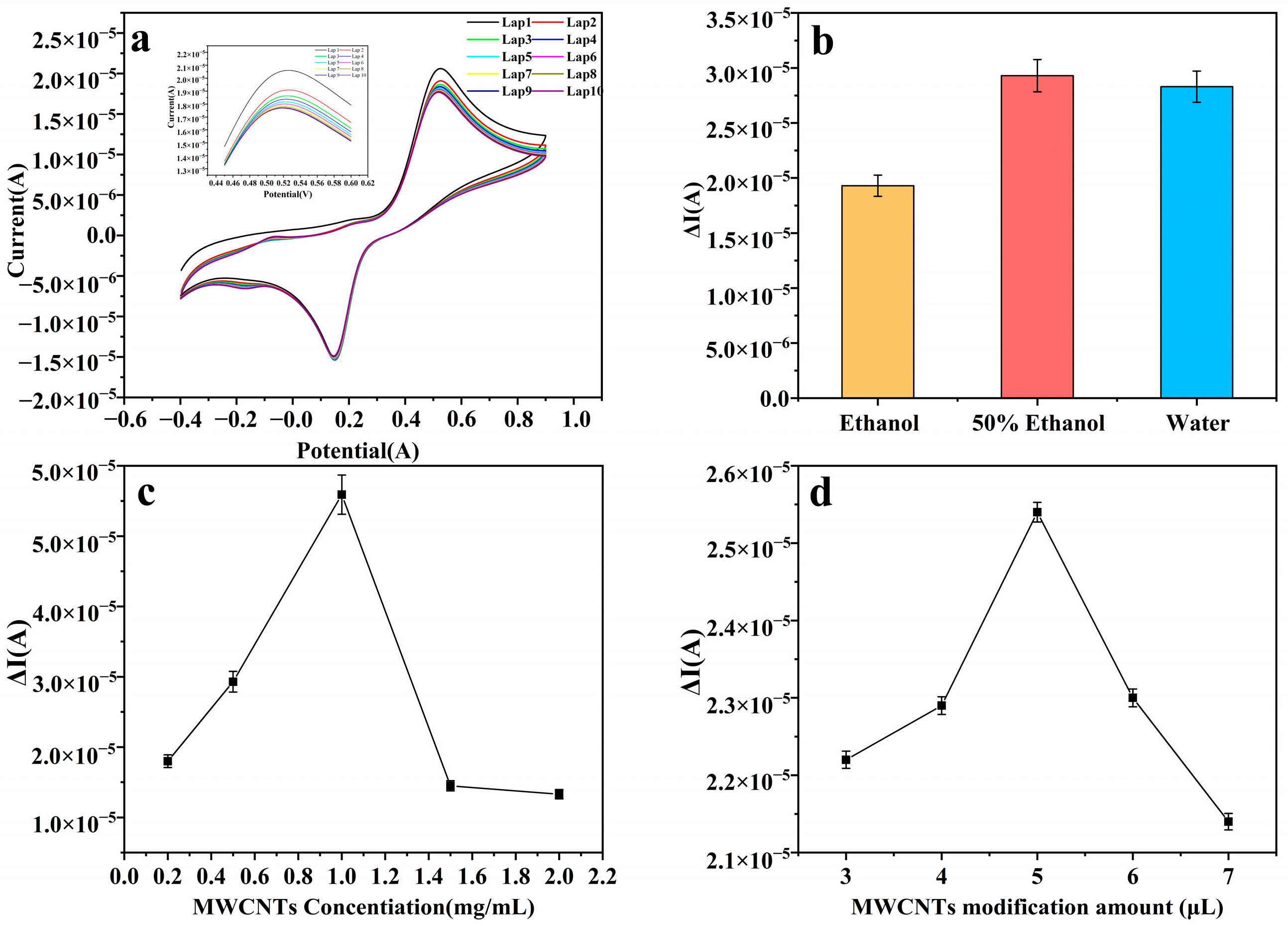
3.1. Optimization of Experimental Conditions
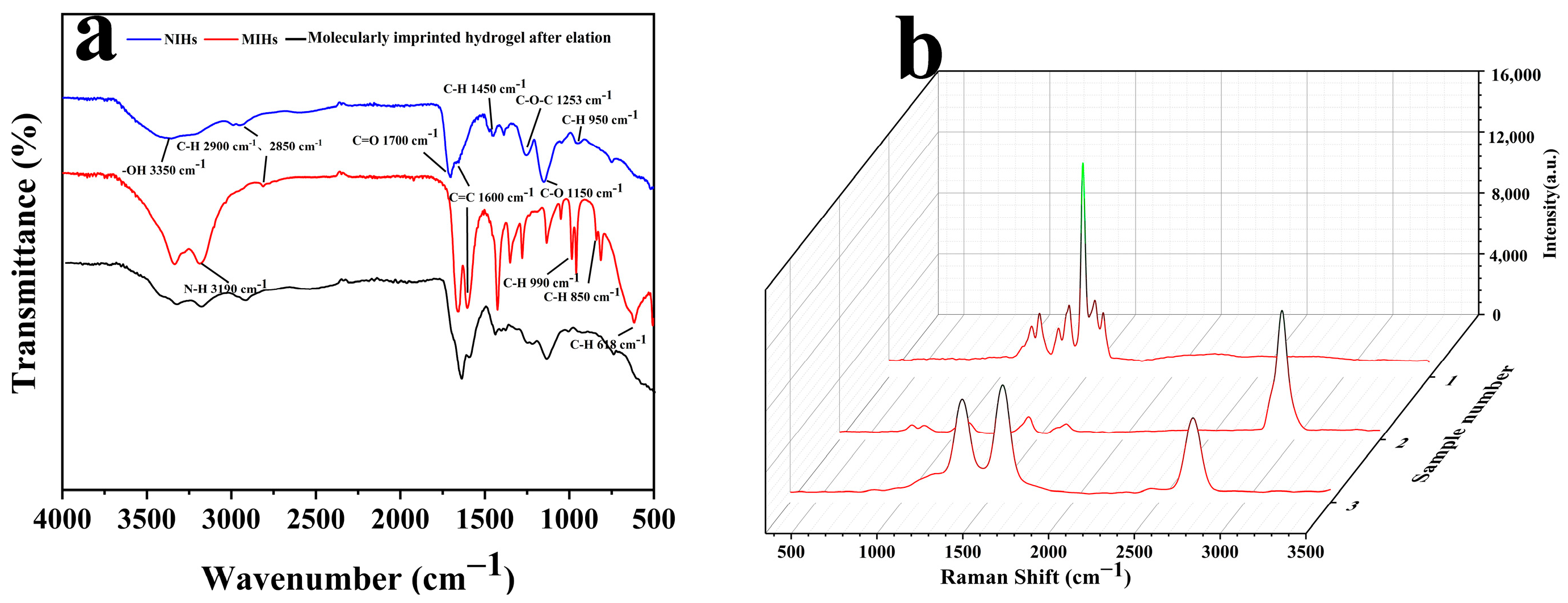
3.2. Characterization
3.3. Characterization of Adsorption Performance
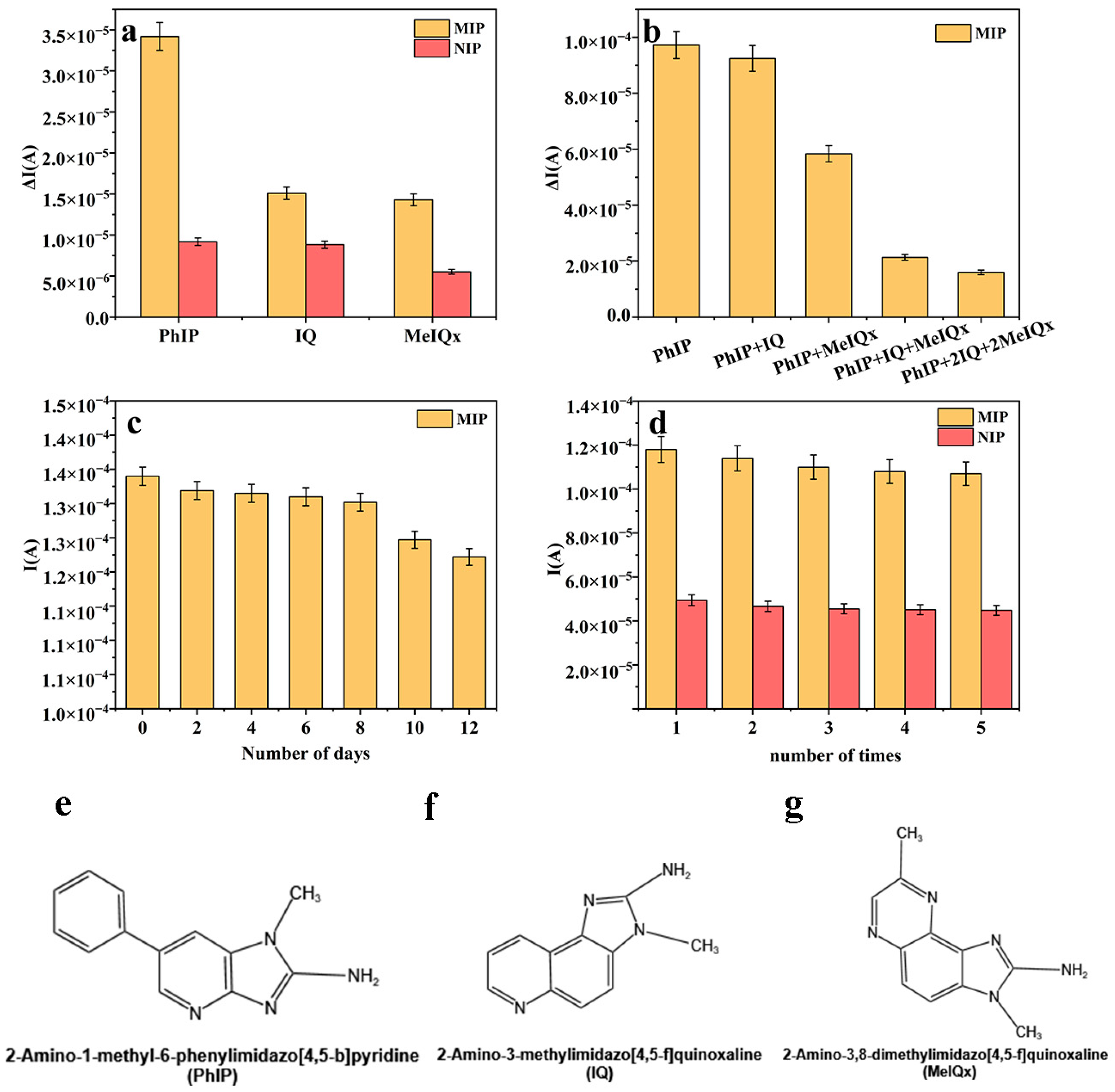
3.4. Selectivity, Stability, and Reproducibility
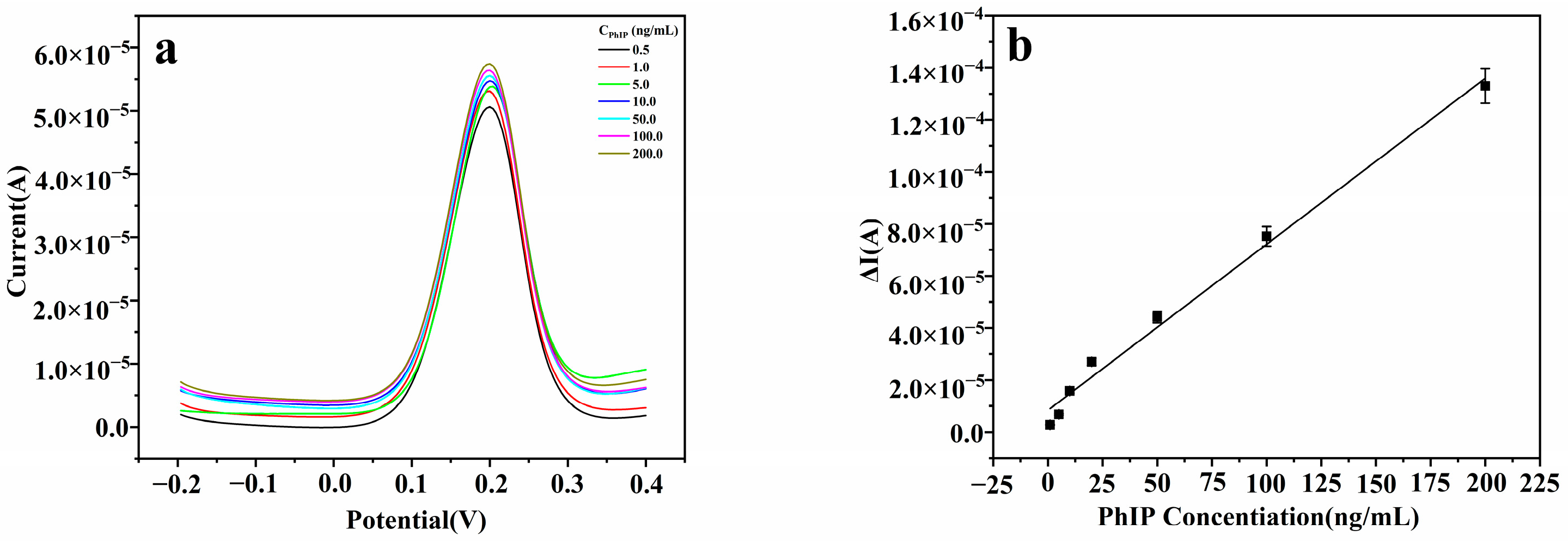
3.5. Applicability of the Method
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Neves, T.D.; da Cunha, D.T.; de Rosso, V.V.; Domene, S.M.Á. Effects of seasoning on the formation of heterocyclic amines and polycyclic aromatic hydrocarbons in meats: A meta-analysis. Compr. Rev. Food Sci. Food Saf. 2021, 20, 526–541. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, V.; Amal, T.C.; Vasanth, K. Food contaminants: Impact of food processing, challenges and mitigation strategies for food security. Food Res. Int. 2024, 191, 114739. [Google Scholar] [CrossRef]
- Oz, E.; Aoudeh, E.; Murkovic, M.; Toldra, F.; Gomez-Zavaglia, A.; Brennan, C.; Proestos, C.; Zeng, M.; Oz, F. Heterocyclic aromatic amines in meat: Formation mechanisms, toxicological implications, occurrence, risk evaluation, and analytical methods. Meat Sci. 2023, 205, 109312. [Google Scholar] [CrossRef] [PubMed]
- Bellamri, M.; Xiao, S.; Murugan, P.; Weight, C.J.; Turesky, R.J. Metabolic Activation of the Cooked Meat Carcinogen 2-Amino-1-Methyl-6-Phenylimidazo[4,5-b]Pyridine in Human Prostate. Toxicol. Sci. 2018, 163, 543–556. [Google Scholar] [CrossRef]
- Gao, H.H.; Gao, X.; Kong, W.Q.; Yuan, J.Y.; Zhang, Y.W.; Wang, X.D.; Liu, H.M.; Qin, Z. Effect of Chinese quince proanthocyanidins on the inhibition of heterocyclic amines and quality of fried chicken meatballs and tofu. J. Food Sci. 2024, 89, 3759–3775. [Google Scholar] [CrossRef]
- Dong, H.; Chen, Q.; Xu, Y.; Li, C.; Bai, W.; Zeng, X.; Wu, Q.; Xu, H.; Deng, J. Effect and mechanism of polyphenols containing m-dihydroxyl structure on 2-amino-1-methyl-6-phenylimidazole [4, 5-b] pyridine (PhIP) formation in chemical models and roast pork patties. Food Chem. X 2024, 23, 101672. [Google Scholar] [CrossRef]
- Wang, H.J.; Chu, X.; Du, P.; He, H.; He, F.; Liu, Y.; Wang, W.; Ma, Y.; Wen, L.; Wang, Y.; et al. Unveiling heterocyclic aromatic amines (HAAs) in thermally processed meat products: Formation, toxicity, and strategies for reduction—A comprehensive review. Food Chem. X 2023, 19, 100833. [Google Scholar] [CrossRef]
- Li, L.; Li, P.; Tang, S.; Xiao, C.; Huang, Y.; Wang, L.; Yang, X.; Chen, X.; Shao, B. Detection of chemical contaminants in heat processed meat products based on UPLC-MS/MS. J. Food Compos. Anal. 2023, 124, 105711. [Google Scholar] [CrossRef]
- Han, T.L.; Wang, T.; Hou, H.; Wang, Z.; Xiao, T.; Gai, S.; Wang, M.; Liu, D. Raw to charred: Changes of precursors and intermediates and their correlation with heterocyclic amines formation in grilled lamb. Meat Sci. 2023, 195, 108999. [Google Scholar] [CrossRef]
- Sheng, W.; Zhang, B.; Zhao, Q.; Wang, S.; Zhang, Y. Preparation of a Broad-Spectrum Heterocyclic Aromatic Amines (HAAs) Antibody and Its Application in Detection of Eight HAAs in Heat Processed Meat. J. Agric. Food Chem. 2020, 68, 15501–15508. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Zhang, Y.X.; Dong, X.W. Determination of heterocyclic amines in braised sauce beef and the effects of different cooking conditions on the formation of heterocyclic amines. J. Sci. Food Agric. 2022, 102, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.L.; Wang, Z.; Hou, J.; Fang, G.; Liu, J.; Wang, S. Detection of heterocyclic amine (PhIP) by fluorescently labelled cucurbit[7]uril. Analyst 2022, 147, 2477–2483. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.J.; Wang, Y.; Yang, S.; Sun, X.; Peng, B.; Pan, L.; Jia, Y.; Zhang, X.; Nie, C. Molecularly Imprinted Polymer Nanospheres with Hydrophilic Shells for Efficient Molecular Recognition of Heterocyclic Aromatic Amines in Aqueous Solution. Molecules 2023, 28, 2052. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.L.; Li, H.; Xue, X.; Tan, F.; Ye, L. Signal amplification in molecular sensing by imprinted polymers. Microchim. Acta 2024, 191, 574. [Google Scholar] [CrossRef]
- Yao, Y.; Xie, W.; Shen, Y.; Ning, K.; Li, H.; Hu, X.Y.; Xu, Q. Carbon felt tailored with artificial recognition elements: An effective membrane for bisphenol A adsorption and removal. J. Mater. Sci. 2023, 58, 18046–18059. [Google Scholar] [CrossRef]
- Azizi, A.; Bottaro, C.S. A critical review of molecularly imprinted polymers for the analysis of organic pollutants in environmental water samples. J. Chromatogr. A 2020, 1614, 460603. [Google Scholar] [CrossRef]
- Yang, S.C.; Liu, Z.; Pan, Y.; Guan, J.; Yang, P.; Asel, M. A Review of Research Progress on the Performance of Intelligent Polymer Gel. Molecules 2023, 28, 4246. [Google Scholar] [CrossRef]
- Sun, Y.C.; Bai, L.; Wang, T.; Cao, S.; Han, C.; Sun, X. Effective scavenging and selective adsorption of salicylic acid from wastewater using a novel deep eutectic solvents-based chitosan-acrylamide surface molecularly imprinted hydrogel. Appl. Surf. Sci. 2023, 608, 155102. [Google Scholar] [CrossRef]
- Sun, B.; Sun, W.; Wang, Z.; Zhao, B.; Yang, S. Highly sensitive electrochemical detection of melatonin based on graphene-assisted molecular imprinting technology. Carbon Lett. 2024, 34, 437–444. [Google Scholar] [CrossRef]
- Garg, M.; Chatterjee, M.; Sharma, A.L.; Singh, S. Label-free approach for electrochemical ferritin sensing using biosurfactant stabilized tungsten disulfide quantum dots. Biosens. Bioelectron. 2020, 151, 111979. [Google Scholar] [CrossRef]
- Bhuvaneswari, K.; Radha, S.; Sreeja, B.S.; Kumar, P.S. Development of in-situ electrochemical heavy metal ion sensor using integrated 1D/0D/1D hybrid by MWCNT and CQDs supported MnO2 nanomaterial. Environ. Res. 2023, 225, 115570. [Google Scholar] [CrossRef] [PubMed]
- Alam, Y.N.; Kumar, L.; Sharma, N. Development of Cu-Exfoliated Graphite Nanoplatelets (xGnP) Metal Matrix Composite by Powder Metallurgy Route. Graphene 2015, 4, 91–111. [Google Scholar] [CrossRef][Green Version]
- Luo, Q.Y.; He, S.; Huang, Y.; Lei, Z.; Qiao, J.; Li, Q.; Xu, D.; Guo, X.; Wu, Y. Non-toxic fluorescent molecularly imprinted hydrogel based on wood-derived cellulose nanocrystals and carbon dots for efficient sorption and sensitive detection of tetracycline. Ind. Crops Prod. 2022, 177, 114528. [Google Scholar] [CrossRef]
- Li, D.; Li, Z.; Dong, L.; Zhang, Y.; Lu, Y.; Wang, J.; Sun, H.; Wang, S. Coffee prevents IQ-induced liver damage by regulating oxidative stress, inflammation, endoplasmic reticulum stress, autophagy, apoptosis, and the MAPK/NF-κB signaling pathway in zebrafish. Food Res. Int. 2023, 169, 112946. [Google Scholar] [CrossRef]
- Song, D.; Guo, R.; Huang, H.; Zheng, P.; Huang, H.; Oyang, Q.; Xiao, X.; Wang, B.; Rong, J.; Rong, L. 2-Amino-3,8-dimethylimidazo[4,5-f]quinoxaline Alters Autophagosome Maturation, Cellular Lipidomic Profiles, and Expression of Core Pluripotent Factors. J. Agric. Food Chem. 2019, 67, 7977–7985. [Google Scholar] [CrossRef]
- Xu, Y.; Li, H.; Liang, J.; Ma, J.; Yang, J.; Zhao, X.; Zhao, W.; Bai, W.; Zeng, X.; Dong, H. High-throughput quantification of eighteen heterocyclic aromatic amines in roasted and pan-fried meat on the basis of high performance liquid chromatography-quadrupole-orbitrap high resolution mass spectrometry. Food Chem. 2021, 361, 130147. [Google Scholar] [CrossRef]
- Reartes, G.A.; Naranjo, R.D.D.P.; Eynard, A.R.; Muñoz, S.E. Cooking methods and the formation of PhIP (2-Amino, 1-methyl, 6-phenylimidazo[4,5-b] pyridine) in the crust of the habitually consumed meat in Argentina. Food Chem. Toxicol. 2016, 92, 88–93. [Google Scholar] [CrossRef]
- Feng, Y.; Shi, Y.; Huang, R.; Wang, P.; Li, G. Simultaneous detection of heterocyclic aromatic amines and acrylamide in thermally processed foods by magnetic solid-phase extraction combined with HPLC-MS/MS based on cysteine-functionalized covalent organic frameworks. Food Chem. 2023, 424, 136349. [Google Scholar] [CrossRef]



| Detection Method | Electrochemical Workstation | High-Performance Liquid Chromatography | ||||
|---|---|---|---|---|---|---|
| Scalar Addition (ng/mL) | Recovery Rate (%) | RSD(%) | Scalar Addition (ng/mL) | Recovery Rate (%) | RSD(%) | |
| Grilled chicken | 1 | 95.00 | 9.68 | 1 | 94.60 | 8.25 |
| 10 | 103.70 | 5.77 | 10 | 110.20 | 8.38 | |
| 20 | 108.80 | 1.95 | 20 | 111.80 | 7.34 | |
| Roast duck meat | 1 | 85.70 | 7.54 | 1 | 87.59 | 4.90 |
| 10 | 75.90 | 9.90 | 10 | 77.00 | 9.49 | |
| 20 | 94.40 | 2.33 | 20 | 96.17 | 6.29 | |
| Analytical Methods | Test Sample | Linear Range (ng/mL) | Recovery Rate (%) | Reference |
|---|---|---|---|---|
| HPLC–Q-Orbitrap-HRMS | Pork, Beef | 0.1–100.0 | 71.3–114.8% | [26] |
| HPLC–DAD–ESI-MS/MS | Pork, Beef, Chicken, Fish | 20–6250 | / | [27] |
| MSPE–HPLC–MS/MS | Bread, cake, French fries | 0.5–100.0 | 98.2–108.2% | [28] |
| Molecularly Imprinted-Hydrogel Electrochemical Sensor | Grilled chicken, Roast duck meat | 1.0–200.0 | 75.9–108.8% | This experiment |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Zhao, X.; Yin, X.; Zhang, S.; He, J. Development and Application of a Rapid Detection Technique for 2-Amino-1-methyl-6-phenyl-imidazo [4,5-b] Pyridine in Meat Products Based on Hydrogel-Molecular-Imprinting Electrochemical Sensing Technology. Foods 2025, 14, 1292. https://doi.org/10.3390/foods14081292
Li C, Zhao X, Yin X, Zhang S, He J. Development and Application of a Rapid Detection Technique for 2-Amino-1-methyl-6-phenyl-imidazo [4,5-b] Pyridine in Meat Products Based on Hydrogel-Molecular-Imprinting Electrochemical Sensing Technology. Foods. 2025; 14(8):1292. https://doi.org/10.3390/foods14081292
Chicago/Turabian StyleLi, Chunxiao, Xiaolei Zhao, Xiaofei Yin, Shuting Zhang, and Jinxing He. 2025. "Development and Application of a Rapid Detection Technique for 2-Amino-1-methyl-6-phenyl-imidazo [4,5-b] Pyridine in Meat Products Based on Hydrogel-Molecular-Imprinting Electrochemical Sensing Technology" Foods 14, no. 8: 1292. https://doi.org/10.3390/foods14081292
APA StyleLi, C., Zhao, X., Yin, X., Zhang, S., & He, J. (2025). Development and Application of a Rapid Detection Technique for 2-Amino-1-methyl-6-phenyl-imidazo [4,5-b] Pyridine in Meat Products Based on Hydrogel-Molecular-Imprinting Electrochemical Sensing Technology. Foods, 14(8), 1292. https://doi.org/10.3390/foods14081292






