1. Introduction
Myofibrillar proteins are essential for the formation of a three-dimensional gel network, which has a crucial role in the texture of meat products, such as chewability, elasticity and slicing ability [
1,
2]. Protein molecules are denatured and unfolded after heating, exposing more relevant groups, which are then aggregated through hydrophobic interactions to form a three-dimensional reticulated protein gel structure. Thus, they support the structure of the meat product and slow down the loss of water from the tissue. Myofibrillar proteins include mainly actin and myosin. These two form myonodes by forming in an overlapping manner, which then form myocytes and further form myogenic fibers. Gel formation occurs primarily through myosin, which is extremely unstable and easily oxidized. In addition to the oxidation that normally occurs, meat products are enriched with lipolytic compounds that contribute to the oxidation process, causing adverse effects on the meat product through the production of reactive oxygen species that lead to protein aggregation and amino acid modification. However, meat products are prone to oxidation during processing, which can lead to alterations in protein structure. This is primarily due to modifications in amino acid side chains, including the formation of protein carbonylation, protein fragmentation, and cross-linking [
3]. This disruption can compromise the integrity of the gel network, leading to disarray, surface irregularities, diminished color vibrancy, and reduced water retention [
4,
5], further reducing the quality of the meat [
6]. It can even trigger oxidative stress within the body following consumption [
7], so resistance to protein oxidation has a very important role.
The addition of polyphenols and polysaccharides can effectively inhibit the oxidation of myofibrillar proteins and enhance their gel properties. Polyphenols are regarded as a safe food additive that inhibits oxidation reactions by lowering oxygen levels, neutralizing reactive oxygen species (ROS), and chelating metal ions. [
8,
9]. Polyphenols can have covalent and non-covalent interactions with proteins. Covalent interactions generally occur under oxidizing conditions, where polyphenols are oxidized to semiquinones or quinones. The polyphenols are then reacted with protein side-chain groups to cross-link to form complexes. Under non-oxidizing conditions, non-covalent cross-links are formed mainly through hydrophobic interactions and hydrogen bond formation. Ferulic acid, a type of plant phenolic acid, markedly diminishes oxidation-induced alterations in MPs and boosts the cross-linking of myofibrillar proteins [
10]. However, the application of FA is constrained by its low solubility in water, inefficient utilization, and susceptibility to instability. Complexation of FA with CDs can solve the problem [
11]. It also has a large concentration dependence, with high concentrations adversely affecting meat products. Cyclodextrin-embedded polyphenols were able to inhibit the oligomerization of proteins while improving the stability of polyphenols. Cyclodextrins possess an inner hydrophobic cavity and an outer hydrophilic surface, enabling them to form noncovalent inclusion complexes with a range of guest molecules. The interaction between the host cyclodextrin and the guest molecules occurs via forces such as van der Waals interactions, hydrophobic effects, and hydrogen bonding, which serve to enhance the solubility and molecular stability of the guest molecules [
12]. Polysaccharides and polyphenols have great affinity for each other and can form complexes through hydrophobic interactions and hydrogen bonding. At the same time, the addition of polysaccharides in gel formation can play the role of water retention and filling, and the gel network formed is more dense.
However, polyphenols are concentration-dependent in gels; when the concentration is too high, it will modify proteins excessively and have a negative effect on the gel [
13]. The addition of metal ions can effectively improve the chemical shifts of protein amino acid residues and the functional properties of protein molecules. It also forms a metal–phenol network structure with polyphenols and improves the properties of the gel. Metal ions prompt proteins to aggregate into larger complexes, leading to a denser and more uniform structure of the gel [
14]. The addition of CaCl
2 enhances the cross-linking of surimi myofibrillar proteins, thereby densifying the structure [
15]. The addition of alkali denatured and aggregated egg white proteins, followed by the introduction of low concentrations of Ca
2+, Zn
2+, and Fe
3+, enhance the gel’s strength and structural ordering [
16]. In the mixed system of pea protein and cod protein, the addition of NaCl and CaCl
2 diminishes the negative charge on the proteins, thereby reducing repulsive forces. This results in the formation of larger aggregates and gels that exhibit enhanced elasticity and adhesion [
17].
The oxidation of meat products leading to deterioration in their gel texture can greatly affect product quality. Although the antioxidant effect of FA on myofibrillar proteins has been reported, the improvement in gel texture varies greatly depending on the concentration and other factors. In contrast, CD is able to encapsulate polyphenols and eliminate the negative effect of excessive cross-linking on the protein in the gel texture. Therefore, it was hypothesized that FA/CD had the same improvement effect on MP gels. Meanwhile, the metal ion Fe(III) was able to form a metallophenolic network structure with polyphenols. Similarly, it is hypothesized that Fe(III) is equally capable of promoting the improvement in MP gel structure. Existing studies mainly discuss this between other individual proteins, but a few discuss the relationship between FA, CD, Fe(III) and MP gel structure and the effect of different composite structures on the gel structure. So the aim of this study was to explore the effects of FA, the addition of CD to FA for encapsulation (MP-FA/CD), and the addition of Fe(III) to form a metal–phenolic network structure (Fe@MP-FA) and metal–cyclodextrin–phenolic acid structure (Fe@MP-FA/CD) on the structure of MP gels. The effect of different coating methods after treating the gels was also considered. Thus, it provides a reference for the improvement of MP gel texture in meat products.
2. Materials and Methods
2.1. Materials and Chemicals
Fresh chicken breasts were purchased from local supermarkets, stored at 4 °C and myofibrillar proteins were extracted within 24 h. NaCl, MgCl2, NaH2PO4, Na2HPO4, NaOH, EGTA, ferulic acid, α-cyclodextrin, FeCl3−6H2O, anhydrous ethanol, urea, β-mercaptoethanol, Caumas Brilliant Blue, and other chemicals (analytical grade) were purchased from Aladdin Biochemistry and Technology Co. Ltd. (Shanghai, China).
2.2. Preparation of Myofibrillar Protein Gel
2.2.1. Extraction of Myofibrillar Protein
The fat and connective tissue were removed from the chicken breasts and placed in a meat grinder. After the meat was minced, it was combined with 4 times the volume (
v/
w) of separation buffer (0.1 M NaCl, 2 mM MgCl
2, 1 mM EGTA, 10 mM NaH
2PO
4/Na
2HPO
4, pH 7.0), homogenized and centrifuged at 10,000
g for 15 min at 4 °C. Then, the precipitate was collected, the above operation was repeated twice and the resulting precipitate was MP [
18]. The protein concentration was determined by BCA method. The extracted MP was stored at 0~4 °C and used up within 48 h.
2.2.2. Ferulic Acid (FA)/Cyclodextrin (CD) Inclusion Complex Preparation
The FA ethanol solution was added dropwise to 2 mM CD solution (molar ratio of CD:FA: 1:1) at 60 °C and 400 rpm and stirred continuously for 2 h, sealed and light protected. Then, it was dried at 60 °C and washed with anhydrous ether 3 times to remove the residual FA. The powder was then dried naturally at room temperature, sealed in a plastic bag and stored in a desiccator.
2.2.3. Preparation of Myofibrillar Protein (MP)-Ferulic Acid (FA) Composite Gel
MP was prepared to a protein concentration of 40 mg/mL using 0.6 M NaCl. The FA and its inclusion complex were subsequently dissolved in the MP solution. This resulted in the final concentration of FA and its inclusion complex reaching 0.04% (
w/
v). A control group was established using MP solution without the addition of FA and its inclusion complex. Following this, the MP solution underwent heating via a secondary method. The samples were initially placed in a water bath maintained at 30 °C for 20 min. Subsequently, the temperature was increased linearly from 30 °C to 70 °C at a rate of 1 °C per minute, and the samples were then held at 70 °C for an additional 20 min. Finally, the gel was promptly cooled in ice water and stored in a refrigerator at 4 °C overnight [
19].
2.2.4. Preparation of Fe(III)-Reinforced Myofibrillar Protein (MP) Gels
Surface coating: the surface construction method of MPN used is referred to in [
20]. Initially, the MP gels were prepared and subsequently submerged in deionized water. Following this, equivalent volumes of FA (4 mg/mL) and FeCl
3−6H
2O (1.2 mg/mL) solutions were sequentially introduced into the system, achieving final concentrations of FA at 0.4 mg/mL and FeCl
3−6H
2O at 0.12 mg/mL. After each addition, the mixture was agitated for 30 s to ensure thorough contact between the FA and FeCl
3−6H
2O solutions and the gel samples, after which the pH was adjusted to 10.0 using NaOH (2 M). Finally, the coating procedure was performed five times, with the gels being rinsed multiple times with deionized water throughout the process. The final gel samples obtained were Fe@MP-FA and Fe@MP-FA/CD.
Internal cross-linking: FA (4 mg/mL) and FeCl3−6H2O (1.2 mg/mL) solutions were added to the MP solution and mixed homogeneously, making the final concentrations of FA and FeCl3−6H2O up to 0.4 mg/mL and 0.12 mg/mL. Then, the pH was adjusted to 10.0 with NaOH (2 M). The secondary heating method was used to prepare the gels using the same steps.
2.3. Textural Properties
The MP gel was measured by p/20 probe at room temperature and the parameters were as follows: using the texture profile analysis (TPA) mode, pre-test speed: 2 mm/s, test speed: 0.8 mm/s, post-test speed: 0.8 mm/s, trigger force: 5.0 g, interval time: 5.0 s, target deformation: 50%.
2.4. Cooking Yield and WHC
First, the MP sol was heated in the beaker with cling film during heating to prevent the surface of the MP gel from drying out. W is the weight of the empty beaker, W1 is the total weight of the beaker and MP sol before the heat-induced treatment. and W2 is the total mass of the beaker and MP gel after the heat-induced treatment. Cooking yield is calculated using the following [
21].
The gel samples were centrifuged at 10,000
g for 10 min at 4 °C. The M
0 is the weight of empty centrifuge tube, M
1 is the total weight of centrifuge tube and MP gel before centrifugation, and M
2 is the total weight of tube and MP gel after centrifugation. WHC is calculated using the following equation [
22]:
2.5. Water Distribution
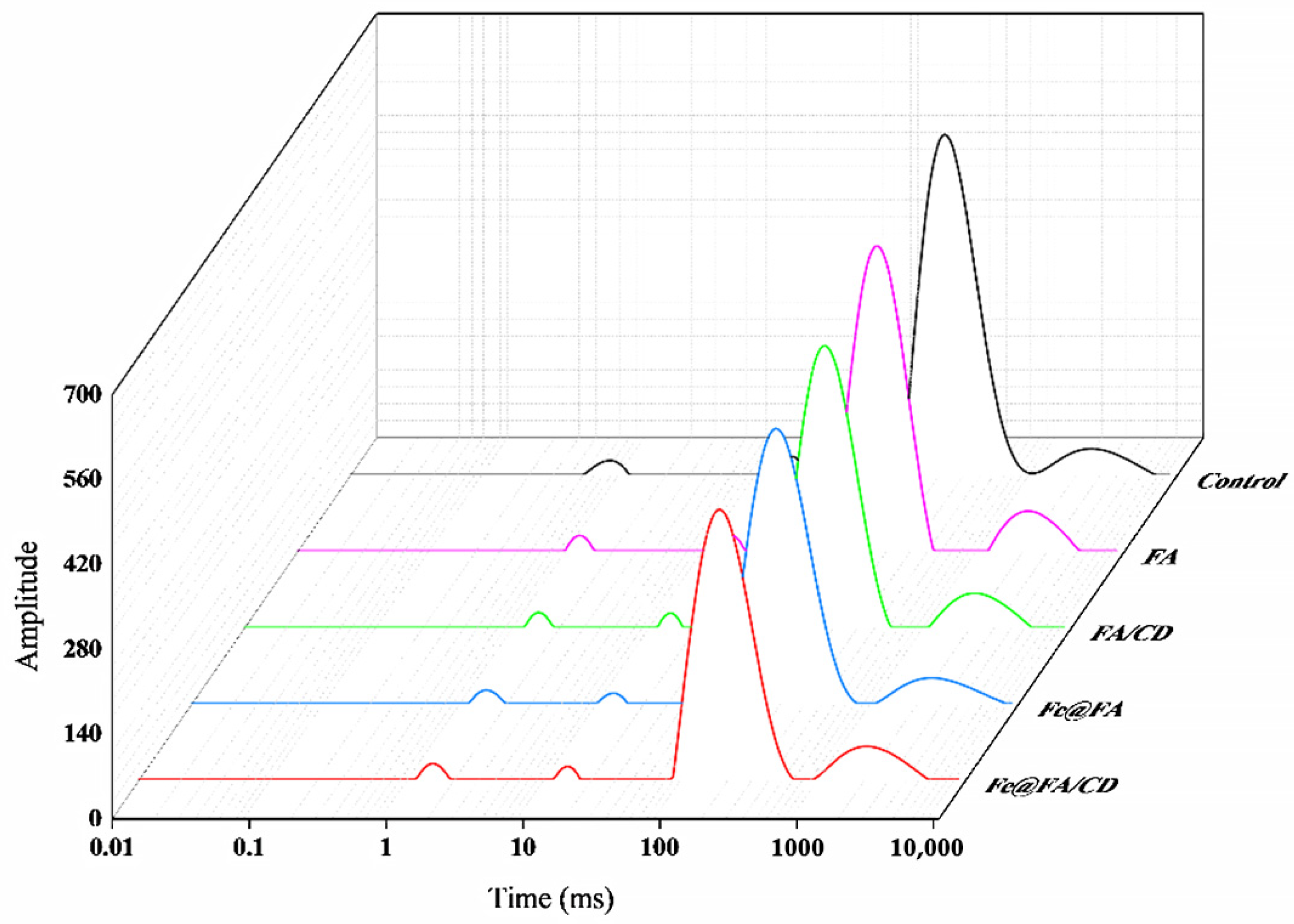
Water composition and distribution of MP gel samples were analyzed using LF-NMR [
23]. First, 2 g of MP gel was put in a glass tube. The spectrum was collected using an LF-NMR analyzer with 21 MHz of resonance frequency at 32 °C. The transverse relaxation time (T
2) was measured using a Carr–Purcell–Meiboom–Gill (CPMG) with 12,000 echoes, 32 scans, 6.5 s between scans, the lengths of the pulse set at 200 μs. Each test was repeated 4 times. The distribution of T2 and the percentage of peak area were calculated for each sample.
2.6. Dynamic Rheological Characterization
Initially, the MP solution was uniformly applied to the center of the carrier table, employing an oscillating temperature scanning test mode with a P20-type rotor for analysis. The parameters were set as follows: the oscillation frequency was 0.1 Hz, the maximum stress was 2%, the gap between the upper and lower plates was 1 mm, and the sample was heated at a rate of 1 °C/min. Finally, the heating curves of the samples from 20 to 80 °C were recorded, along with the values of the energy storage modulus (G’), loss modulus (G”), and phase angle tangent (tanδ).
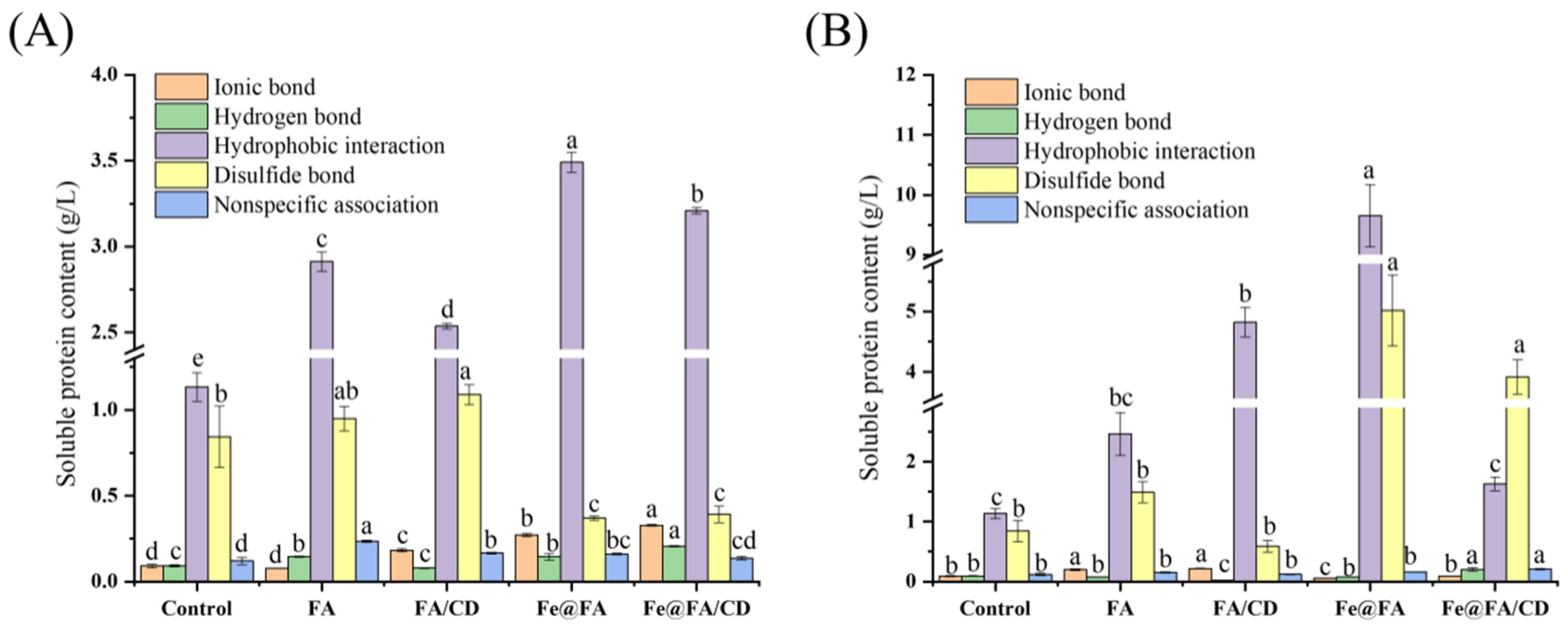
2.7. Covalent and Non-Covalent Interactions
First, 1 g of gel sample was added to solutions, then the following reagents were added separately: 9 mL of 0.05 M NaCl (SA), 0.6 M NaCl (SB), 0.6 M NaCl + 1.5 M urea (SC), 0.6 M NaCl + 8 M urea (SD), 0.6 M NaCl + 8 M urea + 2% β-mercaptoethanol (SE) mixing. After homogenization for 20 s, the samples were centrifuged at 8000×
g for 10 min at 4 °C. Then, the supernatant was taken to determine the protein content by Bradford’s method. The non-specific binding between protein molecules was indicated by the protein content in SA solution. Ionic bonding, hydrogen bonding, hydrophobic interactions, and disulfide bonding were expressed by the difference in protein content between SB and SA solution, SC and SB solution, SD and SC, and SE and SD. Results are expressed as grams of soluble protein per liter of homogenate [
24].
2.8. Secondary Structure
Secondary structure changes in MP samples were analyzed using MOS-500 circular dichroism spectrometer [
25]. MP gels were dissolved in 0.6 M NaCl phosphate buffer (pH 7.0) and centrifuged at 8000×
g for 10 min. Then, the supernatant was removed and the protein concentration was adjusted to 0.2 mg/mL, adding the samples to the 1 mm quartz cuvette (scanning temperature: 25 °C, scanning wavelength: 190~260 nm, scanning rate: 100 nm/min, bandwidth: 1 nm).
2.9. Microstructure
The MP gels were fixed with liquid nitrogen for 24 h, then were freeze-dried and sprayed with gold. The microscope was accelerated at a voltage of 5 kV to observe [
26].
2.10. Elemental Analysis
The distribution of Fe(III) on the surface of MP gels was examined using the SEM-EDS. The MP gels were subjected to liquid nitrogen freezing treatment and then freeze-dried in vacuum using a freeze-dryer. The gels were fixed on the sample stage by conductive adhesive and sprayed with gold coating, and then observed and photographed under an operating voltage of 5 kV.
2.11. Statistical Analysis
All measurements were performed three times and expressed as means ± SD. Statistical analysis was performed using SPSS 19.0 (SPSS, Inc., Chicago, IL, USA) software. Significant differences between data means (p < 0.05) were tested using Duncan (D); Graphs were plotted using Origin 2021 (OriginLab, Co., Ltd., Northampton, MA, USA).
4. Conclusions
FA and Fe(III) improved the MP gel properties to different degrees. The incorporation of FA into the surface-coating process resulted in a substantial enhancement of the hardness, elasticity, chewability, adhesion, and recovery properties of MP gels. Conversely, the internal cross-linking method led to a notable reduction in these same characteristics. The incorporation of FA/CD in either method had minimal impact on the texture of MP, and the introduction of Fe(III) via internal cross-linking further enhanced the textural properties of MP gels. The application of an iron-based surface coating to MP substantially enhanced its water retention capabilities. Furthermore, the incorporation of iron(III) into FA and FA/CD also ameliorated the rheological properties of MP by reducing the gel’s free water content and transforming it into bound, immobile water. Concurrently, hydrophobic interactions, disulfide bonding, and hydrogen bonding are the primary forces responsible for the gelation of MP. By treating MP with internal cross-linking, FA and FA/CD additions reinforced with Fe(III) ions transformed the α-helical structure of the protein to a β-structure or an irregularly coiled structure. However, the treatment of MP by surface coating induced the transformation of the irregularly coiled protein structure to the α-helical structure. Furthermore, the MP gel structure exhibited increased density subsequent to the application of the surface coating, in contrast to the internally cross-linked group. The Fe(III) was evenly dispersed on the internally cross-linked surface, and the MP gel surface exhibited a blocky lamellar structure.