Abstract
Antrodia cinnamomea (A. cinnamomea), a medicinal and edible mushroom endemic to Taiwan, has been traditionally valued as a health tonic. Recent studies have highlighted the diverse specialized metabolites and bioactive potential of this substance, primarily attributed to key secondary metabolites such as benzenoids, maleic and succinic acids, ubiquinone, triterpenoids, and the primary metabolite polysaccharides. These compounds exhibit a broad spectrum of pharmacological properties, including those related to antibacterial, antitumor, anti-inflammation, hepatoprotection, hypoglycaemia, and antioxidant activities, and immunomodulation and gut microbiota regulation. These findings highlight the therapeutic potential of A. cinnamomea and its potential applications in health supplements and functional foods. This review evaluated recent advancements in the cultivation, extraction, and characterization of bioactive compounds from A. cinnamomea, with a particular focus on submerged and solid-state fermentation methods. We hope to provide a comprehensive framework for promoting the efficient and scientific evidence based utilization of A. cinnamomea in novel therapeutic strategies and health-related innovations.
1. Introduction
Antrodia cinnamomea (commonly known as “Niu-Chang-Chih” in Chinese) is a highly esteemed edible and medicinal mushroom native to Taiwan traditionally used as a health tonic by aboriginal tribes and the broader Asian community [1,2]. Wild A. cinnamomea has been used medicinally in Taiwan for a long time, particularly by aboriginal tribes [3]. A. cinnamomea was used to treat abdominal pain, food poisoning, diarrhoea, and itchy skin and to enhance liver function [4]. Recent research efforts have led to the isolation and identification of numerous chemical constituents from A. cinnamomea, and an increasing numbers of human studies have been reported on anticancer, antioxidant, antihypertensive, hypolipidemic, immunomodulatory, and anti-inflammatory functions, which were believed to be due to benzenoids, maleic and succinic acids, ubiquinone, triterpenoids, and polysaccharides [5,6]. The host of the mushroom, the decomposing trunks of Cinnamomum kanehirai, is an endangered tree species found in Taiwan’s broad-leaved forests at altitudes ranging from 450 to 2000 m above sea level [7]. This limited natural habitat, combined with the slow growth rate of A. cinnamomea, has earned it the moniker “ruby of the forest” [3]. Due to its value as a high-quality raw material for furniture and the therapeutic potential of A. cinnamomea cultivated on it, Cinnamomum kanehirai has been subject to extensive logging, and this exploitation has prompted Taiwan to place C. kanehirai on its endangered species list [3]. With the increasing demand, wild A. cinnamomea can no longer meet market needs, driving attention toward artificial cultivation methods of artificial cultivation include basswood cultivation, solid-state cultivation, and submerged fermentation, which closely replicates wild substrate quality [8]. With advancements in research, investigations into A. cinnamomea have progressed beyond crude extracts, focusing on isolated bioactive compounds such as polysaccharides, triterpenoids, and ubiquinone derivatives from its fruiting bodies and cultured mycelia.
2. Cultivation and Extraction Methods for A cinnamomea
2.1. Cultivation Methods
Artificial cultivation methods of A. cinnamomea include basswood cultivation, plate culture, solid-state fermentation, and submerged fermentation (Figure 1 and Figure 2).
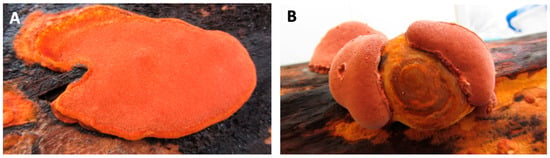
Figure 1.
Pictures of Antrodia cinnamomea. (A) Typical flat type wild fruiting bodies; (B) Horse-hoof-shaped wild fruiting bodies. Pictures of Antrodia cinnamomea. (A) Typical flat type wild fruiting bodies; (B) Horse-hoof-shaped wild fruiting bodies. (Images provided by Dr Chien-Liang Kuo from AgriGADA).
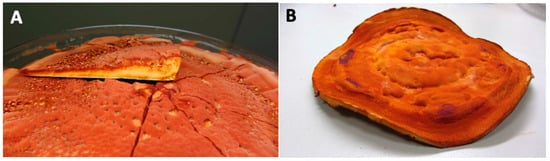
Figure 2.
Pictures of Antrodia cinnamomea dish cultures. (A) Cross section of Antrodia cinnamomea; (B) whole photo of dish cultures. (Images provided by Dr Chien-Liang Kuo from AgriGADA).
2.1.1. Liquid Fermentation
Liquid fermentation is a straightforward and efficient method for cultivating A. cinnamomea mycelium. It utilizes a nutrient-rich liquid medium often enhanced with natural botanical extracts, which is cost-effective, easy to implement, and can enhance secondary metabolite synthesis [9]. The liquid fermentation method offers significantly shorter cultivation periods, typically lasting only a few weeks. This approach generally yields higher polysaccharide content but lower levels of triterpenoids. Specifically, polysaccharide content could be several times higher while the triterpenoid concentration in submerged A. cinnamomea mycelia is approximately 2.3 times lower than that observed in its fruiting body [10].
In addition to some specific metabolites, a study has demonstrated that proteins isolated from an A. cinnamomea strain exhibit promising anticancer activity. The FJ-01 strain was cultured on a potato-dextrose-agar (PDA) slant and incubated at 28 °C for 25 days. Subsequently, segments of the culture were transferred to a liquid seed medium (47.8 g/L corn flour, 31.9 g/L YM medium, pH 5.5) and incubated at 28 °C for an additional 7 days. A novel active protein, termed ACAP, isolated from an A. cinnamomea liquid fermentation medium, exhibited a dose-dependent inhibitory effect on the proliferation of HeLa and HepG2 cells. Notably, ACAP demonstrated superior efficacy compared to the positive control drug 5-Fu at low concentrations, highlighting its potential as a promising anticancer agent [11].
2.1.2. Solid-State Fermentation (SSF)
Solid-state fermentation (SSF) is extensively utilized in both research and commercial production of A. cinnamomea, with the selection of substrates playing a pivotal role in providing essential nutrients and creating a supportive environment for fungal growth, thereby enhancing its bioactivity. Commonly used solid substrates include rice bran, wheat bran, sawdust, and agricultural residues, selected for their nutritional value and ability to simulate natural growth conditions [12]. For example, Yang et al. demonstrated that incorporating various citrus peel extracts into submerged A. cinnamomea cultures significantly promoted mycelial growth and polysaccharide production, and the nutrient composition of cultured mycelia closely matches that of the wild fruiting body and exhibits notable hepatoprotective potential [13]. Specifically, wheat-based solid-state fermented A. cinnamomea (WFAC) was prepared by inoculating liquid-cultivated A. cinnamomea into sterilized wheat mixed with a medium containing 2% sugar, 0.5% malt extract, and 0.5% yeast extract in water, followed by a 4-month incubation at 25 ± 2 °C. The administration of WFAC to rats demonstrated significant protective effects against CCl4-induced liver damage, further highlighting its therapeutic potential [13].
2.2. Extraction Methods for Bioactive Components of A. cinnamomea
Due to its pharmacological activities and significant therapeutic potential, A. cinnamomea has garnered attention for the identification and discovery of its bioactive components including polysaccharides, triterpenoids, ubiquinone derivatives, maleic and succinic acid derivatives, benzenoid derivatives, and glycoproteins. Various extraction technologies have been developed to enhance the yield of these active ingredients; these methods include liquid solvent extraction with ethanol (ETOH-E), supercritical fluid extraction (SFE) using CO2, high-hydrostatic-pressure extraction (HPE), ultrasonic extraction, heat reflux extraction, microwave-assisted extraction with controlled microwave power, and mechanochemical-assisted extraction (Table 1).
Water extraction tends to yield higher levels of water-soluble polysaccharides and small molecules, which are associated with immune-modulating and antioxidant activities. For instance, Tsai et al. demonstrated that the water fraction of A. cinnamomea possesses antioxidant properties, partly due to polysaccharides that upregulate glutathione S-transferase activity, maintain the GSH/GSSG ratio, and scavenge ROS [14]. Additionally, the water-soluble fraction from cultured mycelia of A. cinnamomea exhibits strong anti-inflammatory effects by inhibiting ROS production in human leukocytes at pharmacologically relevant concentrations [15].
Ethanol extraction, using varying ethanol concentrations (e.g., 50%, 70%, 95%), is effective for isolating lipophilic compounds such as polyphenols, triterpenoids, and benzoic acid derivatives. Ethanol extracts are also known for their liver-protective properties, making them suitable for medicinal and health supplement applications. For example, the ethanolic extract of A. cinnamomea mycelium (EMAC) has shown hepatoprotective effects through the activation of HO-1 and phase II enzymes via the MAPKs-mediated Nrf-2 signalling pathway [16]. Ethanol extracts also inhibit cancer cell migration; Chen et al. found that the ethanolic extract of A. cinnamomea fruiting bodies exhibits anti-migration effects in human adenocarcinoma CL1-0 cells through the MAPK and PI3K/AKT signalling pathways [17]. These highlight the value of ethanol extraction for therapeutic applications A. cinnamomea.
The synergistic use of advanced extraction technologies can significantly improve the efficiency and yield of A. cinnamomea bioactive compounds. Ultrasound-assisted extraction enhances solvent penetration, effectively extracting polysaccharides, triterpenoids, and other bioactive compounds on a large scale, while it may sometimes alter compound structures, potentially affecting bioactivity [18]. Enzyme-assisted extraction uses enzymes to break down cell walls, increasing the release of compounds like polysaccharides and proteins [19]. Despite its effectiveness, high enzyme costs limit its application to laboratory research or high-value extractions. Supercritical CO2 extraction, conducted at low temperatures without organic solvents, is ideal for isolating heat-sensitive, lipophilic compounds, such as triterpenoids, yielding high-purity extracts suitable for premium food and pharmaceutical products, although high equipment costs constrain large-scale implementation [20]. High-pressure-assisted extraction (HPE) offers a fast and efficient alternative to improve yield by applying high pressure to disrupt cell structures and enhance solvent penetration and operating at room temperature to preserve heat-sensitive compounds [21]. HPE achieves the desired concentration at 600 MPa in just 3 min, much faster than with ultrasound (1 h) and agitation (8 h).

Table 1.
Using different treatment to enhance the extraction efficiency of the active components from the fruiting bodies of A. cinnamomea.
Table 1.
Using different treatment to enhance the extraction efficiency of the active components from the fruiting bodies of A. cinnamomea.
| Entry | Type | Sample Preparation | Detection Methods | References |
|---|---|---|---|---|
| 1 | Conventional liquid solvent extraction with ethanol | Immersed in ethanol for extraction | HPLC analysis | [16,17,22] |
| 2 | Conventional shake extraction | Shake extraction at 150 rpm for 8 h | HPLC analysis | [23] |
| 3 | Supercritical CO2 | Supercritical carbon dioxide at 35 MPa with as cosolvent | HPLC analysis | [24] |
| 4 | High hydrostatic pressure | 600 MPa (100–700 MPa at 25 °C) high-pressure, liquid/solid ratio of 40:1, 3 min of treatment in a 0.3 L chamber | HPLC analysis | [21,23,25] |
| 5 | Ultrasonic extraction methods | Ultrasonic extraction at 50 Hz for 60 min | HPLC analysis | [18,23,26] |
| 6 | Heat reflux extraction | The dried powder samples were extracted with 3-fold 95% ethanol under heat reflux | UPLC | [27] |
| 7 | Mechanochemical-assisted extraction method (TAEM) | The mixture of powder and NaHCO3 was added into the PM-200 ball mill with steel balls (3 mm diameter, 150 g) at 300 rpm for 20 min before performing extraction by water | HPLC and LC-MS/MS | [28] |
3. Nutritional and Physicochemical Compositions of A. cinnamomea
3.1. Nutritional Composition
The dried biomass of A. cinnamomea contains 6.65% moisture, 56.7% carbohydrates, 25.4% total reducing sugars, 3.92% crude ash, 9.79% crude fat, 20.1% crude fibre, and 9.49% crude protein. Also, A. cinnamomea is rich in sugars, amino acids, triterpenoids, polyphenols, polysaccharides, proteins, flavonoids, lignin and essential oils, enhancing its nutritional and nutraceutical value [29,30]. Supplementing a modified Potato Carrot Agar (PCA) medium with phytohormones and lignin significantly enhanced mycelial growth, increased active ingredient content, and improved antioxidant properties [31].
3.2. Physicochemical Profiles of A. cinnamomea
3.2.1. Benzenoids
Several benzenoid compounds isolated from A. cinnamomea have been identified as possessing potent anti-inflammatory properties and have summarized key information on the preparation methods of A. cinnamomea components from recent studies (Figure 3). Specifically, 4,7-dimethoxy-5-methyl-1,3-benzodioxole (DMB) and antrolone have demonstrated significant anti-inflammatory effects [32,33]. Additionally, coenzyme Q0 (2,3-dimethoxy-5-methyl-1,4-benzoquinone), known for its antitumor and anti-inflammatory activities, further highlights the therapeutic potential of these compounds [34,35,36].
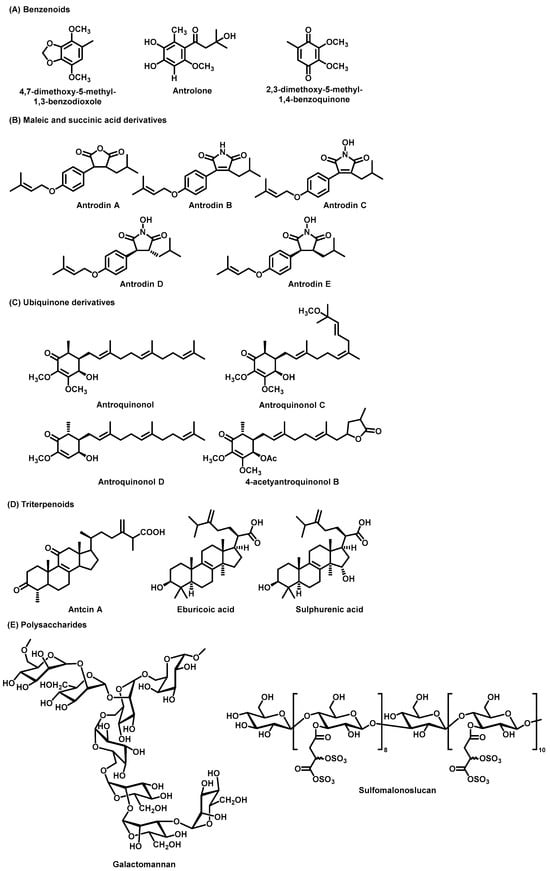
Figure 3.
The chemical structures of the main bioactive components from A. cinnamomea. (A) Benzenoids. (B) Maleic and succinic acid derivatives. (C) Ubiquinone derivatives. (D) Triterpenoids. (E) Polysaccharides.
3.2.2. Maleic and Succinic Acid Derivatives
Succinic acid, historically recognized for its use as a natural antibiotic and therapeutic agent in the 19th century, is a notable compound found in A. cinnamomea, which also serves as a source of maleic and succinic acid derivatives with potential hepatoprotective effects comparable to silymarin [37]. Antrodins A–E (Figure 3), maleic and succinic acid derivatives from A. cinnamomea, have demonstrated selective cytotoxicity against Lewis lung carcinoma (LLC) cells, highlighting their potential as candidates for cancer therapy [38]. Table 2 presents essential information on the preparation methods of maleic and succinic acid derivatives from recent studies. Furthermore, maleic acid derivatives, particularly Antrodin A, have shown protective effects against liver damage, enhancing the antioxidant and anti-inflammatory capacities of the liver while mitigating acute alcoholic liver injury [38]. Tetrodotoxin B has been identified for its anti-hepatic fibrotic effects, and Antrodin C has been found to inhibit the migration and invasion of breast cancer cells by modulating specific signalling pathways [39,40].

Table 2.
Preparation methods of A. cinnamomea components in recent studies.
3.2.3. Ubiquinone Derivatives
Ubiquinone derivatives are key bioactive compounds in A. cinnamomea, with 13 distinct derivatives isolated and characterized to date, including antroquinonol, antroquinonol B, antroquinonol C, antroquinonol D, antroquinonol L, antroquinonol M, antroquinonol Z, antrocamol LT1, antrocamol LT2, antrocamol LT3, 4-acetyantroquinonol B, 4-acetylantrocamol LT3, and antrocinnamone, have been isolated from A. cinnamomea [5,52]. The first ubiquinone compound identified from the solid fermentation culture of A. cinnamomea mycelium was antroquinonol (Figure 3) [53]. Key details on the preparation methods of ubiquinone derivatives from recent studies are summarized in Table 3. These compounds exhibit significant therapeutic potential against cancer, inflammation, periodontal disease, Parkinson’s disease, and cardiovascular disorders, supported by both in vitro models and animal models [5].
4-Acetylantroquinonol B has been evaluated for its anti-inflammatory activity, demonstrating a potent IC50 value of 14.7 μg/mL. Antroquinonol D, successfully synthesized via chemical methods, exhibits selective cytotoxicity against breast cancer cell lines (MCF7, T47D, and MDA-MB-231) with IC50 values of 8.01, 3.57, and 25.08 μM, respectively, while remaining nontoxic to normal cells. Additionally, Antroquinonol C has shown significant anticancer activity. These compounds were selected to emphasize key bioactive ubiquinone derivatives, balancing structural diversity with pharmacological relevance. Ubiquinol-10 supplementation reduces mild fatigue in healthy individuals and improves endothelium-dependent vasodilation in those with mild-to-moderate dyslipidaemia, as demonstrated by human clinical studies [54,55].
3.2.4. Triterpenoids
Triterpenoids, the largest group of phytochemicals in A. cinnamomea, are present in significantly higher concentrations in wild strains compared to submerged-cultured counterparts [56]. Studies have identified 75 ergostanes and 28 landostanes as the predominant types in wild A. cinnamomea, with their levels being 10–30 times higher than those in cultivated strains [57,58], this variation underscores the need for consistent quality control methods to evaluate triterpenoid content. In A. cinnamomea, antcin A is recognized for its potent anti-inflammatory properties. Among the most abundant landostane-type triterpenoids, eburicoic acid, and sulphurenic acid exhibit notable bioactivities, including significant antidiabetic effects [48].
3.2.5. Polysaccharides
Polysaccharides represent a highly promising class of bioactive compounds due to their diverse structural variations and functional versatility. Table 3 summarizes important information on the preparation methods of polysaccharides from recent studies. Polysaccharides isolated from A. cinnamomea mycelia and cultured mycelium have been extensively characterized and evaluated for their broad spectrum of biological activities. These include notable immunomodulatory and anti-inflammatory properties, as well as anticancer, cytotoxic, and anti-angiogenic effects, underscoring their therapeutic potential [59,60]. Additionally, polysaccharides have the potential to be used as natural anticancer agents as they can inhibit tumour growth by restoring or enhancing the immune system [61,62]. Polysaccharides derived from A. cinnamomea also demonstrate potential as natural therapeutic agents for Parkinson’s disease [63]. Chiu et al. identified a novel polysaccharide, Antrodan, isolated from the submerged fermented mycelium of A. cinnamomea [27]. Their study demonstrated that Antrodan, administered at a low dose of 40 mg/kg, exhibits hepatoprotective properties, potentially mediated through the activation of the AMPK/Sirt1/SREBP-1c/PPARγ signalling pathway [28]. Additionally, Antrodan has demonstrated anti-metastatic activity in the treatment of lung cancer and has been shown to mitigate cisplatin-induced renal impairment when co-administered with cisplatin [64,65]. Moreover, Antrodan exhibits promising efficacy in the treatment of non-alcoholic fatty liver disease (NAFLD), with favourable outcomes observed across various administration routes [66]. Additionally, the galactomannan ACP, characterized by an octasaccharide-repeating unit, was first isolated from this species. Perera et al. identified ACP in A. cinnamomea mycelia, confirming its mannose-to-galactose ratio of 75% to 25% [67].

Table 3.
Chemical constituents and their reported activities of A. cinnamomea.
Table 3.
Chemical constituents and their reported activities of A. cinnamomea.
| No. | Chemical Class | Compound Name | Cas | Formula | Biological Activity | Model | Reference |
|---|---|---|---|---|---|---|---|
| 1 | Diterpenes | 19-Hydroxylabda8(17)-en-16,15-olide | 82209-74-3 | C20H32O3 | – | – | [68] |
| 2 | 19-Hydroxylabda8(17),13-dien-16,15-olide | 82209-74-3 | C20H30O3 | – | – | [68] | |
| 3 | 14-Deoxy-11,12-didehydroandrographolide | 42895-58-9 | C20H28O4 | Steatohepatitis and liver Injury activities | in vitro; in vivo | [68] | |
| 4 | 14-Deoxyandrographolide | 4176-97-0 | C20H30O4 | Antimicrobial activity | in vitro | [68] | |
| 5 | Pinusolidic acid | 40433-82-7 | C20H28O4 | Neuroprotective and anti-inflammatory activities | in vitro | [69] | |
| 6 | Terpenoids | Eburicoic acid [58] | 560-66-7 | C31H50O3 | Hepatoprotective effects and anti-inflammatory activities | in vitro; in vivo | [70] |
| 7 | Dehydroeburicoic acid | 1740-19-8 | C20H28O2 | Hepatoprotective, antidiabetic and antihyperlipidemic, anti-inflammatory, anti-insecticidal activities | in vitro; in vivo | [71] | |
| 8 | Sulphurenic acid | – | C31H50O4 | Anti-insecticidal activity | – | [54] | |
| 9 | Dehydrosulphurenic acid | 175615-56-2 | C31H48O3 | Anti-inflammatory activity | in vitro | [4] | |
| 10 | 15α-Acetyl-dehydrosulphurenic acid | 215438 | C34H50O5 | Anti-inflammatory activity | in vitro | [17] | |
| 11 | Versisponic acid D | – | C33H52O5 | Anti-inflammatory activity | in vitro | [72] | |
| 12 | 3β,15α-Dihydroxylanosta-7,9(11),24-trien-21-oic acid | – | C30H46O4 | Anti-inflammatory activity | in vitro | [73] | |
| 13 | 24-Methylenedihydrolanosterol | 14297-52-2 | C31H52O | – | – | [74] | |
| 14 | epi-Friedelinol | 16844-71-6 | C30H52O | – | – | [4] | |
| 15 | Antrocin | – | C15H22O2 | Antioxidant, anti-mutagenic activities | in vitro | [75] | |
| 16 | 19-Hydroxylabda-8(17)-en-16,15-olide | – | C20H32O3 | Neuroprotective activity | in vitro | [70] | |
| 17 | 14-Deoxy-11,12- didehydroandrographolide | 42895-58-9 | C20H28O4 | Neuroprotective activity | in vitro | [76] | |
| 18 | 14-Deoxyandrographolide | 4176-97-0 | C20H30O4 | – | – | [77] | |
| 19 | Pinusolidic acid | – | C20H28O4 | – | – | [78] | |
| 20 | Antcin A | 163597-24-8 | C29H42O4 | Anti-inflammatory, anti-insecticidal activities | in vitro | [52] | |
| 21 | Zhankuic acid A(Antcin B) | 163597-25-9 | C29H40O5 | Anti-inflammatory, anti-insecticidal activities | in vitro | [79] | |
| 22 | Antcin C | – | C29H42O5 | Anti-inflammatory activity | in vitro | [80] | |
| 23 | Antcin D (Zhankuic acid F) | – | C29H40O6 | – | – | [79] | |
| 24 | Antcin E | – | C29H40O4 | – | – | [81] | |
| 25 | Antcin F | – | C29H40O5 | – | – | [69] | |
| 26 | Antcin G | – | C31H44O6 | – | – | [69] | |
| 27 | Zhankuic acid C(Antcin H) | – | C29H42O6 | Anti-inflammatory, anti-insecticidal activities | [73] | ||
| 28 | Antcin I (Zhankuic acid B) | – | C29H42O5 | Anti-inflammatory activity | in vitro | [82] | |
| 29 | Antcin K | 741268-13-3 | C29H44O6 | Anti-inflammatory activity | in vitro | [82] | |
| 30 | Methyl antcinate A | 169477-80-9 | C30H44O4 | – | – | [79] | |
| 31 | Zhankuic acid B | 173221-07-3 | C29H42O5 | – | – | [83] | |
| 32 | Zhankuic acid D(Methyl antcinate B) | – | – | Anti-insecticidal activity | in vitro | [83] | |
| 33 | Zhankuic acid E | – | – | – | – | [83] | |
| 34 | Eburicol (24-methylenedihydrolanosterol) | 6890-88-6 | C31H52O | – | – | [78] | |
| 35 | β-Sitosterol | 83-46-5 | C29H50O | – | – | [84] | |
| 36 | β-Sitostenone | 1058-61-3 | C29H48O | – | – | [84] | |
| 37 | Stigmasterol | 83-48-7 | C29H48O | – | – | [84] | |
| 38 | Ergosta-4,6,8(14),22-tetraen-3-one | 19254-69-4 | C28H40O | – | – | [84] | |
| 39 | Methyl antcinate | 134-20-3 | C8H9NO2 | – | – | [85] | |
| 40 | Methyl antcinate H | 169477-80-9 | C30H44O6 | – | – | [84] | |
| 41 | Eburicol | 6890-88-6 | C31H52O | – | – | [85] | |
| 42 | Benzenoids | Antrocamphin A | – | C15H18O3 | Anti-inflammatory activity | in vitro | [86] |
| 43 | Antrocamphin B | 945622-08-2 | C14H16O4 | Anti-inflammatory activity | in vitro | [86] | |
| 44 | 2,3,4,5-Tetramethoxybenzoyl chloride | 4521-61-3 | (CH3O)3C6H2COCl | Anti-inflammatory activity | in vitro | [86] | |
| 45 | Antrodioxolanone | – | C29H32O9 | Anti-inflammatory activity | in vitro | [86] | |
| 46 | Isobutylphenol | 30749-25-8 | C10H14O | – | – | [86] | |
| 47 | Benzoquinone and its derivatives | 5-Methylbenzodioxole-4,7-dione | 7145-99-5 | C8H6O4 | – | – | [87] |
| 48 | 2,3-Dimethoxy-5-methyl-benzoquinone | 605-94-7 | C9H10O4 | Anti-inflammatory activity | in vitro | [87] | |
| 49 | 2-Methoxy-5- methyl-benzoquinone | 614-13-1 | C8H8O3 | Antioxidant activity | in vitro | [87] | |
| 50 | Maleic anhydrides | Camphorataanhydride A | 656830-24-9 | C19H22O4 | Glycation inhibitors with lipid peroxidation activity | in vitro | [87] |
| 51 | Maleimides | Camphorataimide B | 656830-25-0 | C19H23NO3 | Anti-breast cancer activity | in vitro | [38] |
| 52 | Maleimides | Camphorataimide C | 656830-26-1 | C19H23NO4 | – | – | [38] |
| 53 | Lignans | Sesamin | 607-80-7 | C20H18O6 | – | – | [38] |
| 54 | 4-Hydroxysesamin | 63427-86-1 | C20H18O7 | – | – | [40] | |
| 55 | Succinic acid derivatives | Camphorataimide D | 656830-26-1 | C19H23NO4 | – | – | [40] |
| 56 | Phenyl methanoids | 4,7-Dimethoxy-5-methyl-1,3-benzodioxole | 165816-66-0 | C10H12O4 | Anti-inflammatory and anti-tumour activities | in vitro; in vivo | [88] |
| 57 | Ubiquinone derivatives | Antroquinonol | 1010081-09-0 | C24H38O4 | Anti-inflammatory, anti-HBV activities | in vitro | [42] |
| 58 | antroquinonol B | – | C24H36O6 | Anti-inflammatory activity | in vitro | ||
| 59 | antroquinonol C | – | C25H40O5 | Anti-breast cancer activity | in vitro; in vivo | [16] | |
| 60 | antroquinonol D | – | C23H36O3 | Anti-breast cancer activity | in vitro; in vivo | [46] | |
| 61 | antroquinonol L | – | C23H32O3 | – | – | [53] | |
| 62 | antroquinonol M | – | C23H32O3 | – | – | [48] | |
| 63 | antrocamol LT1 | – | C24H39O5 | Anti-colon cancer, anti-liver cancer, anti-kidney cancer activities | in vitro | [89] | |
| 64 | antrocamol LT2 | – | C26H40O6 | – | – | [89] | |
| 65 | antrocamol LT3 | – | C24H39O5 | – | – | [89] | |
| 66 | 4-acetyantroquinonol B | – | C26H38O7 | Anti-colorectal cancer activity | in vitro | [89] | |
| 67 | 4-acetylantrocamol LT3 | – | C26H40O6 | Anticancer activity | in vitro | [89] | |
| 68 | antrocinnamone | – | C23H32O3 | – | – | [49] | |
| 69 | Tocopherols | α-Tocospiro B | 601490-41-9 | C28H48O4 | – | – | [89] |
4. Pharmacological Studies on Phytochemicals Isolated from A. cinnamomea
To date, extensive research has elucidated the diverse bioactivities of A. cinnamomea. Advanced extraction techniques have facilitated the identification of various pharmacological properties, including anti-inflammatory, antitumor, and antioxidant activities [90]. These pharmacological effects will be explored in detail in the following sections, followed by a comprehensive overview of in vivo studies and human clinical trial results (Table 2 and Table 3).
4.1. Antrolone, 25R-Antcin A, and Versisponic Acid D: Potent Antioxidant and Anti-Inflammatory Agents for Oxidative Stress-Related Diseases
Oxidative stress, driven by reactive oxygen species (ROS), plays a key role in the pathogenesis of various diseases [91]. Antioxidant metabolites mitigate ROS-induced damage by scavenging ROS, suppressing pro-inflammatory cytokines, and modulating critical signalling pathways. Compounds such as antrolone, 25R-Antcin A, and Versisponic Acid D, isolated from A. cinnamomea, exhibit significant antioxidant and anti-inflammatory effects [92]. Their bioactivity underscores the therapeutic potential of A. cinnamomea in mitigating oxidative stress [93].
The benzenoid compound antrolone has been shown to possess significant anti-inflammatory effects by inhibiting LPS-induced production of nitric oxide (NO), prostaglandin E2 (PGE2), pro-inflammatory cytokines, and the chemokine CXCL1 in immune cells [94]. Furthermore, antrolone reduces the expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) proteins. Mechanistically, antrolone modulates the levels of Nrf2 and HO-1, which suppress the activation of NF-κB, MAPK, and AKT signalling pathways, leading to a downregulation of critical inflammatory pathways, particularly MAPK and NF-κB [95].
Moreover, compounds such as 25R-antcin A and versisponic acid D have demonstrated efficacy in reducing LPS-induced inflammation. At a concentration of 10 μM, 25R-antcin A and versisponic acid D exhibited half-inhibitory concentrations (IC50) of 19.61 ± 0.8 and 17.16 ± 1.0 μM, respectively [22]. In addition to benzenoids and triterpenoids, polyphenols isolated from A. cinnamomea exhibit potent in vitro antioxidant activity, with maximum scavenging rates of 94.10%, 83.34%, and 95.42% against DPPH, ABTS+, and hydroxyl radicals, respectively, at a concentration of 0.1 mg/mL [96]. The corresponding IC50 values—0.01 mg/mL for DPPH, 0.014 mg/mL for ABTS+, and 0.007 mg/mL for hydroxyl radicals—demonstrate the remarkable antioxidant efficacy of these polyphenols [97,98]. These findings underscore the therapeutic potential of A. cinnamomea in relief oxidative stress and inflammation which are the underlining symptoms of all diseases (Figure 4).
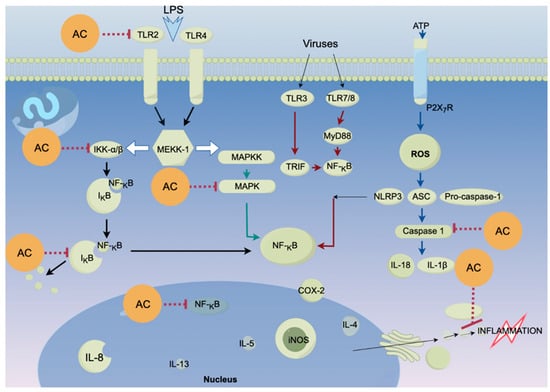
Figure 4.
Proposed mechanisms of action of Antrodia cinnamomea on ROS induced oxidation. AC, Antrodia cinnamomea extracts. The “…I” indicates that A. cinnamomea exhibits anti-inflammatory bioactivity by down-regulating these proteins/molecules. Arrows mean promoting or upregulating activity. The figure was generated using Figdraw (www.figdraw.com).
4.2. 4-Acetylantroquinonol B, Dehydroeburicoic Acid, and Antcins: Potent Antitumor Agents for Cancer Therapy
A. cinnamomea extracts have diverse anticancer properties across various cell lines in vitro. For example, the ethanol extract (EEAC) inhibited the growth of A549 lung cancer cells, with an approximate IC50 of 170 μg/mL after 24 h of treatment, and migration via suppression of cav-1 expression and activation of p-AMPK, p21, and p27, and EEAC also suppresses ovarian carcinoma (SKOV-3) cell proliferation through HER-2/neu pathway inhibition (IC50 = 196 μg/mL) and demonstrates cytotoxic effects on breast cancer cells (MDA-MB-231: IC50 = 136 μg/mL; MCF-7: IC50 = 316 μg/mL) [78,99,100]. Based on the high IC50 values, the activity is moderate and this may be due to the presence of components that have no activity. Further purification of the active compounds would reduce the IC50 values. Indeed, the ubiquinone derivative 4-acetylantroquinonol B (4-AAQB) enhances dendritic cell-mediated immunity, inhibits liver cancer stem cells, and arrests HepG2 cell proliferation (IC50 = 0.1 μg/mL) by modulating p53, p21, and p27 expression, while reducing hepatocellular carcinoma metastasis [101]. Furthermore, triterpenoids and sesquiterpenoids, such as dehydroeburicoic acid (IC50 = 3.4–25.7 μM), antcins B, H, K, and methyl antcinates demonstrate cytotoxicity against liver (HepG2, Huh7, Hep3B), colon (HCT-116), oral (SCC-9), and breast (MCF-7, MDA-MB-231) cancer cells (IC50 = 22.3–78.0 μM) via apoptosis induction, endoplasmic reticulum stress, and the inhibition of integrin-mediated adhesion and migration [58]. These findings underscore A. cinnamomea’s potential as a multifunctional therapeutic agent for cancer treatment (Figure 5).
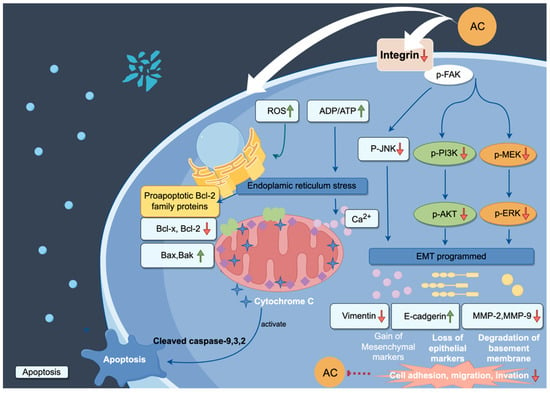
Figure 5.
Proposed mechanisms of action of bioactive compounds from A. cinnamomea (AC) in the suppression of cancer. Blue arrows mean promotion. Green arrows mean up-regulation, orange arrows mean down-regulation. The figure was generated using Figdraw (www.figdraw.com).
4.3. Antcin K and Dehydroeburicoic Acid and Eburicoic Acid as Potent Antidiabetic Agents
Diabetes mellitus stands as a significant cause of morbidity and mortality worldwide [102]. Purified triterpenoids from A. cinnamomea extracts, including antcin K and dehydroeburicoic acid have demonstrated potent α-glucosidase inhibitory activity, with exhibited stronger effects (EC50 = 0.025–0.21 mg/mL) compared to acarbose (EC50 = 0.278 mg/mL). These findings establish these active compounds as powerful mushroom-derived α-glucosidase inhibitors by blocking the conversion of maltose into glucose, thereby helping to regulate blood glucose levels and prevent spikes in the bloodstream [102]. Furthermore, eburicoic acid treatment increased hepatic PPARα expression, enhancing fatty acid oxidation, reducing lipid accumulation while reducing FAS expression and SREBP-1c levels, and leading to lower blood triglycerides and improved hepatic health. These studies suggest that A. cinnamomea mycelium powder or fruiting bodies may effectively improve insulin resistance for treating type 2 diabetes (T2D) [51].
4.4. Other Compounds from A. cinnamomea Extracts as Potential Therapeutic Agents for Neurological Disorders and SARS-CoV-2
Coenzyme Q0 induces ROS-mediated apoptotic and autophagic cell death, highlighting its potential effectiveness in treating central nervous system disease glioblastoma multiforme by inhibiting growth and suppressing colony formation by activating caspase-3, cleaving PARP, and dysregulating the Bax/Bcl-2 expression, also inhibits the PI3K/AKT/mTOR signalling pathway, which is crucial in apoptosis and autophagy mechanisms [79]. Furthermore, Antcin-A, Antcin-B, Antcin-C, and Antcin-I strongly show anti-acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by reducing human ACE2 levels in HT-29 cells with a moderate reduction was observed in Antcin-H treated cells (5.91 ng/mL) [69].
4.5. In Vivo Study in Animal Models with Crude Extract
Recent research on A. cinnamomea extracts and pure compounds demonstrates promising anticancer effects across various cancer types and preclinical models (Table 4) [22]. A. cinnamomea dropping pills (ACDPs) at high (400 mg/kg/day) and low (200 mg/kg/day) doses dose-dependently inhibited tumour growth, reducing tumour weight by 48% and 67% and decreasing tumour volume without noticeable toxicity [74]. These effects were mediated by the inhibition of the PI3K/AKT signalling pathway in hepatocellular carcinoma (HCC). In leukaemia models, oral administration of an ethanol extract of A. cinnamomea fruiting bodies (0.9 g/kg for two weeks) reduced p-ERK1/2, p-Akt, and MMP-9 expression while increasing p21 and p27, markers of cell cycle arrest [77]. In lung cancer, ethanolic A. cinnamomea extracts (0.25 and 0.5 g/kg) reduced tumour size by 30–58% after 7 days and 68–76% after 14 days [78]. Compounds from A. cinnamomea also exhibit potent anticancer effects, 4-acetylantroquinonol B (2.5 mg/kg, intraperitoneally for 6 weeks) inhibited colorectal cancer xenograft growth by regulating the Lgr5/Wnt/β-catenin and JAK-STAT pathways, and dehydroeburicoic acid suppressed tumour growth in HL60 xenograft mice through DNA damage and mitochondrial dysfunction [103]. It also mitigated non-alcoholic fatty liver disease (NAFLD) by enhancing ALDH2 activity, promoting ROS elimination, and suppressing lipogenesis [70].

Table 4.
In vivo bioactivity of A. cinnamomea extracts.
A. cinnamomea extracts also show therapeutic potential for nervous system disorders. CoQ0 (1.5 mg/kg, subcutaneously every two days) induced autophagy and exerted an antimetastatic effect in models of nervous system tumours [35]. In oesophageal cancer, the oral administration of A. cinnamomea-MFB (1.0 mg/mL for 34 days) enhanced the effectiveness of radiation therapy, delaying tumour growth [104]. These findings demonstrate A. cinnamomea potential as a multi-targeted therapeutic agent, with anticancer and neuroprotective effects mediated through mechanisms such as PI3K/AKT inhibition, cell cycle regulation, autophagy induction, and mitochondrial dysfunction.
4.6. Human Clinical Trials on Antrodia cinnamomea Extracts
Some clinical trials have highlighted the therapeutic potential of A. cinnamomea across multiple health domains (Table 5). A. cinnamomea extracts and metabolites exhibit significant immunomodulatory, hepatoprotective, antihypertensive, and cholesterol-lowering effects, supporting its broad medicinal applications. A Phase I study of LEAC-102, an A. cinnamomea-derived extract, assessed its safety and immunomodulatory effects in 18 healthy adults (20–44 years) with escalating doses up to 2988 mg/day [105]. LEAC-102 showed a favourable safety profile with no serious adverse effects and enhanced T-cell activation, upregulating PD-1 expression, suggesting potential utility in cancer immunotherapy.

Table 5.
Human trials bioactivity of A. cinnamomea extracts.
Daily supplementation with A. cinnamomea mycelium resulted in significant reductions in systolic and diastolic blood pressure, attributed to plasma renin activity inhibition and reduced oxidative stress, indicating its potential as an antihypertensive agent. Another study of 28 participants with non-alcoholic steatohepatitis (NASH) showed that 420 mg A. cinnamomea mycelium daily for 3–6 months significantly reduced liver steatosis and TNF-α levels without adverse events, demonstrating its hepatoprotective efficacy [106,107]. For cholesterol management, 36 participants consuming 380 mg of AC mycelium (LAC) capsules twice daily for 3 months showed modest reductions in total cholesterol without adverse effects on liver or renal function [108]. Additionally, 44 Japanese adults taking 250 mg A. cinnamomea mycelium extract daily for 12 weeks experienced improved liver health, particularly in individuals with regular alcohol consumption [109].
These studies underscore the safety and efficacy of A. cinnamomea as a therapeutic agent with broad applications in immune modulation, liver protection, blood pressure regulation, and cholesterol management, providing a strong foundation for further clinical research.
5. Industrial Applications and Quality Control Issues of A. cinnamomea Extract Products
The development of natural products with verified bioactivity, such as A. cinnamomea, offers significant potential for advancing public health. Industrial applications of A. cinnamomea span a wide range of functional foods and nutraceuticals, including wines (CN104017703A), tablets (CN103784482B), dripping pills (WO2016184147A1), tea (TW201221065A), and soft confectioneries (CN104186880A) enriched with its bioactive constituents. Furthermore, A. cinnamomea extracts have demonstrated potential therapeutic effects, including the inhibition of cariogenic and periodontopathic bacteria such as Streptococcus mutans and Porphyromonas gingivalis, thereby mitigating dental plaque formation and offering oral health benefits(CN104127355A).
Despite its broad applications, the commercialization of A. cinnamomea products is hindered by significant quality control challenges. The content of bioactive triterpenoids, a key indicator of product efficacy, varies substantially between wild and cultivated strains. Additionally, many commercial products are derived from lower-quality mycelium rather than fruiting bodies, resulting in diminished bioactive compound profiles. Current quality control practices, often limited to assessing a narrow range of marker compounds (e.g., antcin A, B, C, K) using HPLC, fail to address broader variations and may lack sensitivity. Issues such as insufficient standardization exacerbate the inconsistency and unreliability of A. cinnamomea products. These shortcomings highlight the urgent need for comprehensive and robust quality control methodologies to ensure consistency, authenticity, and efficacy across A. cinnamomea-based products. To ensure the quality and consistency of A. cinnamomea-derived products on the market, always recommend including key triterpenoids as marker compounds. Due to the significant presence of terpenoids, which account for 63% of the composition in the fruiting bodies of A. cinnamomea, this group of natural compounds has become the subject of numerous phytochemical studies. The identified triterpenoids—antcin K, (25S)-antcin H, (25R)-antcin H, (25R)-antcin C, (25S)-antcin C, (25R)-antcin A, 15α-acetyl-dehydrosulphurenic acid, versisponic acid D, dehydroeburicoic acid, and eburicoic acid—serve as essential indicators of bioactive content in A. cinnamomea. These compounds were selected for their significant pharmacological activities, particularly their promising anti-inflammatory effects, and can serve as reliable indicators of bioactive content in commercial formulations. To ensure reliable quantification, also had study developed validated HPLC-UV method demonstrating high sensitivity, linearity, precision, and accuracy [110]. Incorporating these triterpenoids into the quality control framework can help mitigate the variability often observed in the market and enhance the standardization and reliability of A. cinnamomea products. We hope this method offers a rigorous solution to the quality control challenges associated with the highly valued A. cinnamomea food and nutraceutical products, which often exhibit significant variability and inconsistency, thereby contributing to the standardization and reliability of these products in the market.
6. Summary and Future Perspectives
Pharmacologically active natural compounds have long been recognized for their roles in drug discovery and development. A. cinnamomea, an emerging medicinal fungus, is known for its diverse biological activities and therapeutic potential. Despite significant progress in isolating bioactive compounds from its fruiting bodies and cultured mycelia, several challenges persist, hindering its full application in nutraceuticals and pharmaceuticals.
A primary limitation lies in the inefficiency of conventional solvent extraction methods, which are often characterized by high solvent consumption, low yields, and adverse environmental impacts. The health risks associated with organic solvents further emphasize the need for sustainable and efficient extraction technologies. Future studies should focus on developing environmentally friendly solvents and optimizing extraction processes to enhance yield while minimizing ecological and health concerns. The systematic identification of characteristic metabolites in A. cinnamomea remains inadequate, with current techniques for isolation, purification, and characterization limiting the development of high-quality products. Establishing advanced and standardized methodologies for metabolite profiling is critical to facilitate the industrial application of A. cinnamomea as a reliable source of bioactive compounds. Finally, the mechanisms underlying the pharmacological activities of specific A. cinnamomea compounds remain poorly understood. The research to date has largely relied on crude extracts or compound mixtures, complicating the identification of individual roles. Understanding the synergistic, antagonistic, or independent effects of these bioactive compounds is essential to ensure efficacy and safety. The isolation and characterization of pure compounds will enable the elucidation of their mechanisms of action, facilitating their development as targeted therapeutic agents.
Addressing these challenges through innovative approaches in extraction technologies, metabolite profiling, and mechanism-based studies will unlock the full potential of A. cinnamomea as a nutraceutical and pharmaceutical resource. Furthermore, integrating sustainable practices, such as waste valorization, will enhance its environmental and economic viability. These advancements will not only support the evidence-based application of A. cinnamomea but also contribute to the development of next-generation nutraceuticals and therapeutics for global health challenges.
Author Contributions
C.X.: Writing manuscript. Q.X.: Improving the manuscript. C.-L.K.: Investigation, X.Y.: Conceptualization, Writing—review and editing, Project administration. D.H.: Supervision, Conceptualization, Writing—review and editing, Project administration, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Singapore Ministry of Education, grant number [R160-000-B04-114] as well as internal fund from National University of Singapore (Suzhou) Research Institute for a grant under Peak of Excellence Scheme to Huang Dejian, National University of Singapore. This work was supported by Science and Technology Project of Jiangsu Province, grant number: BZ2022056.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, H.-X.; Wang, J.-J.; Lu, C.-L.; Gao, Y.-J.; Gao, L.; Yang, Z.-Q. Review of Bioactivity, Isolation, and Identification of Active Compounds from Antrodia cinnamomea. Bioengineering 2022, 9, 494. [Google Scholar] [CrossRef]
- Ganesan, N.; Baskaran, R.; Velmurugan, B.K.; Thanh, N.C. Antrodia cinnamomea—An updated minireview of its bioactive components and biological activity. J. Food Biochem. 2019, 43, e12936. [Google Scholar] [CrossRef]
- Lu, M.-C.; El-Shazly, M.; Wu, T.-Y.; Du, Y.-C.; Chang, T.-T.; Chen, C.-F.; Hsu, Y.-M.; Lai, K.-H.; Chiu, C.-P.; Chang, F.-R.; et al. Recent research and development of Antrodia cinnamomea. Pharmacol. Ther. 2013, 139, 124–156. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.J.S.; Wang, S.-Y. Antioxidant Properties of Antrodia cinnamomea: An Extremely Rare and Coveted Medicinal Mushroom Endemic to Taiwan. In Medicinal Plants and Fungi: Recent Advances in Research and Development; Agrawal, D.C., Tsay, H.-S., Shyur, L.-F., Wu, Y.-C., Wang, S.-Y., Eds.; Springer: Singapore, 2017; pp. 135–164. [Google Scholar]
- Zhang, B.-B.; Hu, P.-F.; Huang, J.; Hu, Y.-D.; Chen, L.; Xu, G.-R. Current advances on the structure, bioactivity, synthesis, and metabolic regulation of novel ubiquinone derivatives in the edible and medicinal mushroom Antrodia cinnamomea. J. Agric. Food Chem. 2017, 65, 10395–10405. [Google Scholar]
- Tsai, Y.-J.; Huang, J.-W.; Wu, M.-D.; Cheng, M.-J. A New Ubiquinone Derivative from the Fungus of Antrodia cinnamomea. Chem. Nat. Compd. 2023, 59, 269–271. [Google Scholar] [CrossRef]
- Chen, C.Y.; Yeh, H.C.; Li, H.T.; Liu, S.L.; Cheng, M.J. Secondary Metabolites of Cinnamomum kanehirae. Chem. Nat. Compd. 2024, 60, 36–38. [Google Scholar] [CrossRef]
- Luo, Z.-S.; Lu, Z.-M.; Hu, M.-M.; Li, X.-Y.; Xu, G.-Q.; Gong, J.-S.; Shi, J.-S.; Xu, Z.-H. Characterization of Cinnamomum kanehirae Extract-Stimulated Triterpenoids Synthesis in Submerged Fermentation of Antrodia camphorata via Untargeted Metabolomics. J. Agric. Food Chem. 2023, 71, 9175–9186. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, A.S.; Sharma, N.; Bhatia, S.; Verma, A.; Soni, S.; Batra, N. Insights on sustainable approaches for production and applications of value added products. Chemosphere 2022, 286, 131623. [Google Scholar] [CrossRef]
- Zhang, B.-B.; Guan, Y.-Y.; Hu, P.-F.; Chen, L.; Xu, G.-R.; Liu, L.; Cheung, P.C.K. Production of bioactive metabolites by submerged fermentation of the medicinal mushroom Antrodia cinnamomea: Recent advances and future development. Crit. Rev. Biotechnol. 2019, 39, 541–554. [Google Scholar] [CrossRef]
- Li, Y.; Ge, J.; Li, Y.; Zheng, S.; Liu, Y.; Liang, Y.; Mei, Y. Isolation, Purification, and Antitumor Activity of a Novel Active Protein from Antrodia cinnamomea Liquid Fermentation Mycelia. Fermentation 2023, 9, 185. [Google Scholar] [CrossRef]
- Liu, D.-Z.; Liang, Y.-C.; Lin, S.-Y.; Lin, Y.-S.; Wu, W.-C.; Hou, W.-C.; Su, C.-H. Antihypertensive activities of a solid-state culture of Taiwanofungus camphoratus (Chang-Chih) in spontaneously hypertensive rats. Biosci. Biotechnol. Biochem. 2007, 71, 23–30. [Google Scholar]
- Yang, F.-C.; Ma, T.-W.; Chuang, Y.-T. Medium modification to enhance the formation of bioactive metabolites in shake flask cultures of Antrodia cinnamomea by adding citrus peel extract. Bioprocess Biosyst. Eng. 2012, 35, 1251–1258. [Google Scholar]
- Tsai, M.-C.; Song, T.-Y.; Shih, P.-H.; Yen, G.-C. Antioxidant properties of water-soluble polysaccharides from Antrodia cinnamomea in submerged culture. Food Chem. 2007, 104, 1115–1122. [Google Scholar]
- Shen, Y.-C.; Chou, C.-J.; Wang, Y.-H.; Chen, C.-F.; Chou, Y.-C.; Lu, M.-K. Anti-inflammatory activity of the extracts from mycelia of Antrodia camphorata cultured with water-soluble fractions from five different Cinnamomum species. FEMS Microbiol. Lett. 2004, 231, 137–143. [Google Scholar]
- Kumar, K.S.; Chu, F.-H.; Hsieh, H.-W.; Liao, J.-W.; Li, W.-H.; Lin, J.C.-C.; Shaw, J.-F.; Wang, S.-Y. Antroquinonol from ethanolic extract of mycelium of Antrodia cinnamomea protects hepatic cells from ethanol-induced oxidative stress through Nrf-2 activation. J. Ethnopharmacol. 2011, 136, 168–177. [Google Scholar] [PubMed]
- Chen, Y.-Y.; Chou, P.-Y.; Chien, Y.-C.; Wu, C.-H.; Wu, T.-S.; Sheu, M.-J. Ethanol extracts of fruiting bodies of Antrodia cinnamomea exhibit anti-migration action in human adenocarcinoma CL1-0 cells through the MAPK and PI3K/AKT signaling pathways. Phytomedicine 2012, 19, 768–778. [Google Scholar] [PubMed]
- Vilkhu, K.; Mawson, R.; Simons, L.; Bates, D. Applications and opportunities for ultrasound assisted extraction in the food industry—A review. Innov. Food Sci. Emerg. Technol. 2008, 9, 161–169. [Google Scholar]
- Puri, M.; Sharma, D.; Barrow, C.J. Enzyme-assisted extraction of bioactives from plants. Trends Biotechnol. 2012, 30, 37–44. [Google Scholar]
- Corzzini, S.C.; Barros, H.D.; Grimaldi, R.; Cabral, F.A. Extraction of edible avocado oil using supercritical CO2 and a CO2/ethanol mixture as solvents. J. Food Eng. 2017, 194, 40–45. [Google Scholar]
- Huang, H.-W.; Hsu, C.-P.; Yang, B.B.; Wang, C.-Y. Advances in the extraction of natural ingredients by high pressure extraction technology. Trends Food Sci. Technol. 2013, 33, 54–62. [Google Scholar]
- Yang, X.; Wang, X.; Lin, J.; Lim, S.; Cao, Y.; Chen, S.; Xu, P.; Xu, C.; Zheng, H.; Fu, K.-C. Structure and anti-inflammatory activity relationship of ergostanes and lanostanes in Antrodia cinnamomea. Foods 2022, 11, 1831. [Google Scholar] [CrossRef]
- Huang, H.W.; Chen, B.Y.; Wang, C.Y. Extraction of bioactive ingredients from fruiting bodies of Antrodia cinnamomea assisted by high hydrostatic pressure. Foods 2019, 8, 588. [Google Scholar] [CrossRef] [PubMed]
- Lien, H.M.; Chiu, C.H.; Chen, C.C.; Chang, W.L.; Chyau, C.C.; Peng, R.Y. Comparison of the apoptotic effects of supercritical fluid extracts of Antrodia cinnamomea mycelia on hepatocellular carcinoma cells. Molecules 2014, 19, 9033–9050. [Google Scholar] [CrossRef]
- Huang, Y.-J.; Yadav, V.K.; Srivastava, P.; Wu, A.T.; Huynh, T.-T.; Wei, P.-L.; Huang, C.-Y.F.; Huang, T.-H. Antrodia cinnamomea enhances chemo-sensitivity of 5-FU and suppresses colon tumorigenesis and cancer stemness via up-regulation of tumor suppressor miR-142-3p. Biomolecules 2019, 9, 306. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ma, H.; He, R.; Ren, X.; Zhou, C. Prospects and application of ultrasound and magnetic fields in the fermentation of rare edible fungi. Ultrason. Sonochem. 2021, 76, 105613. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.-H.; Peng, C.-C.; Ker, Y.-B.; Chen, C.-C.; Lee, A.; Chang, W.-L.; Chyau, C.-C.; Peng, R.Y. Physicochemical characteristics and anti-inflammatory activities of antrodan, a novel glycoprotein isolated from Antrodia cinnamomea mycelia. Molecules 2013, 19, 22–40. [Google Scholar] [CrossRef]
- He, R.; Wu, K.; Zhang, A.; Xie, Z.; Sun, P. Mechanochemical-assisted extraction and pharmacological study of triterpenoids from Antrodia camphorata. Appl. Sci. 2019, 9, 4281. [Google Scholar] [CrossRef]
- Chang, H.-L.; Chao, G.-R.; Chen, C.-C.; Mau, J.-L. Non-volatile taste components of Agaricus blazei, Antrodia camphorata and Cordyceps militaris mycelia. Food Chem. 2001, 74, 203–207. [Google Scholar]
- Liu, X.; Yu, S.; Zhang, Y.; Zhang, W.; Zhong, H.; Lu, X.; Guan, R. A review on the protective effect of active components in Antrodia camphorata against alcoholic liver injury. J. Ethnopharmacol. 2023, 300, 115740. [Google Scholar]
- LI, Y.-F. Effects of phytohormones and lignin on growth of Antrodia cinnamomea mycelium. Chin. Tradit. Herb. Drugs 2019, 24, 1453–1460. [Google Scholar]
- You, R.-I.; Lee, Y.-P.; Su, T.-Y.; Lin, C.-C.; Chen, C.-S.; Chu, C.-L. A Benzenoid 4, 7-Dimethoxy-5-Methyl-L, 3-Benzodioxole from Antrodia cinnamomea attenuates dendritic cell-mediated th2 allergic responses. Am. J. Chin. Med. 2019, 47, 1271–1287. [Google Scholar] [CrossRef]
- Yen, I.-C.; Shi, L.-S.; Chung, M.-C.; Ahmetaj-Shala, B.; Chang, T.-C.; Lee, S.-Y. Antrolone, a novel benzoid derived from Antrodia cinnamomea, inhibits the LPS-induced inflammatory response in RAW264. 7 macrophage cells by balancing the NF-κ B and Nrf2 pathways. Am. J. Chin. Med. 2018, 46, 1297–1313. [Google Scholar] [PubMed]
- Hseu, Y.-C.; Tsai, T.-J.; Korivi, M.; Liu, J.-Y.; Chen, H.-J.; Lin, C.-M.; Shen, Y.-C.; Yang, H.-L. Antitumor properties of Coenzyme Q0 against human ovarian carcinoma cells via induction of ROS-mediated apoptosis and cytoprotective autophagy. Sci. Rep. 2017, 7, 8062. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-M.; Yang, H.-L.; Thiyagarajan, V.; Huang, T.-H.; Huang, P.-J.; Chen, S.-C.; Liu, J.-Y.; Hsu, L.-S.; Chang, H.-W.; Hseu, Y.-C. Coenzyme Q0 enhances ultraviolet B–induced apoptosis in human estrogen receptor–positive breast (MCF-7) cancer cells. Integr. Cancer Ther. 2017, 16, 385–396. [Google Scholar] [CrossRef]
- Chou, W.-L.; Lee, T.-H.; Huang, T.-H.; Wang, P.-W.; Chen, Y.-P.; Chen, C.-C.; Chang, Z.-Y.; Fang, J.-Y.; Yang, S.-C. Coenzyme Q0 from Antrodia cinnamomea exhibits drug-resistant bacteria eradication and keratinocyte inflammation mitigation to ameliorate infected atopic dermatitis in mouse. Front. Pharmacol. 2019, 10, 1445. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.S.; Kim, B.; Shin, J.H.; Choi, Y.J.; Choi, S.; Song, C.W.; Lee, J.; Park, H.G.; Lee, S.Y. Bio-based production of C2–C6 platform chemicals. Biotechnol. Bioeng. 2012, 109, 2437–2459. [Google Scholar]
- Nakamura, N.; Hirakawa, A.; Gao, J.-J.; Kakuda, H.; Shiro, M.; Komatsu, Y.; Sheu, C.-c.; Hattori, M. Five New Maleic and Succinic Acid Derivatives from the Mycelium of Antrodia c amphorata and Their Cytotoxic Effects on LLC Tumor Cell Line. J. Nat. Prod. 2004, 67, 46–48. [Google Scholar] [CrossRef]
- Geng, Y.; Wang, J.; Sun, Q.; Xie, M.; Lu, Z.M.; Xu, H.y.; Shi, J.S.; Xu, Z.H. Identification of antrodin B from Antrodia camphorata as a new anti-hepatofibrotic compound using a rapid cell screening method and biological evaluation. Hepatol. Res. 2016, 46, E15–E25. [Google Scholar] [CrossRef]
- Wu, M.-D.; Cheng, M.-J.; Wang, B.-C.; Yech, Y.-J.; Lai, J.-T.; Kuo, Y.-H.; Yuan, G.-F.; Chen, I.-S. Maleimide and maleic anhydride derivatives from the mycelia of Antrodia cinnamomea and their nitric oxide inhibitory activities in macrophages. J. Nat. Prod. 2008, 71, 1258–1261. [Google Scholar] [CrossRef]
- Shie, P.-H.; Wang, S.-Y.; Lay, H.-L.; Huang, G.-J. 4, 7-Dimethoxy-5-methyl-1, 3-benzodioxole from Antrodia camphorata inhibits LPS-induced inflammation via suppression of NF-κB and induction HO-1 in RAW264. 7 cells. Int. Immunopharmacol. 2016, 31, 186–194. [Google Scholar] [CrossRef]
- Yi, Z.; Liu, X.; Liang, L.; Wang, G.; Xiong, Z.; Zhang, H.; Song, X.; Ai, L.; Xia, Y. Antrodin A from Antrodia camphorata modulates the gut microbiome and liver metabolome in mice exposed to acute alcohol intake. Food Funct. 2021, 12, 2925–2937. [Google Scholar]
- Hsieh, Y.-Y.; Lee, K.-C.; Cheng, K.-C.; Lee, K.-F.; Yang, Y.-L.; Chu, H.-T.; Lin, T.-W.; Chen, C.-C.; Hsieh, M.-C.; Huang, C.-Y. Antrodin C Isolated from Antrodia Cinnamomea Induced Apoptosis through ROS/AKT/ERK/P38 Signaling Pathway and Epigenetic Histone Acetylation of TNFα in Colorectal Cancer Cells. Antioxidants 2023, 12, 764. [Google Scholar] [CrossRef]
- Lee, T.-H.; Lee, C.-K.; Tsou, W.-L.; Liu, S.-Y.; Kuo, M.-T.; Wen, W.-C. A new cytotoxic agent from solid-state fermented mycelium of Antrodia camphorata. Planta Medica 2007, 73, 1412–1415. [Google Scholar] [CrossRef]
- Sulake, R.S.; Jiang, Y.-F.; Lin, H.-H.; Chen, C. Total synthesis of (±)-antroquinonol d. J. Org. Chem. 2014, 79, 10820–10828. [Google Scholar]
- Wang, S.-C.; Lee, T.-H.; Hsu, C.-H.; Chang, Y.-J.; Chang, M.-S.; Wang, Y.-C.; Ho, Y.-S.; Wen, W.-C.; Lin, R.-K. Antroquinonol D, isolated from Antrodia camphorata, with DNA demethylation and anticancer potential. J. Agric. Food Chem. 2014, 62, 5625–5635. [Google Scholar] [PubMed]
- Lin, Y.W.; Pan, J.H.; Liu, R.H.; Kuo, Y.H.; Sheen, L.Y.; Chiang, B.H. The 4-acetylantroquinonol B isolated from mycelium of Antrodia cinnamomea inhibits proliferation of hepatoma cells. J. Sci. Food Agric. 2010, 90, 1739–1744. [Google Scholar]
- Chen, Y.-c.; Liu, Y.-l.; Li, F.-y.; Chang, C.-I.; Wang, S.-y.; Lee, K.-y.; Li, S.-l.; Chen, Y.-p.; Jinn, T.-r.; Tzen, J.T. Antcin A, a steroid-like compound from Antrodia camphorata, exerts anti-inflammatory effect via mimicking glucocorticoids. Acta Pharmacol. Sin. 2011, 32, 904–911. [Google Scholar]
- Lin, C.-H.; Kuo, Y.-H.; Shih, C.-C. Eburicoic acid, a triterpenoid compound from Antrodia camphorata, displays antidiabetic and antihyperlipidemic effects in palmitate-treated C2C12 myotubes and in high-fat diet-fed mice. Int. J. Mol. Sci. 2017, 18, 2314. [Google Scholar] [CrossRef]
- Lin, C.-H.; Hsiao, L.-W.; Kuo, Y.-H.; Shih, C.-C. Antidiabetic and antihyperlipidemic effects of sulphurenic acid, a triterpenoid compound from Antrodia camphorata, in streptozotocin-induced diabetic mice. Int. J. Mol. Sci. 2019, 20, 4897. [Google Scholar] [CrossRef]
- Perera, N.; Yang, F.-L.; Chang, C.-M.; Lu, Y.-T.; Zhan, S.-H.; Tsai, Y.-T.; Hsieh, J.-F.; Li, L.-H.; Hua, K.-F.; Wu, S.-H. Galactomannan from Antrodia cinnamomea Enhances the Phagocytic Activity of Macrophages. Org. Lett. 2017, 19, 3486–3489. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Wu, H.-C.; Chang, J.-M.; Ko, H.-H.; Lin, C.-H.; Chang, H.-S. Chemical investigations and cytotoxic effects of metabolites from Antrodia camphorata against human hepatocellular carcinoma cells. Nat. Prod. Res. 2022, 37, 560–570. [Google Scholar]
- Lin, H.-C.; Lin, M.-H.; Liao, J.-H.; Wu, T.-H.; Lee, T.-H.; Mi, F.-L.; Wu, C.-H.; Chen, K.-C.; Cheng, C.-H.; Lin, C.-W. Antroquinonol, a ubiquinone derivative from the mushroom Antrodia camphorata, inhibits colon cancer stem cell-like properties: Insights into the molecular mechanism and inhibitory targets. J. Agric. Food Chem. 2017, 65, 51–59. [Google Scholar]
- Mizuno, K.; Sasaki, A.T.; Watanabe, K.; Watanabe, Y. Ubiquinol-10 intake is effective in relieving mild fatigue in healthy individuals. Nutrients 2020, 12, 1640. [Google Scholar] [CrossRef]
- Sabbatinelli, J.; Orlando, P.; Galeazzi, R.; Silvestri, S.; Cirilli, I.; Marcheggiani, F.; Dludla, P.V.; Giuliani, A.; Bonfigli, A.R.; Mazzanti, L. Ubiquinol ameliorates endothelial dysfunction in subjects with mild-to-moderate dyslipidemia: A randomized clinical trial. Nutrients 2020, 12, 1098. [Google Scholar] [CrossRef]
- Du, Y.-C.; Chang, F.-R.; Wu, T.-Y.; Hsu, Y.-M.; El-Shazly, M.; Chen, C.-F.; Sung, P.-J.; Lin, Y.-Y.; Lin, Y.-H.; Wu, Y.-C. Antileukemia component, dehydroeburicoic acid from Antrodia camphorata induces DNA damage and apoptosis in vitro and in vivo models. Phytomedicine 2012, 19, 788–796. [Google Scholar]
- Qiao, X.; Wang, Q.; Ji, S.; Huang, Y.; Zhang, Z.-x.; Bo, T.; Tzeng, Y.-m.; Guo, D.-a.; Ye, M. Metabolites identification and multi-component pharmacokinetics of ergostane and lanostane triterpenoids in the anticancer mushroom Antrodia cinnamomea. J. Pharm. Biomed. Anal. 2015, 111, 266–276. [Google Scholar]
- Kuang, Y.; Li, B.; Wang, Z.; Qiao, X.; Ye, M. Terpenoids from the medicinal mushroom Antrodia camphorata: Chemistry and medicinal potential. Nat. Prod. Rep. 2021, 38, 83–102. [Google Scholar]
- Liu, Y.; Ding, Y.; Ye, M.; Zhu, T.; Tian, D.; Ding, K. A novel heterogalactan from Antrodia camphorata and anti-angiogenic activity of its sulfated derivative. Polymers 2017, 9, 228. [Google Scholar] [CrossRef]
- Lin, C.-C.; Pan, I.-H.; Li, Y.-R.; Pan, Y.-G.; Lin, M.-K.; Lu, Y.-H.; Wu, H.-C.; Chu, C.-L. The adjuvant effects of high-molecule-weight polysaccharides purified from Antrodia cinnamomea on dendritic cell function and DNA vaccines. PLoS ONE 2015, 10, e0116191. [Google Scholar]
- Borchers, A.T.; Keen, C.L.; Gershwin, M.E. Mushrooms, tumors, and immunity: An update. Exp. Biol. Med. 2004, 229, 393–406. [Google Scholar]
- Ooi, V.E. Antitumor and immunomodulatory activities of mushroom polysaccharides. In Mushrooms as Functional Foods; Wiley: Hoboken, NJ, USA, 2008; pp. 147–198. [Google Scholar]
- Han, C.; Guo, L.; Yang, Y.; Li, W.; Sheng, Y.; Wang, J.; Guan, Q.; Zhang, X. Study on antrodia camphorata polysaccharide in alleviating the neuroethology of PD mice by decreasing the expression of NLRP3 inflammasome. Phytother. Res. 2019, 33, 2288–2297. [Google Scholar]
- Chen, P.-C.; Chen, C.-C.; Ker, Y.-B.; Chang, C.-H.; Chyau, C.-C.; Hu, M.-L. Anti-metastatic effects of antrodan with and without cisplatin on Lewis lung carcinomas in a mouse xenograft model. Int. J. Mol. Sci. 2018, 19, 1565. [Google Scholar] [CrossRef] [PubMed]
- Fa, K.-N.; Yang, C.-M.; Chen, P.-C.; Lee, Y.-Y.; Chyau, C.-C.; Hu, M.-L. Anti-metastatic effects of antrodan, the Antrodia cinnamomea mycelia glycoprotein, in lung carcinoma cells. Int. J. Biol. Macromol. 2015, 74, 476–482. [Google Scholar]
- Chyau, C.-C.; Wang, H.-F.; Zhang, W.-J.; Chen, C.-C.; Huang, S.-H.; Chang, C.-C.; Peng, R.Y. Antrodan alleviates high-fat and high-fructose diet-induced fatty liver disease in C57BL/6 mice model via AMPK/Sirt1/SREBP-1c/PPARγ pathway. Int. J. Mol. Sci. 2020, 21, 360. [Google Scholar] [CrossRef]
- Perera, N.; Yang, F.-L.; Lu, Y.-T.; Li, L.-H.; Hua, K.-F.; Wu, S.-H. Antrodia cinnamomea galactomannan elicits immuno-stimulatory activity through Toll-like receptor 4. Int. J. Biol. Sci. 2018, 14, 1378. [Google Scholar]
- Wu, D.P.; Chiang, H.C. Constituents of Antrodia cinnamomea. J. Chin. Chem. Soc. 1995, 42, 797–800. [Google Scholar]
- Senthil Kumar, K.; Gokila Vani, M.; Hsieh, H.-W.; Lin, C.-C.; Wang, S.-Y. Antcins from Antrodia cinnamomea and Antrodia salmonea inhibit angiotensin-converting enzyme 2 (ACE2) in epithelial cells: Can be potential candidates for the development of SARS-CoV-2 prophylactic agents. Plants 2021, 10, 1736. [Google Scholar] [CrossRef]
- Cao, Y.-n.; Yue, S.-s.; Wang, A.-y.; Xu, L.; Hu, Y.-t.; Qiao, X.; Wu, T.-Y.; Ye, M.; Wu, Y.-C.; Qi, R. Antrodia cinnamomea and its compound dehydroeburicoic acid attenuate nonalcoholic fatty liver disease by upregulating ALDH2 activity. J. Ethnopharmacol. 2022, 292, 115146. [Google Scholar]
- Deng, J.-Y.; Chen, S.-J.; Jow, G.-M.; Hsueh, C.-W.; Jeng, C.-J. Dehydroeburicoic acid induces calcium-and calpain-dependent necrosis in human U87MG glioblastomas. Chem. Res. Toxicol. 2009, 22, 1817–1826. [Google Scholar]
- Lu, Z.-M.; Geng, Y.; Li, H.-X.; Sun, Q.; Shi, J.-S.; Xu, Z.-H. Alpha-terpineol promotes triterpenoid production of Antrodia cinnamomea in submerged culture. FEMS Microbiol. Lett. 2014, 358, 36–43. [Google Scholar] [PubMed]
- Huo, Y.; Win, S.; Than, T.A.; Yin, S.; Ye, M.; Hu, H.; Kaplowitz, N. Antcin H protects against acute liver injury through disruption of the interaction of c-Jun-N-terminal kinase with mitochondria. Antioxid. Redox Signal. 2017, 26, 207–220. [Google Scholar]
- Zhang, Y.; Lv, P.; Ma, J.; Chen, N.; Guo, H.; Chen, Y.; Gan, X.; Wang, R.; Liu, X.; Fan, S. Antrodia cinnamomea exerts an anti-hepatoma effect by targeting PI3K/AKT-mediated cell cycle progression in vitro and in vivo. Acta Pharm. Sin. B 2022, 12, 890–906. [Google Scholar]
- Su, Y.-H.; Wu, J.-S.; Dai, Y.-Z.; Chen, Y.-T.; Lin, Y.-X.; Tzeng, Y.-M.; Liao, J.-W. Anti-Oxidant, Anti-Mutagenic Activity and Safety Evaluation of Antrocin. Toxics 2023, 11, 547. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, R.; Li, L.; Dong, R.; Yin, H.; Wang, Y.; Yang, A.; Wang, J.; Li, C.; Wang, D. The triterpenoids-enriched extracts from Antrodia cinnamomea mycelia attenuate alcohol-induced chronic liver injury via suppression lipid accumulation in C57BL/6 mice. Food Sci. Hum. Wellness 2021, 10, 497–507. [Google Scholar]
- Liu, F.-C.; Lai, M.-T.; Chen, Y.-Y.; Lin, W.-H.; Chang, S.-J.; Sheu, M.-J.; Wu, C.-H. Elucidating the inhibitory mechanisms of the ethanolic extract of the fruiting body of the mushroom Antrodia cinnamomea on the proliferation and migration of murine leukemia WEHI-3 cells and their tumorigenicity in a BALB/c allograft tumor model. Phytomedicine 2013, 20, 874–882. [Google Scholar]
- Wu, C.-H.; Liu, F.-C.; Pan, C.-H.; Lai, M.-T.; Lan, S.-J.; Wu, C.-H.; Sheu, M.-J. Suppression of cell growth, migration and drug resistance by ethanolic extract of Antrodia cinnamomea in human lung cancer A549 cells and C57BL/6J allograft tumor model. Int. J. Mol. Sci. 2018, 19, 791. [Google Scholar] [CrossRef]
- Lu, C.-W.; Wu, W.-J.; Nguyen, T.K.N.; Shen, S.-C.; Wu, Y.-B.; Liang, H.-J.; Wu, C.-H. Alleviating Effects of Ovatodiolide and Antcin K Supplements on High-Fat Diet-Induced Cardiovascular Dysfunction in ApoE-Knockout Mice by Attenuating Oxidative Stress. Nutrients 2023, 15, 4074. [Google Scholar] [CrossRef]
- Gokila Vani, M.; Kumar, K.; Liao, J.-W.; Chien, S.-C.; Mau, J.-L.; Chiang, S.-S.; Lin, C.-C.; Kuo, Y.-H.; Wang, S.-Y. Antcin C from Antrodia cinnamomea protects liver cells against free radical-induced oxidative stress and apoptosis in vitro and in vivo through Nrf2-dependent mechanism. Evid.-Based Complement. Altern. Med. 2013, 2013, 296082. [Google Scholar]
- Wang, Y.-J.; Lee, S.-C.; Hsu, C.-H.; Kuo, Y.-H.; Yang, C.-C.; Lin, F.-J. Antcins, triterpenoids from Antrodia cinnamomea, as new agonists for peroxisome proliferator-activated receptor α. J. Food Drug Anal. 2019, 27, 295–304. [Google Scholar]
- Kumar, K.S.; Vani, M.G.; Chen, C.-Y.; Hsiao, W.-W.; Li, J.; Lin, Z.-x.; Chu, F.-H.; Yen, G.-C.; Wang, S.-Y. A mechanistic and empirical review of antcins, a new class of phytosterols of formosan fungi origin. J. Food Drug Anal. 2020, 28, 38–59. [Google Scholar]
- Shen, Y.-C.; Wang, Y.-H.; Chou, Y.-C.; Chen, C.-F.; Lin, L.-C.; Chang, T.-T.; Tien, J.-H.; Chou, C.-J. Evaluation of the anti-inflammatory activity of zhankuic acids isolated from the fruiting bodies of Antrodia camphorata. Planta Medica 2004, 70, 310–314. [Google Scholar]
- Wu, S.-H.; Ryvarden, L. Antrodia camphorata (" niu-chang-chih"), new combination of a medicinal fungus in Taiwan. Bot. Bull. -Acad. Sin. Taipei 1997, 38, 273–276. [Google Scholar]
- Jung, Y.-S.; Joe, B.-Y.; Cho, S.J.; Konishi, Y. 2, 3-Dimethoxy-5-methyl-1, 4-benzoquinones and 2-methyl-1, 4-naphthoquinones: Glycation inhibitors with lipid peroxidation activity. Bioorganic Med. Chem. Lett. 2005, 15, 1125–1129. [Google Scholar]
- Chen, J.-J.; Lin, W.-J.; Liao, C.-H.; Shieh, P.-C. Anti-inflammatory benzenoids from Antrodia camphorata. J. Nat. Prod. 2007, 70, 989–992. [Google Scholar]
- Pan, X.; Li, W.; Fang, Y.; Zhang, H.; Xiao, Y.; Molokeev, M.; Ping Jiang, S.; Liu, Y.; Lei, B. Semi-artificial photosynthetic system based on TiO2/Chlorophyll composite and microalgae for N2 fixation. Chem. Eng. J. 2023, 475, 146179. [Google Scholar] [CrossRef]
- Xi, W.; Zhang, G.; Jiang, D.; Zhou, Z. Phenolic compositions and antioxidant activities of grapefruit (Citrus paradisi Macfadyen) varieties cultivated in China. Int. J. Food Sci. Nutr. 2015, 66, 858–866. [Google Scholar]
- Angamuthu, V.; Shanmugavadivu, M.; Nagarajan, G.; Velmurugan, B.K. Pharmacological activities of antroquinonol-Mini review. Chem.-Biol. Interact. 2019, 297, 8–15. [Google Scholar]
- Liang, D.; Zhang, Y.; Wu, Z.; Chen, Y.J.; Yang, X.; Sun, M.; Ni, R.; Bian, J.; Huang, D. A near infrared singlet oxygen probe and its applications in in vivo imaging and measurement of singlet oxygen quenching activity of flavonoids. Sens. Actuators B Chem. 2018, 266, 645–654. [Google Scholar]
- Yang, X.; Huang, D. One-step synthesis of biflavones mediated by peroxynitrite oxidative coupling of flavone monomers. In Proceedings of the Abstracts of Papers of The American Chemical Society; American Chemical Society: Washington, DC, USA, 2019. [Google Scholar]
- Yang, X.; Huang, D.; Du, K.; Toy, Y.H.J. Methods to Synthesize Flavonoid Dimers and Oligomers and the Use Thereof. U.S. Patent 18/256,596, 16 June 2022. [Google Scholar]
- Wang, X.; Cao, Y.; Jing, L.; Chen, S.; Leng, B.; Yang, X.; Wu, Z.; Bian, J.; Banjerdpongchai, R.; Poofery, J. Three-Dimensional RAW264. 7 cell model on electrohydrodynamic printed poly (ε-Caprolactone) scaffolds for in vitro study of anti-inflammatory compounds. ACS Appl. Bio Mater. 2021, 4, 7967–7978. [Google Scholar]
- Alsaiari, S.K.; Eshaghi, B.; Du, B.; Kanelli, M.; Li, G.; Wu, X.; Zhang, L.; Chaddah, M.; Lau, A.; Yang, X. CRISPR–Cas9 delivery strategies for the modulation of immune and non-immune cells. Nat. Rev. Mater. 2025, 10, 44–61. [Google Scholar]
- Wang, X.; Cao, Y.; Chen, S.; Yang, X.; Bian, J.; Huang, D. Antioxidant and anti-inflammatory effects of 6, 3’, 4-and 7, 3, 4-Trihydroxyflavone on 2D and 3D RAW264. 7 Models. Antioxidants 2023, 12, 204. [Google Scholar] [CrossRef]
- Sun, M.; Wang, T.; Yang, X.; Yu, H.; Wang, S.; Huang, D. Facile mitochondria localized fluorescent probe for viscosity detection in living cells. Talanta 2021, 225, 121996. [Google Scholar] [CrossRef]
- Sun, M.; Yang, X.; Zhang, Y.; Wang, S.; Wong, M.W.; Ni, R.; Huang, D. Rapid and Visual detection and quantitation of ethylene released from ripening fruits: The new use of Grubbs catalyst. J. Agric. Food Chem. 2019, 67, 507–513. [Google Scholar]
- Lin, Y.; Yang, X.; Lu, Y.; Liang, D.; Huang, D. Isothiocyanates as H2S donors triggered by cysteine: Reaction mechanism and structure and activity relationship. Org. Lett. 2019, 21, 5977–5980. [Google Scholar] [CrossRef]
- Su, L.; Jing, L.; Zeng, X.; Chen, T.; Liu, H.; Kong, Y.; Wang, X.; Yang, X.; Fu, C.; Sun, J. 3D-Printed prolamin scaffolds for cell-based meat culture. Adv. Mater. 2023, 35, 2207397. [Google Scholar] [CrossRef]
- Yang, H.L.; Lin, K.Y.; Juan, Y.C.; Kumar, K.S.; Way, T.D.; Shen, P.C.; Chen, S.C.; Hseu, Y.C. The anti-cancer activity of Antrodia camphorata against human ovarian carcinoma (SKOV-3) cells via modulation of HER-2/neu signaling pathway. J. Ethnopharmacol. 2013, 148, 254–265. [Google Scholar] [CrossRef]
- Li, T.Y.; Chiang, B.H. 4-Acetylantroquinonol B from Antrodia cinnamomea Enhances Immune Function of Dendritic Cells Against Liver Cancer Stem Cells. Biomedicines 2019, 109, 2262–2269. [Google Scholar] [CrossRef]
- Huang, H.T.; Wang, S.-L.; Nguyen, V.B.; Kuo, Y.-H. Isolation and identification of potent antidiabetic compounds from Antrodia cinnamomea—An edible Taiwanese mushroom. Molecules 2018, 23, 2864. [Google Scholar] [CrossRef]
- Chang, T.C.; Yeh, C.T.; Adebayo, B.O.; Lin, Y.C.; Deng, L.; Rao, Y.K.; Tzeng, Y.M. 4-Acetylantroquinonol B Inhibits Colorectal Cancer Tumorigenesis and Suppresses Cancer Stem-Like Phenotype. Toxicol. Appl. Pharmacol. 2015, 288, 258–268. [Google Scholar] [CrossRef]
- Liu, Y.-M.; Liu, Y.-K.; Wang, L.-W.; Huang, Y.-C.; Huang, P.-I.; Tsai, T.-H.; Chen, Y.-J. The medicinal fungus Antrodia cinnamomea regulates DNA repair and enhances the radiosensitivity of human esophageal cancer cells. OncoTargets Ther. 2016, 9, 6651–6661. [Google Scholar] [CrossRef]
- Liao, Y.-T.; Huang, K.-W.; Chen, W.-J.; Lai, T.-H. A botanical drug extracted from Antrodia cinnamomea: A first-in-human phase I study in healthy volunteers. J. Am. Nutr. Assoc. 2023, 42, 274–284. [Google Scholar]
- Chiou, Y.-L.; Chyau, C.-C.; Li, T.-J.; Kuo, C.-F.; Kang, Y.-Y.; Chen, C.-C.; Ko, W.-S. Hepatoprotective effect of Antrodia cinnamomea mycelium in patients with nonalcoholic steatohepatitis: A randomized, double-blind, placebo-controlled trial. J. Am. Coll. Nutr. 2021, 40, 349–357. [Google Scholar] [CrossRef]
- Chen, C.-C.; Li, I.-C.; Lin, T.-W.; Chang, H.-L.; Lin, W.-H.; Shen, Y.-C. Efficacy and safety of oral Antrodia cinnamomea mycelium in mildly hypertensive adults: A randomized controlled pilot clinical study. Eur. J. Integr. Med. 2016, 8, 654–660. [Google Scholar] [CrossRef]
- Chen, W.-J.; Chang, F.-W. A Pilot Study to Assess Food Safety and Potential Cholesterol-Lowering Efficacy of Antrodia cinnamomea Solid-State Cultivated Mycelium in Healthy Adults. Evid. -Based Complement. Altern. Med. 2020, 2020, 5865764. [Google Scholar] [CrossRef]
- Ho, C.-Y.; Kuan, C.-M.; Hsu, P.-K. Hepatoprotective effect of Antrodia Cinnamomea mycelia extract in subhealth Japanese adults: A randomized, double-blind, placebo-controlled clinical study. J. Diet. Suppl. 2023, 20, 939–949. [Google Scholar] [CrossRef]
- Xu, C.; Yang, X.; Wang, X.; Jing, L.; Zhou, Z.; Cao, Y.; Zheng, H.; Kuo, C.-L.; Huang, D. Authentication of ten distinctive triterpenoids in Antrodia cinnamomea serves as a crucial aspect for ensuring the quality control of associated nutraceutical products. Curr. Res. Food Sci. 2024, 8, 100721. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).