Exploring the Composition of Blueberry-Based Functional Products: Polyphenolic and Elemental Characterization and Quantification
Abstract
1. Introduction
2. Materials and Methods
- Resveratrol (3,4,4′-triidrossi-trans-stilbene; Karlsruhe, Carl Roth GmbH + Co. KG, Mühlburg, Germany);
- Piceatannol (3,3′,4,5′-Tetrahydroxy-trans-stilbene; MCE, MedChemExpress, Monmouth Junction, NJ, USA);
- Astringin (3,3′,4,5′-Tetrahydroxy stilbene 3′-glucoside; MCE, MedChemExpress, Monmouth Junction, NJ, USA);
- Catechin ((+)—Cyanidanol, D-Catechin; MCE, MedChemExpress, Monmouth Junction, NJ, USA);
- Epicatechin ((−) Epicatechin, cis-D-Catechin; MCE, MedChemExpress, Monmouth Junction, NJ, USA);
- Rutin (Quercetin 3-rhamnoglucoside; Karlsruhe, Carl Roth GmbH + Co. KG, Mühlburg, Germany);
- Isoquercitrin (Quercetin 3-β-D-glucoside; Extrasynthese, Genay, France);
- Quercitrin (Quercetin 3-rhamnoside; Karlsruhe, Carl Roth GmbH + Co. KG, Mühlburg, Germany);
- Quercetin (Quercetin hydrate; Karlsruhe, Carl Roth GmbH + Co. KG, Mühlburg, Germany);
- Kampferol (3,4′,5,7-Tetrahydroxy flavone; MCE, MedChemExpress, Monmouth Junction, NJ, USA);
- Hesperetin (3′,5,7-Trihydroxy-4′-methoxy flavanone; Extrasynthese, Genay, France);
- Luteolin (3′,4′,5,7-Tetrahydrox flavone; Karlsruhe, Carl Roth GmbH + Co. KG, Mühlburg, Germany);
- Multi-element standard solution for ICP-MS calibration (1.000 ± 0.005 mg/L As, Al, Ba, Be, Bi, Cd, Ce, Co, Cr, Cs, Cu, Ga, La, Li, Mn, Mo, Nb, Ni, Pb, Rb, Sb, Se, Sn, Te, Ti, Tl, U, V, W and Zr; 10.00 ± 0.05 mg/L Fe and Zn; 50.00 ± 0.25 mg/L P and Si; 55.00 ± 0.25 mg/L B and Sr; 500.0 ± 2.5 mg/L K, Mg and Na; 1000 ± 5 mg/L Ca and S in 5% HNO3, VWR International S.r.l., Milan, Italy);
- Single standard solution for ICP-MS internal standards (1000 ± 2 mg/L Y, Panreac Química, Barcelona, Spain; 1000 ± 5 mg/L Sc, Rh, In and Th, Merck KGaA, Darmstadt, Germany) to monitor matrix effects and sensitivity drifts;
- Multi-standard stock solution of Ba, Be, Ce, Co, In, Pb, Mg, Tl and Th (10.00 ± 0.05 mg/L, Spectro Pure, Ricca Chemical Company, Arlington, TX, USA) for testing ICP-MS performance;
- NIST 1643f trace elements in water (National Institute of Standards and Technology, NIST; Gaithersburg, MD, USA) were used to assess the elemental analysis accuracy.
- Nitric acid (HNO3, 70%, super-pure) (Carlo Erba Reagents S.r.l., Milal, Italy);
- Methanol (CH3OH, MeOH) (Carlo Erba Reagents, Milan, Italy);
- Acetonitrile (AcN) (Romil-UpSTM Ultra Purity Solvents, London, UK);
- Formic acid (HCOOH) (Carlo Erba Reagents, Milan, Italy);
- Water Milli-Q (Millipore Corporation, Burlington, MA, USA);
- Ethanol (CH3CH2OH, EtOH) (Carlo Erba Reagents, Milan, Italy);
- Sodium nitrite (NaNO2) (Carlo Erba Reagents, Milan, Italy);
- Aluminum trichloride (AlCl3) (Carlo Erba Reagents, Milan, Italy);
- Sodium hydroxide (NaOH) (Carlo Erba Reagents, Milan, Italy);
- Sodium carbonate (Na2CO3) (Sigma-Aldrich Co., Saint Louis, MO, USA);
- Folin–Ciocâlteau reagent (Sigma-Aldrich Co., Saint Louis, MO, USA);
- Sodium persulfate (NaS2O8) (Carlo Erba Reagents, Milan, Italy);
- Sodium hydrogen phosphate (Na2HPO4) (Sigma-Aldrich Co., Saint Louis, MO, USA);
- 2,2′-azino-bis (3-eylbenzothiazoline-6 sulfonic acid (ABTS) (Sigma-Aldrich Co., Saint Louis, MO, USA);
- 2,2-Dipheny/L-picrylhydrazyl (DPPH) (Sigma-Aldrich Co., Saint Louis, MO, USA).
2.1. Samples
2.2. Sample Pre-Treatment
2.2.1. Fresh Blueberries
2.2.2. Dried Blueberries
2.2.3. Supplement
2.2.4. Herbal Tea
2.3. Total Phenolics Content (TPC)
2.4. Total Flavonoids Content (TFC)
2.5. Total Antioxidant Capacity
2.5.1. DPPH Radical Scavenging Activity
2.5.2. ABTS Radical Cation Decolorization Activity
2.6. Analysis of the Extracted Elements
2.7. Analysis of Polyphenols
2.8. Quality Assurance
2.9. Calculation
2.9.1. Polyphenol/Flavonoid Daily Intake
2.9.2. Estimated Daily Intake of Elements
2.9.3. Non-Carcinogenic Risk
2.9.4. Carcinogenic Risk
3. Results
3.1. Optimized Operating Conditions in HPLC-ESI-MS/MS
3.2. Validation Parameters
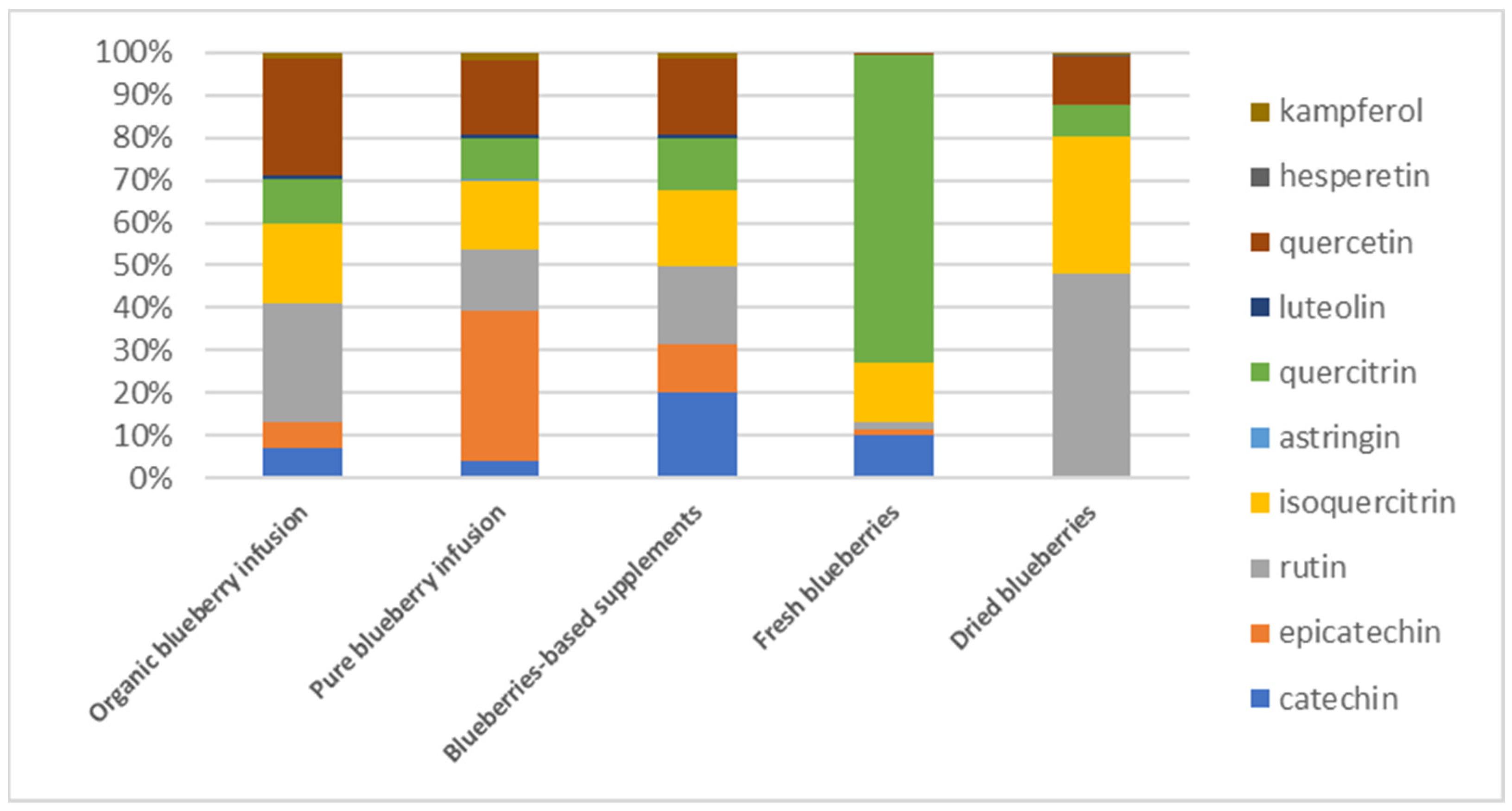
3.3. Polyphenolic Profile in Blueberry-Based Samples
3.4. TPC and TFC Test Results
3.5. DPPH and ABTS Method Results
3.6. Intake Based on Nutraceutical Content of Blueberry-Based Samples
3.7. Element Levels
3.8. Health Risk Assessment
3.8.1. Estimated Daily Intake (EDI) of Elements in Blueberry-Based Samples
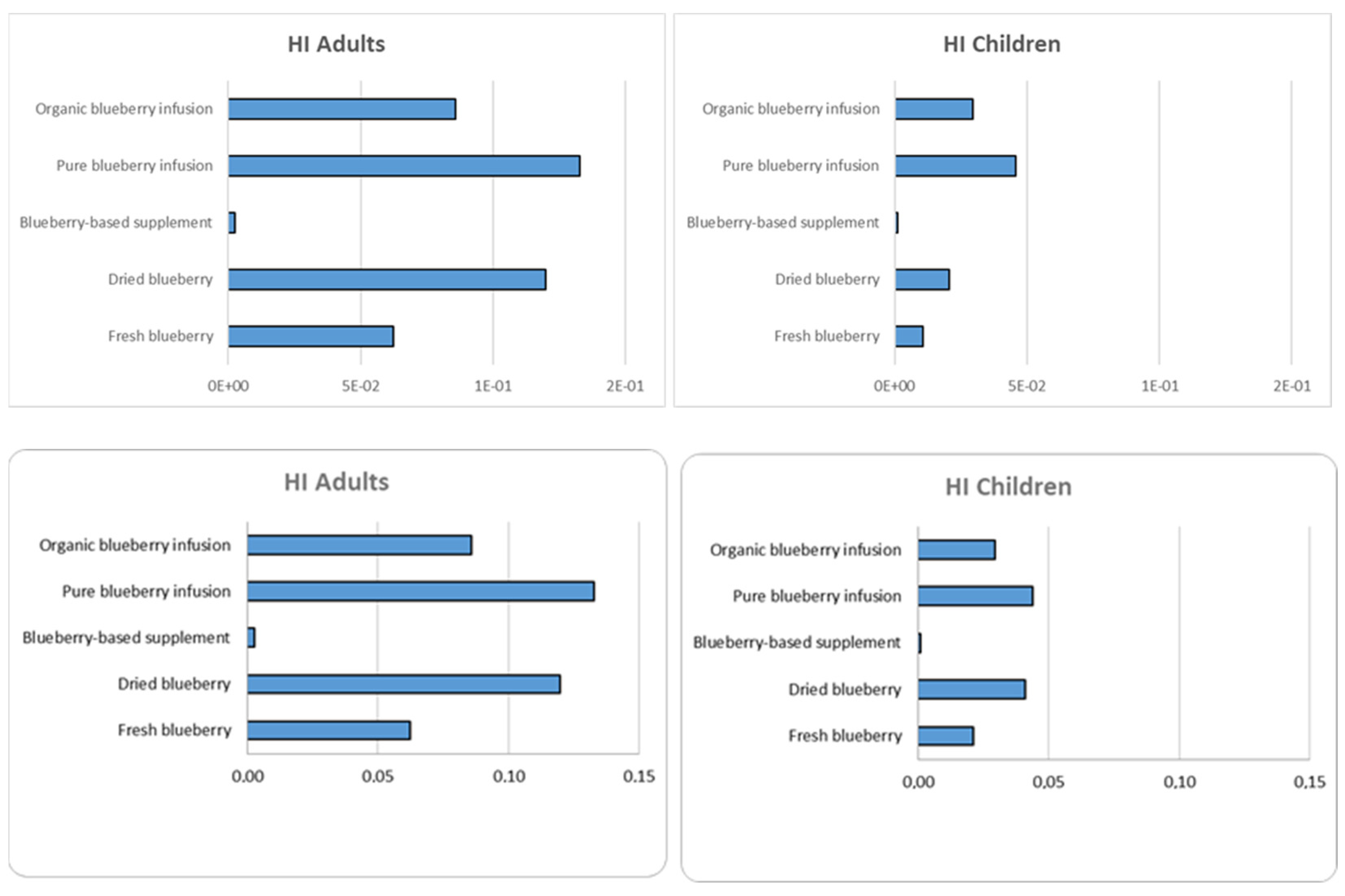
3.8.2. Non-Carcinogenic Risk
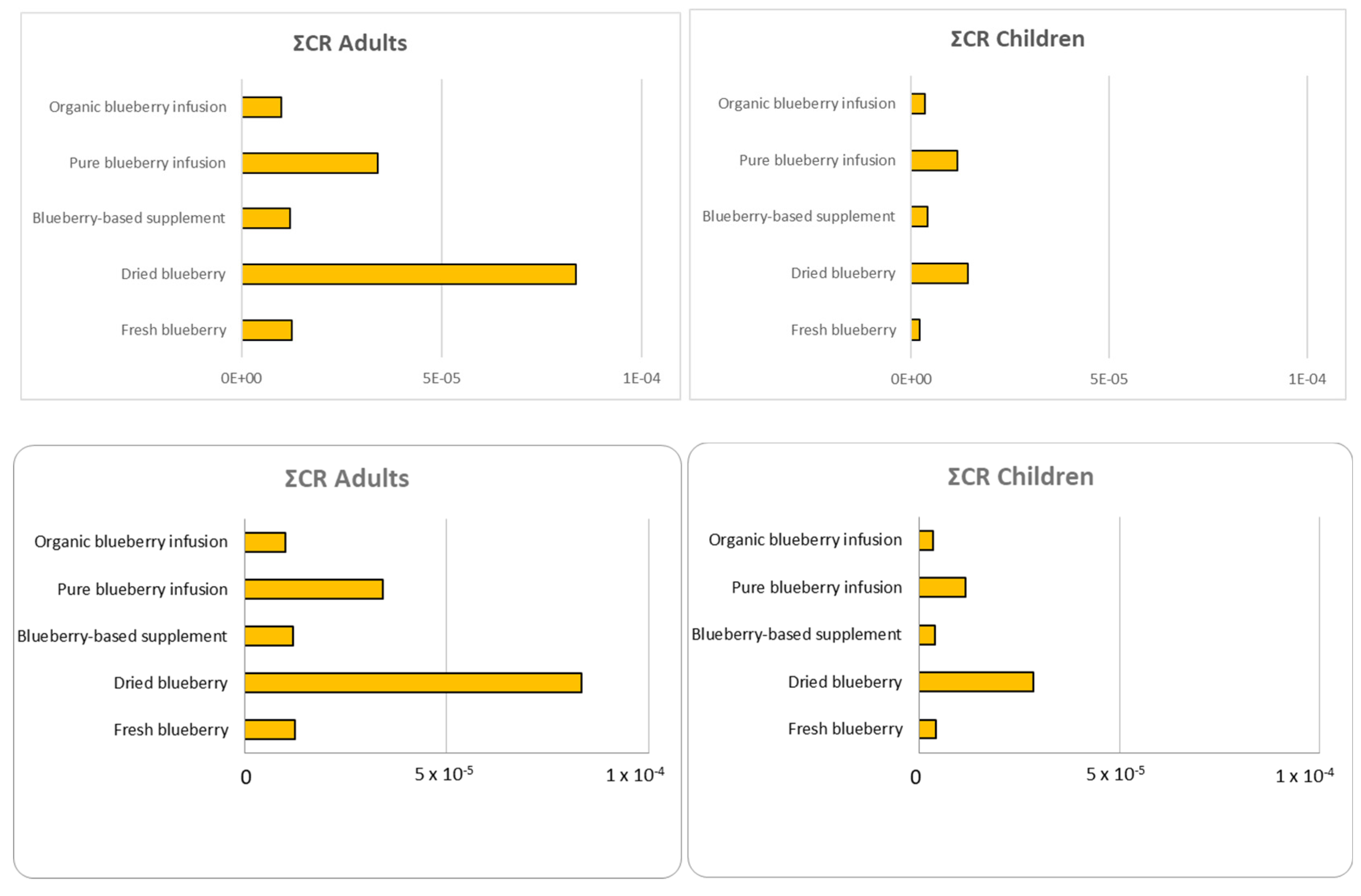
3.8.3. Carcinogenic Risk
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Template, J.N. A rational definition for functional food: A perspective. Front. Nutr. 2022, 9, 957516. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.; Silva, A.M. Plant flavonoids: Chemical characteristics and biological activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Zhang, H.; Tsao, R. Dietary polyphenols. oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Steele, C.M.; Lalies, M.; Ioannides, C. Inhibition of the mutagenicity of aromatic amines by the plant flavonoid (+)-catechin. Cancer Res. 1985, 45, 3573–3577. [Google Scholar]
- Francis, A.R.; Shetty, T.K.; Bhattacharya, R.K. Modifying role of dietary factors on the mutagenicity of aflatoxin B1: In vitro effect of plant flavonoids. Mutat. Res./Genet. Toxicol. 1989, 222, 393–401. [Google Scholar] [CrossRef]
- Das, A.; Wang, J.H.; Lien, E.J. Carcinogenicity, mutagenicity and cancer preventing activities of flavonoids: A structure-system-activity relationship (SSAR) analysis. Prog. Drug Res. 1994, 42, 133–166. [Google Scholar] [CrossRef] [PubMed]
- Romagnolo, D.F.; Selmin, O.I. Flavonoids and cancer prevention: A review of the evidence. J. Nutr. Gerontol. Geriatr. 2012, 31, 206–238. [Google Scholar] [CrossRef]
- Batra, P.; Sharma, A.K. Anti-cancer potential of flavonoids: Recent trends and future perspectives. 3 Biotech 2013, 3, 439–459. [Google Scholar] [CrossRef]
- Ren, W.; Qiao, Z.; Wang, H.; Zhu, L.; Zhang, L. Flavonoids: Promising anticancer agents. Med. Res. Rev. 2003, 23, 519–534. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Cushnie, T.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.H.; Jiang, Y.M.; Shi, J.; Tomas-Barberan, F.A.; Datta, N.; Singanusong, R.; Chen, S.S. Flavonoids in food and their health benefits. Plant Foods Hum. Nutr. 2004, 59, 113–122. [Google Scholar] [CrossRef]
- Costa, A.S.G.; Nunes, M.A.; Almeida, I.M.C.; Carvalho, M.R.; Barroso, M.F.; Alves, R.C.; Oliveira, M.B. Teas, dietary supplements and fruit juices: A comparative study regarding antioxidant activity and bioactive compounds. LWT Food Sci. Technol. 2012, 49, 324–328. [Google Scholar] [CrossRef]
- Wang, S.P.; Huang, K.J. Determination of flavonoids by high-performance liquid chromatography and capillary electrophoresis. J. Chromatogr. A 2004, 1032, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Feng, J.; Huang, W.Y.; An, X.T. Composition of polyphenols and antioxidant activity of rabbiteye blueberry (Vaccinium ashei) in Nanjing. J. Agric. Food Chem. 2013, 61, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Vrhovsek, U.; Masuero, D.; Palmieri, L.; Mattivi, F. Identification and quantification of flavonol glycosides in cultivated blueberry cultivars. J. Food Compos. Anal. 2012, 25, 9–16. [Google Scholar] [CrossRef]
- Stull, A.J.; Cassidy, A.; Djousse, L.; Johnson, S.A.; Krikorian, R.; Lampe, J.W.; Tangney, C. The state of the science on the health benefits of blueberries: A perspective. Front. Nutr. 2024, 11, 1415737. [Google Scholar] [CrossRef]
- Ignat, I.; Volf, I.; Popa, V.I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef]
- Li, D.; Li, B.; Ma, Y.; Sun, X.; Lin, Y.; Meng, X. Polyphenols. anthocyanins. and flavonoids contents and the antioxidant capacity of various cultivars of highbush and half-high blueberries. J. Food Compos. Anal. 2017, 62, 84–93. [Google Scholar] [CrossRef]
- Wang, T.; Guo, N.; Wang, S.X.; Kou, P.; Zhao, C.J.; Fu, Y.J. Ultrasound-negative pressure cavitation extraction of phenolic compounds from blueberry leaves and evaluation of its DPPH radical scavenging activity. Food Bioprod. Process. 2018, 108, 69–80. [Google Scholar] [CrossRef]
- Huang, W.Y.; Zhang, H.C.; Liu, W.X.; Li, C.Y. Survey of antioxidant capacity and phenolic composition of blueberry, blackberry, and strawberry in Nanjing. J. Zhejiang Univ. Sci. B 2012, 13, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Shannon, E.; Jaiswal, A.K.; Abu-Ghannam, N. Polyphenolic content and antioxidant capacity of white, green, black, and herbal teas: A kinetic study. Food Res. 2017, 2, 1–11. [Google Scholar] [CrossRef]
- Rodrigues, E.; Poerner, N.; Rockenbach, I.I.; Gonzaga, L.V.; Mendes, C.R.; Fett, R. Phenolic compounds and antioxidant activity of blueberry cultivars grown in Brazil. Food Sci. Technol. 2011, 31, 911–917. [Google Scholar] [CrossRef]
- Oh, J.; Jo, H.; Cho, A.R.; Kim, S.J.; Han, J. Antioxidant and antimicrobial activities of various leafy herbal teas. Food Control 2013, 31, 403–409. [Google Scholar] [CrossRef]
- Astolfi, M.L.; Marconi, E.; Protano, C.; Canepari, S. Comparative elemental analysis of dairy milk and plant-based milk alternatives. Food Control 2020, 116, 107327. [Google Scholar] [CrossRef]
- De La Cruz, L.N.P.; Giorgione, R.; Marini, F.; Astolfi, M.L. Rice sample preparation method for ICP-MS and CV-AFS analysis: Elemental levels and estimated intakes. Food Chem. 2024, 461, 140831. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Chee Kong, Y.; Mohd Zain, N. A Effect of cadmium and copper exposure on growth, secondary metabolites and antioxidant activity in the medicinal plant Sambung Nyawa (Gynura procumbens (Lour.) Merr). Molecules 2017, 22, 1623. [Google Scholar] [CrossRef] [PubMed]
- Einolghozati, M.; Talebi-Ghane, E.; Khazaei, M.; Mehri, F. The level of heavy metal in fresh and processed fruits: A study meta-analysis, systematic review, and health risk assessment. Biol. Trace Elem. Res. 2023, 201, 2582–2596. [Google Scholar] [CrossRef]
- Simonetti, G.; Buiarelli, F.; Bernardini, F.; Di Filippo, P.; Riccardi, C.; Pomata, D. Profile of free and conjugated quercetin content in different Italian wines. Food Chem. 2022, 382, 132377. [Google Scholar] [CrossRef]
- European Parliament. Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002 on the approximation of the laws of the Member States relating to food supplements. Off. J. Eur. Communities 2002, 45, 51–57. Available online: http://data.europa.eu/eli/dir/2002/46/oj (accessed on 1 January 2025).
- Abdullah, S.S.S.; Mazlan, A.N. Quantification of polyphenols and antioxidant activity in several herbal and green tea products in Malaysia. Mater. Today Proc. 2020, 31, A106–A113. [Google Scholar] [CrossRef]
- Chan, E.W.C.; Lye, P.Y.; Tan, L.N. Analysis and evaluation of antioxidant properties of Thai herbal teas. Int. J. Adv. Sci. Arts 2011, 2, 8–15. [Google Scholar]
- Astolfi, M.L.; Marconi, E.; Vitiello, G.; Massimi, L. An optimized method for sample preparation and elemental analysis of extra-virgin olive oil by inductively coupled plasma mass spectrometry. Food Chem. 2021, 360, 130027. [Google Scholar] [CrossRef]
- Curtis, P.J.; Van Der Velpen, V.; Berends, L.; Jennings, A.; Feelisch, M.; Umpleby, A.M.; Cassidy, A. Blueberries improve biomarkers of cardiometabolic function in participants with metabolic syndrome—Results from a 6-month. double-blind, randomized controlled trial. Am. J. Clin. Nutr. 2019, 109, 1535–1545. [Google Scholar] [CrossRef]
- Patrick-Iwuanyanwu, K.C.; Udowelle, N.A. Monitoring of essential and toxic metals in imported herbal teas marketed in selected cities in Southern Nigeria: A health risk assessment study. J. Appl. Sci. Environ. Manag. 2017, 21, 1189–1196. [Google Scholar] [CrossRef][Green Version]
- Taiwo, A.M.; Olowookere, Z.A.; Bada, B.S.; Akinhanmi, T.F.; Oyedepo, J.A. Contamination and health risk assessments of metals in selected fruits from Abeokuta. Southwestern Nigeria. J. Food Compos. Anal. 2022, 114, 104801. [Google Scholar] [CrossRef]
- Vuong, Q.V.; Pham, H.N.T.; Negus, C. From herbal tea bag to infusion—Impact of brewing on polyphenols and antioxidant capacity. Beverages 2022, 8, 81. [Google Scholar] [CrossRef]
- Mustafa, A.M.; Angeloni, S.; Abouelenein, D.; Acquaticci, L.; Xiao, J.; Sagratini, G.; Caprioli, G. A new HPLC-MS/MS method for the simultaneous determination of 36 polyphenols in blueberry. strawberry and their commercial products and determination of antioxidant activity. Food Chem. 2022, 367, 130743. [Google Scholar] [CrossRef]
- Giovanelli, G.; Buratti, S. Comparison of polyphenolic composition and antioxidant activity of wild Italian blueberries and some cultivated varieties. Food Chem. 2009, 112, 903–908. [Google Scholar] [CrossRef]
- Peluso, I.; Palmery, M. Flavonoids at the pharma-nutrition interface: Is a therapeutic index in demand? Biomed. Pharmacother. 2015, 71, 102–107. [Google Scholar] [CrossRef]
- Gibson, L.; Rupasinghe, H.V.; Forney, C.F.; Eaton, L. Characterization of changes in polyphenols, antioxidant capacity and physico-chemical parameters during lowbush blueberry fruit ripening. Antioxidants 2013, 2, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Codex Alimentarius Commission. Codex Alimentarius: Food Hygiene—Basic Texts. Joint FAO/WHO Food Standards Programme. World Health Organization. 2001. Available online: https://www.fao.org/fao-who-codexalimentarius/codex-texts/list-standards/en/ (accessed on 1 January 2025).
- Codex Alimentarius Commission. Codex Alimentarius: Food Hygiene—Basic Texts. Joint FAO/WHO Food Standards Programme. World Health Organization. 1991. Available online: https://www.fao.org/fao-who-codexalimentarius/codex-texts/list-standards/en/ (accessed on 1 January 2025).
- European Commission (EC). Commission regulation (EU) 2023/915 of 25 April 2023 on maximum levels for certain contaminants in food and repealing regulation (EC) No 1881/2006. Off. J. Eur. Union 2023, L119, 103–157. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32023R0915 (accessed on 1 January 2025).
- Jairoun, A.A.; Shahwan, M.; Zyoud, S.H. Heavy metal contamination of dietary supplements products available in the UAE markets and the associated risk. Sci. Rep. 2020, 10, 18824. [Google Scholar] [CrossRef]

| Samples | Type | Composition |
|---|---|---|
| Blueberries | Fresh fruit | Fresh blueberries |
| Blueberries | Dried fruit | Dried blueberries |
| Blueberry-based supplements | Capsule | A 200 mg capsule of dry blueberry extract (Vaccinium myrtillus L.) |
| Herbal teas | Organic blueberry infusion | Rose hip, blueberry berries and leaves 20%, karcadé, elderberries |
| Pure blueberry infusion | Blueberry leaves (Vaccinium myrtillus L.) 50% and blueberry berries (Vaccinium myrtillus L.) 50% |
| Compound | Molecular Weight (g/mol) | Precursor Ion [M-H]− (m/z) | Product Ions (m/z) * |
|---|---|---|---|
| Catechin | 290 | 289 | 109, 125 |
| Epicatechin | 290 | 289 | 109, 123 |
| Astringin | 406 | 405 | 243, 201 |
| Rutin | 610 | 609 | 300, 271 |
| Isoquercitrin | 464 | 463 | 300, 271 |
| Piceatannol | 244 | 243 | 201, 159 |
| Quercitrin | 448 | 447 | 300, 270 |
| Resveratrol | 228 | 227 | 143, 185 |
| Luteolin | 286 | 285 | 133, 151 |
| Quercetin | 302 | 301 | 151, 107 |
| Hesperetin | 302 | 301 | 164, 151 |
| Kaempferol | 286 | 285 | 93, 159 |
| Compound | CUR (psi) | CAD (psi) | IS-V (V) | DP (V) | EP (V) | FP (V) | CE (eV) | CXP (V) |
|---|---|---|---|---|---|---|---|---|
| Catechin | 20 | 3 | −4200 | −20 | −10 | −300 | −30 | −15 |
| Epicatechin | 20 | 3 | −4200 | −20 | −10 | −350 | −30 | −15 |
| Astringin | 30 | 2 | −4500 | −20 | −10 | −330 | −25 | −10 |
| Rutin | 20 | 2 | −4300 | −20 | −10 | −330 | −48 | −10 |
| Isoquercitrin | 20 | 2 | −4300 | −20 | −10 | −320 | −35 | −10 |
| Piceatannol | 20 | 2 | −4000 | −20 | −10 | −300 | −32 | −15 |
| Quercitrin | 20 | 2 | −4300 | −20 | −10 | −330 | −35 | −15 |
| Resveratrol | 20 | 5 | −4200 | −25 | −10 | −330 | −33 | −10 |
| Luteolin | 25 | 2 | −4500 | −20 | −10 | −320 | −40 | −15 |
| Quercetin | 20 | 2 | −4500 | −20 | −10 | −300 | −30 | −15 |
| Hesperetin | 25 | 2 | −4300 | −20 | −10 | −350 | −35 | −15 |
| Kaempferol | 20 | 2 | −4500 | −20 | −10 | −330 | −48 | −15 |
| Polyphenols | Calibration Curve Equations | R2 |
|---|---|---|
| Catechin | y = 14,500x − 1807.4 | 0.9981 |
| Epicatechin | y = 15,760x − 1581.7 | 0.9991 |
| Astringin | y = 154,019x + 11,410 | 0.9978 |
| Rutin | y = 52,459x – 15,139 | 0.9964 |
| Isoquercitrin | y = 62,777x – 18,383 | 0.9989 |
| Piceatannol | y = 14,018x − 1692.2 | 0.9985 |
| Quercitrin | y = 50,154x − 12,299 | 0.9954 |
| Resveratrol | y = 11,826x − 118.15 | 0.9997 |
| Luteolin | y = 54,169x – 14,876 | 0.9978 |
| Quercetin | y = 16,767x + 2160.7 | 0.9972 |
| Hesperetin | y = 40,797x + 1938.3 | 0.9986 |
| Kaempferol | y = 8145.3x − 2010.4 | 0.9996 |
| Polyphenols | LOD (μg/mL) | LOQ (μg/mL) | Intra-Day Repeatability (RSD) | Inter-Day Repeatability (RSD) |
|---|---|---|---|---|
| Catechin | 0.08 | 0.15 | 1 | 2 |
| Epicatechin | 0.08 | 0.15 | 1 | 2 |
| Astringin | 0.04 | 0.08 | 3 | 4 |
| Rutin | 0.08 | 0.15 | 2 | 10 |
| Isoquercitrin | 0.15 | 0.31 | 3 | 7 |
| Piceatannol | 0.31 | 0.62 | 5 | 10 |
| Quercitrin | 0.08 | 0.15 | 1 | 2 |
| Resveratrol | 0.08 | 0.15 | 3 | 2 |
| Luteolin | 0.08 | 0.15 | 2 | 3 |
| Quercetin | 0.04 | 0.08 | 1 | 4 |
| Hesperetin | 0.02 | 0.04 | 5 | 8 |
| Kaempferol | 0.31 | 0.62 | 2 | 4 |
| Organic Blueberry Infusion | Pure Blueberry Infusion | Blueberry-Based Supplement | Dried Blueberries | Fresh Blueberries | |
|---|---|---|---|---|---|
| Σ flavonoids (mg/g) | 8.020 ± 0.010 | 6.310 ± 0.008 | 3.570 ± 0.010 | 1.060 ± 0.002 | 0.420 ± 0.002 |
| Samples | Type | TPC | TFC |
|---|---|---|---|
| mg GAE/g sample | |||
| Blueberries | Fresh fruit | 0.9 ± 0.1 | 0.40 ± 0.06 |
| Blueberries | Dried fruit | 1.2 ± 0.2 | 0.9 ± 1.1 |
| Blueberry-based supplements | Capsule | 5.8 ± 0.8 | 3.0 ± 1.3 |
| Herbal teas | Organic blueberry infusion | 37.1 ± 1.1 | 13.4 ± 1.3 |
| Pure blueberry infusion | 31.4 ± 2.4 | 12.2 ± 1.9 | |
| Samples | Type | DPPH mg Trolox/g sample | ABTS | DPPH | ABTS |
|---|---|---|---|---|---|
| % Reduction | |||||
| Blueberries | Fresh fruit | 0.70 ± 0.01 | 0.7 | 35.8 | 55.2 |
| Blueberries | Dried fruit | 0.80 ± 0.02 | 0.7 | 33.5 | 46.9 |
| Blueberry-based supplements | Capsule | 7.410 ± 0.002 | 7.0 | 34.2 | 54.1 |
| Herbal teas | Organic blueberry infusion | 10.3 ± 0.1 | 11.7 | 41.9 | 70.1 |
| Pure blueberry infusion | 6.3 ± 1.2 | 11.6 | 46.1 | 68.8 | |
| Samples | Flavonoids Daily Intake (mg/day) | Polyphenols Daily Intake (mg/day) | Average Daily Intake of Each Sample |
|---|---|---|---|
| Fresh blueberries | 22–45 | 67–135 | 75–150 g/day |
| Dried blueberries | 52–105 | 90–180 | |
| Blueberry-based supplements | 2.7 | 5.2 | 0.9 g/day |
| Organic blueberry infusion | 94 | 260 | 7 g/day (one tea bag/day) |
| Pure blueberry infusion | 86 | 220 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buiarelli, F.; Presutti, M.; Astolfi, M.L.; Riccardi, C.; Pomata, D.; Fricano, A.; Simonetti, G.; Di Filippo, P. Exploring the Composition of Blueberry-Based Functional Products: Polyphenolic and Elemental Characterization and Quantification. Foods 2025, 14, 1210. https://doi.org/10.3390/foods14071210
Buiarelli F, Presutti M, Astolfi ML, Riccardi C, Pomata D, Fricano A, Simonetti G, Di Filippo P. Exploring the Composition of Blueberry-Based Functional Products: Polyphenolic and Elemental Characterization and Quantification. Foods. 2025; 14(7):1210. https://doi.org/10.3390/foods14071210
Chicago/Turabian StyleBuiarelli, Francesca, Maria Presutti, Maria Luisa Astolfi, Carmela Riccardi, Donatella Pomata, Andrea Fricano, Giulia Simonetti, and Patrizia Di Filippo. 2025. "Exploring the Composition of Blueberry-Based Functional Products: Polyphenolic and Elemental Characterization and Quantification" Foods 14, no. 7: 1210. https://doi.org/10.3390/foods14071210
APA StyleBuiarelli, F., Presutti, M., Astolfi, M. L., Riccardi, C., Pomata, D., Fricano, A., Simonetti, G., & Di Filippo, P. (2025). Exploring the Composition of Blueberry-Based Functional Products: Polyphenolic and Elemental Characterization and Quantification. Foods, 14(7), 1210. https://doi.org/10.3390/foods14071210









