The Fungal Microbiome in the Vineyard Ecosystem Plays a Key Role in Shaping the Regional Characteristics of Wine
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Soil Analysis
2.3. Analysis of Volatile Compounds in Wine
2.4. DNA Extraction and Sequencing
2.5. Data Analysis
3. Results
3.1. Wine Metabolite Profile Can Distinguish Vineyards
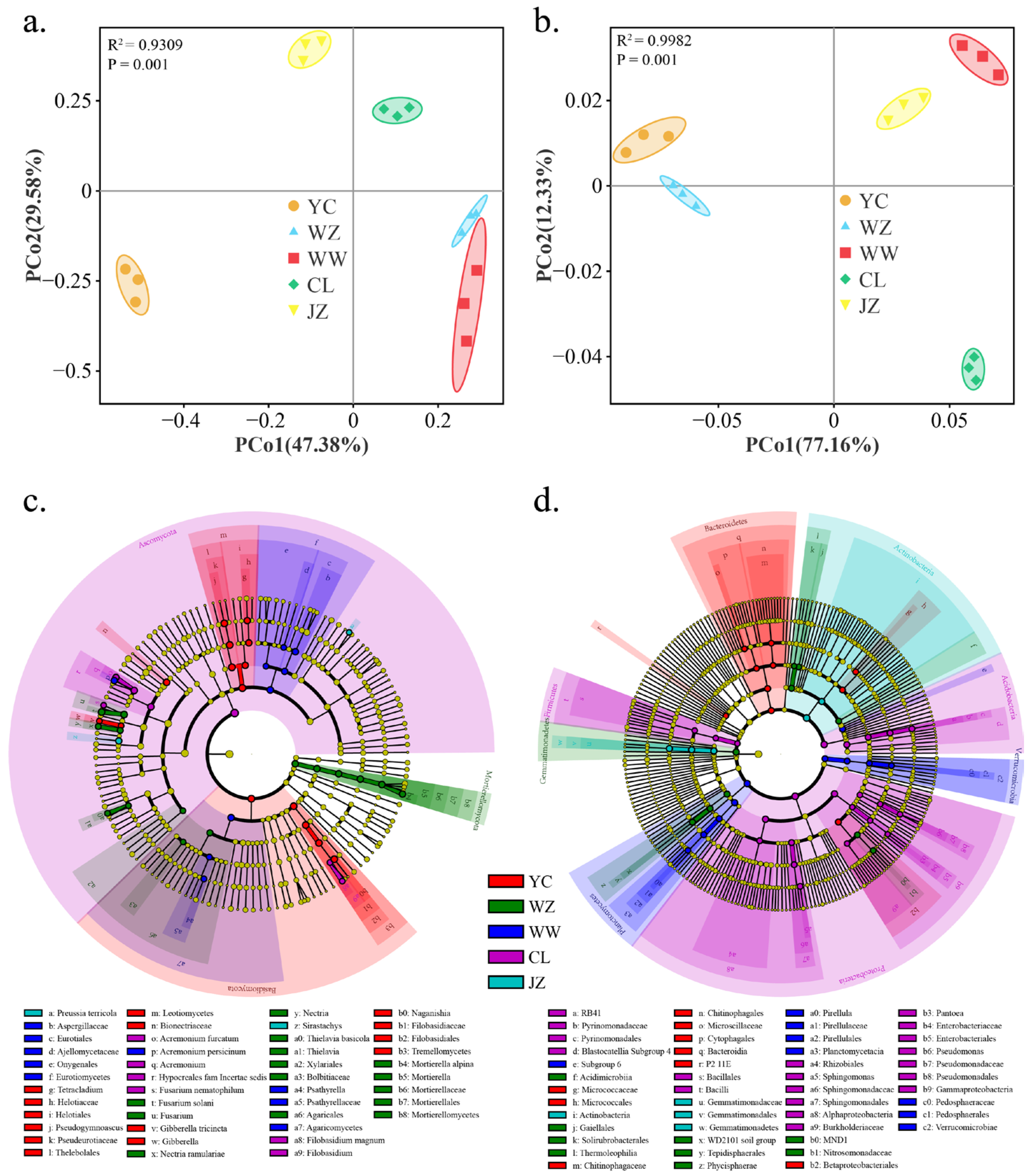
3.2. Geographical Origin of Vineyards Leads to Differences in Soil Microbial Diversity
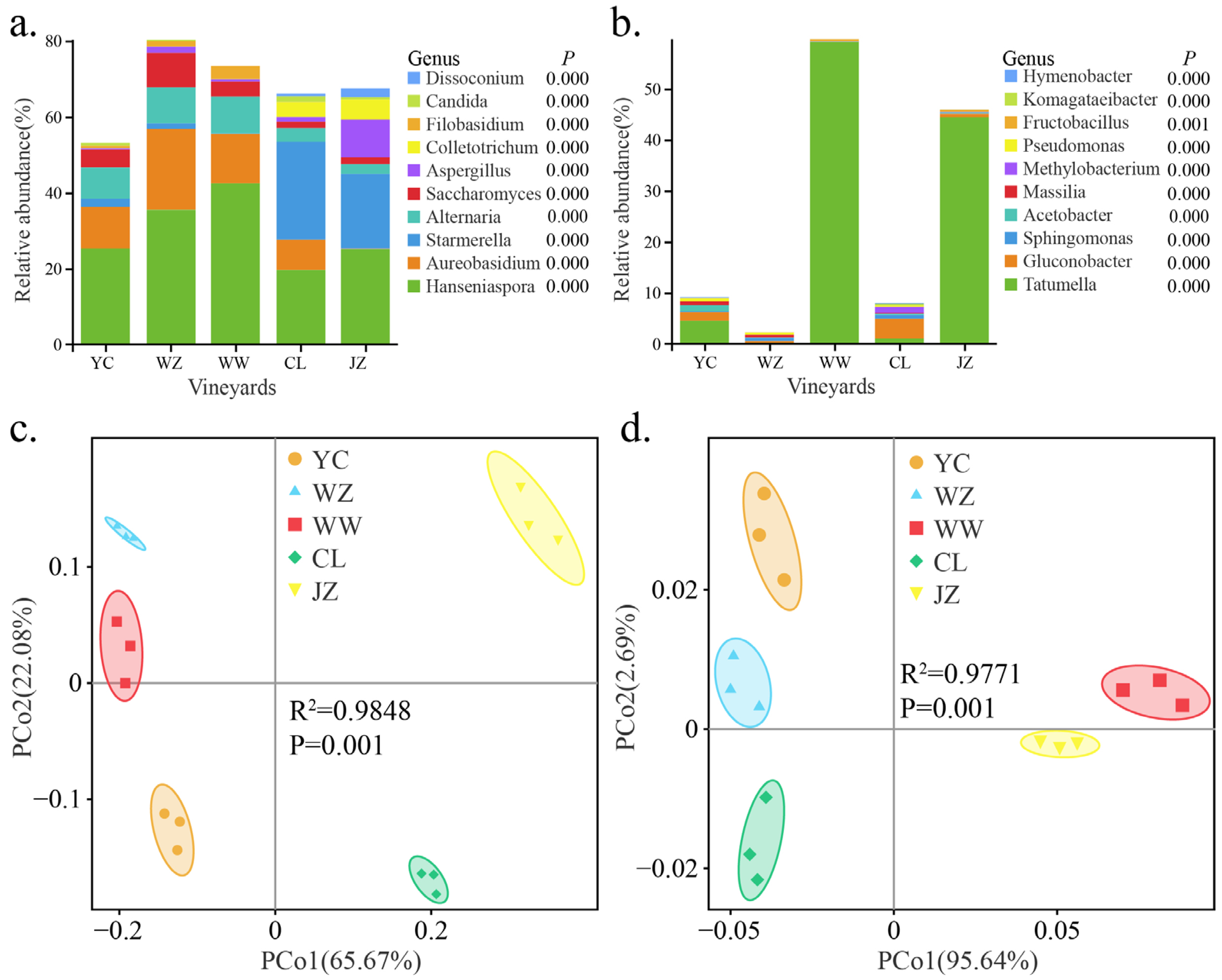
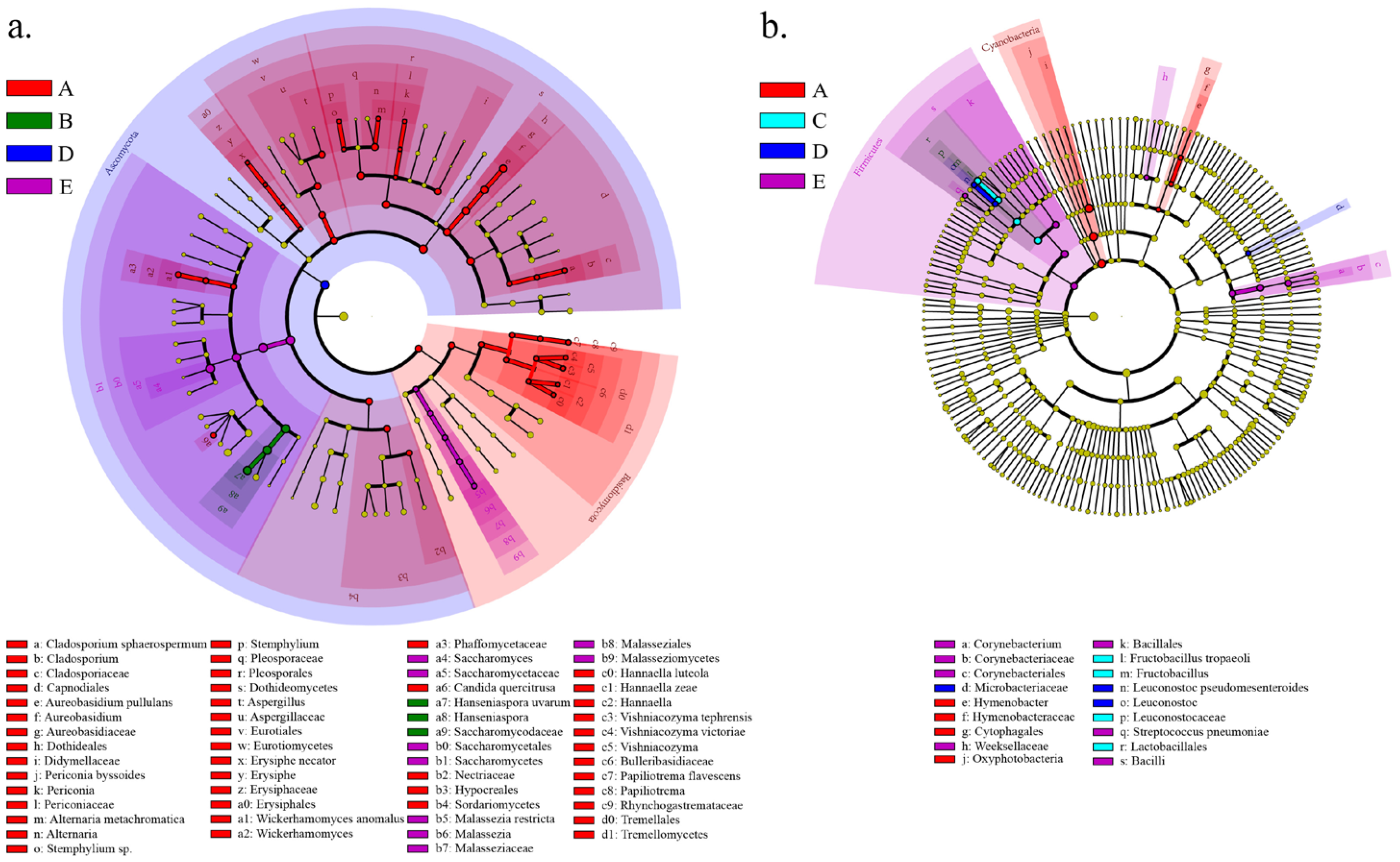
3.3. The Geographical Origin of Vineyards Leads to Differences in Winery Microbial Diversity
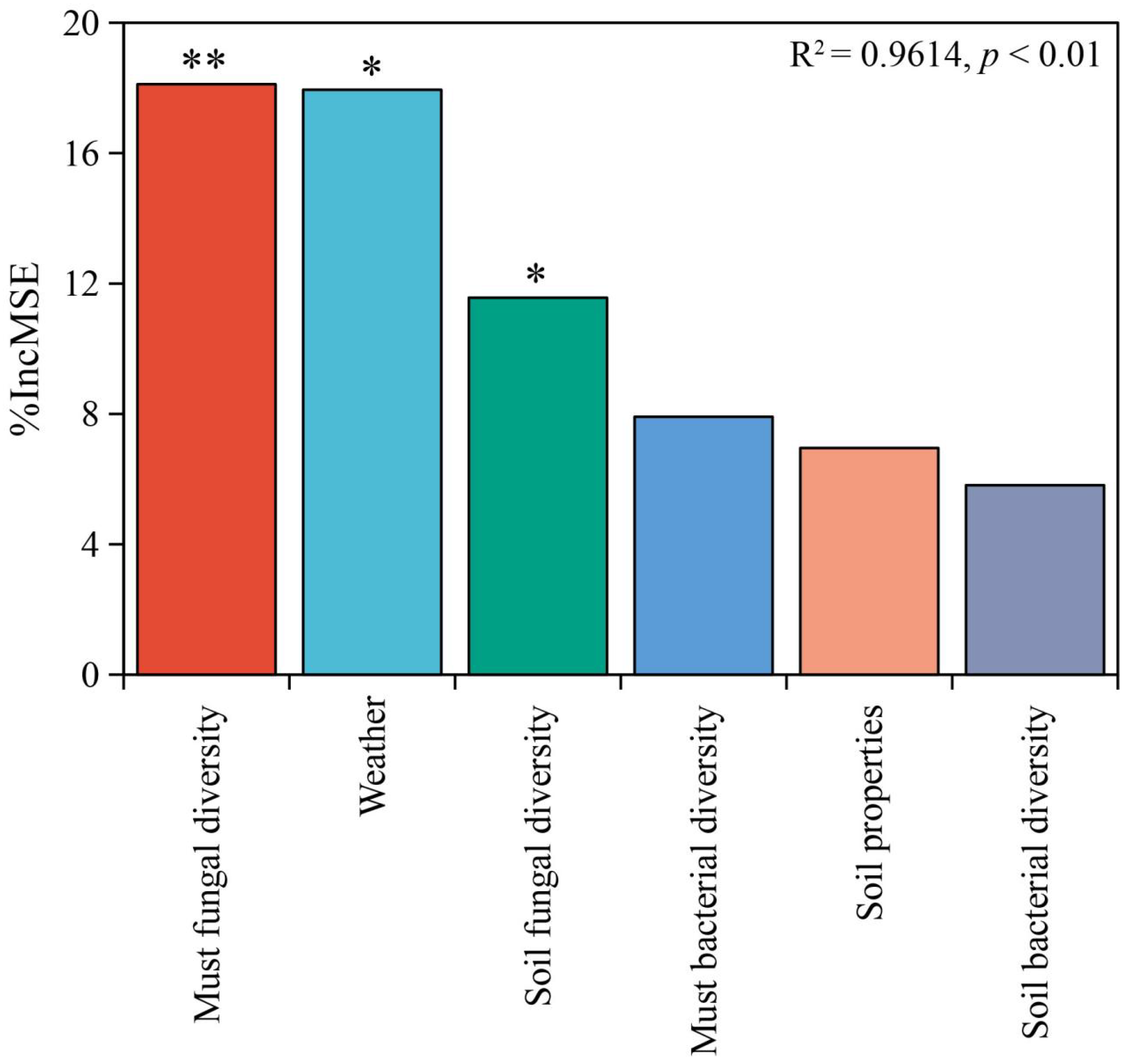
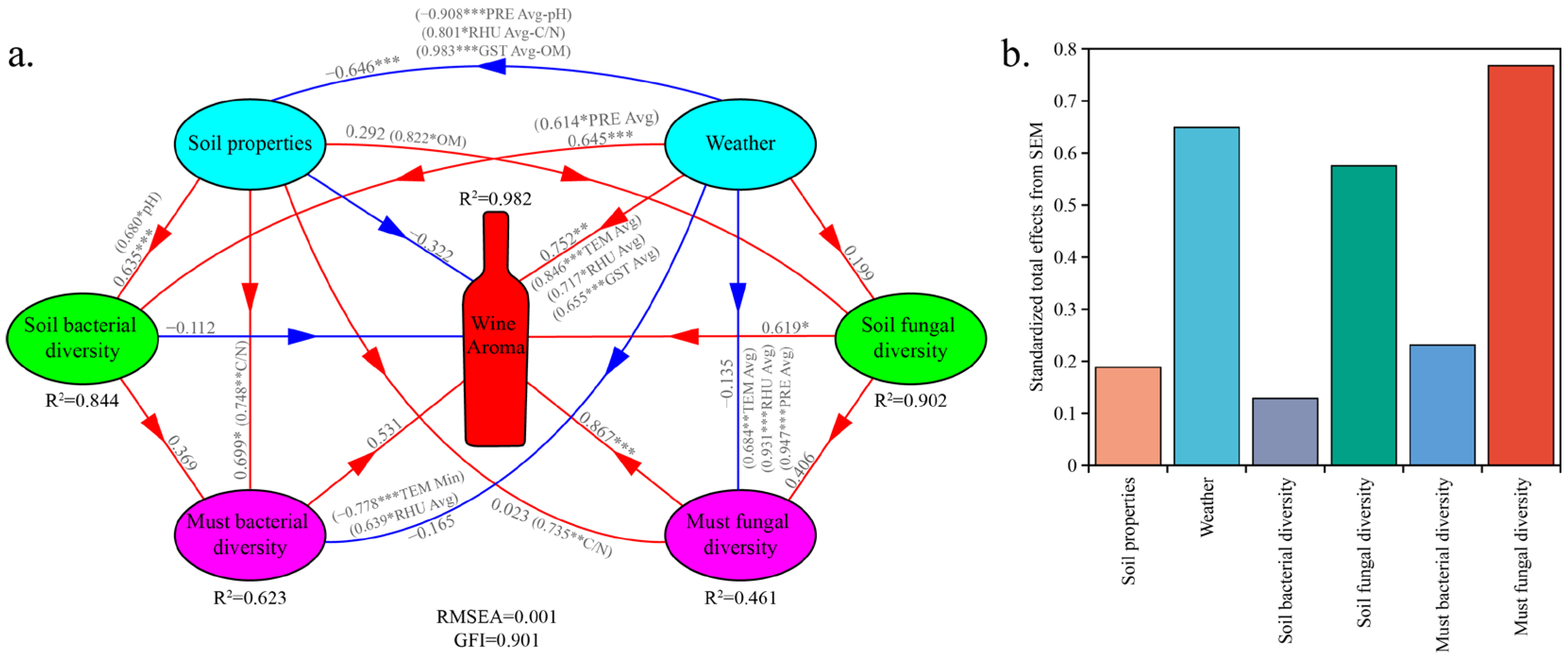
3.4. Multiple Factors Influence the Metabolite Profiles of Wine
4. Discussion
4.1. Microorganisms as Integral Components of Wine Terroir
4.2. Mycobiota Shape the Regionality of Wine
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, D.; Zhang, P.; Chen, D.; Howell, K. From the Vineyard to the Winery: How Microbial Ecology Drives Regional Distinctiveness of Wine. Front. Microbiol. 2019, 10, 2679. [Google Scholar] [CrossRef]
- Franco, G.C.; Leiva, J.; Nand, S.; Lee, D.M.; Hajkowski, M.; Dick, K.; Withers, B.; Soto, L.; Mingoa, B.-R.; Acholonu, M.; et al. Soil Microbial Communities and Wine Terroir: Research Gaps and Data Needs. Foods 2024, 13, 2475. [Google Scholar] [CrossRef]
- Leeuwen, C.V.; Seguin, G. The Concept of Terroir in Viticulture. J. Wine Res. 2006, 17, 1–10. [Google Scholar] [CrossRef]
- Griggs, R.G.; Steenwerth, K.L.; Mills, D.A.; Cantu, D.; Bokulich, N.A. Sources and Assembly of Microbial Communities in Vineyards as a Functional Component of Winegrowing. Front. Microbiol. 2021, 12, 673810. [Google Scholar] [CrossRef]
- Bettenfeld, P.; Canals JC i Jacquens, L.; Fernandez, O.; Fontaine, F.; Schaik E v Courty, P.-E.; Trouvelot, S. The Microbiota of the Grapevine Holobiont: A Key Component of Plant Health. J. Adv. Res. 2022, 40, 1–15. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Thorngate, J.H.; Richardson, P.M.; Mills, D.A. Microbial Biogeography of Wine Grapes is Conditioned by Cultivar, Vintage, and Climate. Proc. Natl. Acad. Sci. USA 2013, 111, E139–E148. [Google Scholar] [CrossRef] [PubMed]
- Pinto, C.; Pinho, D.; Cardoso, R.; Custódio, V.; Fernandes, J.; Sousa, S.; Pinheiro, M.; Egas, C.; Gomes, A.C. Wine Fermentation Microbiome: A Landscape from Different Portuguese Wine Appellations. Front. Microbiol. 2015, 6, 905. [Google Scholar] [CrossRef]
- Gayevskiy, V.; Goddard, M.R. Geographic Delineations of Yeast Communities and Populations Associated with Vines and Wines in New Zealand. ISME J. 2012, 6, 1281–1290. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Collins, T.S.; Masarweh, C.; Allen, G.; Heymann, H.; Ebeler, S.E.; Mills, D.A. Associations among Wine Grape Microbiome, Metabolome, and Fermentation Behavior Suggest Microbial Contribution to Regional Wine Characteristics. mBio 2016, 7, 00631-16. [Google Scholar] [CrossRef]
- Knight, S.; Klaere, S.; Fedrizzi, B.; Goddard, M.R. Regional Microbial Signatures Positively Correlate with Differential Wine Phenotypes: Evidence for a Microbial Aspect to Terroir. Sci. Rep. 2015, 5, 14233. [Google Scholar] [CrossRef]
- Wei, R.; Ding, Y.; Chen, N.; Wang, L.; Gao, F.; Zhang, L.; Song, R.; Liu, Y.; Li, H.; Wang, H. Diversity and Dynamics of Microbial Communities During Spontaneous Fermentation of Cabernet Sauvignon (Vitis vinifera L.) from Different Regions of China and Their Relationship with the Volatile Components in the Wine. Food Res. Int. 2022, 156, 111372. [Google Scholar] [CrossRef] [PubMed]
- Habran, A.; Commisso, M.; Helwi, P.; Hilbert, G.; Negri, S.; Ollat, N.; Gomès, E.; Leeuwen C v Guzzo, F.; Delrot, S. Roostocks/Scion/Nitrogen Interactions Affect Secondary Metabolism in the Grape Berry. Front. Plant Sci. 2016, 7, 1134. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yuan, M.; Meng, N.; Li, H.; Sun, J.; Sun, B. Influence of Nitrogen Status on Fermentation Performances of Non-Saccharomyces Yeasts: A Review. Food Sci. Hum. Well 2024, 13, 556–567. [Google Scholar] [CrossRef]
- Rillig, M.C.; Lehmann, A.; Lehmann, J.; Camenzind, T.; Rauh, C. Soil Biodiversity Effects from Field to Fork. Trends Plant Sci. 2018, 23, 17–24. [Google Scholar] [CrossRef]
- Huang, L.; Hu, H.; Bao, W.; Hu, B.; Liu, J.; Li, F. Shifting Soil Nutrient Stoichiometry with Soil of Variable Rock Fragment Contents and Different Vegetation Types. Catena 2023, 220, 106717. [Google Scholar] [CrossRef]
- Singh, B.K.; Bardgett, R.D.; Smith, P.; Reay, D.S. Microorganisms and Climate Change: Terrestrial Feedbacks and Mitigation Options. Nat. Rev. Microbiol. 2010, 8, 779–790. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Jin, G.-J.; Xu, Y.-H.; Xue, S.-J.; Qiao, S.-J.; Teng, Y.-X.; Tao, Y.-S. Enhancing Wine Ester Biosynthesis in Mixed Hanseniaspora uvarum/Saccharomyces cerevisiae Fermentation by Nitrogen Nutrient Addition. Food Res. Int. 2019, 123, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Lin, M.; Guo, S.; Yang, S.; Han, X.; Ren, M.; Song, Y.; Du, L.; You, Y.; Zhan, J.; et al. A Fundamental Landscape of Fungal Biogeographical Patterns Across the Main Chinese Wine-producing Regions and the Dominating Shaping Factors. Food Res. Int. 2021, 150, 110736. [Google Scholar] [CrossRef]
- Gobbi, A.; Acedo, A.; Imam, N.; Santini, R.G.; Ortiz-Álvarez, R.; Ellegaard-Jensen, L.; Belda, I.; Hansen, L.H. A Global Microbiome Survey of Vineyard Soils Highlights the Microbial Dimension of Viticultural terroirs. Commu Biol. 2022, 5, 241. [Google Scholar] [CrossRef]
- Zarraonaindia, I.; Owens, S.M.; Weisenhorn, P.; West, K.; Hampton-Marcell, J.; Lax, S.; Bokulich, N.A.; Mills, D.A.; Martin, G.; Taghavi, S.; et al. The Soil Microbiome Influences Grapevine-associated Microbiota. mBio 2015, 6, e02527-14. [Google Scholar] [CrossRef]
- Rivas, G.A.; Guillade, A.C.; Semorile, L.C.; Delfederico, L. Influence of Climate on Soil and Wine Bacterial Diversity on a Vineyard in a Non-traditional Wine Region in Argentina. Front. Microbiol. 2021, 12, 726384. [Google Scholar] [CrossRef]
- Morrison-Whittle, P.; Goddard, M.R. From Vineyard to Winery: A Source Map of Microbial Diversity Driving Wine Fermentation. Environ. Microbiol. 2018, 20, 75–84. [Google Scholar] [CrossRef]
- Wei, R.; Ding, Y.; Gao, F.; Zhang, L.; Wang, L.; Li, H.; Wang, H. Community Succession of the Grape Epidermis Microbes of Cabernet Sauvignon (Vitis vinifera L.) from Different Regions in China during Fruit Development. Int. J. Food Microbiol. 2022, 362, 109475. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Chen, Q.; Zhang, P.; Chen, D.; Howell, K.S. The Fungal Microbiome Is an Important Component of Vineyard Ecosystems and Correlates with Regional Distinctiveness of Wine. mSphere 2020, 5, e00534-20. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Li, Y.; Zhu, Z.; Zhang, L.; Wang, H.; Li, H. Vineyard Reclamation Alters Soil Properties and Microbial Community in Desertified Land. Catena 2024, 246, 108399. [Google Scholar] [CrossRef]
- Khaled, F.; Sayed, A. Soil pH and Its Influence on Nutrient Availability and Plant Health. Inter. J. Adv. Chem. Res. 2023, 5, 68–70. [Google Scholar] [CrossRef]
- Xiong, R.; He, X.; Gao, N.; Li, Q.; Qiu, Z.; Hou, Y.; Shen, W. Soil pH Amendment Alters the Abundance, Diversity, and Composition of Microbial Communities in Two Contrasting Agricultural Soils. Microbiol. Spectr. 2024, 12, e0416523. [Google Scholar] [CrossRef]
- Wu, K.; Xu, W.; Yang, W. Effects of Precipitation Changes on Soil Bacterial Community Composition and Diversity in the Junggar Desert of Xinjiang, China. PeerJ 2020, 8, e8433. [Google Scholar] [CrossRef]
- Gao, Q.; Ma, L.; Fang, Y.; Zhang, A.; Li, G.; Wang, J.; Wu, D.; Wu, W.; Du, Z. Conservation Tillage for 17 Years Alters the Molecular Composition of Organic Matter in Soil Profile. Sci. Total Environ. 2021, 762, 143116. [Google Scholar] [CrossRef]
- Liu, Y.; Rousseaux, S.; Tourdot-Maréchal, R.; Sadoudi, M.; Gougeon, R.; Schmitt-Kopplin, P.; Alexandre, H. Wine Microbiome: A Dynamic World of Microbial Interactions. Crit. Rev. Food Sci. Nutr. 2017, 57, 856–873. [Google Scholar] [CrossRef]
- Carpena, M.; Fraga-Corral, M.; Otero, P.; Nogueira, R.A.; Garcia-Oliveira, P.; Prieto, M.A.; Simal-Gandara, J. Secondary Aroma: Influence of Wine Microorganisms in Their Aroma Profile. Foods 2020, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Barata, A.; Malfeito-Ferreira, M.; Loureiro, V. The microbial ecology of wine grape berries. Int. J. Food Microbiol. 2012, 153, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Börlin, M.; Venet, P.; Claisse, O.; Salin, F.; Legras, J.-L.; Masneuf-Pomarede, I. Cellar-Associated Saccharomyces cerevisiae Population Structure Revealed High-Level Diversity and Perennial Persistence at Sauternes Wine Estates. Appl. Environ. Microbiol. 2016, 82, 2909–2918. [Google Scholar] [CrossRef] [PubMed]
- Valero, E.; Schuller, D.; Cambon, B.; Casal, M.; Dequin, S. Dissemination and Survival of Commercial Wine Yeast in the Vineyard: A Large-scale, Three-years Ttudy. FEMS Yeast Res. 2005, 5, 959–969. [Google Scholar] [CrossRef]
- Liu, D.; Howell, K. Community Succession of the Grapevine Fungal Microbiome in the Annual Growth Cycle. Environ. Microbiol. 2020, 23, 1842–1857. [Google Scholar] [CrossRef]
- Mandl, K.; Schieck, J.; Silhavy-Richter, K.; Prange, A.; Schneider, V.; Schmidt, H.-P. Vines Take Up Yeasts From Soil and Transport Them Through the Vine to the Stem and Skins of Grapes. Ithaka J. 2015, 2015, 349–355. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, C.; Yao, Y.; Wang, H.; Li, H.; Wei, R. The Fungal Microbiome in the Vineyard Ecosystem Plays a Key Role in Shaping the Regional Characteristics of Wine. Foods 2025, 14, 1211. https://doi.org/10.3390/foods14071211
Bai C, Yao Y, Wang H, Li H, Wei R. The Fungal Microbiome in the Vineyard Ecosystem Plays a Key Role in Shaping the Regional Characteristics of Wine. Foods. 2025; 14(7):1211. https://doi.org/10.3390/foods14071211
Chicago/Turabian StyleBai, Chunyan, Yuan Yao, Hua Wang, Hua Li, and Ruteng Wei. 2025. "The Fungal Microbiome in the Vineyard Ecosystem Plays a Key Role in Shaping the Regional Characteristics of Wine" Foods 14, no. 7: 1211. https://doi.org/10.3390/foods14071211
APA StyleBai, C., Yao, Y., Wang, H., Li, H., & Wei, R. (2025). The Fungal Microbiome in the Vineyard Ecosystem Plays a Key Role in Shaping the Regional Characteristics of Wine. Foods, 14(7), 1211. https://doi.org/10.3390/foods14071211




